Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The liver is composed of three systems: the hepatocyte, concerned with metabolic reactions, macromolecular (especially protein) synthesis, and degradation and metabolism of xenobiotics (e.g., drugs); the biliary system, involved with the metabolism of bilirubin and bile salts; and the reticuloendothelial system, concerned with the immune system and the production of heme and globin metabolites (e.g., bilirubin).
The function of each of these systems can be measured conveniently and virtually noninvasively by determining the serum levels of specific analytes, in the so-called liver function test profile.
One of the most common causes of acute liver injury is viral hepatitis, mainly hepatitis A, B, and C, all of which induce acute elevations of serum alanine and aspartate aminotransferase.
Diagnosis of viral hepatitis can be made by screening for viral antigens, especially in hepatitis B, and for immunoglobulin M and G directed against specific viral antigens. Confirmation of the diagnosis of a particular form of viral hepatitis is carried out using suitable molecular diagnostic techniques such as real-time polymerase chain reaction using primers encoding specific viral gene sequences.
Treatment of hepatitis C has changed dramatically recently. The standard treatment of pegylated interferon and ribavirin has now been replaced with specific viral protease (e.g., NS3 protein) inhibitors such as telaprevir, simeprevir, and boceprevir.
The diagnosis of specific liver diseases, including hepatitis, cirrhosis, chronic passive congestion, acute biliary obstruction, space-occupying lesions, autoimmune diseases, and fulminant hepatic failure, can be made from specific patterns of serum liver function tests and from the presence of specific antibodies in serum.
The liver is the largest and most complex organ of the gastrointestinal tract. Overall, it comprises three systems: first, the biochemical hepatocytic system, which is responsible for the vast majority of all metabolic activities in the body, including protein synthesis; aerobic and anaerobic metabolism of glucose and other sugars; glycogen synthesis and breakdown; amino acid and nucleic acid metabolism; amino acid and dicarboxylic acid interconversions via transaminases (aminotransferases); lipoprotein synthesis and metabolism; xenobiotic metabolism (e.g., drug metabolism), usually involving the cytochrome P450 oxidation system; storage of iron and vitamins such as A, D, and B 12 ; and synthesis of hormones such as angiotensinogen, insulin-like growth factor I, and triiodothyronine. It is also the site of clearance of many other hormones such as insulin, parathyroid hormone, estrogens, and cortisol. Uniquely, the liver is the site of metabolism of ammonia to urea.
Albumin in the body is synthesized in the liver, as are all coagulation factor proteins with the exception of von Willebrand factor, which is synthesized in endothelial cells and megakaryocytes. Patients with liver disease may have signs or symptoms related to disturbance of any of the functions outlined earlier.
