Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
A great number of changes occur in the bone marrow during and after therapy for malignancy. Although proper interpretation of these changes requires knowledge of the patient's original disease process, some general features are common to all cases. It may be assumed that the purpose of bone marrow studies performed during or after therapy is to evaluate disease involvement; however, other important information can be obtained from these specimens. In the case of acute leukemias, with effective high-dose chemotherapy, the marrow is completely ablated, and bone marrow studies may be performed to confirm obliteration of the neoplastic process. Later in the patient's disease course, a bone marrow examination may be performed to confirm the presence of regenerating hematopoiesis. In these cases, a comment on bone marrow cellularity and the presence of maturing trilineage marrow elements (i.e., granulocytes, erythroid precursors, megakaryocytes) is important. Following hematopoietic cell transplantation (which includes bone marrow transplantation, cord blood, or peripheral blood cell transplantation), an examination may be performed to confirm engraftment, and descriptions of bone marrow cellularity and trilineage hematopoiesis are of primary importance. Therefore, complete clinical information—including information about the primary disease process, type of treatment, and time interval since treatment—should be submitted with the bone marrow specimen.
Several studies have evaluated bone marrow changes in acute leukemias after high-dose chemotherapy or chemotherapy with radiation, or after hematopoietic cell transplantation, and there are many similarities in the findings ( Box 57-1 ; Fig. 57-1 ). These changes are also similar to the toxic changes resulting from drug injury of the bone marrow. The changes expected in the first week after treatment are those of complete marrow aplasia. The marrow cellularity is often nearly zero, with an absence of normal marrow fat. There is prominent edema, with dilated marrow sinuses. Scattered stromal cells, histiocytes, plasma cells, and lymphocytes may be present. Deposition of pink proteinaceous material may be seen, which can mimic fat serous atrophy (gelatinous transformation), but the presence of the eosinophilic gelatinous material of serous atrophy has only rarely been reported after chemotherapy. Normal hematopoietic cells, such as maturing granulocytes, nucleated red blood cells, and megakaryocytes, are often not identifiable. Histiocytes containing cellular debris are often present, and acellular areas of pink-staining fibrin and fibrinoid necrosis often predominate. Rare cases may also show zonal areas of tumor cell necrosis. The reappearance of fat cells and development of mild reticulin fibrosis follow these changes. The early fat in regenerating bone marrow is often loculated. Although the marrow remains markedly hypocellular, the fat is associated with focal areas of early hematopoiesis in the second week after treatment. This may be represented by islands of erythroid cells, alone or in combination with areas of left-shifted granulocytes. Both elements are usually present after 2 weeks. Megakaryocytes, often occurring in clusters with atypical or hypolobated nuclei, occur later in this process but are usually easily identified by the third week. In some patients, particularly children, early regeneration may be accompanied by an increase in precursor B cells, or hematogones. The features of these cells are discussed later in the chapter.
Marrow aplasia
Absence of fat cells
Edema
Fibrinoid necrosis
Dilated sinuses
Rare stromal cells, histiocytes, lymphocytes, and plasma cells
Reappearance of fat, often lobulated
Mild reticulin fibrosis
Foci of left-shifted erythroid and granulocyte islands
Increase in precursor B cells on smears
Resolution of reticulin fibrosis
Appearance of small megakaryocytes in clusters
Normal or slightly increased marrow cellularity
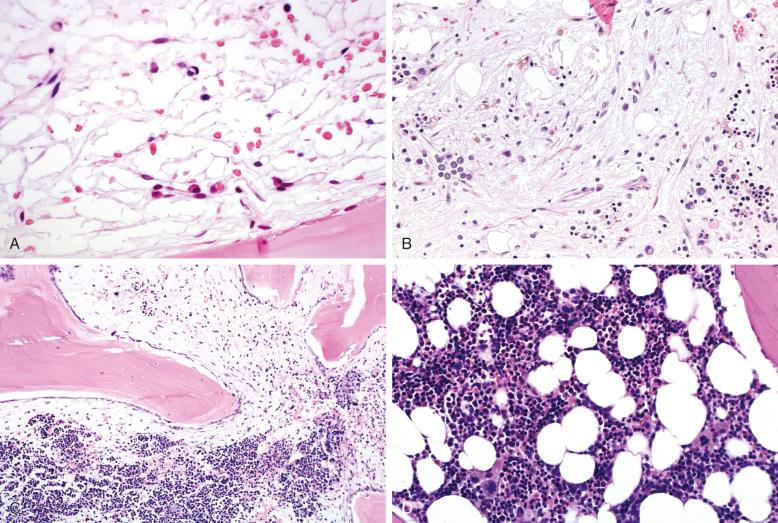
As the bone marrow continues to repopulate and returns to normal cellularity, loss of the mild reticulin fibrosis of early regeneration and an even slightly increased marrow cellularity may be seen. All three normal marrow cell lines are present, although a left shift of granulocytes and erythroid cells and atypical megakaryocyte clustering may persist for some time.
