Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The posterior fossa is the infratentorial compartment of the cranial vault, which houses the cerebellum and the bulk of the brainstem. Tumors that occur in this region can arise from, or spread to, any of the adjacent structures. The brainstem, which is composed of the midbrain, pons, and medulla, may develop or seed tumors that are intrinsic and contained within the brainstem, intrinsic with exophytic components, or extraaxial. Tumors in the posterior fossa may produce general symptoms caused by increased intracranial pressure, localizing symptoms due to compression of specific nerves or nuclei, or a combination of both general and localizing symptoms .
The posterior fossa is a small compartment compared with the supratentorium, and the cerebrospinal fluid (CSF) outflow tracts in this region are much smaller and less tolerant of compression. Even a small tumor in the midbrain region may produce significant aqueductal stenosis and obstructive hydrocephalus which may present with early morning awakening with headache, recumbent headaches, nausea, vomiting, and personality, mood, or mental capacity changes. Other tumors may produce symptoms specific to the tumor location. Tumors in the proximal brainstem commonly present with unilateral cranial nerve (CN) VI and VII palsies or with tectal involvement and Perinaud’s syndrome. Tumors intrinsic to the pons typically present with a short clinical history, bilateral CN VI and VII dysfunction, ataxia, and long tract signs. Tumors in the medulla may cause deficits in cranial nerves VII, IX, and X early on, while obstructive symptoms including headaches or papilledema tend to occur later as the lesion compresses the fourth ventricle. Midline cerebellar tumors can cause truncal and gait ataxia, whereas lateral cerebellar hemispheric tumors tend to cause unilateral appendicular ataxia, commonly worse in the arm compared with the leg. Children with lateral cerebellar hemispheric tumors may be found tilting their heads away from the side of the tumor.
Across all ages, brain tumors are predominantly located supratentorially. However, in children 3–18 years old, infratentorial tumors are more prevalent than supratentorial tumors, accounting for more than 60% of all CNS cancers in this age group. The differential diagnosis for lesions found in the posterior fossa, therefore, is highly age-dependent. The index of suspicion for a tumor in an adult with a posterior fossa lesion on imaging, in whom the most common expansile lesion in this location is a subacute stroke followed by cerebellar metastasis, is different compared with children, in whom primary brain tumors are the most common solid tumor.
CT and MRI are the primary neuroimaging techniques used to investigate posterior fossa lesions. For most posterior fossa tumors, gadolinium-enhanced MRI is the modality of choice because of its superior anatomic resolution and sensitivity in detecting pathologic alterations relative to normal brain architecture. The benefit of CT studies, however, is that they are quicker, less expensive, more widely available at small and medium-sized hospitals than MRI, and can more easily be obtained without sedation. Non-enhanced CT can rapidly exclude life-threatening emergencies such as hydrocephalus, hemorrhage, or significant mass effect. Although CT can be used to screen for hydrocephalus, MRI is superior at isolating a posterior fossa lesion that may be causing obstruction of the cerebral aqueduct or the fourth ventricle. Though an acute hemorrhage associated with a brain tumor can be visualized on CT as a hyperdense lesion, a subacute hemorrhage is more often isodense to normal brain and harder to detect by CT alone; subacute hemorrhage is visible on MRI as it produces a bright signal on both T1- and T2-weighted sequences. Additionally, whereas contrast-enhanced CT and MRI can localize a tumor that has disrupted the blood-brain barrier and is, thus, contrast-enhancing, non-enhancing tumors are often isodense to brain, making MRI scans significantly more sensitive for detecting such lesions. Finally, advanced MRI techniques, such as diffusion-weighted imaging (DWI), perfusion-weighted imaging (PWI), MR-PET, and dynamic contrast-enhanced studies increase diagnostic confidence and may be helpful in distinguishing different tumor types and histological grades.