The second major hepatic system is the hepatobiliary system, which is concerned with the metabolism of bilirubin, a process that involves transport of bilirubin into the hepatocyte and its conjugation to glucuronic acid and its secretion into bile canaliculi and the enterohepatic system. Last is the reticuloendothelial system—that is, Kupffer cells. These are a form of macrophage involved (a) with the immune system, including being a major site of defense against intestinal bacteria and the primary location for removal of antigen–antibody complexes from the circulation, and (b) with the breakdown of hemoglobin from dead erythrocytes, giving rise to bilirubin, which, together with bilirubin from the spleen, enters the hepatocyte.
Because clinical symptoms in liver disease often lag behind the progression of disease, it is important to detect the presence and even the onset of these conditions. Fortunately, evaluation of liver function can often be achieved by determination of serum analytes in a test profile known as liver function tests , many of whose components are not unique to the liver but, when evaluated together, allow for accurate diagnosis of abnormalities of liver function. An outline of liver function tests and their interpretation has been presented in Chapter 9 .
This chapter reviews the most common laboratory tests for evaluation of liver function and injury, methods used for their measurement, testing for causes of liver injury, and patterns of laboratory abnormalities seen in specific liver diseases.
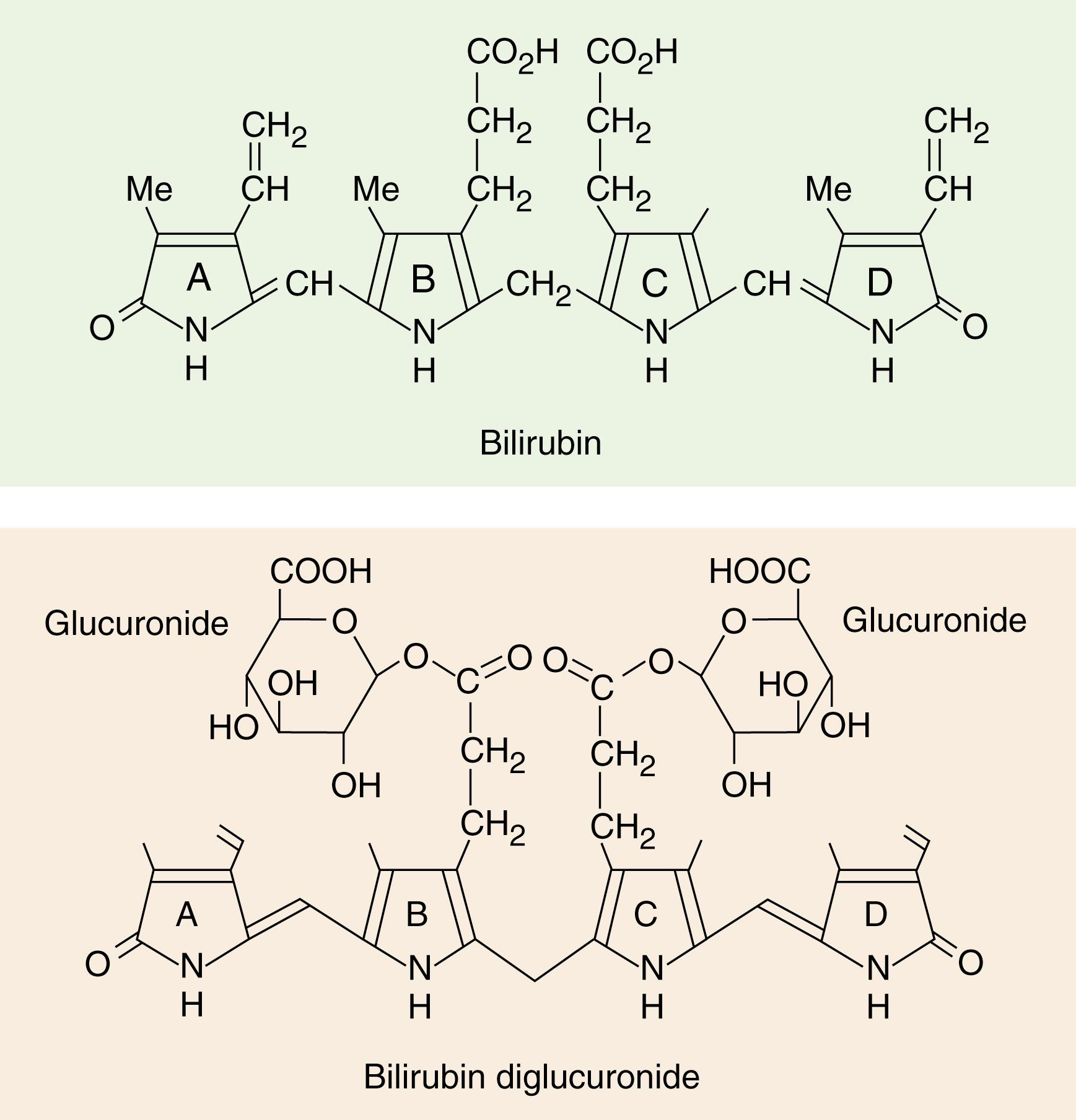
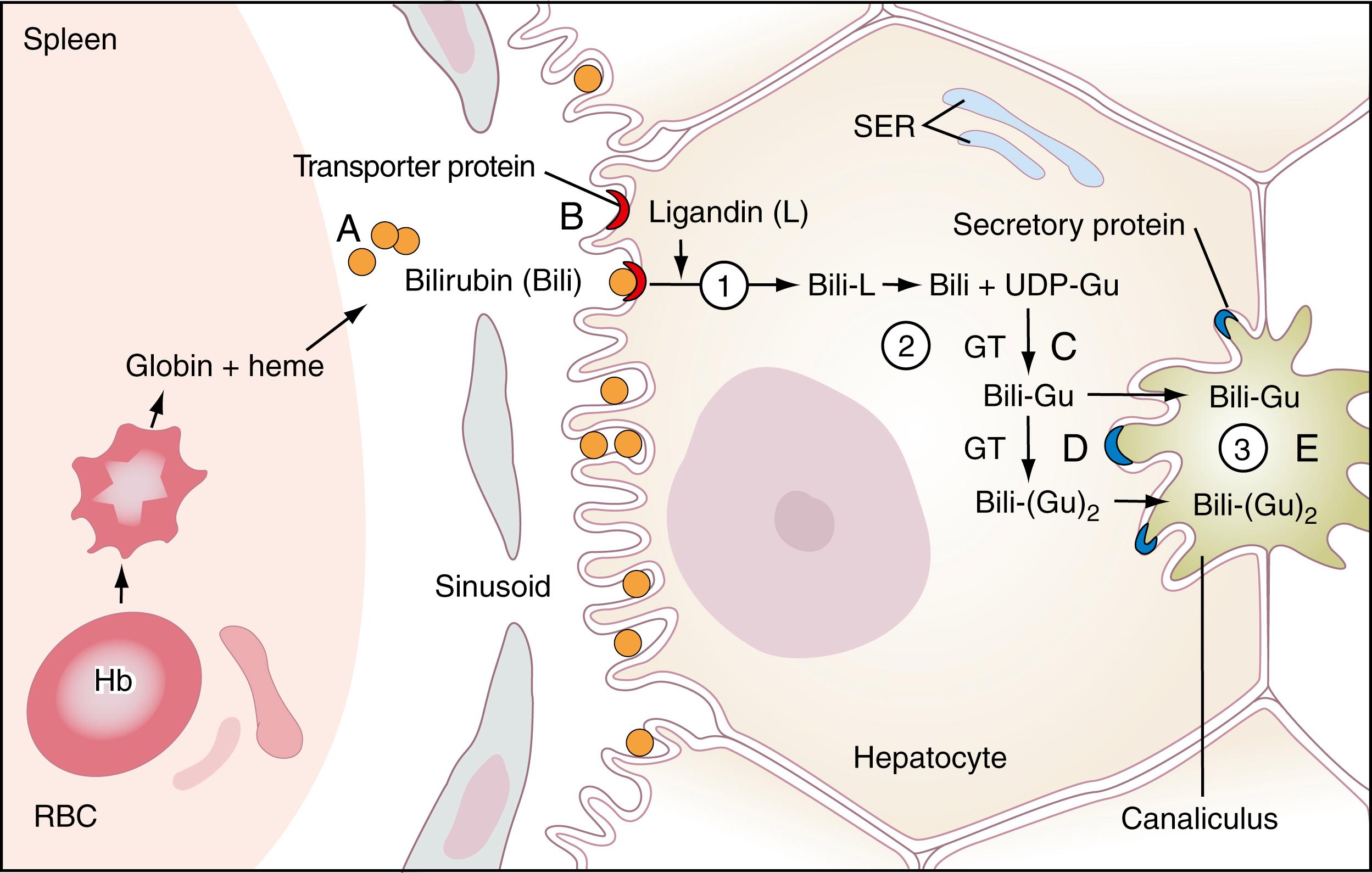
Bilirubin is the major metabolite of heme, the iron-binding tetrapyrrole ring found in hemoglobin, myoglobin, and cytochromes. Approximately 250 to 350 mg of bilirubin is produced daily in healthy adults, about 85% of which is derived from turnover of senescent red blood cells ( ; ; ). In macrophages mainly in the spleen, methemoglobin from red cells is split to give free globin chains and heme. The porphyrin ring of heme is oxidized by microsomal heme oxygenase, producing the straight-chain compound biliverdin and releasing iron. In this ring-opening reaction, 1 mole of carbon monoxide is released, which is transported ultimately as carboxyhemoglobin, whose serum levels can be useful in the diagnosis of hemolytic anemia, as discussed in Chapter 9 . Biliverdin is then reduced to bilirubin ( Fig. 22.1 ; ) by the nicotinamide adenine dinucleotide phosphate (NADPH)-dependent enzyme, biliverdin reductase. Bilirubin, bound mainly to albumin, is then transported mainly in the portal system to the liver, where it enters the hepatocyte through its membrane surface in contact with the sinusoids, as shown in Figure 22.2 . Specific transporters on the sinusoidal (basolateral) plasma membrane of hepatocytes help mediate the transport and uptake of bile acids and non-bile acid organic anions such as bilirubin, sulfobromophthalein (BSP), various drugs, and hormones. The fenestrated sinusoidal endothelium of the liver permits these compounds to have access to hepatocytes. These transporters include organic anion transport proteins (oatps), a family of 12 transmembrane domain glycoproteins and Na+/taurocholate transporting protein (ntcp), a seven transmembrane domain glycoprotein ( ; Russell, 2007).
As free bilirubin enters hepatocytes, additional bilirubin dissociates from albumin. This process is highly efficient; clearance of unconjugated bilirubin at normal values is about 5 mg/kg/day or, for a 75-kg individual, about 400 mg/day ( ). The half-life of unconjugated bilirubin is short; 60% of labeled bilirubin appears within hepatocytes within 5 minutes of injection ( ). The clearance rate increases with an increasing concentration of unconjugated bilirubin up to at least 4 mg/dL ( ).