Some additional bone marrow changes may be observed in patients who have undergone high-dose therapy followed by hematopoietic cell transplantation ( Box 57-2 ). Foci of regeneration may occur earlier in patients treated with stem cell infusion. Although clusters of regenerating marrow elements usually show a spectrum of maturation, these islands of cells may have a more monotonous appearance, without obvious maturation, after transplantation. This is most commonly seen with erythroid precursors. In addition, the topographic pattern of regenerating cells may differ after transplantation. In the normal bone marrow and in normally regenerating marrow after cytotoxic therapy, immature granulocyte islands of blast cells and promyelocytes usually occur adjacent to bony trabeculae. The presence of such islands away from the bone is considered an abnormal feature, referred to as abnormal localization of immature precursors (ALIP), and is described as a feature of myelodysplastic syndrome on biopsy sections. After hematopoietic cell transplantation, these immature cell islands often occur away from the bone, and this feature should not be considered evidence of recurrent or impending myelodysplastic syndrome ( Fig. 57-2 ).
Regenerative islands with a monotonous, immature appearance
Localization of immature cell precursors away from bony trabeculae
Increased storage iron and siderotic iron with or without ring sideroblasts
Prolonged variable cellularity with or without cytopenias
Prolonged aplasia due to engraftment failure
Increased large granular lymphocytes
Increases in bone marrow iron storage or siderotic iron incorporation are also common findings following hematopoietic cell transplantation. This is usually apparent by an increase in hemosiderin-laden macrophages on both aspirate smears and trephine biopsy sections. Although the increase in siderotic iron is usually less uniform than that seen in refractory anemia with ring sideroblasts, in some cases the pattern of iron staining may be similar or identical to that of sideroblastic anemia; therefore, iron stains must be interpreted with caution in post–marrow transplant patients. Finally, after hematopoietic cell transplantation, bone marrow cellularity may never return to the normal range. These patients frequently exhibit variability in marrow cellularity and often have a persistently hypocellular marrow that may be accompanied by mild peripheral blood cytopenias for many years. Prolonged impairment of hematopoiesis has been demonstrated following standard as well as high-dose chemotherapy followed by bone marrow transplantation, and this may explain the differences in cellularity in post-transplantation marrows.
A hypocellular post-treatment marrow may result from bone marrow failure after solid organ or hematopoietic cell transplantation, failure to engraft after transplantation, or delayed engraftment after transplantation. The bone marrow in these patients is similar and shows signs of aplasia even after several weeks. Histiocytes, stromal cells, lymphocytes, and plasma cells predominate. Delayed engraftment may occur in patients with marked marrow fibrosis before transplantation, and diffuse histiocytic proliferations have been reported with delayed engraftment. Graft failure after hematopoietic cell transplantation or bone marrow failure after solid organ transplantation may occur secondary to viral infection, reactivation of virus, or hemophagocytic syndrome. Late marrow failure may also occur as a terminal event of post-therapy myelodysplastic syndrome.
The immunodeficiency associated with chemotherapy also increases these patients' risk for infectious diseases. Examination of the bone marrow is one means of identifying infection. If an infectious disease is suspected, fresh bone marrow aspirate material should be sent for microbiology studies. Histochemical stains for acid-fast and fungal organisms should be performed on all biopsy specimens containing granulomas.
The optimal materials for evaluating post-therapy changes include the peripheral blood smear, bone marrow aspirate, and touch preparations (imprints), as well as trephine biopsy sections. However, clot biopsy sections may also be useful, in particular if further molecular DNA or RNA studies are performed; however, focal lesions that cannot be aspirated may not be present on such sections. Aspirates and imprints generally offer the best cytologic detail and are helpful in evaluating residual blast cells after therapy. Biopsy material is useful for showing the pattern of blast cells, once they are identified on the aspirate material, as well as the presence and pattern of residual lymphoma or solid tumor involvement.
