Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Anemia may result from decreased marrow production or shortened red cell survival.
Nonmarrow diseases, such as endocrine, renal, and inflammatory disorders, significantly influence bone marrow function.
Anemias associated with nutritional deficiencies may affect tissues other than the bone marrow.
Stem cell disorders, such as inherited and acquired aplastic anemias, and paroxysmal nocturnal hemoglobinuria usually affect more than one cell line.
Hemolytic anemia may be caused by extrinsic factors, usually acquired, such as chemical agents or antibodies, or intrinsic factors, usually inherited, such as disorders of the red cell membrane or enzymes, or hemoglobinopathy.
Polycythemia is defined by laboratory parameters and may be absolute with an increase in total red cell mass or relative as a result of a decrease in plasma volume.
Porphyrias are inherited and acquired disorders of heme biosynthetic pathways with clinical manifestations resulting from accumulation of intermediate heme synthetic products.
Anemia is considered to be present if the hemoglobin (Hb) concentration or the hematocrit (Hct) is below the lower limit of the 95% reference interval for the individual’s age, sex, and geographic location (altitude; Table 33.1 ). This means that 2.5% of normal individuals will be classified as anemic. Conversely, individuals whose Hb falls within the reference intervals for age and sex yet significantly below their own usual values should be considered anemic.
| Age, Years | Hb, g/dL |
|---|---|
| Both Sexes | |
| 1–2 | 11 |
| 3–5 | 11.2 |
| 6–11 | 11.8 |
| Females | |
| 12–15 | 11.9 |
| 16–69 | 12 |
| ≥70 | 11.8 |
| Males | |
| 12–15 | 12.6 |
| 16–19 | 13.6 |
| 20–49 | 13.7 |
| 50–69 | 13.3 |
| ≥70 | 12.4 |
Anemia may be absolute when red blood cell (RBC) mass is decreased or relative when associated with a higher plasma volume. Causes of absolute anemia fall into two major pathophysiologic categories: impaired red cell production and increased erythrocyte destruction (or loss in excess of the ability of the marrow to replace these losses). Several authors have included anemia following acute blood loss in the latter category ( ; ). The presence of anemia may be a sign of an underlying disorder of which the cause should be identified because correction may be very important to the health of the individual. Relative anemia may occur with pregnancy, with macroglobulinemia, and in postflight astronauts. Recent studies suggest that prolonged exposure to weightlessness, which causes hemoconcentration due to decreased plasma volume, as well as the return to sea level after a period of high-altitude acclimatization, are associated with selective loss of young red cells, also known as neocytes , as a result of erythropoietin (EPO) withdrawal, a process known as neocytolysis. It is thought that EPO withdrawal may cause decreased expression of certain molecules, such as CD55 and CD59, which are protective against red cell destruction by macrophages ( ).
Anemia may also be classified by red cell morphology as macrocytic, normocytic, or microcytic—an approach that is useful in differential diagnosis (see later discussion). Both pathophysiologic and morphologic classifications should be understood. Some anemias (e.g., blood loss anemia) have more than one pathogenetic mechanism and go through more than one morphologic stage.
Clinical signs and symptoms result from diminished delivery of oxygen (O 2 ) to the tissues; therefore, they are related to the lowered Hb concentration and blood volume and are dependent on the rate of these changes. Modifying factors include compensatory adjustments in cardiac output, respiratory rate, and O 2 affinity of Hb. When anemia develops slowly in a patient who is not otherwise severely ill, Hb concentrations as low as 6 g/dL may develop without producing any discomfort or physical signs as long as the patient is at rest.
In general, the anemic patient complains of easy fatigability and dyspnea on exertion, and often of faintness, vertigo, palpitations, and headache. The more common physical findings are pallor, a rapid bounding pulse, low blood pressure, slight fever, some dependent edema, and systolic murmurs. In addition to these general signs and symptoms, certain clinical findings are characteristic of the specific type of anemia.
Iron is an essential component of Hb, of myoglobin (in muscle cells), and of certain enzymes (in most body cells). The major “pools” of iron in the body are illustrated in Figure 33.1 . Two-thirds or more of the body’s total iron is in the erythron (normoblasts and erythrocytes); each milliliter of red cells contains about 1 mg of iron. Storage iron is present in macrophages of the reticuloendothelial system in two forms: ferritin and hemosiderin. Ferritin is a water-soluble complex of ferric salt and a protein, apoferritin. Apoferritin has a molecular weight of approximately 450,000 Da and consists of 24 subunits with a variable ratio of H (heavy) and L (light) types. Apoferritin forms a shell around a crystalline core of predominantly ferric oxyhydroxide (FeOOH). The genes for the H and L subunits have been located on chromosomes 11 and 19, respectively. The H monomers have ferroxidase activity, allowing them to become more efficient in taking up and releasing iron. Hemosiderin is water insoluble and consists mostly of aggregates of FeOOH core crystals with partially or completely degraded protein shells. Protein degradation usually occurs in lysosomes. The iron in hemosiderin is much more difficult to release than that of ferritin. Most of the iron utilized in Hb synthesis is that recently released from degraded Hb in macrophages and transported to the normoblasts by plasma transferrin (a β-globulin, molecular weight 80,000 Da, gene located on chromosome 3). Each molecule of apotransferrin binds two atoms of ferric iron. Subsequently, transferrin binds to transferrin receptor-1 (TfR-1) (CD71) on the cell membrane of erythroid precursors, reticulocytes, and most body cells. The transferrin–transferrin receptor complex is rapidly internalized as an endosome, iron is released following pH decline of the endosome, and apotransferrin returns to the circulation and binds more iron. The freed iron is reduced to the ferrous form by STEAP3 (six-transmembrane epithelial antigen of prostate 3) and transported into the cytosol by DMT1 (divalent metal transporter 1, also known as NRAMP2, SLC11A2). Iron is imported into the mitochondria by a solute carrier known as mitoferrin-1, where iron is used to synthesize heme, iron-sulphur clusters, and other proteins ( ). Experimental studies in mice indicate that TfR-1 is essential for brain, heart, gut, and skeletal muscle development as well as immune system function. While TfR-1 is widely expressed, only hepatocytes and erythroid precursors express a related molecule designated as transferrin receptor-2 (TfR-2). Recent evidence suggests that TfR-2 may work in concert with HFE protein in hepatocytes to control iron absorption. Mutations in the TfR-2 gene have been associated with type 3 hemochromatosis (see later discussion).