In its most common isomeric ( trans- ) form, bilirubin is highly insoluble in water, and most of it is transported bound to albumin, with only a small fraction of free bilirubin. Light can cause photoisomerization of bilirubin, from a trans- form to a more compact cis- form, making it much more water soluble and allowing it to be excreted in urine ( ). This forms the basis for phototherapy in the treatment of neonatal (unconjugated) hyperbilirubinemia. The pathway for clearance of bilirubin by the liver is illustrated in Figure 22.2 . Note that unconjugated bilirubin enters the hepatocyte at the membrane surface adjacent to the sinusoids, opposite the face that is in contact with bile canaliculi.
Bilirubin enters hepatocytes by two mechanisms: passive diffusion and receptor-mediated endocytosis. As summarized in Figure 22.2 , once in the hepatocyte, bilirubin is “handed-off” from one protein complex to another in a chain. First, it complexes with the so-called Y and Z proteins; then, it binds sequentially to a protein complex called ligandin . From this complex, it is transported to the smooth endoplasmic reticulum (SER). In the SER, bilirubin becomes the substrate of the enzyme glucuronyl transferase, encoded by the UGT1A1 gene, which catalyzes the esterification of the propionic acid side chains of bilirubin with glucuronic acid (present as uridine diphosphoglucuronic acid) to form mainly the diglucuronide conjugate shown in Figure 22.1 (bottom) ( ). Some monoglucuronide and a small amount of triglucuronide also form. The ratio of monoconjugated to diconjugated pigment in bile is 1:4, whereas the ratio is nearly 1:1 in plasma, suggesting that monoconjugates reflux into plasma more readily.
Both monoconjugated and diconjugated pigments undergo ATP-dependent transport via an ABC cassette that is mediated by multidrug resistance protein (MRP1). Additionally, there is a distinct canalicular isoform (MRP2). Both MRP1 and MRP2 are ATP-dependent export pumps. This transporter function is deficient in Dubin-Johnson syndrome ( ), discussed later.
As shown in Figure 22.2 , an energy-dependent mechanism transports the conjugated bilirubin to the canalicular face of the hepatocyte, at which it is directly secreted into the canaliculi. Only conjugated bilirubin can be directly excreted into the canaliculi; unconjugated bilirubin cannot traverse this membrane.
Once bilirubin is excreted into the canaliculi and ultimately into the intestinal tract, it is further metabolized by intestinal bacteria, which effect its deconjugation and oxidation or reduction with the formation of compounds collectively called urobilinogen and urobilin , which can then be reabsorbed from the gut. Most urobilinogen absorbed is reexcreted by the liver. A minor fraction may be excreted in the urine. Larger quantities are found in the urine in conditions leading to hyperbilirubinemia or in conditions in which the liver cannot readily secrete urobilinogen absorbed from the gut. Ultimately, intestinal urobilinogen is converted to stool pigments such as stercobilin. Their absence leads to clay-colored stools, often an early sign of impaired bilirubin metabolism.