Ancillary studies are often of critical importance in the evaluation of post-therapy bone marrow specimens, particularly in the assessment of residual disease. These studies include flow cytometry, immunohistochemistry, and cytogenetic and molecular genetic studies. With the exception of immunohistochemistry and some molecular genetic tests, these ancillary methods require additional fresh bone marrow aspirate, and material must be saved at the time of specimen submission for these studies. The utility of each of these methods is discussed in the sections on the specific disease processes.
Once the bone marrow has begun to regain its cellularity with normal hematopoietic cells, the pathologist is faced with the challenge of evaluating for residual or recurrent disease. It is well established that the presence of minimal residual disease (MRD) that is undetectable by morphologic methods is a powerful predictor of recurrence. Although different types of acute leukemia offer their own unique problems, some general features are common to all cases. A blast cell count of 5% in the bone marrow is the historic cutoff for delineating the presence of residual or recurrent leukemia. However, this cutoff is arbitrary, and the current goal is to detect the presence of neoplastic clones as early as possible. The use of multiparameter flow cytometry and molecular genetic techniques to detect MRD is redefining remission in many diseases. A general overview of the sensitivity of the methods used to detect MRD is given in Table 57-1 .
| Method | Sensitivity (%) |
|---|---|
| Morphology | 1-5 |
| Cytogenetic karyotype analysis | 3-5 |
| FISH | 1-5 |
| Immunohistochemistry | 0.1-5 |
| Consensus primer PCR for gene rearrangements | 0.1-1 |
| Flow cytometry | 0.01-1 |
| Next-generation sequencing | 0.0001-5 |
| PCR and RT-PCR for specific translocations | 0.001-0.01 |
| Patient-specific PCR and RT-PCR | 0.001 |
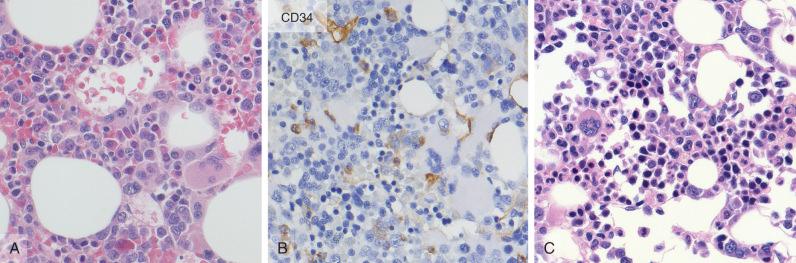
The common use of growth factors (discussed in more detail later) creates the problem of regenerative blast cell increases above 5% in some cases. Therefore, decisions regarding relapse or remission should not be based on blast cell counts alone. When the blast cell population in post-therapy bone marrow is suspicious for residual disease, comparison to the original acute leukemia is often helpful, and the presence of unique morphologic features such as Auer rods, distinctive cytoplasmic granules, prominent nucleoli, or nuclear irregularities that were identified in the original disease can be useful. In addition, the detection of an aberrant immunophenotype by flow cytometry is helpful, although this may require knowledge of the original immunophenotype of the tumor. The immunohistochemical detection of immature CD34-positive or terminal deoxynucleotidyl transferase (TdT)–positive cells in clusters in a bone marrow biopsy is also helpful, because immature cells in regenerating bone marrow do not normally show the clustering seen in recurrent leukemia specimens.
The detection of clonal cytogenetic abnormalities that were present in the patient's original leukemia can also be helpful in the evaluation of residual disease. Routine karyotype analysis or fluorescence in situ hybridization (FISH) studies are the most commonly performed genetic tests, but specific molecular genetic tests with polymerase chain reaction (PCR) may be useful as well.
For a specimen to be optimally assessed for residual or recurrent disease, it is important that the original disease process be diagnosed and evaluated appropriately. Immunophenotyping for residual disease is more expensive owing to the larger panel of antibodies needed, and it is often less rewarding when information about the original leukemia is not available. Molecular genetic testing is usually not justified when the original karyotypic abnormality is not known. Ancillary testing is not necessary on all follow-up specimens. If residual or recurrent disease is suspected in the absence of material for these tests, such suspicion should be relayed to the treating physician. A repeat bone marrow evaluation after 1 or 2 weeks is often helpful to determine a change in the number of blasts, which would be expected to increase with recurrent disease, and appropriate ancillary tests can be performed on the second specimen. In contrast, increased immature cells in an early phase of marrow regeneration would be expected to show more mature precursors in the second bone marrow study.