Computed tomography . Certain characteristics of tumors imaged with CT can provide clues as to tumor type. Tumors with high cellularity, such as high-grade gliomas (HGGs), metastases, medulloblastoma, ependymoma, and atypical teratoid rhabdoid tumor (ATRT), may appear hyperdense or isodense compared with surrounding tissue, whereas tumors with low cellularity, such as low-grade gliomas (LGGs), are likely to appear hypodense. LGGs may be associated with intratumoral cysts that can be seen on CT, though cysts are not unique to LGGs, as medulloblastomas and hemangioblastomas can also have cystic components. Calcifications, which are better visualized with CT versus MRI, may be found up to 20% of cases of medulloblastoma and 50% of cases of ependymoma.
Magnetic resonance imaging . MRI features can help differentiate posterior fossa tumor types, though there is significant heterogeneity of imaging characteristics of all tumors. The pattern of T1 hypointensity and T2 hyperintensity is common among LGG and HGG, ATRT, many medulloblastoma, hemangioblastoma, and gangliocytoma, whereas ependymoma tends to be T1 hyperintense and T2 hypointense. Contrast enhancement patterns can also be useful. Contrast enhancement is highly variable in medulloblastoma, with distinct molecular subgroups of medulloblastoma expressing variable levels of gadolinium enhancement. Choroid plexus tumors tend to enhance brightly. Gliomas often enhance heterogeneously, with LGGs frequently enhancing at the cyst wall or the mural portion, and higher-grade gliomas showing more ring-like enhancement patterns. Importantly, DWI is becoming increasingly recognized as a method for differentiating different tumor types. Medulloblastoma, ATRT, and embryonal tumor with multilayered rosettes all typically show restricted diffusion, whereas ependymomas most often do not restrict diffusion (they appear darker on DWI imaging). , Other advanced MRI techniques, including PWI, diffusion tensor imaging, and MR spectroscopy, have been evaluated as a means to differentiate brain metastases and other lesions such as HGG, CNS lymphoma, and abscess. Although no one technique can completely accurately discriminate a brain metastasis from other lesions, differences in peritumoral edema characteristics (purely vasogenic with metastases versus vasogenic edema plus infiltrative neoplastic cells in HGGs) may provide helpful clues.
Imaging differential of posterior fossa lesion . Non-malignant lesions of the posterior fossa can also be characterized with CT and/or MRI imaging. Demyelination, either from a post-infectious cause or due to an underlying progressive neurologic disorder, often appears as hyperintense lesions on T2-weighted imaging. These lesions may be focal or more diffuse, depending on the underlying pathophysiology. Similarly, inflammation of structures in the posterior fossa most frequently causes hyperintensity on fluid attenuated inversion recovery (FLAIR)/T2 weighted images, though MRI features can be incredibly heterogeneous depending on the underlying cause of inflammation, its chronicity, and other complications such as abscess, meningitis, or autoimmune disease.
Case. A 4-year-old previously healthy male presented with 4 weeks of progressive headaches, nausea, and vomiting. The headaches were initially attributed to streptococcal pharyngitis diagnosed by throat culture; however, the headaches worsened in frequency and severity, and became progressively associated with emesis. Headaches and vomiting tended to worsen throughout the day. By history, he was not exhibiting any lack of coordination, nor did he have any weakness or reported tingling. Additionally, family denied fevers, chills, rashes, chest pain or palpitations, shortness of breath, abdominal discomfort, or change in vision. He did not have papilledema on examination, and the remainder of the neurological examination was grossly normal. Given the progressive nature of his symptoms, a head CT was obtained ( Fig. 7.1A,B ), which revealed an isodense midline posterior fossa tumor, expanding into the fourth ventricle, and causing obstructive hydrocephalus of the lateral and third ventricles. There were no visible calcifications within the tumor. This prompted an urgent referral to a pediatric emergency department (ED), where brain MRI was obtained ( Fig. 7.1C–G ). MRI revealed a T1 hypointense, T2 mildly hyperintense, heterogeneously enhancing mass centered in the fourth ventricle, appearing to arise from the inferior roof of the fourth ventricle, measuring 3.4 × 4.0 × 4.1 cm in anterior-posterior, transverse, and craniocaudal dimensions, respectively. The mass demonstrated restricted diffusion.
