Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Congenital pituitary hormone deficiencies can be caused by maldevelopment of central brain structures, acute injury, and pituitary gene mutation.
The anterior pituitary is composed of four distinct cell types that produce five different hormones.
Understanding the role of each of these hormones allows more timely diagnosis of pituitary hormone deficiencies.
Treatment is typically replacement of missing hormones and can be safely accomplished, allowing children to grow and develop properly.
The diagnosis of pituitary hormone deficiency or congenital hypopituitarism in the neonate can often be a challenge to the provider. Patients may present either extremely ill in an intensive care setting or apparently healthy in the newborn nursery or pediatric office without apparent signs or symptoms suggesting deficiency. The provider, therefore, requires adequate knowledge of the multiple roles of the pituitary, symptoms of pituitary hormone deficiency, and/or select phenotypes that may suggest a risk for pituitary abnormalities.
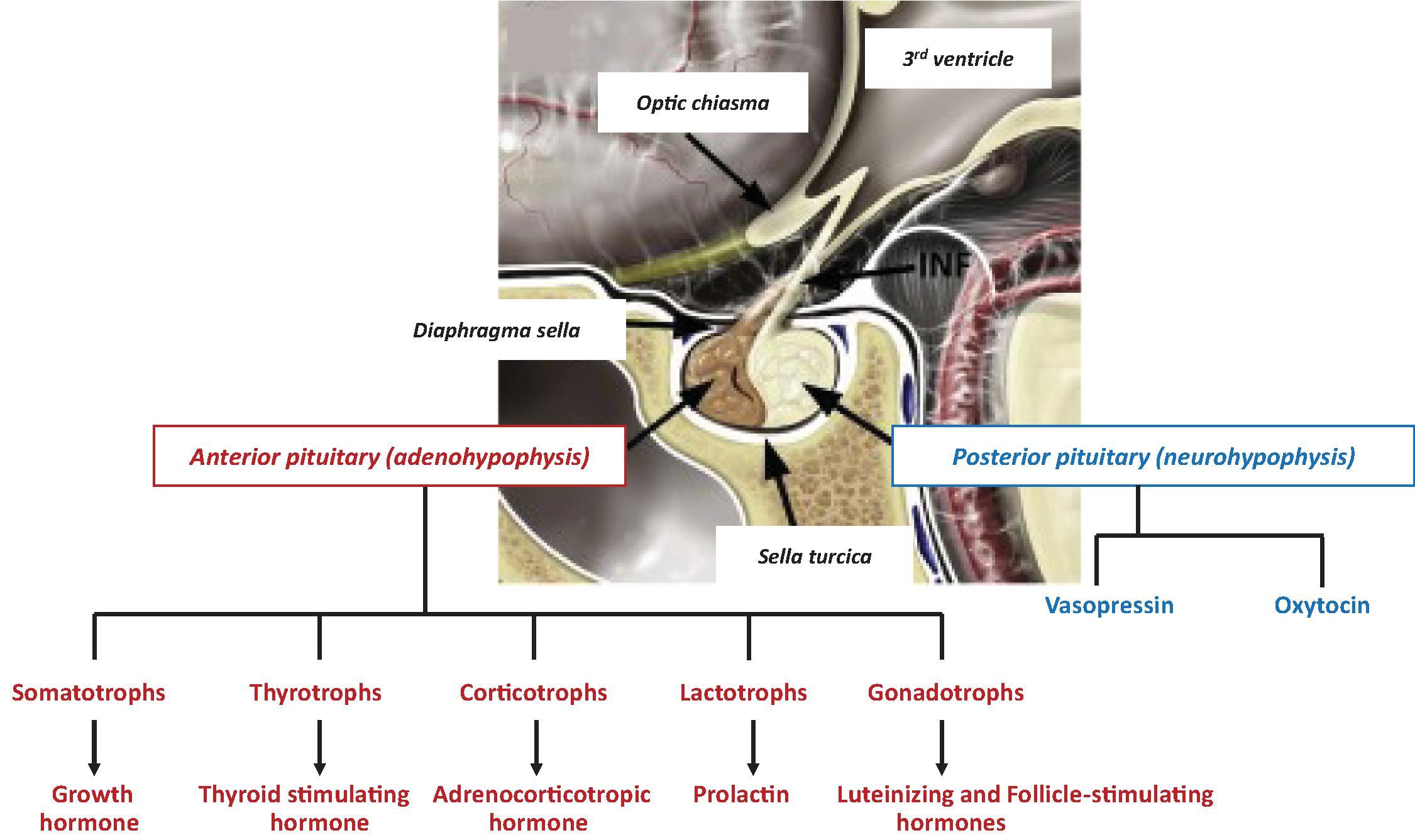
The development of the pituitary gland is a complex process that is achieved through an orchestrated expression of transcription factors and signaling molecules. The pituitary gland is composed of an anterior lobe (adenohypophysis) and a posterior lobe (neurohypophysis) ( Fig. 25.1 ). The adenohypophysis contains five distinct cell types that produce six different hormones. These include somatotrophs, which produce growth hormone (GH); thyrotrophs, which produce thyroid stimulating hormone (TSH); lactotrophs, which produce prolactin; corticotrophs, which produce adrenocorticotrophic hormone (ACTH); and gonadotrophs, which produce both luteinizing hormone (LH) and follicle-stimulating hormone (FSH). The neurohypophysis does not contain hormone-producing cells but instead is a reservoir for both antidiuretic hormone and oxytocin. These hormones are produced in cells in the paraventricular and supraoptic nuclei of the hypothalamus; they are then transported to the posterior pituitary within the axons of these cells, which form the posterior pituitary.
The true incidence of congenital hypopituitarism is not entirely clear, but reports have cited estimates of 1 in 3500 to 1 in 10,000 or even less in patients diagnosed with a single or multiple hormone deficiencies. , Congenital hypopituitarism may occur as an isolated disorder, although in some cases, pituitary disease can occur as part of a syndrome. In addition, breech delivery or perinatal insult may be associated with idiopathic hypopituitarism. , Once one or more deficiencies are established, hormone replacement is typically successful in bringing levels to normal and providing a means for continuation of normal growth and development.
