Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
American College of Radiology
computed tomography
diffusion-weighted imaging
focal nodular hyperplasia
hepatocellular carcinoma
hepatocyte nuclear factor 1α
Liver Imaging Reporting and Data System
magnetic resonance
Organ Procurement and Transplantation Network
Focal hepatic masses may be encountered during routine medical imaging done for other reasons, staging for extrahepatic malignancies, and surveillance for primary hepatic malignancies. Advances in technology allow improved detection and characterization of these masses. The ability to distinguish many of these masses as definitively benign or malignant by imaging alone can eliminate unnecessary biopsy or costly follow-up in many patients and help determine the appropriate treatment strategy for malignant masses. This chapter includes a description of imaging modalities used for evaluation of hepatic masses followed by a discussion of benign and malignant lesions, including hemangioma, focal nodular hyperplasia (FNH), hepatic adenoma, hepatic cyst, bile duct hamartoma, biliary cystadenoma, hepatic abscess, hepatocellular carcinoma (HCC), cholangiocarcinoma, and metastases.
The main imaging studies performed for hepatic mass evaluation are ultrasonography, computed tomography (CT), and magnetic resonance (MR) imaging. Ultrasonography is often used as an initial imaging study because of its low cost, wide availability, and lack of radiation exposure. Limitations of ultrasonography include operator dependence, suboptimal assessment in patients with large body habitus or difficulty following breath-holding instructions, and decreased through penetration of the sound beam in the presence of fatty or fibrofatty infiltration. Ultrasonography can readily diagnose cysts and classic hemangiomas but is limited in the characterization of solid hepatic masses. Lack of FDA-approved contrast agents for use with abdominal ultrasonography in the United States reduces the full potential of ultrasonography for the detection and characterization of liver masses. CT or MR imaging is often required for further characterization when a solid mass is detected by ultrasonography.
CT offers many advantages, including wide availability, speed, moderate cost, and improved ability to detect and characterize liver masses. One important disadvantage particularly in younger patients is radiation exposure, because repeated scanning during multiple phases is necessary for accurate liver mass characterization. Another limitation is decreased specificity compared with MR imaging, as interpretation of MR imaging relies not only on the enhancement pattern of a mass but also on its appearance on multiple additional sequences, allowing better tissue characterization and more complete evaluation.
Detection and characterization of a mass on contrast-enhanced CT is based on blood flow differential between the lesion and the adjacent liver parenchyma. A triphasic CT scan may consist of unenhanced images followed by repeated imaging during hepatic arterial and portal venous phases. Hepatic arterial phase imaging is performed to detect lesions such as HCC that typically demonstrate hyperenhancement during the arterial phase due to vascular supply from the hepatic arterial system. HCC and other hypervascular lesions may then become isodense or hypodense relative to the adjacent liver during the portal venous phase. Therefore some lesions could be missed on a routine CT scan of the abdomen, in which images are typically acquired only in the portal venous phase. In other instances, a delayed scan at 5 to 15 minutes could be considered for lesions such as cholangiocarcinoma to assess them for delayed enhancement or hemangiomas to assess them for progressive filling by contrast agent, although this is often not required for diagnosis.
MR imaging is the best modality for characterizing liver masses. The American College of Radiology (ACR) Appropriateness Criteria assign contrast-enhanced MR imaging the highest rating for characterization of an indeterminate liver lesion, and it is the recommended imaging modality if there are no contraindications. The advantages of MR imaging over CT and ultrasonography include better characterization of lesions or pseudolesions detected on ultrasonography and CT, lack of ionizing radiation, superior contrast resolution, biliary tree depiction, and the ability to use extracellular or hepatocyte-specific contrast agents. Evaluation of signal characteristics of a mass on multiple sequences, such as routine T1- and T2-weighted images, chemical shift imaging (in- and opposed-phase images used for detection of internal fat, calcification, or iron content), diffusion-weighted imaging (DWI), and contrast-enhanced images, allows more specific characterization of lesions than is possible with CT and ultrasonography. The disadvantages include higher cost, longer imaging time, contraindications in certain patients (e.g., pacemakers that are not MR imaging compatible), and the need for patient cooperation such as breath-holding to minimize motion artifact.