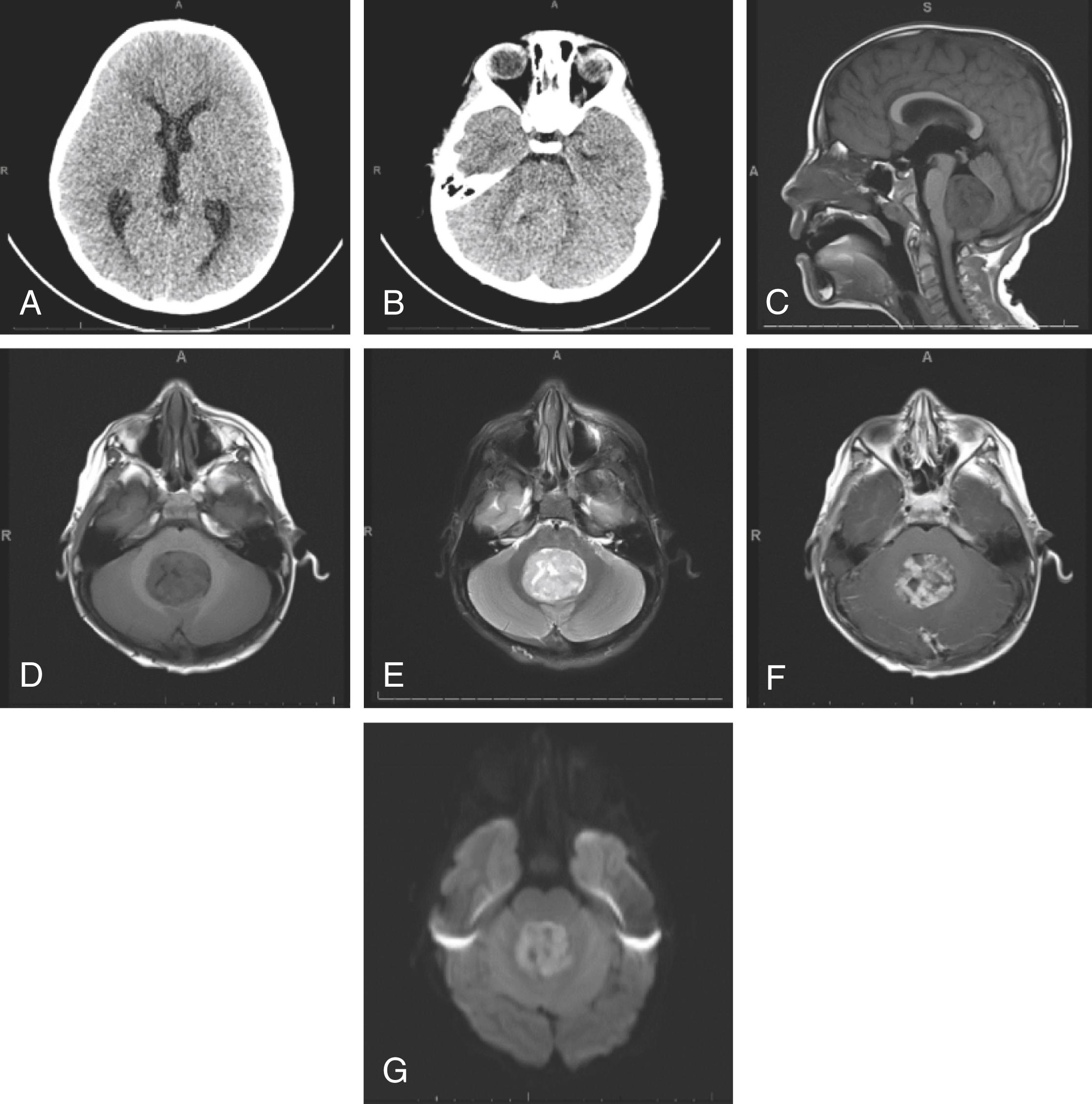
Teaching Points: Differential Diagnosis for Midline Fourth Ventricular Lesion. The patient’s young age and absence of systemic complaints immediately raises the suspicion for a primary brain tumor. The tumor’s specific location within the posterior fossa—midline and arising from or within the fourth ventricle—is most consistent with medulloblastoma or ependymoma. Other possible tumors in this location include tumors arising from the choroid plexus (choroid plexus papilloma or choroid plexus carcinoma) and exophytic LGGs growing from the lower brainstem/medulla oblongata. In contrast, tumors arising just lateral to midline, at the cerebellopontine angle or at the Foramen of Luschka, are more likely to be ATRT or a favorable subtype of medulloblastoma called WNT subtype. The CT findings of an isodense lesion correlates with either medulloblastoma or ependymoma. MRI is helpful in differentiating between medulloblastoma and ependymoma here, with the T1 hypointense and T2 hyperintense pattern with heterogeneous contrast enhancement favoring medulloblastoma. Finally, the restricted diffusion also suggests medulloblastoma. Ultimately, however, the diagnosis must be made pathologically, not radiographically.
