Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
As each day goes by, the medical literature expands almost exponentially, and the literature in organ transplantation is no different. Some 27 million medical articles have been listed on PubMed and there are well over 220,000 articles on organ transplantation alone. Seeking evidence in informing our practice in organ transplantation, be it the value of a particular immunosuppressive drug or a surgical intervention, such as the prophylactic use of a stent in the ureter in kidney transplantation, is therefore challenging for an inexperienced literature searcher. Thus competence in searching the medical literature is essential for members of the transplant community (or indeed any clinician).
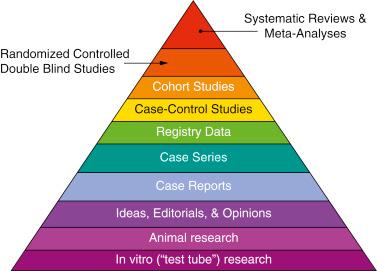
Evidence is ranked according to levels, which prioritize study designs on the basis of internal validity ( Table 42.1 ; Fig. 42.1 ). Systematic reviews and meta-analyses of randomized controlled trials (RCTs) provide level I evidence, with individual RCTs providing level II evidence. Level I and II studies provide the most valuable evidence to allow us to arrive at a management decision of our patient population during and after organ transplantation or indeed of the individual patient.
| Level I | Systematic review and meta-analysis of randomized controlled trials |
| Level II | Randomized controlled trials |
| Level III |
|
| Level IV | Case series |
RCTs are the most desirable form of evidence to answer a question about an intervention, with many advantages due to the ability to minimize risk of bias ( Table 42.2 ). However, there are a number of disadvantages that mean that a randomized trial is not always the best study design to answer every clinical question. Inclusion and exclusion criteria are often restrictive in clinical trials, and this can limit the generalizability of the results to external populations. Cost constraints and recruitment issues often mean a small sample size, limiting the statistical power to detect differences in infrequent outcomes. In addition, cost often limits the length of follow-up, which does not allow the study of the long-term efficacy and safety outcomes.
| Advantages | Disadvantages |
|---|---|
| Prospective study | Relatively small numbers—limited power for rare outcomes |
| Contemporary, well matched, control group | Poor generalizability of results if inclusion/exclusion criteria are too strict |
| Minimal risk of bias | Relatively short follow-up |
| Specific question in a well-defined population | Quality of trials often substandard |
| Complex to implement, coordinate and manage | |
| Not always ethical to randomize to an intervention | |
| Expensive |
Even RCTs vary in methodologic quality. In a previous analysis of the methodology of reporting of trials in organ transplantation by our group, just over one-third of trials were considered to be of good quality, showing a significant risk of bias in many studies that limit their value in informing practice.
Other levels of evidence, although perhaps not as robust, do have advantages in certain circumstances. One such source of evidence is transplant registry data. In our specialty there are a number of national, regional, and international registries for organ transplantation, for example, United Network for Organ Sharing (UNOS) in the US ( www.unos.org ), the international Collaborative Transplant Study ( www.ctstransplant.org/ ), the ANZDATA registry for Australia and New Zealand ( www.anzdata.org.au/ ), and the Eurotransplant registry ( www.eurotransplant.org ). National registries also exist, such as that maintained by National Health Service Blood and Transplantation (NHSBT) for the United Kingdom ( www.odt.nhs.uk ).
Transplant registry data have the advantages of large numbers and diverse real-world populations, which mean that findings may be more generalizable than those from clinical trials with restrictive inclusion criteria ( Table 42.3 ). They not only identify current practice but the large numbers will allow detection of rare events that smaller clinical trials may not be powered to investigate. The major disadvantages of registry data are that data are often recorded retrospectively with lack of validation, data may be incomplete, and one cannot exclude a selection bias in analysis. To minimize the disadvantages of registry data, an effort should be made to require the return of all data to registries; national registries should be able to achieve that.
| Advantages | Disadvantages |
|---|---|
| Large numbers | Risk of selection bias in comparative studies |
| Heterogeneous population reflecting real-world practice—generalizable results | No validation of data entry into registry |
| Long follow-up and facilitates long-term follow-up of RCT participants | Incomplete data |
| Useful for hypothesis generation | Retrospective data entry—risk of recall bias |
| Useful to identify rare adverse events | Limited data fields collected |
| Useful to identify variations in current practice | |
| Support planning of future RCTs regarding sample size calculations and implementation | |
| Relatively cheap and easy to conduct as data are already available |
The debate over the role of RCTs versus registry data should not be regarded as a contest. Both sources supply useful data, and they should be regarded as complementary. Used together they can provide more robust evidence for interventions in transplantation. For example, registry data can be used to generate hypotheses that need to be tested in properly designed RCTs or identify patients that are expected to benefit from an intervention. Well-run registries provide the facility for long-term follow-up of participants in trials. For example, in the large 3C study in the UK of alemtuzumab versus basiliximab induction with subsequent randomization to sirolimus or tacrolimus at 6 months, all recipients have been registered with the national registry at NHSBT to enable long-term follow up, and there are other examples too.