The two main categories of contrast agents used for MR imaging are (1) standard agents with an extracellular distribution and (2) hepatocyte-specific agents. The standard extracellular agents are the most widely used and best documented. These rely on blood flow differential between the liver parenchyma and focal lesions for detection and characterization analogous to an iodinated contrast agent for CT. Contrast-enhanced sequences are performed in the arterial, portal venous, equilibrium, and delayed phases. The ability to perform multiple contrast-enhanced sequences without added radiation is a major benefit over CT. Hepatocyte-specific contrast agents provide information about a lesion similar to extracellular contrast agents during the early contrast phases but also add unique functional information on additional delayed images as contrast agent is taken up by hepatocytes and excreted through the biliary system. Delayed hepatocyte phase images are obtained at 20 minutes for gadoxetate disodium (Eovist; Bayer HealthCare, Whippany, NJ, United States). Hepatocyte-specific contrast agents are helpful in the differentiation of FNH from hepatic adenoma and can be helpful in the staging of metastatic disease because of improved detection of smaller metastases. The disadvantages of hepatocyte-specific agents over standard extracellular agents include higher cost, difficulty in obtaining peak arterial phase enhancement in part due to a lower FDA-approved dose than with extracellular agents, and impaired hepatic uptake and biliary excretion in patients with hepatocyte dysfunction and obstructive jaundice. MR imaging is often routinely performed with extracellular contrast agents, whereas hepatocyte-specific agents are reserved for select indications (e.g., FNH vs. adenoma); however, hepatocyte-specific agents may be used more often depending on institution preference.
DWI is a technique that has been increasingly implemented in routine abdominal MR imaging examinations. DWI signal in biologic tissues is derived from the random motion of water molecules in the intracellular, extracellular, and intravascular spaces. The degree of restriction of water diffusion depends on tissue cellularity and cell membrane integrity. The ability of DWI to depict restricted diffusion in areas of high cellularity such as tumors allows improved lesion detection, which may be especially helpful in patients who cannot receive intravenous contrast agents. Common benign hepatic lesions generally show a lesser degree of restricted diffusion than malignant lesions. However, significant overlap in quantitative values of restricted diffusion limits the ability of DWI to differentiate solid benign and malignant lesions.
Hemangioma is a common benign hepatic mass with a reported prevalence of up to 20%. Such masses comprise numerous vascular channels, each lined with a single layer of epithelial cells and separated by fibrous septa. They occur more frequently in females (ratio of 2 : 1 to 5 : 1), are usually asymptomatic, and are often multiple. Hemangiomas are usually incidentally discovered and are of no clinical consequence.
On ultrasonography, typical hemangiomas are well-defined round or mildly lobulated masses that are uniformly hyperechoic relative to adjacent liver parenchyma, demonstrate posterior acoustic enhancement, and lack detectable internal color flow ( Fig. 11-1, A ). They are often peripherally located within the right hepatic lobe, although they can occur anywhere in the liver. The most common atypical appearance of hemangioma on ultrasonography is that of a hypoechoic or isoechoic mass with a peripheral hyperechoic rim. Hemangiomas can also present as hypoechoic masses in the setting of hepatic steatosis, whereby they appear hypoechoic relative to the echogenic fatty liver ( Fig. 11-2 ).
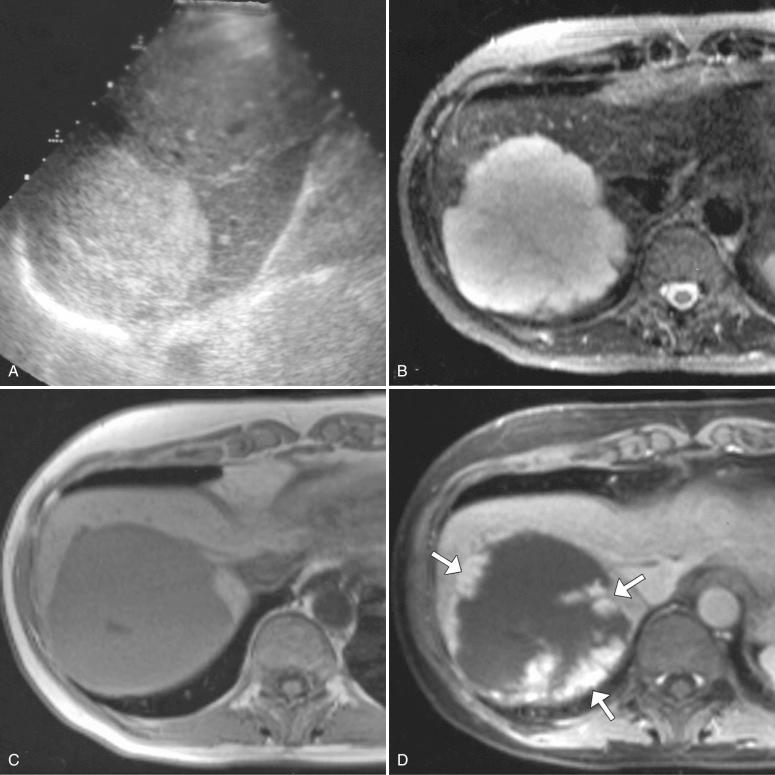
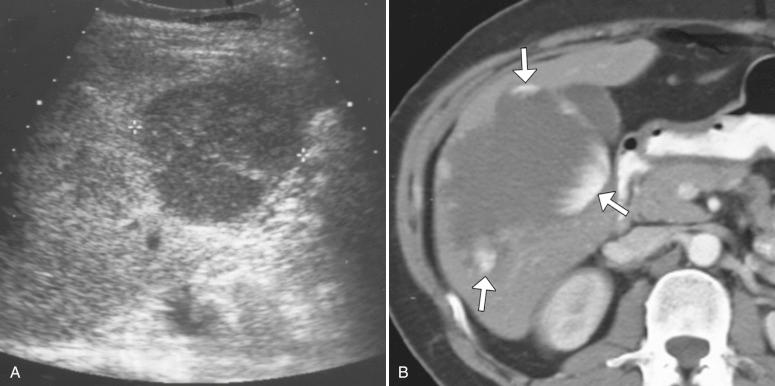