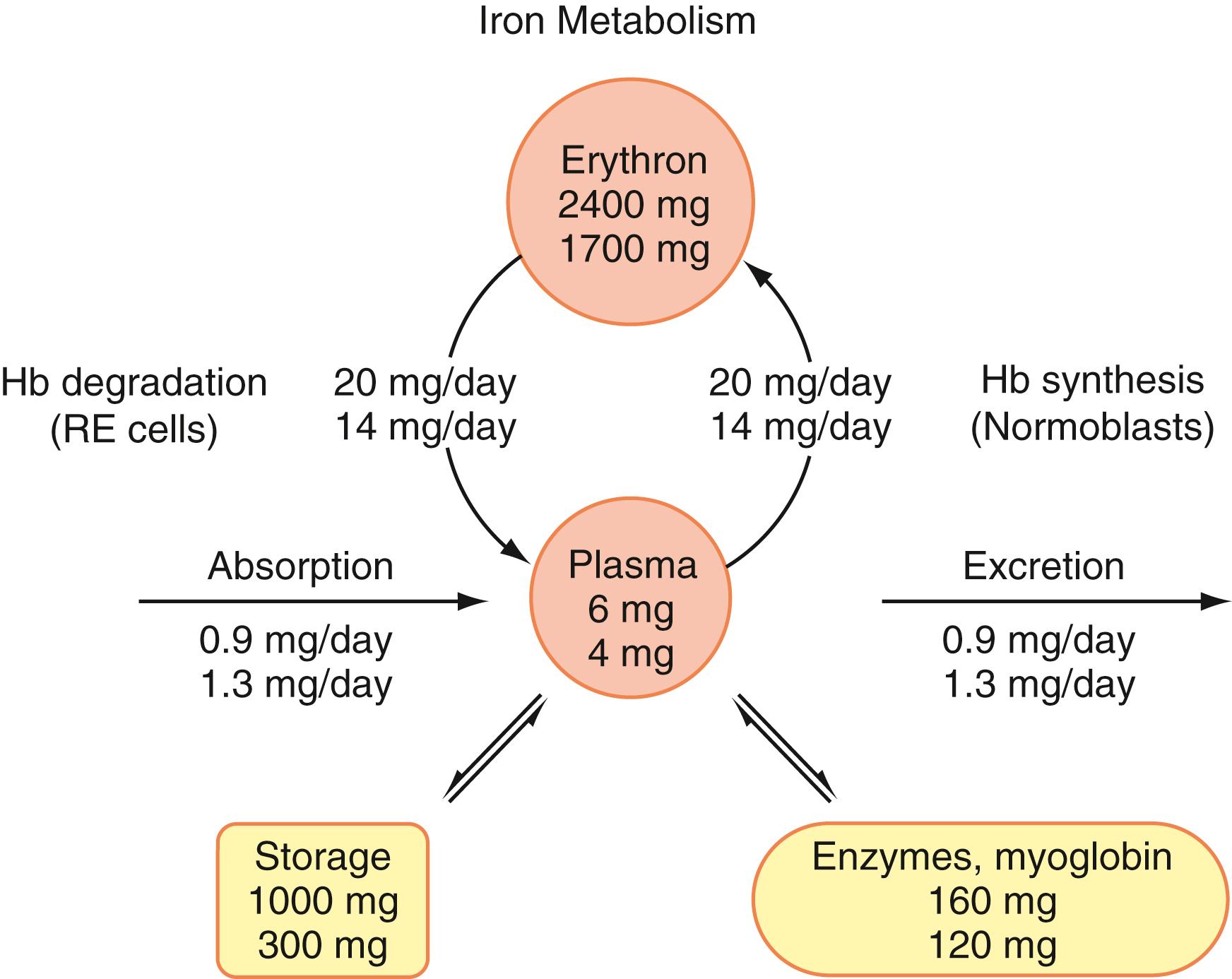
In humans, approximately 20 mg of iron is needed daily in order to generate in excess of 200 billion RBCs. The majority of the daily iron needs (80%–90%) is provided by recycling of iron from senescent red cells and the remainder through daily intake of iron. Very little iron is lost from the body; this small iron loss occurs mainly as loss of cells from the gastrointestinal (GI) tract and to a lesser extent from the skin and through the urine. The iron lost in women averages more, because of menstrual blood loss, than that excreted in men. About 1 mg is lost each day, except in menstruating females, whose iron loss averages about 2 mg/day. Iron balance is maintained by control of absorption. In the United States, dietary iron averages 15 mg/day with 7% absorption in men and 11 mg/day with 13% absorption in women. Absorption can be increased in iron deficiency, but only to about 20% of ingested iron in meat-containing diets and less in vegetarian diets ( ). Absorption takes place largely in the small intestine, most efficiently in the duodenum and upper jejunum, with heme iron absorbed more efficiently than inorganic iron. Iron absorption is facilitated by ascorbate and citrate and is inhibited by phytates and tannins. Acid production by the stomach lowers the pH in the duodenum, thus enhancing the solubility and uptake of nonheme ferric iron. Iron entry into mucosal cells of the upper GI tract seems to involve more than one pathway, including an independent mechanism for heme absorption. A heme carrier protein 1 (HCP1) has been described. However, recent evidence suggests that it is identical to proton-coupled folate transporter (PCFT), which is involved rather exclusively in folate transport (Beutler, 2010; ). A dual role of PCFT in folate and heme iron uptake is being investigated.