When conjugated bilirubin is present in serum, it can become covalently bound to albumin, producing biliprotein or delta-bilirubin ( ; ). Although conjugated bilirubin has a half-life of less than 24 hours, delta-bilirubin has a half-life similar to that for albumin at 17 days ( ), causing prolonged jaundice during recovery from hepatocellular injury ( ) or biliary obstruction ( ). Conjugated bilirubin, being water soluble, can be filtered by the glomerulus and can appear in urine, where it may be detected by dipstick examination. Urobilinogen measurement, however, adds little to standard tests of liver function or injury ( ). Urinary bilirubin is elevated in most patients with increased serum conjugated bilirubin ( ).
As shown in Figure 22.2 , in each step in the processing of bilirubin, a possible lesion leads to elevated serum levels of unconjugated or conjugated bilirubin. Each of these is discussed in turn.
Hemolysis. As discussed in Chapter 9 , in hemolytic anemias, unconjugated bilirubin rises as a result of abnormally high levels of hemoglobin released from erythrocytes. If the rate of bilirubin formation exceeds the rate of liver clearance (i.e., a state of overproduction of bilirubin), there will be a rise in the bilirubin level in serum. Virtually all of this bilirubin will be unconjugated bilirubin. This is particularly likely to occur in neonates, whose glucuronyl transferase activity is low. Thus, one manner of confirming a diagnosis of hemolytic anemia is the finding, in adults, of elevated indirect bilirubin levels in serum. Usually, these levels are not dramatically elevated and are generally in the 1.5 to 3.0 mg/dL range.
Gilbert syndrome and Crigler-Najjar syndrome are caused by gene mutations and deletions. In Gilbert syndrome, characterized by a mild unconjugated hyperbilirubinemia, the most common genetic lesion appears to be the insertion of two bases into the promoter region of the UGT1A1 gene that encodes glucuronyl transferase, resulting in lower transcriptional rates ( ; ) and overall lower enzymatic activity (reduced to about 30% of normal). In the more serious Crigler-Najjar syndrome, frequently characterized by high serum levels of unconjugated bilirubin, multiple mutations are found to occur in this gene, including shifts in the reading frames, stop codons, and critical amino acid substitutions, all of which give rise to a spectrum of dysfunctional proteins, from mildly dysfunctional to completely nonfunctional ( ).
In Gilbert syndrome, which occurs in a significant fraction (3%–5%) of the population, the genetic defect may be necessary but not sufficient because, in an earlier study ( ), a significant percentage of males with this defect were found to have hyperbilirubinemia, but no females with this enzyme deficit were found to have elevated serum bilirubin levels ( ). In some patients with Gilbert syndrome, the rate of organic anion uptake has been found to correlate negatively with serum bilirubin ( ), suggesting that an additional defect may be present to cause hyperbilirubinemia that may be related to a transport deficit in the sinusoidal membrane of the hepatocyte. In this condition, total bilirubin, virtually all of which is unconjugated, is typically elevated to 2 to 3 mg/dL; levels can increase further with fasting but seldom exceed 5 mg/dL. Because passive diffusion of bilirubin into hepatocytes occurs, this condition is rarely serious and may result in mild elevations of bilirubin such as those seen in hemolytic anemia, as described previously.
Gilbert syndrome has perhaps been overdiagnosed; it is most frequently diagnosed in young adults ranging in age from 20 to 30 years. However, normal bilirubin ranges are age dependent and actually reach their highest levels in adolescents and young adults ( ), as discussed further later. Diagnosis of UGT1A1 mutations have an important practical value, not just for the accurate diagnosis of Gilbert syndrome, but also because carriers of various genotypes of UGT1A1 differ in their metabolism of a number of common medications. This may assist in adjusting the dosage of these medications when considering a patient’s individual genetics. Recent studies show that DNA melting curve analysis is an effective method for rapidly diagnosing Gilbert syndrome ( ).
In the more serious or type I form of the Crigler-Najjar syndrome (i.e., homozygously nonfunctioning proteins), the unconjugated hyperbilirubinemia becomes marked, almost always exceeding 5 mg/dL and causing jaundice and sometimes exceeding 20 mg/dL. Affected infants develop severe unconjugated hyperbilirubinemia, which typically leads to kernicterus, deposition of bilirubin in the brain, particularly affecting the basal ganglia, mainly the lenticular nucleus, causing severe motor dysfunction and retardation. In the less severe type II form, enzyme activity is approximately 10% of normal, and survival to adulthood is possible ( ). The danger of kernicterus is a certainty at levels exceeding 20 mg/dL. It is vital to treat these infants with phototherapy, as discussed earlier, to cause excretion of the unconjugated bilirubin.
Excretion deficits: Dubin-Johnson syndrome. In another inborn error of metabolism, called Dubin-Johnson syndrome , there is a blockade of the excretion of bilirubin into the canaliculi, caused by defects in the adenosine triphosphate (ATP)-binding cassette (ABC) canalicular multispecific organic anion transporter, MRP2/cMOAT/ABCC2 ( ; ; ). This protein is a member of a family of approximately 100 different transporter proteins that share homology within the ABC region and contain transmembrane domains involved in recognition of substrates, which are transported across, into, and out of cell membranes and include proteins involved in multiple-drug resistance to chemotherapeutic agents in cancer treatment. Some protein members utilize ABCs to regulate ion channels. Several genetic diseases result from transporter mutations, including Dubin-Johnson syndrome, cystic fibrosis, age-related macular degeneration, Tangier disease, and progressive familial intrahepatic cholestasis ( ).