Searching for evidence can be a challenging task. There is an abundance of resources available and a rapidly increasing number of citations. This section provides a brief introduction of how to search for evidence in commonly used resources.
A search for evidence starts with a well-constructed, answerable question. For questions about interventions, the PICO format, which defines the question across four domains: Population, Intervention, Comparator, and Outcomes of interest, helps define the clinical question and aids the identification of relevant evidence ( Table 42.4 ).
| Considerations | Example | |
|---|---|---|
| Population | Age, sex, underlying condition/pathology, comorbidities | Adult renal transplant recipients at the time of transplantation |
| Intervention | Drug or procedure, dose or duration | Complete steroid avoidance or withdrawal at any time after transplantation |
| Comparator | No intervention, current gold standard, placebo | Maintenance steroids |
| Outcomes | Efficacy (acute rejection, graft/patient survival, graft function) and safety (infection, malignancy, specific side effects) | Acute rejection, graft function, graft and patient survival Steroid-related side effects (serum lipids, hypertension, new-onset diabetes, leukopenia, infection, cataracts) |
Once a clinical question has been defined it should be translated to bibliographic search. The initial search should be broad, including only two or three elements of the PICO question. Combining too many search terms in the first instance increases the risk of overdefining the search, which can result in eliminating relevant search results.
There are two approaches to identify relevant articles. First, one can search using keywords, which involves selecting words or terms to describe the key elements of the clinical question. Second, one can search using the thesaurus or controlled vocabulary of a database. The thesaurus is used to index articles according to fixed terminology, ensuring that articles on the same topic are indexed under the same standardized terms. This ensures greater precision when searching. MEDLINE’s thesaurus is called Medical Subject Headings (MeSH), and Embase’s thesaurus is called Emtree.
To refine search results most databases allow inclusion of limits such as date of publication, language, publication type, or patient age.
Boolean operators “AND,” “OR,” and “NOT” can be used to combine concepts, find synonymous terms, or exclude words from the search results. Care must be taken when using the Boolean operators because inappropriate use could exclude relevant search results.
There is an increasing number of resources available when undertaking searches for evidence, and a brief description of the most commonly used resources follows.
Google Scholar ( http://scholar.google.com ) is a search engine that is a subset of Google. It is easy to search and gives quick results, especially when using its “cited by” feature, which lists all other references that cited an article, allowing the user to rapidly focus on a topic. The Web search engine includes both basic and advanced search options, the latter allowing the user to search by keywords, author name, source name, and date of publication. Google Scholar comprises academic and gray literature, including peer-reviewed articles, books, theses, and other publications from societies and universities. Users should note that Google Scholar displays only the first 1000 search results according to an undisclosed algorithm. Google Scholar has been criticized for a number of other issues, such the inability to search MeSH terms, partial inclusion of MEDLINE records, and infrequent updates.
PubMed is a free database of the US National Library of Medicine ( www.pubmed.gov ). It is the most comprehensive biomedical bibliographic database and contains more than 27 million fully indexed or in process articles ahead of print from MEDLINE, life science journals, and online books. PubMed has the following features:
The basic, intuitive search allows one to search by word or phrase, author, or journal.
Searches can be combined using the Boolean operators.
Searches can be limited by, for example, date, type of article, language, or age.
The advanced search allows one to use the search “Builder” and “History.” Using the search Builder one can design a search using Boolean operators.
A useful feature of PubMed is that by default PubMed will try to map a search term to a MeSH term (automatic term mapping).
Search details can be found on the search results pages in the right-hand column. It shows which search and/or MeSH terms were used, and users can directly add or omit terms in the search details box.
The single-citation matcher is a useful feature to find an article when only part of the citation is known.
Clinical queries are highly sensitive, built-in search filters to easily retrieve articles on clinical study categories (etiology, diagnosis, therapy, prognosis, and guidelines), systematic reviews, and medical genetics.
Helpful tutorials are available on the PubMed website explaining all of these search options in greater detail.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here