Nonheme iron is transported across the mucosal membrane through a channel called the divalent metal transporter , which is regulated by iron regulatory proteins 1 and 2 (IRP-1 and IRP-2). These regulatory proteins, particularly IRP-2, affect the translation of several proteins that are involved in iron metabolism, with a net result of increasing intracellular iron. Messenger RNA (mRNA) of these proteins have IREs (internal ribosome entry sites), which may increase or decrease translation when bound to IRPs. For example, iron deficiency increases the translation of DMT1 and TfR-1 while it decreases the translation of erythroid aminolevulinic acid synthetase and apoferritin ( ). DMT1 regulates iron transport from intestinal surface to inside the cell. This process is aided by duodenal cytochrome b –like ferroreductase, which reduces iron to the ferrous form. DMT1 also transports other metals, such as lead, cobalt, and nickel. The export of iron from enterocytes, macrophages, and hepatocytes to plasma involves another transport system, which includes ferroportin-1 and hephaestin ( ). Ferroportin-1 is located at the basolateral membrane of apical enterocytes and functions as a transport protein delivering iron in the ferrous state to the plasma. Ferroportin-1 is also expressed on macrophages. Hephaestin, named after the Greek god Hephaestus , who forged iron, has a ferroxidase activity that contributes to iron transport by transforming iron to the ferric form to enable its uptake by circulating apotransferrin. Ceruloplasmin also has ferroxidase activity and is involved as well in the release of iron from the cells. Because both ceruloplasmin and hephaestin are copper-containing ferroxidases, copper deficiency affects iron release and can produce iron deficiency anemia. Another 25-amino-acid antimicrobial peptide, hepcidin, has been shown to play a major role in iron homeostasis through a hormonal effect. Hepcidin is produced by the liver, is filtered by the kidney, and accumulates in urine. Hepcidin negatively controls the release of iron from cells, such as intestinal epithelium and macrophages, to the plasma. Hepcidin combines with ferroportin-1, leading to internalization and lysosomal degradation of both proteins. Thus, it limits the release of intracellular iron into the plasma ( ). It has been recently reported that hepcidin also physically occludes the site of iron export in the central cavity of ferroportin ( ). Hepcidin is the product of the HAMP (hepcidin/hepatic antimicrobial peptide) gene located on chromosome 19q13.1. At least 4 mechanisms are involved in the regulation of hepcidin secretion: inflammation, systemic iron availability through hepatocyte iron-sensing system, erythropoietic activity, and hypoxia. Increased marrow erythropoietic activity has been associated with decreased hepcidin and increased iron absorption. A hormone named erythroferrone (ERFE) has been recently identified. ERFE is secreted by erythroblasts in response to erythropoietin and causes suppression of hepcidin release ( ). The current evidence suggests that human hemochromatosis protein (HFE), TfR-2, and hemojuvelin (HJV) interact with each other to form a hepatocyte “iron-sensing complex.” When transferrin is highly saturated, HFE is displaced from TfR-1 and interacts with TfR-2 and HJV to activate HAMP transcription pathways. Matriptase-2 (MT-2) is a membrane serine protease expressed predominantly in hepatocytes. It acts to cleave and inactivate HJV, producing a lower level of hepcidin ( ). Recent studies suggest that the early steps of iron sensing actually occur in the liver sinusoidal endothelial cells, which transmit signals to the hepatocytes for further steps of control ( ). In the plasma, total iron averages 110 μg/dL (19.7 μ mol/L). The great majority of this is bound to the transferrin, which has a capacity to bind 330 μg of iron per deciliter (or 59.1 μmol/L) and, therefore, is about one-third saturated. A very small amount of iron in plasma is in ferritin. Plasma (or serum) ferritin averages about 100 μg/L in men (less in women—about 50 μg/L).
When iron loss exceeds iron intake for a time long enough to deplete the body’s iron stores, insufficient iron is available for normal Hb production. When well developed, iron deficiency is characterized by a hypochromic microcytic anemia.
Iron deficiency typically results when the need for iron is increased (e.g., during rapid growth in infancy and childhood, during pregnancy) or when excessive loss of blood has reduced the body’s reserves of iron (e.g., following repeated hemorrhages, excessive menstruation, or multiple pregnancies).
Iron deficiency is probably the most common cause of anemia, affecting at least 1.2 billion individuals worldwide ( ). Children between the ages of 6 and 24 months are particularly susceptible. It is caused by insufficient dietary iron to meet the needs of rapid growth. After the first 4 to 6 months of life, the iron stores present from birth have been exhausted, and the infant depends on dietary iron. An infant maintained on milk and carbohydrates without supplements of iron-containing foods is likely to develop iron deficiency anemia—one component of the “milk anemia” of infancy. This anemia is also frequently related to intestinal blood loss due to cow’s milk protein intolerance in young children when cow’s milk is introduced too early. In a study in the United States, IDA was reported in 3% of toddlers aged 1 to 2 years and in 2% to 5% of adolescent girls and women of childbearing age, with iron deficiency (absent anemia) noted in higher (>9%) proportions ( ). IDA also affects 2% to 5% of pregnant women. Defective absorption of iron and eventual IDA occur after total gastrectomy or even subtotal gastrectomy. Prolonged treatment of peptic ulcer and acid reflux by H 2 blockers and acid pump blockers may cause defective iron absorption. Except for the sprue syndrome, causes of malabsorption of iron are extremely rare. Because iron deficiency increases the rate of absorption of both iron and lead, lead intoxication may accompany iron deficiency.