Dubin-Johnson syndrome is associated with increased plasma conjugated bilirubin, typically with mild jaundice (total bilirubin, 2–5 mg/dL), and intense dark pigmentation of the liver due to accumulation of lipofuscin pigment, brown granules from liposomal degradation of lipids. Thus, conjugated bilirubin accumulates within the hepatocyte and eventually back-diffuses into the circulation, where it is detected in serum. This inborn error can sometimes be confused with Rotor syndrome, possibly of viral origin, where there is also a block in the excretion of conjugated bilirubin but without liver pigmentation ( ). In these cases, liver biopsy often will reveal cytosolic inclusion bodies within hepatocytes.
Biliary obstruction. In adults, cholelithiasis is the most common cause of hyperbilirubinemia. This condition results from the presence of bile stones (composed of bilirubin or cholesterol), most commonly in the common bile duct (choledocholithiasis). Most frequently, patients presenting with this condition are parous white females in early middle age (giving rise to the alliterative, semi-mnemonic, “fair, fecund, fortyish female”). Biliary obstruction due to cholelithiasis results in elevation of total bilirubin, with over 90% being direct bilirubin. In more than 90% of such patients, a concomitant rise in alkaline phosphatase (ALP) occurs. The levels of this enzyme are variable but are frequently above 300 international units (IU)/L.
Inflammatory conditions of the biliary tract, such as ascending cholangitis, also give rise to elevated serum levels of direct bilirubin and ALP, as discussed later in this chapter. The rise in direct bilirubin often exceeds 5 mg/dL. In gram-negative sepsis, there can be what appears to be a mild inflammation of the biliary tract, resulting in mild elevation of direct bilirubin to levels of 2 to 3 mg/dL. A concomitant elevation of ALP to levels of 200 to 300 IU/L is also observed.
In hepatitis, in which toxic destruction of hepatocytes is due to viral, chemical, or traumatic causes, focal necrosis and/or cellular injury results both in blocking conjugation of bilirubin and in excretion of conjugated bilirubin. Thus, elevation of both direct and indirect bilirubin occurs. Serum levels of bilirubin are variable, depending on the severity of infection and the extent of disease. In viral hepatitis, such as hepatitis B, as discussed subsequently, serum bilirubin levels often reach levels of 5 to 10 mg/dL or greater.
Aside from liver disease, elevations of conjugated bilirubin may occur with a few other disorders. Septicemia (as noted previously), total parenteral nutrition, and certain drugs such as androgens commonly cause increased conjugated bilirubin, but the mechanism is not understood ( ; ). Fasting causes increases in unconjugated bilirubin in normal individuals but to a lesser degree than is seen in Gilbert syndrome.
Bilirubin is typically measured using diazotized sulfanilic acid, which forms a conjugated azo compound with the porphyrin rings of bilirubin, resulting in reaction products that absorb strongly at 540 nm (see Chapter 28 ). Because unconjugated bilirubin reacts slowly, accelerants such as caffeine or methanol are used to measure total bilirubin. Deletion of these accelerants allows determination of direct-reacting, or direct, bilirubin.
Until the early 1980s, it was accepted that direct bilirubin was equal to conjugated bilirubin. The introduction of dry-slide technology, using differential spectrophotometry to measure conjugated and unconjugated bilirubin separately, led to the observation that the sum of these two entities did not equal total bilirubin and to the characterization of delta-bilirubin. Approximately 70% to 80% of conjugated bilirubin and delta-bilirubin and a small percentage of unconjugated bilirubin are measured in the direct bilirubin assay ( ; ). Although good data support the measurement of conjugated bilirubin instead of estimating it from direct bilirubin ( ; ), the direct bilirubin assay is still widely used. The accuracy of direct bilirubin assays is dependent on sample handling and reagent composition. Prolonged exposure to light causes photoisomerization, increasing direct-reacting bilirubin ( ; ). Use of wetting agents or incorrect pH buffers increases the amount of unconjugated bilirubin measured as direct bilirubin ( ). Typically, direct bilirubin should measure 0 to 0.1 mg/dL in normal individuals, with rare values of 0.2 mg/dL in the absence of liver or biliary tract disease.
Reference values for total bilirubin are both age and gender dependent. Bilirubin levels typically reach peak values at around ages 14 to 18 years, falling to stable adult levels by age 25 years ( ; ; ). Values are higher in males than in females at all ages ( ; ; ; ; ). Strenuous exercise causes a significant increase in bilirubin values compared with those seen in sedentary individuals or those engaging in chronic exercise ( ; ; ; ). African Americans have bilirubin levels significantly lower than those of other ethnic groups.
This critical and toxic compound is metabolized exclusively in the liver. Ammonia is derived mainly from amino acid and nucleic acid metabolism. Some ammonia is also produced from metabolic reactions such as the action of the enzyme glutaminase on glutamine, resulting in the production of glutamic acid and ammonia. As it happens, ammonia can be metabolized only in the liver because the liver uniquely contains the critical enzymes for the Krebs-Henseleit or urea cycle, in which ammonia, a toxic substance, is ultimately converted into urea, a nontoxic compound that is readily excreted. In this cycle, ammonia, with the enzyme carbamoyl phosphate synthetase, is condensed with carbon dioxide (CO 2 ) and ATP to form carbamoyl phosphate that then, in the rate-determining step, carboxamidates the delta-amino group of ornithine to form citrulline using the enzyme ornithine carbamoyltransferase (OCT), an enzyme that is unique to the liver. Congenital deficiency of this or other urea cycle enzymes leads to increased levels of ammonia in serum and in cerebrospinal fluid ( ).