This chapter will focus on reviewing the presenting signs and symptoms of pituitary hormone deficiency, diagnostic tools used to assess deficiencies, and the treatment required. Given the complexity of pituitary disease and the fact that single or multiple hormone deficiencies can occur, we will present each hormone deficiency individually for clarity. The provider must understand, however, that an evaluation of the pituitary should involve diagnostic measures to assess the function of as many of the cell types as possible simultaneously in order to guide the provider with appropriate treatments. This also helps with counseling the parents. By helping the provider to better understand each deficiency, early recognition and an appropriate diagnosis will expedite treatment that can ultimately prevent comorbidities and mortality associated with congenital hypopituitarism.
Congenital pituitary disease, whether it originates from a structural abnormality, an isolated hormone deficiency, or both, often has a genetic basis. Alternatively, perinatal stress such as ischemia could presumably injure the pituitary or impact its development without an underlying genetic abnormality. Despite advances in genetic screening, however, identifying a precise etiology of pituitary disease remains a challenge. The identification of specific genetic mutations leading to hypopituitarism continues to be a rare occurrence, with reports citing less than 5% of cases. Nevertheless, our understanding of pituitary development has expanded tremendously over the past several decades. We now recognize that pituitary development is a complex, temporally regulated cascade of events orchestrated through the expression of various transcription factors and signaling molecules.
Given the complexity of pituitary development and the numerous genes associated with pituitary hormone deficiency, this chapter will not discuss the topic in detail. The clinician should be aware, however, that improvement in genetic technology has enabled easier access and affordable options for studies such as whole exome sequencing. , Some research centers have also developed panels to screen for various genetic mutations involved in pituitary development and function. Currently, however, there are limited resources for pituitary genetic screening to be done commercially.
Various syndromes have been associated with a risk for pituitary hormone deficiency. In addition, given the location of the pituitary gland, an infant noted to have midline defects such as cleft lip, cleft palate, and holoprosencephaly should be considered for evaluation for pituitary hormone deficiencies. Abnormal pituitary magnetic resonance imaging (MRI) findings, such as seen in pituitary stalk interruption syndrome, should alert the provider to screen for pituitary dysfunction. In cases of isolated GH deficiency (GHD), some have commented on the utility of pituitary MRI findings.