If an adult male had absolutely no iron intake or absorption (which would be extremely unlikely), his body iron stores of 1000 mg would last for 3 to 4 years before he would even begin to become iron deficient. Therefore, almost all cases of IDA in adult males are due to chronic blood loss. Hemorrhagic lesions—such as benign and malignant tumors, chronic ingestion of some medications, and helminthic infections—are common causes of iron deficiency in males and postmenopausal females.
The sequence of events in developing IDA is usually as follows ( ): When blood loss exceeds absorption, a negative iron balance exists. Iron is mobilized from stores, storage iron decreases, plasma ferritin decreases, iron absorption increases, and plasma iron-binding capacity (transferrin) increases. This stage is known as iron depletion . After iron stores are depleted, the plasma iron concentration falls, saturation of transferrin falls below 15%, and the percentage of sideroblasts decreases in the marrow. As a result of lack of iron for heme synthesis, red cell protoporphyrin increases. This second stage is iron-deficient erythropoiesis ; anemia may not yet be present. The third stage is IDA ; in addition to the abnormalities just discussed, anemia is detectable. The anemia at first is normochromic and normocytic, gradually becomes microcytic, and finally becomes microcytic and hypochromic.
Clinical findings may be due to the underlying cause of the blood loss itself, to the general manifestations of anemia (see previous discussion), or to iron deficiency. Those that are probably attributable to lack of tissue iron include paresthesias, such as numbness and tingling; restless leg syndrome; atrophy of epithelium of the tongue with burning or soreness; fissures or ulcers at the corners of the mouth (angular stomatitis); chronic gastritis, which leads to decreased gastric secretions but few symptoms; “pica,” which is the craving to eat unusual substances such as dirt or ice; concave or spoon-shaped nails (koilonychia); and difficulty swallowing due to “webs” of tissue or partial strictures at the junction of the esophagus and hypopharynx. The latter two findings are relatively uncommon. The combination of glossitis, sore mouth, dysphagia, and iron deficiency is called Plummer-Vinson syndrome. Splenomegaly may occur in iron deficiency but is uncommon. In young children, lower test scores and delayed motor development have been reported in association with IDA.
Blood. In early IDA, the stained blood film often shows normochromic normocytic erythrocytes ( ). In later stages, the picture is one of microcytosis, anisocytosis, poikilocytosis (including elliptical and elongated cells), and varying degrees of hypochromia. The thin red cells (leptocytes) have faint red color compared to normal. The plasma membranes of iron-deficient cells are abnormally stiff. This abnormality contributes to the development of poikilocytes, particularly elongated hypochromic elliptocytes (pencil cells). Anisocytosis may be identified by automated blood counters as increased red cell distribution width (RDW). This finding, however, is not specific for IDA. A high RBC count is commonly encountered in children under the age of 4 years. Reticulocytes are usually decreased in absolute numbers, except following iron therapy. Also, reticulocyte hemoglobin content (Ret-He, CHr) is reduced. The mean corpuscular volume (MCV) is low, and Hb and Hct are relatively lower than the erythrocyte count. Osmotic fragility may be decreased because the red cells are thinner than normal (see Figs. 31.10 and 33.2 ).
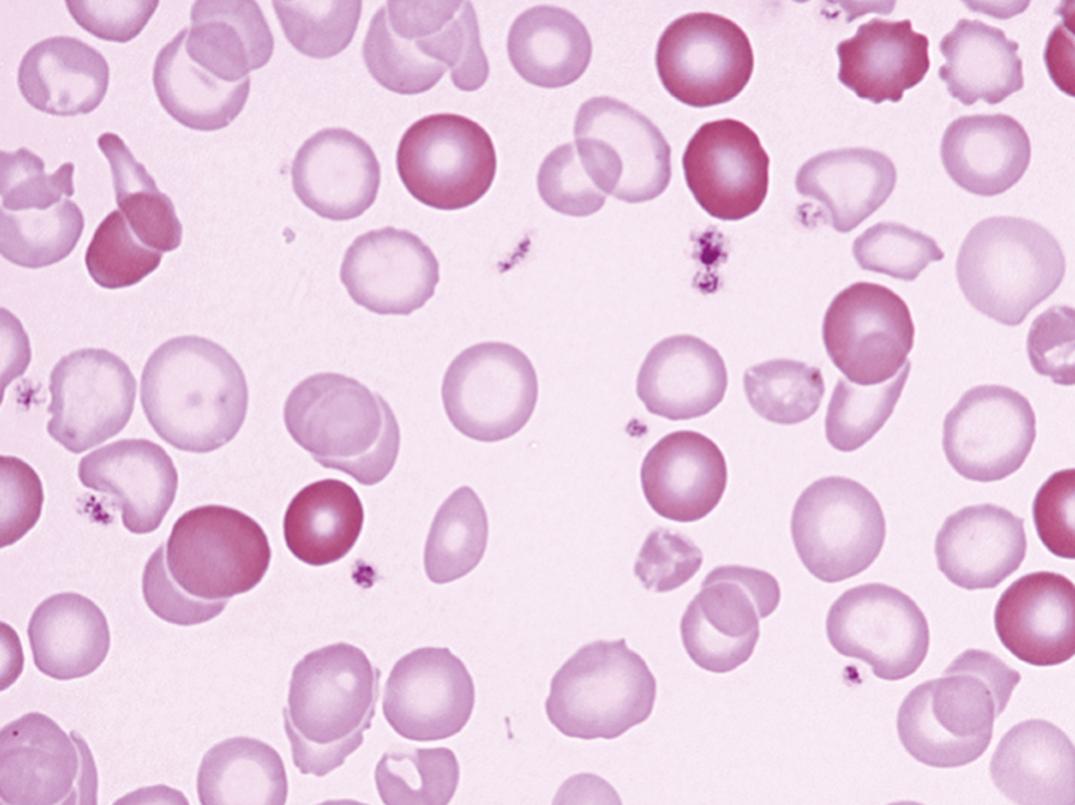
The leukocyte count is normal or slightly lowered. Granulocytopenia and a small number of hypersegmented neutrophils may be present. Megaloblastic changes in severe iron deficiency may be related to decreased activity of the enzyme ribonucleotide reductase, which contains an essential nonheme iron atom ( ). However, the detection of hypersegmented neutrophils should raise suspicion for a mild folate deficiency, which may become more overt after iron therapy ( ). Platelets may be increased whether the lack of iron is due to blood loss or dietary deficiency, but tend to be decreased in severe anemia. When microcytosis is severe, automated platelet count may not be accurate due to some degree of interference by very small erythrocytes.