A unique feature of liver tissue is its ability to regenerate. To abolish liver tissue function, more than 80% of the liver must be destroyed. If most of the liver is destroyed by such conditions as cirrhosis ( ) or, less commonly, acute fulminant hepatic failure, including Reye syndrome ( ; ), urea cycle enzymes are no longer present. This leads to a toxic buildup of ammonia and some of the amino acid intermediates in the urea cycle, such as arginine, which has known neurotoxic effects. The result is an increase in ammonia and these amino acid intermediates in the circulation and in the central nervous system (CNS), giving rise to hepatic encephalopathy. In addition, in most cirrhotics, intrahepatic portal-systemic shunting occurs, causing ammonia to bypass the liver and resulting in elevated serum ammonia concentrations. Elevated serum levels of ammonia therefore often indicate some form of liver failure, although other conditions can also induce increases in serum ammonia levels.
In patients with cirrhosis or fulminant hepatic failure, there has been some dispute as to whether ammonia itself is the cause of the observed metabolic encephalopathy; possibly other toxins that accumulate as a result of absent hepatic detoxification are the cause. One of the arguments often used is that there is no clear correlation between the severity of the encephalopathy and serum ammonia concentrations ( ). Countering this argument is the finding that, although venous ammonia levels do not correlate with the degree of encephalopathy ( ), arterial levels of ammonia do generally correlate with the degree of encephalopathy. Furthermore, in patients with cirrhosis or fulminant hepatic failure, lowering serum ammonia invariably diminishes the severity of the encephalopathy ( ). Furthermore, idiopathic hyperammonemia, not related to liver disease, also induces lethal encephalopathy ( ; ). An important mechanism by which ammonia can cause toxicity to the CNS is its ability to lower the concentration of γ-aminobutyric acid (GABA), a critically important neurotransmitter in the CNS, by reacting with glutamic acid to form glutamine via reversal of the glutaminase-catalyzed reaction ( ). This depletes glutamic acid in the CNS. However, GABA is formed directly from the decarboxylation of glutamic acid; thus, GABA levels consequently decrease, with potentially serious effects on neurotransmission (see Chapter 24 ). Because ammonia causes accumulation of glutamine in the CNS, there is the suggestion that, at least in valproic acid–induced hyperammonemia, cerebrospinal fluid levels of glutamine can be used in the diagnosis and management of hepatic encephalopathy ( ). More recently, besides evidence that ammonia in the CNS is directly toxic to astrocytes, there is growing recognition that there is a complex and synergistic relationship between ammonia, inflammation, and oxidative stress in the pathogenesis of hepatic encephalopathy. Neutrophil dysfunction results in the generation of reactive oxygen species contributing to oxidative stress and inflammation, with lowered ability of the CNS to block infectious agents. In accordance with these findings, strategies targeting systemic inflammation, such as rifaximin-α and other anti-inflammatory agents being studied, are quickly becoming the new mainstay in the treatment of hepatic encephalopathy ( ; ). One major new finding put forth by these studies is that treatment of hepatic encephalopathy with suitable anti-inflammatory agents may be effective.
At present, most commonly, elevated serum ammonia concentrations in hepatic encephalopathy are reduced by the agent lactulose, which is metabolized by a wide range of gut bacteria to lactic acid ( ). The acid so produced in the intestinal lumen traps ammonia as ammonium ion, which can no longer diffuse across the intestinal membrane and is thus excreted. Ammonia-producing bacteria in the intestine are removed by treatment with antibiotics such as neomycin.
Ammonia is typically measured by enzymatic assays using glutamate dehydrogenase, which catalyzes the reaction of α-ketoglutarate and ammonia to form glutamate, with oxidation of NADPH to NADP as the indicator (decrease in absorbance at 340 nm, as described in Chapter 21 ). Ammonia is also measured via a dry-slide method (e.g., on the Ortho Diagnostics Vitros systems) using alkaline pH buffers to convert all ammonium ions to ammonia gas, with bromophenol blue as the indicator ( ). Because ammonia is a product of cellular metabolism, methods used in specimen collection and transportation are critical in preventing artifactually increased levels. Arterial blood is the preferred specimen for measurement of ammonia. Although venous blood is not recommended, if used, tourniquets should be used minimally, and fist clenching and relaxing should be avoided during collection. Specimens should be kept in ice water until separation of cells from plasma occurs ( ; ).
Because the liver is vital in lipoprotein synthesis and interconversions, hepatic disorders often cause derangements in lipoprotein metabolism. Although none of these abnormalities is used to diagnose liver pathology, it is important to recognize that they may result from liver disease. In severe liver injury, including cirrhosis, these abnormalities include a decrease in high-density lipoprotein (HDL), particularly the HDL 3 (but often not the HDL 2 ) subfraction, and in other altered lipoprotein distributions, caused in part by deficiencies of lecithin/cholesterol acyltransferase (LCAT, the enzyme that esterifies cholesterol) and of lipoprotein lipases, resulting in elevated levels of unesterified cholesterol and hypertriglyceridemia (triglyceride levels ranging from 250 to 500 mg/dL), respectively. In addition, there are increased levels of phospholipids, including lecithins, in blood and in the very-low-density lipoprotein (VLDL) fraction. Overall, the resulting lipoprotein pattern is that of the so-called abnormally migrating β-lipoprotein, typical of type III hyperlipoproteinemia (see Chapter 18 ). However, in cirrhotics with poor nutrition, despite critical enzyme deficiencies, low levels of cholesterol (<100 mg/dL) may be found.