One particular phenotype the clinician should recognize is septo-optic dysplasia. A diagnosis is made by identifying two of the following: pituitary hypoplasia/hormone deficiency, midline forebrain defects (e.g., absence of the septum pellucidum), and optic nerve hypoplasia. A clinical clue to optic nerve hypoplasia is an infant who presents with nystagmus along with symptoms suggesting pituitary hormone deficiency. This condition may also present with other neurologic manifestations such as schizencephaly, which may be a risk for the development of seizures. Although mutations in several transcription factors known to be important in pituitary development have been implicated as the etiology, such as HESX1 , the identification of a genetic mutation is rare. , Table 25.1 summarizes several possible clinical syndromes or phenotypes that may be diagnosed in the newborn period and have been associated with pituitary disease. Although a genetic etiology is not always identified nor is pituitary dysfunction diagnosed, the clinician should have high suspicion for possible pituitary hormone deficiency in these patients.
| Diagnosis | Gene(s) Defect | Clinical Description |
|---|---|---|
| Axenfeld-Rieger syndrome | PTX2 | Ocular anterior compartment abnormalities, craniofacial abnormalities, cardiac defects, variable anterior pituitary hormone deficiencies |
| Central hypothyroidism/macroorchidism | IGSF-1 | X-linked syndrome of central hypothyroidism delayed puberty despite testicular enlargement, GH, and PRL deficiencies |
| CHARGE syndrome | CHD7 | Eye coloboma, heart defects, choanal atresia, retardation of growth, hypogonadism, ear abnormalities, may also be MPHD |
| DAVID (deficient anterior pituitary with variable immune deficiency) | NFKB2 | Anterior pituitary hormone deficiency and common variable immunodeficiency—hypogammaglobulinemia, ACTH deficiency is less common |
| Eye development abnormalities (microphthalmia, anophthalmia, optic nerve maldevelopment) | PAX6 , OTX2 , RAX | Abnormalities in eye or optic nerve development associated with isolated or MPHD, anterior pituitary hypoplasia |
| Holoprosencephaly | SHH , GLI2 | Cephalic disorder where forebrain fails to develop two hemispheres, severe midline defects, polydactyly, MPHD |
| Moebius syndrome | Usually unknown; reported genes: PLXND1 , REV3L , TUBB3 | Neurologic disorder with weakness/paralysis of cranial nerves, pituitary hormone deficiencies (GH, ACTH, LH/FSH), hypoplastic optic disc |
| Pallister-Hall syndrome | GLI3 | Hypothalamic hamartoma, polydactyly, bifid epiglottis |
| Pituitary stalk interruption syndrome | HESX1 , LHX4 , OTX2 , SOX3 , PROKR2 | Pituitary stalk, ectopic posterior pituitary gland, hypoplastic anterior pituitary, IGHD, MPHD |
| Septo-optic dysplasia | HESX1 , SOX2 , SOX3 , OTX2 , FGFR1 | Complex midline brain defects (absent septum pellucidum/corpus callosum, optic nerve dysplasia, MPHD |
a Listed are various syndromes or clinical phenotypes that are associated with congenital hypopituitarism. Some of these have gene defects associated with the diagnosis, although quite often a genetic etiology may not be available. Pituitary hormone deficiency(s) may also not be detectable at the time of diagnosis or may develop over time. Therefore the clinician should be vigilant to consider evaluating pituitary function. In addition, future follow-up to ensure appropriate growth and development is recommended given the risk of pituitary disease. ACTH , Adrenocorticotrophic hormone; CHARGE , Coloboma, Heart defects, Atresia choanae, Retardation of mental and somatic development, Growth retardation/Genital abnormalities, and Ear abnormalities; FSH , follicle-stimulating hormone; GH , growth hormone; IGHD , isolated Growth hormone deficiency; LH , luteinizing hormone; MPHD , multiple pituitary hormone deficiency; PRL , prolactin.