Published guidelines for the morphologic definition of remission in patients treated for acute myeloid leukemia (AML) require peripheral blood neutrophil counts of greater than 1.0 × 10 9 /L, platelet counts of at least 100 × 10 9 /L, and less than 5% blast cells without Auer rods. Before these changes are demonstrated, however, several morphologic features of the peripheral blood and bone marrow have prognostic significance. Failure to demonstrate a reduction in blast cells and cellularity at day 6 of induction chemotherapy usually results in a change in or augmentation of induction chemotherapy. Not surprisingly, the presence of residual leukemic cells at the end of induction chemotherapy is a poor prognostic indicator. Even after meeting the criteria for remission, patients with bone marrow hypercellularity, anemia, bone marrow blast cell counts of 1% or more, or peripheral blood blast cell counts exceeding 3% have a shortened duration of remission and shortened survival. Therefore, more detailed evaluation of bone marrow and peripheral blood samples is needed than is suggested by the remission criteria.
The presence of an increased number of blast cells with features similar to the original AML or myelodysplastic syndrome should be regarded with suspicion. Auer rods (rod-shaped cytoplasmic aggregates of granules) are not a feature of regenerating or non-neoplastic myeloblasts and should be considered evidence of residual disease. Auer rods may rarely be encountered in maturing granulocytes but are nevertheless considered abnormal. Regenerating blast cells are usually admixed with promyelocytes and maturing granulocytes, and the presence of sheets of blasts on a smear is a sign of recurrent disease. In contrast, specimens with equal or fewer numbers of blast cells compared with promyelocytes usually represent regeneration. Clustering of blast cells is often difficult to interpret on hematoxylin-eosin (H&E)–stained biopsy specimens, and aggregates of regeneration may be difficult to differentiate from leukemic blast cell aggregates. Regeneration usually occurs adjacent to bony trabeculae, and the presence of immature cell aggregates away from the bone is considered abnormal. This abnormal localization of immature cell precursors has been used as a feature of myelodysplasia, but, as mentioned, caution should be applied when these criteria are used in patients who have received hematopoietic cell transplants. After transplantation, the normal bone marrow architecture may change, and regenerating immature precursors may be present away from the bone on H&E-stained sections.
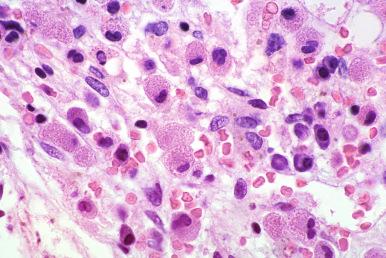
AML with myelodysplasia-related changes and myelodysplastic syndrome may exhibit multilineage dysplasia before an increase in blast cells at relapse. Again, the features of the original multilineage dysplasia should be reviewed, and care should be taken not to overestimate multilineage dysplasia during or immediately after therapy. Dyserythropoietic changes are common during chemotherapy and often include a left shift of erythroid precursors and multinucleation of erythroid cells ( Fig. 57-3 ). In addition, some regenerating megakaryocytes can be small and can cluster during or immediately after chemotherapy; however, numerous megakaryocytes with separate nuclear lobes or a predominance and increase of micromegakaryocytes with absence of nuclear segmentation can be useful in identifying residual disease. Granulocyte changes after therapy are usually restricted to a left shift without hypogranulation or nuclear abnormalities commonly seen in association with myelodysplastic syndrome. Therefore, dysplastic changes of maturing granulocytes are also more reliable in identifying recurrent AML with multilineage dysplasia during or immediately after chemotherapy than are erythroid abnormalities alone ( Fig. 57-4 ).