Diagnostic workup. Urgent referral to a pediatric neurosurgeon should be made. Virtually all children with a posterior fossa mass will undergo a craniotomy, as opposed to stereotactic or open biopsy. The goal of this surgery is threefold: to relieve mass effect (including any obstructive hydrocephalus), to obtain tissue for pathologic diagnosis, and to debulk the tumor. Nearly all tumors in this region in children would benefit from maximal safe resection, though there are some nuances. Whereas the prognosis of medulloblastoma is unchanged if there remains less than 1.5 cm 2 of tissue, the prognosis of ependymoma is dismal in the absence of gross total resection. LGGs are cured by gross total resection, but those that are partially resected to spare surgical morbidity may still have long-term survival, though often at the expense of needing recurrent surgeries or multiple courses of adjuvant chemotherapy and/or radiation therapy. In this particular case, with the most likely etiologies being medulloblastoma and ependymoma, an attempt at near total or gross total resection should be made. The patient successfully underwent gross total resection, and did not need any CSF diversion following tumor resection.
Following surgery of a posterior fossa tumor, there is time to wait for definitive pathologic diagnosis while the patient recovers. Staging and treatment planning entirely depend on the tumor type, so waiting for pathology while the patient recovers from craniotomy is reasonable. Approximately 10 days after surgery, a final pathologic diagnosis confirmed medulloblastoma, WHO grade IV, without nodular, desmoplastic, anaplastic, or large cell features. Over the past 15 years, integrative genomic and methylomic studies have shown that medulloblastoma is not a single entity, but rather a heterogeneous group of diseases with distinct clinical, radiographic, molecular, and prognostic features. Testing, including immunohistochemistry for nuclear beta-catenin, p53, MYC, and NMYC, all confirmed that this patient had a non-WNT, non-Sonic Hedgehog (SHH), group 3/4 non-MYC amplified, non-p53-mutated, medulloblastoma. Of note, upward of 25% of patients with SHH medulloblastoma have a germline mutation in cancer predisposition genes including TP53 or BRCA, so patients with this medulloblastoma subtype benefit from genetic counseling.
Staging of medulloblastoma . With the pathologic diagnosis in hand, staging workup can proceed. Typically, medulloblastoma only spreads within the CNS, and this workup is done with a full spine MRI and lumbar puncture. Ideally, both of those are done at least 2 weeks following surgery, as blood products in the subarachnoid space can be misinterpreted as metastatic tumor. The patient underwent sedated spine MRI and lumbar puncture, both of which were negative, confirming non-metastatic medulloblastoma. The combination of non-metastatic group 3/4 medulloblastoma that is non-MYC amplified is considered standard risk and is associated with an overall survival of 75–90%.
Patient management . The mainstay of treatment for standard risk medulloblastoma is surgery, followed by craniospinal radiation with a posterior fossa boost along with concurrent vincristine, followed by multi-agent cytotoxic chemotherapy. Mainly for social reasons, this patient opted to undergo conventional photon intensity-modulated radiation therapy (as opposed to proton beam radiation, which is increasingly becoming standard-of-care for most children with medulloblastoma). The entire treatment course is long: from surgery through radiation and chemotherapy, the course takes over 1 year. Survivors of medulloblastoma will also often experience long-term sequelae of therapy, including neurocognitive deficits, endocrinopathies, hearing loss, and secondary malignancy risk. At last follow up, the patient is 2 years off-therapy, doing well overall but has an individualized education plan in school to accommodate for some neurocognitive deficits.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here