GH is a polypeptide produced by somatotrophs in the anterior pituitary. Its mode of action is both anabolic and mitogenic with a primary role in growth during childhood. These actions are mediated through insulin-like growth factors (IGFs), of which peripheral and hepatic IGF-1 is the most important in growth. GH is secreted in a pulsatile pattern under the control of two hypothalamic antagonistic hormones, growth hormone–releasing hormone and somatotropin release-inhibiting factor (also known as somatostatin).
In contrast to later in life, neonates demonstrate a nonpulsatile secretion pattern of GH such that serum levels are often detectable. It may be several months before an infant develops the pulsatile pattern of GH secretion characteristic of later life. This physiologic pattern provides some convenience when attempting to test the GH axis in neonates.
Infants with GHD do not typically present with below-average length at birth, although some have argued that in those with severe GHD there may be abnormal growth as an infant. In part, this is because maternal factors play a more vital role in gestational growth. In addition, the GH receptor is poorly expressed in the infant, explaining why infants with GHD may not be easily detected at birth. These receptors may take up to 6 months to become functional and typically it may be several years until growth deceleration is detected. Therefore size and growth may not be a sign suggesting GHD in neonates and infants. However, GH also has a metabolic role as a counterregulatory hormone for glycemic control. Hypoglycemia is a common symptom in infants with GHD. This is in contrast with older children and adults, where isolated GHD rarely causes hypoglycemia. Hypoglycemia is also a symptom of cortisol deficiency; therefore hypoglycemia in the neonate should prompt a suspicion for pituitary hormone deficiency. This presentation leads to the request of a critical sample that can often be helpful in documenting pituitary dysfunction ( Fig. 25.2 ).
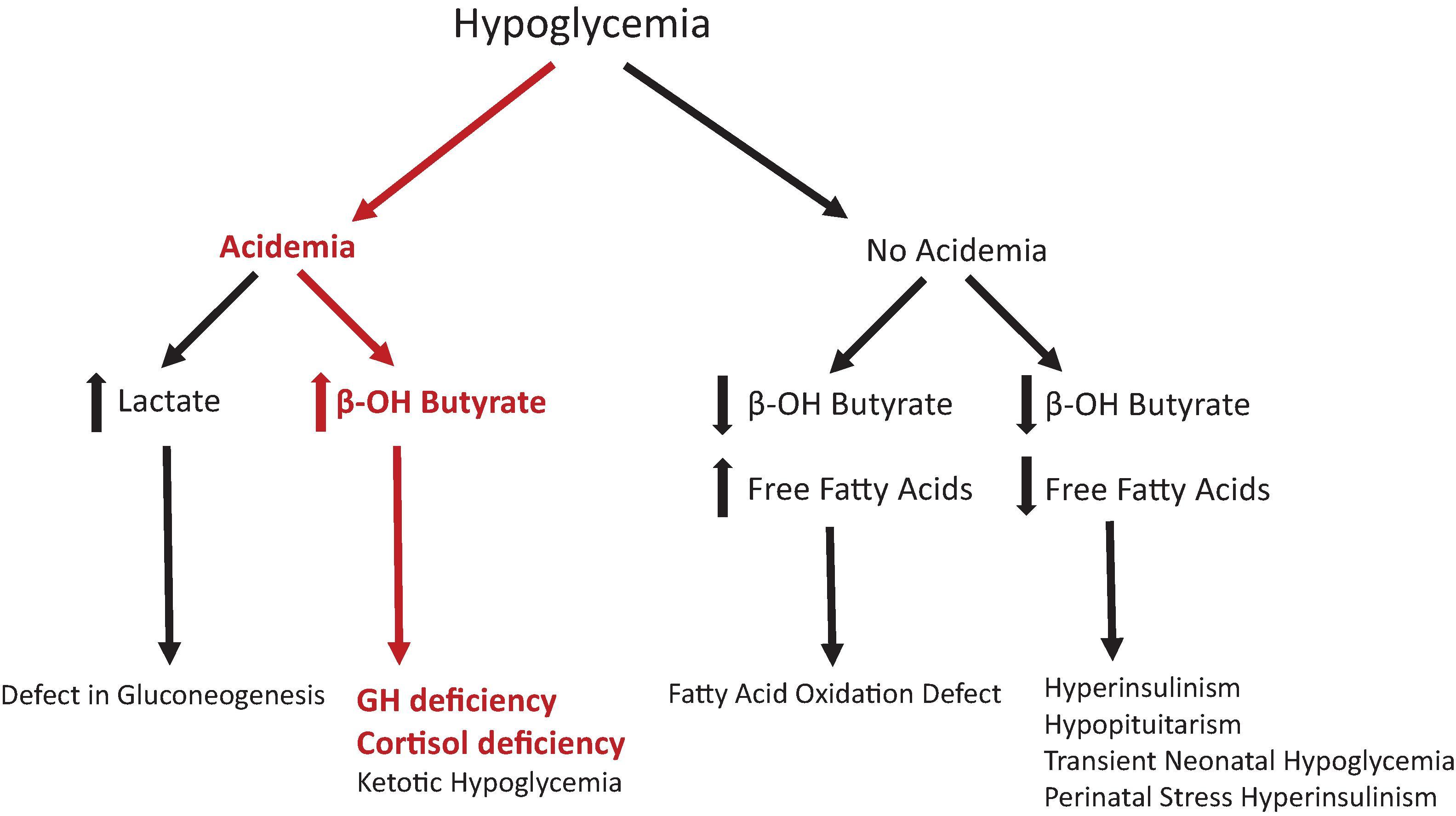
In both the intensive care unit and newborn nursery, the definition of hypoglycemia varies depending on the day of life and can sometimes be controversial. The Pediatric Endocrine Society recently published data helping to better define expectations of glycemic control in term infants given the known risks for complications of neurodevelopment among infants with chronic hypoglycemia. , Strict definitions of hypoglycemia become challenging when prematurity and illness complicate the medical picture. These recommendations state that blood glucose levels should be >50 mg/dL in the first 48 hours of life and >60 mg/dL afterward. The clinician should be aware that if neonatal hypoglycemia is detected, an evaluation for possible GHD should be considered, along with the other causes of neonatal hypoglycemia. Fig. 25.2 delineates the investigations to diagnose the etiology of hypoglycemia.
Phenotypically, another associated finding with severe GHD in a male infant is micropenis. A stretched penile length that measures more than 2.5 SD below the mean should raise suspicion for either GHD or gonadotropin deficiency. In addition, prolonged conjugated hyperbilirubinemia has also been noted in infants with GHD.