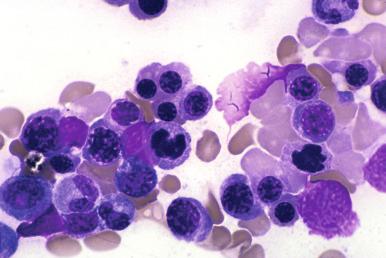
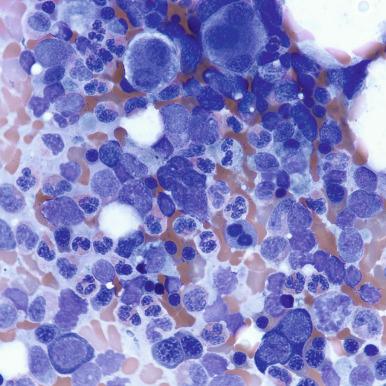
Currently, most patients with acute promyelocytic leukemia are treated with both standard chemotherapy and all- trans -retinoic acid (ATRA) or combinations of ATRA with arsenic trioxide, and their bone marrow changes are usually similar those seen in other AML samples. However, some patients treated with ATRA or combination chemotherapy without ATRA may not show an initial bone marrow aplasia. The bone marrow in these patients may remain hypercellular, with markedly elevated numbers of promyelocytes ( Fig. 57-5 ). These cells usually undergo slow maturation secondary to the therapy, with loss of the t(15;17) cytogenetic abnormality associated with acute promyelocytic leukemia. In this subgroup of patients, it should be understood that the presence of sheets of promyelocytes may not indicate treatment failure, and they should be followed closely with additional bone marrow examinations to confirm that maturational changes are occurring.
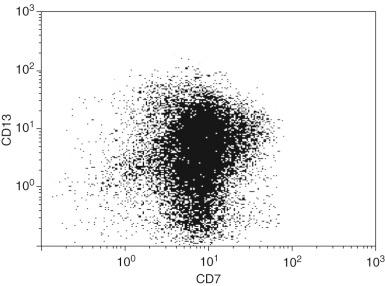
Immunophenotyping studies in AML are often useful because a significant percentage of cases show aberrant expression of lymphoid-associated antigens, which can be used as an immunophenotypic “fingerprint” for residual disease testing. Lymphoid antigen expression is the most easily detected immunophenotypic abnormality, occurring in up to 48% of adult cases of AML. Some aberrant immunophenotypes are commonly associated with a specific disease, such as the high frequency of CD2 expression in the microgranular variant of acute promyelocytic leukemia and in AML with inv(16)(p13.1q22) or t(16;16)(p13.1;q22), loss of HLA-DR and CD34 in the hypergranular variant of acute promyelocytic leukemia, and aberrant expression of CD19 in AML with t(8;21)(q22;q22.3). However, one of the most common aberrant immunophenotypes in AML is expression of CD7 on myeloblasts ( Fig. 57-6 ), which does not correlate with a specific disease type. More complex aberrant immunophenotypes have also been described, including overexpression of CD33 and CD34 on blast cells, abnormal light scatter of blast cells on flow cytometry, and asynchronous expression of mature markers on blast cells ( Box 57-3 ).
CD7 +
CD2 +
CD5 +
CD33 ++
CD34 ++
CD33 ++ /CD34 +
CD33 ++ /HLA-DR − /CD34 − /CD14 − /CD15 −
CD33 + /CD34 + /HLA-DR −
CD33 + /CD13 −
CD33 + /CD117 + /CD34 − /CD15 −
CD33 + /CD117 + /HLA-DR −
CD33 − /CD13 +
CD33 − /CD14 + /HLA-DR +
CD33 − /CD15 + /HLA-DR +
CD34 + /CD11b +
CD34 + /CD69 +
CD34 + /CD15 + /HLA-DR +
CD34 + /CD38 −
CD117 + /CD34 − /CD15 −
CD117 + /CD11b +
CD117 + /CD15 +
Knowledge of these aberrant immunophenotypes in the original leukemia specimen allows the use of a relatively small flow cytometry panel to evaluate for a residual population of blast cells. Detection of aberrant immunophenotypes after therapy for AML has prognostic significance, even in the absence of an increase in blast cells. Patients with only 1 aberrant cell in 100 to 1000 cells had a cumulative relapse rate of 50% in one study, and detecting residual disease at a level of 3.5 × 10 −4 or higher at the end of consolidation chemotherapy is reportedly a significant predictor of relapse. The evaluation of CD117 and CD11b expression on promyelocytes can be useful in the detection of residual acute promyelocytic leukemia versus regenerating promyelocytes. The leukemic promyelocytes express CD117 but not CD11b, whereas regenerating cells are CD11b positive and CD117 negative.
Paraffin section immunohistochemistry may be of value in selected cases, particularly in the presence of left-shifted cell aggregates on H&E-stained sections. Immunophenotyping can show that the immature cell aggregates of regeneration represent a spectrum of left-shifted cells that are not exclusively blast cells, whereas recurrent leukemia blast cell aggregates are a more uniform population of neoplastic cells. Therefore, the identification of clusters of cells expressing the immature cell antigen CD34 or an aberrant combination of markers that were present in the original leukemia favors residual or recurrent disease.