In contrast, in alcohol-induced liver injury, alcohol induces increased expression of apolipoprotein (apo) A-I protein. Thus, HDL—especially HDL 3 —may be elevated if alcohol ingestion continues. Because apoA-I protein decreases in cirrhosis, serum levels of this protein have been used to diagnose this disease using the so-called PGA index ( ), discussed later in this chapter, a combination of prothrombin time (PT), which increases, with γ-glutamyl transferase activity (discussed later), which also increases, and apoA-I protein. This index differs for alcoholic hepatitis, enabling the distinction to be made between these two conditions without the necessity of liver biopsy ( ).
In cholestasis, regurgitation of biliary contents into the bloodstream results in the buildup of lipoprotein X (LpX; discussed in Chapter 18 ) and elevation of biliary lipids. Because LpX carries high levels of unesterified cholesterol, cholesterol levels in serum can become markedly elevated ( ).
Bile salts, which are products of cholesterol metabolism, facilitate absorption of fat from the intestine. They are stored in the gallbladder and released to the intestine after meals through gallbladder contraction mediated by cholecystokinin. They are not usually used in the diagnosis of abnormal liver function but are important in that they constitute a substantial amount of bile in bilirubin excretion and can therefore be of use in diagnosing cholestasis. Also, in severe biliary obstruction, the buildup of bile salts in serum causes symptomatic illness in the form of intractable itching, although this has been disputed ( ). The primary bile salts, cholate and chenodeoxycholate, are produced in the liver and excreted into the biliary and enterohepatic systems. In the intestinal lumen, bacteria utilize 7-α-dehydroxylation to produce secondary bile salts (i.e., lithocholate, deoxycholate, and ursodeoxycholate) ( ). Ursodeoxycholate, an end-product of bile salt metabolism in humans, is produced by isomerization of secondary bile salts and has been found to be therapeutic in cholestatic diseases ( ). These bile salts are conjugated, in the microsomal system discussed later, to glycine and taurine and are also sulfated and glucuronidated. Conjugation of bile salts to taurine and sulfates increases with the severity of cholestasis in conditions causing obstruction to bile outflow. Recirculation of bile salts to the liver occurs by reabsorption from the terminal ileum, where deoxycholate is almost completely reabsorbed, and chenodeoxycholate is about 75% reabsorbed. In cirrhosis, a disproportionate decrease in cholic acid is seen, along with a reduced ratio of primary to secondary bile salts. With cholestasis, secondary bile salts are not formed; thus, the ratio of primary to secondary bile salts is markedly increased.
Renal clearance of bile salts is negligible in normal patients, but in cholestasis, renal excretion of bile salts, mainly in the form of sulfates and glucuronides, is enhanced. Fasting bile salts, when normal, can exclude the presence of parenchymal liver disease in patients with Gilbert syndrome ( ), as discussed previously. It should also be recognized that defective production of bile salts, which helps solubilize the contents of bile, in the liver may predispose to the formation of bilirubinate or cholesterol stones and posthepatic biliary obstruction.
Analysis of bile salts must be performed on serum taken from patients who are in the fasting state or on serum taken at a specified time after meals, because food ingestion causes a significant increase in bile acid levels. Bile salts can be measured by many techniques, but chromatographic methods, particularly high-performance liquid chromatography, as discussed in Chapter 24 , are most widely used and allow separation of different bile salts.
Many xenobiotics, such as drugs, are metabolized in the liver, mainly in the microsomes of hepatocytes. Complex series of reactions occur, many of which are dependent on cytochrome P450, which is involved in the oxidation of these compounds. Whether specific exogenous compounds are converted to metabolites depends on the isoforms of cytochrome P450, such as CYP1A and CYP2B (cytochrome P450 1A and 2B, respectively). Often, the conversion of xenobiotics into metabolites using this system involves two phases: Phase I reactions involve oxidations/hydroxylations, and phase II reactions conjugate the metabolite (or parent compound) to polar compounds, such as glucuronic acid, glycine, taurine, and sulfate. In more severe liver disease, which involves microsomal damage, this ability to metabolize xenobiotics is compromised. Thus, the ability of hepatocytes to metabolize drugs can be used to measure liver damage.
This is generally accomplished by administering a known dose of isotopically labeled (usually 13 C-labeled) drug and measuring the 13 CO 2 exhaled over time in a patient’s breath. Two categories of breath tests have been developed based on the rate-limiting step in metabolism. The first group includes drugs such as aminopyrine, caffeine, and diazepam, which are metabolized at rates that are independent of hepatic blood flow to the liver and depend only on the enzymatic activity of different cytochromes P450 (e.g., CYP1A). The second group is composed of drugs such as methacetin, phenacetin, and erythromycin, whose rates of metabolism depend on the rate of blood flow (i.e., their rates of metabolism are fast compared with their rates of delivery to the liver). These types of dynamic tests appear not to be so useful in the initial diagnosis of hepatic disease; rather, they are more useful in estimating the extent of liver damage in known liver disease ( ).
Some interferences that complicate interpretation of the results of these tests include dependence of the demethylation of aminopyrine (the methyl group is oxidized to CO 2 ) on vitamin B 12 . In cases of vitamin B 12 deficiency, less than normal amounts of 13 CO 2 will be exhaled because of low B 12 levels, not necessarily because of liver damage. Rates of caffeine metabolism generally decrease with increasing age but are increased by smoking; these findings can complicate interpretation of test results.