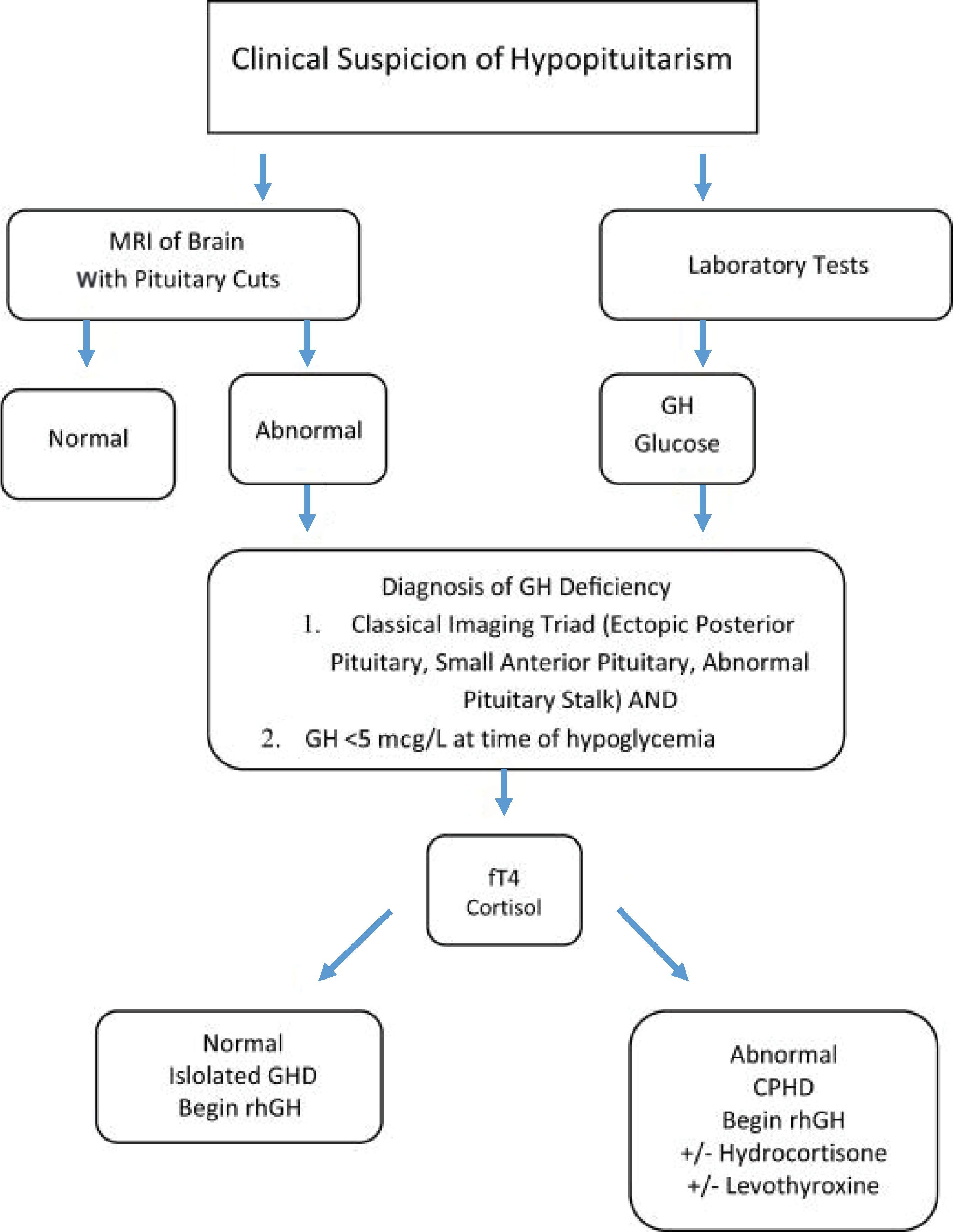
An accurate diagnosis of GHD continues to be a clinical challenge in infants. Classically, stimulation tests that utilize provocative agents to recreate a GH pulse are used in an attempt to assess whether a child can appropriately produce GH. These stimulation tests are not standardized and many pose the risk of adverse effects, especially in younger patients. Furthermore, there is low specificity and reproducibility with the currently available pharmacologic agents used during testing (including clonidine, arginine, levodopa, and glucagon). Fig. 25.3 shows a simplified algorithm for such considerations.
In the neonate, however, guidelines suggest a diagnosis can be made without GH provocative testing. There is increased GH production in the first few days of life and/or at times hypoglycemia, providing an opportunity to more accurately detect levels. , These levels can range from 20 to 50 ng/mL in the first few weeks of life and then gradually decline. Therefore a measured GH level less than 10 ng/mL would be considered diagnostic for GHD. However, by day 15, reports suggest the mean falls to 5.5±3.7 ng/mL. More recently, research efforts are being made to determine whether dried blood spots, similar to newborn screening, can be used to diagnosis deficiency. Results are reported as a possible alternative option for testing GH levels but have not yet been validated.