Cytogenetic studies are not performed on all post-therapy samples but may be of value in some situations. Although some cases of AML are associated with normal karyotypes, most show a clonal cytogenetic abnormality. Identification of that abnormality in a follow-up sample is highly supportive of relapsed disease. Karyotype analysis routinely includes the study of 20 cells; therefore, this method is not optimal for the detection of MRD when blast cells are below 5%. Most cytogenetic laboratories also perform FISH, which allows the screening of several hundred cells for a specific abnormality. This method may increase the detection rate of residual disease over karyotype analysis alone, but it requires knowledge of the karyotypic abnormality of the original disease, as well as probes specific for that abnormality. FISH probes are useful in identifying monosomies, trisomies, and masked 11q23.3 abnormalities, in addition to balanced chromosomal translocations. The detection of numerical chromosomal abnormalities by FISH during clinical remission has been shown to correlate with an increased risk for disease recurrence. Another use of karyotype analysis and FISH is to evaluate for XX/XY chimerism. If a patient receives an allogeneic transplant from a donor of the opposite sex, karyotype and FISH studies can reveal the presence of residual host cells in the bone marrow, even if they are of a normal karyotype. In addition, human leukocyte antigen (HLA)–based chimerism studies are used in a similar fashion. However, the detection of non-donor cells in the marrow is less predictive of recurrent disease than is the detection of a leukemia-specific abnormality.
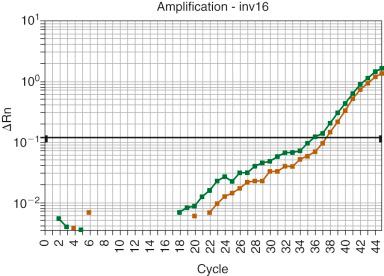
The use of other molecular studies in the evaluation of residual disease is more controversial. These methods generally use PCR and can potentially detect 1 abnormal cell in 100,000 cells. Efforts have been made to standardize these procedures, but there are still great differences in methodology and targets of study among laboratories. Similar to FISH analysis, PCR testing requires knowledge of the original karyotypic abnormality, as well as primers and probes for that abnormality. Because PCR tests are generally directed against balanced cytogenetic abnormalities, they cannot be used to detect the addition (trisomy) or deletion (monosomy) of chromosomes. However, because most de novo AML karyotypic abnormalities involve balanced translocations, PCR tests appear to be ideal for the identification of very low levels of disease. The PCR tests most often offered for AML are those for RUNX1-RUNX1T1 (also known as AML1-MTG8 or AML1-ETO ) of t(8;21)(q22;q22.3), PML-RARA of t(15;17)(q24.1;q21.2), CBFB-MYH11 of inv(16)(p13.1q22)-t(16;16)(p13.1q22), KMT2A translocations involving 11q23.3, and mutated NPM1, FLT3, and biallelic mutations of CEBPA . The PML-RARA RT-PCR test is useful in the early detection of residual disease in acute promyelocytic leukemia and as a predictor of relapse of that disease. Most assays for PML-RARA detect 1 translocated cell in 10,000 to 100,000 cells; however, currently molecular remission is defined as absence of the PML-RARA fusion transcript using RT-PCR methods with a sensitivity threshold of at least 10 −3 or 10 −4 . Ultrasensitive tests have also been developed that can detect 1 mutated cell in 1,000,000, though such sensitivity has been reported to be positive in patients who are in long-term remission and does not appear to be clinically relevant. The RUNX1-RUNX1T1 and CBFB-MYH11 tests are even more problematic. It appears that other genetic aberrations, which may not be detectable by karyotype analysis, are necessary for these types of leukemia to develop. In patients treated for AML with t(8;21)(q22;q22) or AML with inv(16)-(p13.1q22) or t(16;16)(p13.1;q22), standard qualitative PCR testing may detect low levels of these fusions during remission that often do not correlate with disease relapse ( Fig. 57-7 ). These PCR tests suggest that clonal cells with these fusions may persist indefinitely and that qualitative PCR for RUNX1-RUNX1T1 and CBFB-MYH11 is not clinically useful for residual disease testing. It is generally assumed, however, that quantitative PCR has clinical significance by showing that an increasing number of cells with RUNX1-RUNX1T1 or CBFB-MYH11 over time correlates with disease relapse. Several studies appear to confirm this assumption. Quantitative assays for FLT3 gene mutations and Wilms' tumor gene (WT1) expression have also been used to detect residual disease in AML. Mutations occurring in exon 12 of the nucleophosmin gene (NPM1) are the most frequent abnormality (60%) of normal-karyotype AML. PCR and reverse transcriptase PCR (RT-PCR) assays for the detection of NPM1 mutations have been successful in detecting MRD with good sensitivity, although clonal evolution with loss of the mutation at relapse occurs in a small subset of patients. Such clonal evolution and mutational shift can limit the utility of mutational assays to monitor genes such as RAS, WT1, CEBPA, and FLT3, which depend on mutational stability in assessing the diagnostic specimens and later biopsies.