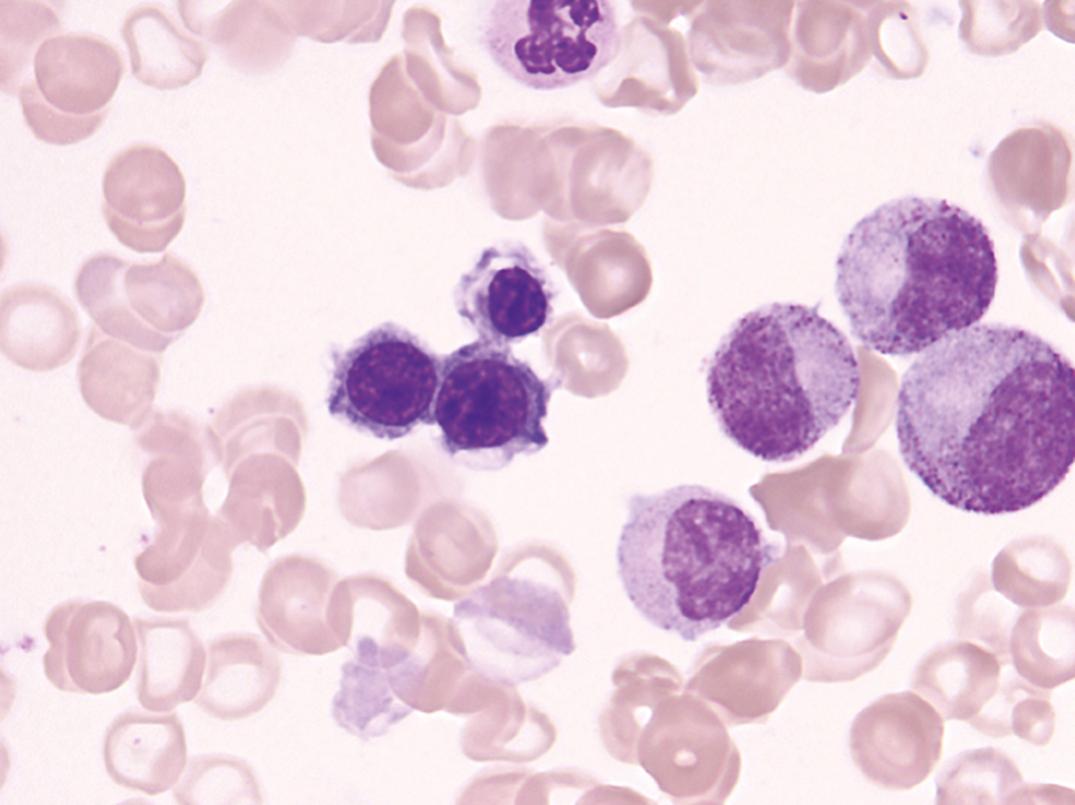
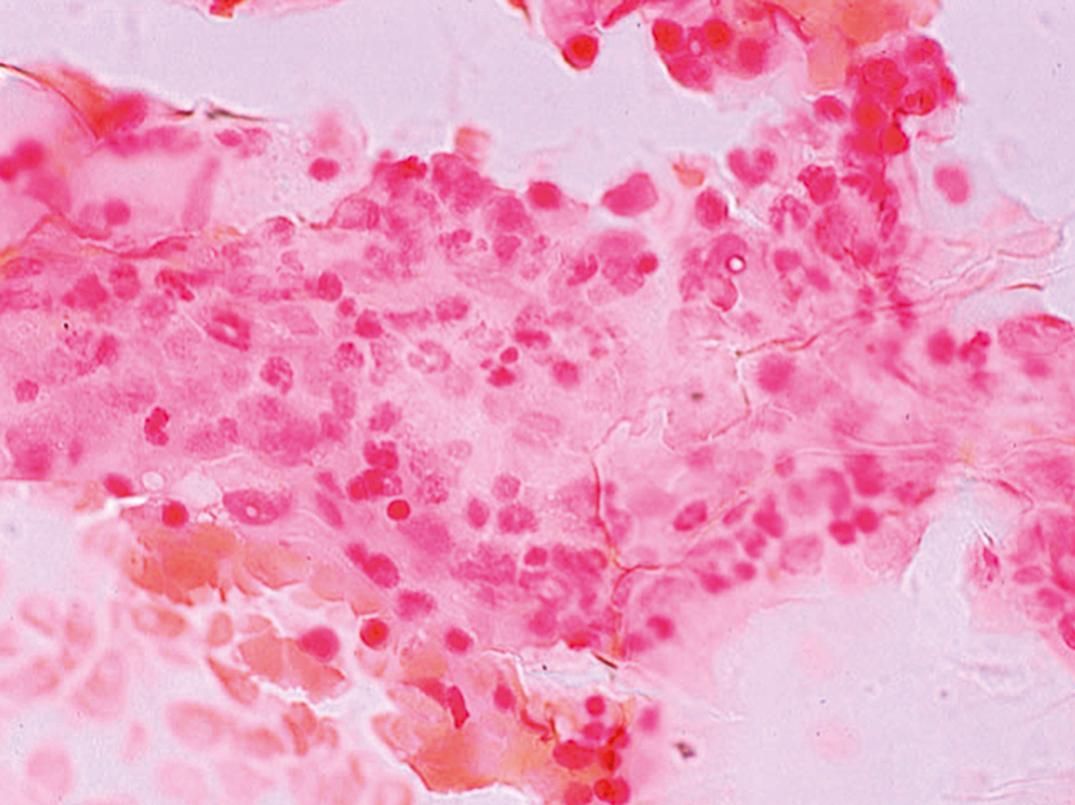
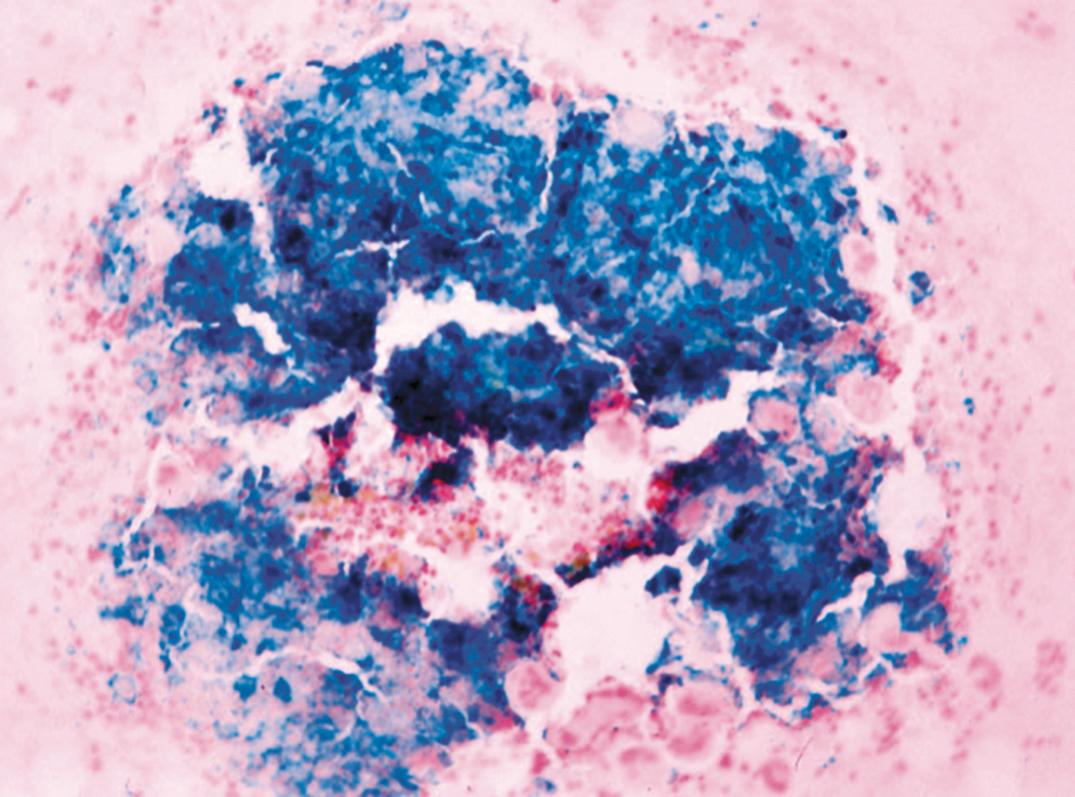
Marrow. Erythroid hyperplasia occurs early; however, in later stages, the limiting effect of severe iron deficiency restricts erythropoiesis to the basal level. The erythroblasts are smaller than normal, deficient in the amount of Hb in the cytoplasm, and irregular in shape, with frayed margins ( Fig. 33.3 ). Giant neutrophil bands or metamyelocytes, if present, are rarely due to iron deficiency per se; usually, they indicate an associated cobalamin or folate deficiency (see later discussion under Megaloblastic Anemia). Iron stains should be performed routinely on marrow aspirate films ( Figs. 33.4 and 33.5 ). Storage iron is absent, unless iron has recently been administered in some form. The proportion of erythroblasts that are sideroblasts is decreased (<20%). This proportion is usually about the same as the percent saturation of transferrin (or total iron-binding capacity [TIBC]) and is a measure of iron delivery to the erythroblasts.
Serum Iron. The reference interval is 50 to 160 μg/dL (9–29 μmol/L) in adults. The level is lower in iron deficiency and in infection and anemia of chronic disease.
Serum (Total) Iron-Binding Capacity. The reference interval for adults is 250 to 400 μg/dL (45–72 μmol/L). In IDA, the serum TIBC is increased. It is normal or decreased in the anemia of chronic disease. If chronic infection coexists with chronic blood loss, the TIBC may not be increased even though the patient is iron deficient. Some laboratories measure transferrin level as an alternative.
Percent Saturation of TIBC. The ratio of serum iron to TIBC is the percent saturation of the TIBC. Normally, this is 20% to 55%; values below 15% indicate iron-deficient erythropoiesis.