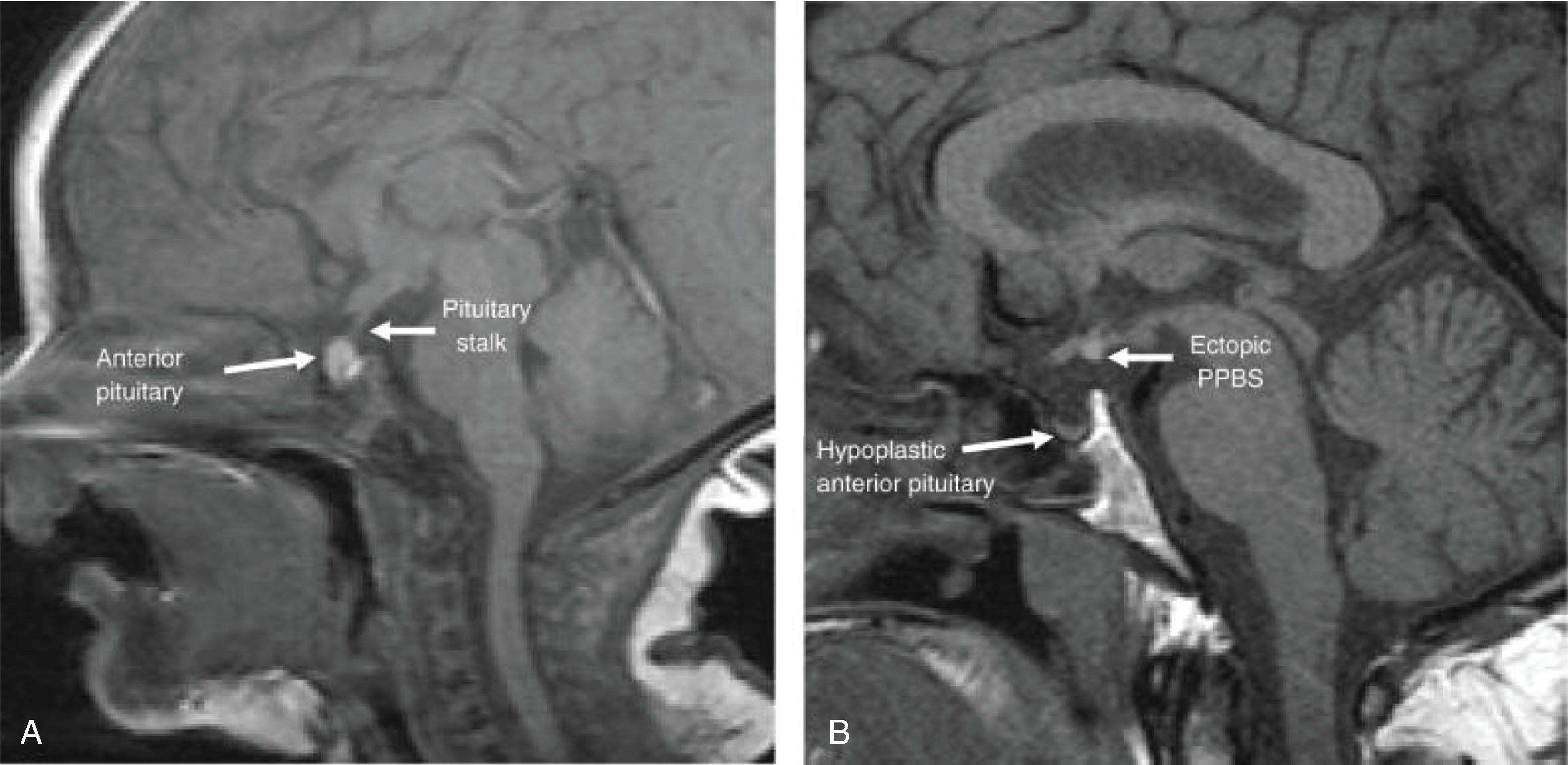
Diagnostic imaging with MRI of the pituitary can sometimes assist to confirm the risk for pituitary hormone dysfunction if an anatomic abnormality is identified ( Fig. 25.4 ). This can include abnormalities such as anterior pituitary hypoplasia, ectopic posterior pituitary, interrupted pituitary stalk, or absence of the corpus callosum. The ability to accurately image a neonate or infant, however, may be limited and require the risk of sedation. Therefore the authors do not feel imaging is absolutely necessary at first for diagnosis. These patients may also have been diagnosed with another pituitary hormone deficiency, therefore already signifying a risk for other hormone deficiencies.
Replacement with recombinant growth hormone therapy is the standard of treatment for infants diagnosed with deficiency. Guidelines for growth hormone treatment established by the Pediatric Endocrine Society state that an initial GH dose should be 0.16 to 0.24 mg/kg/week or 22 to 35 mcg/kg/day. Some manufacturers have recommended higher doses up to 0.3 mg/kg/week in prepubertal children, but the provider should recognize that a decision to treat an infant with recombinant growth hormone is in part dictated by preventing ongoing hypoglycemia and not just to promote normal growth; the higher doses are generally not required to alleviate hypoglycemia.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here