In more recent years, next-generation sequencing (NGS) has begun to emerge as a technology with a high sensitivity and specificity for monitoring MRD. The detection limit of NGS assays depends heavily on the overall approach (i.e., genome sequencing, exome sequencing, targeted sequencing) as well as the computer alignment and mutational variant calling software used to identify mutations. Although most standard targeted NGS assays and pipelines can detect as few as 1 to 5 mutated cells in 100, the detection limit of NGS assays can be combined with various other approaches to increase efficiency and in some instances identify as few as 1 mutated cell in 1,000,000. NGS and other technologies are discussed elsewhere in more depth.
Because of the morphologic overlap with myeloblasts, many of the features that are useful in distinguishing leukemic myeloblasts from regenerating myeloblasts also apply to lymphoblasts. Comparison to the original leukemia blasts is useful to identify distinctive features, such as variation in blast cell size and cytoplasm, cytoplasmic vacuoles, nucleoli, and nuclear convolutions. Some lymphoblasts may contain cytoplasmic granules, but Auer rods are not seen. Distinguishing lymphoblasts from normal precursor B cells or hematogones may create diagnostic difficulties and is discussed in detail later. Not surprisingly, the early clearance of blast cells from peripheral blood (by day 7) and bone marrow (by day 14 or 15) in acute lymphoblastic leukemia (ALL) is associated with an improved prognosis in both adults and children. Even the detection of very low levels of bone marrow lymphoblasts by morphologic evaluation is significant. Sandlund and colleagues demonstrated a significantly worse 5-year survival rate in children with 1% to 4% lymphoblasts by morphology on day 15 and by bone marrow aspirate examination on days 22 to 25 compared with children having less than 1% blast cells. Therefore, the morphologic examination for residual blast cells, even at low levels, is of clinical importance.
Bone marrow biopsy morphology can also help detect residual leukemia in ALL. As with leukemic myeloblasts, residual or recurrent lymphoblasts tend to cluster and form aggregates on biopsy material. Such suspicious aggregates can be evaluated by immunohistochemistry, as described later.
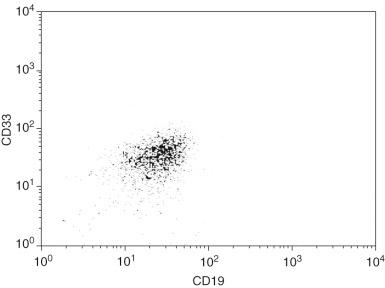
Detection of aberrant immunophenotypes can be extremely helpful in the evaluation for residual ALL. Up to 46% of ALL cases show aberrant expression of myeloid-associated markers ( Fig. 57-8 ). The most common aberrant immunophenotypes are CD13, CD33, and CD38 expression in precursor B-cell ALL with the t(9;22)(q34.1;q11.2) translocation, and CD15 and CD65 expression but lack of CD10 expression in pro–B-cell ALL with KMT2A translocations, particularly t(4;11)(q21;q23.3) ; however, aberrant expression of myeloid antigens may occur in other ALL types. Other immunophenotypic abnormalities, such as dyssynchronous expression of B-cell or T-cell antigens for the stage of lymphocyte development, may be used for the evaluation of residual disease ( Box 57-4 ). Because of the presence of normal precursor B cells in the bone marrow, detection of a small population of CD19-positive, CD10-positive, TdT-positive cells in the bone marrow without other abnormalities is not sufficient for an interpretation of residual precursor B-cell ALL. However, precursor T cells should not be identified in the bone marrow, and the detection of any cytoplasmic CD3-positive and TdT-positive population is evidence of residual T-cell ALL.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here