A marked diurnal variation in serum iron by as much as 30% normally occurs, with highest values in the morning and lowest values late in the day. Consequently, fasting morning blood specimens are preferred for the diagnosis of iron deficiency. The TIBC remains relatively constant in a normal individual. Pregnancy and oral contraceptives increase TIBC.
Serum iron is usually higher in the first 90 days of life. It then dips to somewhat lower reference intervals for the second month of life, and the value gradually increases with age until it reaches the adult range approximately at the age of 15 years ( ; ). On the other hand, the TIBC gradually rises with age until it reaches values that are comparable to those of adults at the age of 15 years ( ). Similarly, percent saturation in children is less than in adults; it reaches the adult value between the ages of 15 and 18 years ( ).
Serum Ferritin. In adults, the reference values are 12 to 300 μg/L, with higher values in men than in women. Serum ferritin appears to be in equilibrium with tissue ferritin and is a good reflection of storage iron in normal subjects and in most disorders. The equivalence of 1 μg/L of serum ferritin with 8 to 10 mg storage iron has been suggested. In patients with some hepatocellular diseases, malignancies, and inflammatory diseases, serum ferritin provides a disproportionately high estimate of storage iron because serum ferritin is an acute-phase reactant. In such disorders, IDA may exist with a normal serum ferritin concentration. In the presence of inflammation, persons with a serum ferritin level of less than 50 to 60 μg/L are likely to respond to iron therapy. On the other hand, hypothyroidism and ascorbate deficiency may lower plasma ferritin levels independent of iron stores (Brittenham, 2009).
In infancy and childhood, between the ages of 6 months and 15 years, the reference interval for serum ferritin is somewhat lower than in early infancy or adult life ( ). In men, serum ferritin gradually rises between the ages of 18 and 30 years, whereas in women, it does not. However, postmenopausal women have a much higher ferritin level than premenopausal women, and it is comparable with that of men ( ). Serum ferritin levels do not display diurnal variation.
Erythrocyte Porphyrins. Because heme is formed by insertion of iron into protoporphyrin IX, the latter is increased in iron-deficient erythropoiesis, whether owing to iron deficiency or to anemia of chronic disease. It is also increased in lead poisoning and in some cases of sideroblastic anemia but is normal in thalassemia. Zinc usually becomes attached to protoporphyrin, forming zinc protoporphyrin (ZPP). A relatively simple micromethod measuring ZPP in whole blood has been shown to be useful in distinguishing microcytosis due to iron deficiency from that due to β-thalassemia minor ( ). The normal reference interval was 10 to 99 μg/dL of erythrocytes; in iron deficiency, erythrocyte porphyrins became elevated before the development of anemia, which may be one of the earliest indicators of iron deficiency ( ).
Serum Transferrin Receptors. TfRs also exist in a soluble form in the circulation. Serum TfRs (STfRs) are produced by shedding of membrane TfRs during erythrocyte maturation. STfRs vary with the rate of erythropoiesis. Patients with aplastic anemia (AA) have lower than normal levels of STfRs, and patients with autoimmune hemolytic anemia have higher values. IDA is associated with increased serum levels of TfRs, probably as a result of increased membrane TfR synthesis and expression secondary to iron starvation. Unlike serum ferritin, STfRs are usually not affected by inflammation ( ).
Serum Transferrin Receptor–to–Serum Ferritin Ratio. The serum transferrin receptor – to – serum ferritin (STfR/F) ratio has been suggested as a new approach to estimate total body iron stores. However, it appears to have limited value in identifying anemia of chronic disease (ACD). The STfR/F ratio may be better utilized in identifying IDA coexisting with ACD ( ).
Reticulocyte Hemoglobin Content. Some automated hematology analyzers offer an assay of Hb content within reticulocytes (Ret-He, CHr; in pg/cell). By measuring this parameter, the status of erythropoiesis in the previous 3 to 4 days may be assessed using reticulocytes as a guide ( ).
Hepcidin Level. Measuring plasma hepcidin may serve as a valuable tool in the study of abnormalities of iron metabolism, hemochromatosis, anemia of inflammation, and iron deficiency. Hepcidin level measurement is currently undergoing numerous studies.
Anemia due to iron deficiency usually needs to be distinguished from other causes of microcytosis with or without anemia. These include the thalassemia traits, long-standing ACD, and the sideroblastic anemias (see later discussions of these entities). Bone marrow storage iron and serum ferritin will be decreased in iron deficiency and normal or elevated in all others. In thalassemia trait , the anemia, if present, is very mild, the ZPP is normal, serum iron is normal, and the condition is present in family members. In β-thalassemia trait, HbA 2 and sometimes fetal hemoglobin (HbF) are increased. Yet, HbA 2 is often decreased in iron deficiency. In ACD (chronic infection, inflammatory conditions such as rheumatoid arthritis, or neoplastic disease), although the serum iron is low, as in iron deficiency, the TIBC is low or normal. In the sideroblastic anemias , which include chronic lead poisoning, the serum iron and percent TIBC saturation are increased, and pathologic “ring” sideroblasts are present in the marrow.
The first principle in therapy of IDA is that the underlying cause can be identified and corrected. Ferrous iron is given orally—at about 200 mg/day—in three doses between meals. This will provide 40 to 60 mg of absorbed iron per day, which, with the iron produced by turnover of senescent red cells, will be sufficient to increase production to two or three times normal ( ). The reticulocyte count will reach a maximum at 5 to 10 days and then will gradually decrease toward normal. Monitoring the Hb is best; Hb should increase by 0.1 to 0.2 g/dL per day after the fifth day and by at least 2 g/dL for each of the subsequent 3 weeks. After the Hb has returned to normal, iron therapy should be continued for at least 2 months to replenish storage iron. Patients refractory to treatment need to be investigated for continued underlying diseases, particularly chronic gastritis and Helicobacter pylori gastritis ( ). In recent years, there has been significant improvement in iron supplements available through intravenous route. Some of these products, such as low-molecular-weight iron dextran, have a stable carbohydrate shell that prevents the release of free iron and enhances their safety ( ).