On noncontrast CT, hemangiomas are generally hypodense and appear similar in attenuation to blood vessels because of their composition of vascular channels. However, they may appear isodense or hyperdense in the setting of hepatic steatosis, where the liver itself demonstrates diffusely low attenuation. On postcontrast imaging, hemangiomas classically demonstrate discontinuous peripheral nodular enhancement on early images, with progressive filling by contrast agent on delayed images.
MR imaging is the preferred modality to evaluate hemangiomas, which appear hyperintense on T2-weighted images because of their blood-filled spaces and the resulting long T2 relaxation time. This T2 hyperintense signal is one of the most reliable findings in the diagnosis of hemangiomas. Hemangiomas are hypointense on T1-weighted images and, similarly to what is found in contrast-enhanced CT examinations, demonstrate typical discontinuous peripheral nodular enhancement with progressive filling on postcontrast images (see Fig. 11-1 ).
Small hemangiomas may demonstrate rapid uniform enhancement on arterial phase images, being termed flash-filling hemangiomas. These are often associated with an adjacent peripheral wedge-shaped area of transient hyperenhancement during the arterial phase. Flash-filling hemangiomas can be differentiated from other hypervascular lesions by their characteristic T2 hyperintense signal, retention of contrast agent on delayed images, and appearance similar to blood pool on all postcontrast phases of imaging.
Giant hemangiomas are those that are larger than 5 cm. They may look more complex on T2-weighted images, with multiple hypointense septa or a central T2 hyperintense cleft from cystic degeneration. Giant hemangiomas also demonstrate discontinuous peripheral nodular or a more flame-shaped pattern of enhancement with progressive filling but may remain incompletely filled on delayed images because of their size and the presence of central scarring or regions of thrombosis; however, their signal intensity and characteristic enhancement pattern allow a correct diagnosis.
Sclerosed hemangiomas are those that have undergone degeneration and fibrosis, resulting in obliteration and replacement of their vascular channels responsible for their typical features on imaging. Findings that may suggest sclerosed hemangioma include T2 hyperintense signal, well-defined margins, associated volume loss or capsular retraction, internal nodular foci of arterial phase hyperenhancement, adjacent peripheral wedge-shaped area of transient arterial phase hyperenhancement, and presence of typical hemangiomas elsewhere in the liver. The diagnosis of hemangiomas in cirrhotic livers is less common and may be even more challenging as they may sclerose over time. Obtaining prior imaging can be helpful to diagnose sclerosed hemangiomas because they may show a more typical hemangioma appearance on older studies.
If MR imaging is performed with hepatocyte-specific contrast agents rather than standard extracellular agents, the diagnosis of hemangioma can be more challenging in select cases. Hemangiomas will appear hypointense on the delayed 20-minute phase images because they contain vascular channels and not hepatocytes. However, redistribution of contrast agent is gradual, and contrast agent removal from the vascular spaces begins before the time when 20 minute–delayed images are obtained. With flash-filling hemangiomas, this results in a pseudo-washout appearance that can make diagnosis difficult unless one also observes a similar decrease in signal intensity of the blood pool in adjacent vessels. Some small hemangiomas lack early enhancement and are diagnosed only by observation of delayed filling of the lesion. Given that the characteristic delayed contrast fill-in is absent with hepatocyte-specific contrast agents, correct diagnosis in this setting can also prove challenging. Therefore if a hemangioma is suspected on initial imaging, MR imaging using a standard extracellular contrast agent is preferred to make the diagnosis.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here