The liver is the site of synthesis for most plasma proteins. Major exceptions include immunoglobulin (Ig) and von Willebrand factor. Synthesis of more than 90% of all protein and 100% of albumin occurs in the liver. Thus, extensive destruction of liver tissue will result in low serum levels of total protein and albumin. In cirrhosis, besides hepatocyte destruction, another cause of diminished protein production is portal hypertension, which decreases delivery of amino acids to the liver. Two vital measurements of liver function, therefore, are total protein and albumin levels in serum. However, it should always be kept in mind that other major causes of low serum total protein and albumin have been identified. These include renal disease, malnutrition, protein-losing enteropathy, and, less commonly, chronic inflammatory disease. These alternative causes must always be considered when liver function status is evaluated.
In liver disease with widespread injury or necrosis, such as fulminant hepatic failure and cirrhosis, plasma levels of liver-synthesized proteins fall such that proteins with longer half-lives tend to decrease more slowly. Albumin has a half-life of about 20 days; thus, decreases in its serum levels occur more slowly than those of proteins with shorter half-lives. Among the liver-produced proteins with shorter half-lives are factor VII (4–6 hours), transthyretin (1–2 days), and transferrin (6 days).
This is based usually on the biuret method as described in Chapter 28 . This method reflects the ability of the peptide backbone C=O groups of proteins to form color complexes with copper that absorb strongly at 540 nm. Some methods utilize a dye-binding method in which the proteins form a complex with the dye Coomassie blue. Albumin forms a unique color complex with the dyes bromocresol green and bromocresol purple, such that they absorb maximally at slightly different wavelengths, thus allowing direct spectrophotometric quantitation ( ; ; ; ). Bromocresol purple tends to react more exclusively with albumin than does bromocresol green (which reacts to a minor extent with some globulins); thus, serum albumin levels may be slightly lower when determined with bromocresol purple. The reference range for total serum protein levels is generally in the 6.0 to 7.8 g/dL range. At least 60% of this should be albumin, the normal range for which is about 3.5 to 5.0 g/dL.
Serum protein electrophoresis and quantitative Ig may reveal characteristic changes in liver disease, as discussed in Chapter 20 . Typically, in cirrhosis, albumin is significantly decreased, as are the α-1, α-2, and β (principally transferrin) bands. However, a polyclonal increase in Ig, which is seen frequently, produces the characteristic β-γ bridging pattern, as discussed in Chapter 20 . In autoimmune hepatitis, albumin is typically decreased; this is accompanied by a marked polyclonal increase in IgG. Primary biliary cirrhosis, an autoimmune disease, is accompanied by a polyclonal increase in IgM. However, as noted later, this polyclonal IgM does not react with the mitochondrial antigens against which the autoimmune antibodies in this disease do react ( ).
Albumin is the major protein produced by the liver. Its synthesis is increased by low plasma oncotic pressure and is decreased by cytokines, particularly interleukin-6. Although normal albumin synthesis occurs at about 120 mg/kg/day, the rate of synthesis can approximately double with low oncotic pressure. A decrease in albumin is one of the major prognostic features in patients with cirrhosis. Albumin measurements were discussed previously in Chapter 20 and are further discussed in Chapter 28 .
Albumin is a transport protein for many substances, both endogenous (e.g., bilirubin, thyroid hormone) and exogenous (e.g., drugs). Low serum albumin levels due to liver disease are almost always caused by massive destruction of liver tissue and are seen primarily in cirrhosis, most often secondary to alcoholism. The diminution in albumin is paralleled by a fall in total serum protein. Because albumin is the osmotically active intravascular colloid, hypoalbuminemia often results in edema. In cirrhosis, in which increased resistance to blood flow in the sinusoids causes portal hypertension, the combined effect of elevated hydrostatic pressure in the portal system and low colloid osmotic pressure results in ascites, a frequent finding in cirrhosis. These same changes may also be seen acutely in fulminant hepatic failure ( ; ).
Although most of the proteins discussed in Chapter 20 are produced by the liver, two bear special importance in the detection of congenital liver disorders.
α 1 -antitrypsin (AAT), the most abundant α 1 -globulin, is the most important protease inhibitor in plasma. Although its name indicates that it inhibits trypsin, it also is an inhibitor of other serine proteases, such as elastin. AAT is coded for by the Pi gene on chromosome 14; several genetic variants are due to point mutations, leading to single amino acid substitutions ( ). The most common variant, M, is associated with normal serum AAT levels. The mutations present in the S and Z variants prevent normal protein glycation, leading to accumulation of AAT within hepatocytes and reduced plasma AAT levels ( ; ; ; ; ). In the United States, the overwhelming genotype is PiMM, where Pi is the protease inhibitor. The other genotypes—PiZZ, PiSS, PiSZ, PiMZ, and PiMS—all contain measurable activity of antiprotease, except a rare null genotype, Pi — . If the antiprotease activity of the MM phenotype is used as the reference, then the activity in phenotype ZZ is 15%, SS is 60%, MZ is 57.5%, and MS is 80%. Adults with PiZZ are most prone to develop emphysema relatively early in life as a result of uninhibited trypsin activity on alveolar wall elastin. Patients with PiZZ tend to accumulate the Z protein in periportal hepatocytes, where it forms discrete cytoplasmic bodies and may also develop neonatal hepatitis. Curiously, although infants may die of hepatic injury, it resolves in most infants and progresses to cirrhosis in only about 3% ( ). In adults, the likelihood of liver injury is increased in patients heterozygous or homozygous for the Z variant of AAT. This may be due to accumulation of AAT in the endoplasmic reticulum that induces autophagy and apoptosis of hepatocytes ( ). AAT phenotyping can be performed using isoelectric focusing ( ; ). Because AAT is an acute-phase reactant, its serum levels can be normal in MZ heterozygotes.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here