This is a recently described hereditary recessive microcytic anemia, which does not respond to oral iron therapy. Initial studies suggest that IRIDA is caused by a mutation in the TMPRSS6 gene (mapped to chromosome 22q12-13) encoding a transmembrane serine protease known as matriptase-2 (MT-2). As mentioned before, MT-2 is involved in the downregulation of hepcidin through modulating the HJV pathway. IRIDA patients have inappropriately high hepcidin levels, preventing iron absorption and release. Patients may show a slow or partial response to parenteral iron ( ).
Macrocytic anemias that are not megaloblastic may be due to early release of erythrocytes from the marrow, so-called shift reticulocytes . This may occur in response to acute blood loss, hemolysis, bone marrow infiltration, and high levels of EPO associated with bone marrow failure diseases such as aplastic anemia, refractory anemia, and inherited bone marrow failure syndromes, including Diamond-Blackfan anemia. Nonmegaloblastic macrocytosis is also found in hypothyroidism, in individuals with excessive alcohol intake, and in liver disease ( ).
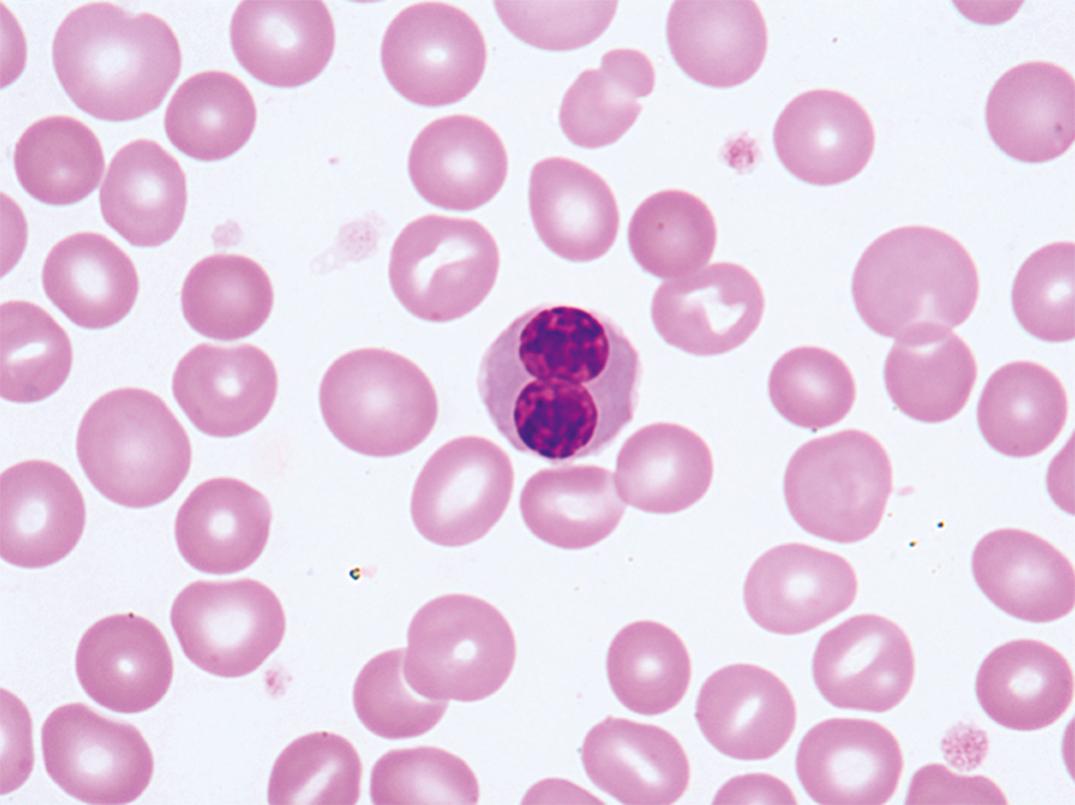
Macrocytic anemias associated with megaloblastosis differ from nonmegaloblastic macrocytic anemia in that macro-ovalocytes and giant hypersegmented neutrophils are present in the blood ( Figs. 31.11, 31.15 , and 33.6 ). Pancytopenia is the rule. The anemia is macrocytic with an elevated MCV and is characterized by macro-ovalocytes and often extreme degrees of anisocytosis and poikilocytosis, including red cell fragments. Microcytes and dacrocytes are common. Basophilic stippling, multiple Howell-Jolly bodies, nucleated red cells with karyorrhexis, and even megaloblasts may be seen in the peripheral blood. Leukopenia is present. Granulocytes have increased numbers of lobes (hypersegmentation), presumably as a result of abnormal nuclear maturation. Five lobes in more than 5% of the neutrophils constitute hypersegmentation ( ), as do any neutrophils with six or more lobes. Thrombocytopenia is usually encountered and on rare occasions is sufficiently severe to be responsible for bleeding. It is worth noting that significant morphologic changes may occur in the blood in the absence of anemia and that neurologic symptoms may be present in the absence of anemia.
Marrow. Megaloblastic anemia is characterized by enlargement of all rapidly proliferating cells of the body, including marrow cells. The major abnormality is the diminished capacity for deoxyribonucleic acid (DNA) synthesis. In cobalamin or folate deficiency, there is marked reduction in intracellular 5,10-methylene tetrahydrofolate, which is required to transform deoxyuridine monophosphate to deoxythymidine monophosphate. This reaction is mediated by thymidylate synthase and is essential to maintain the normal rate of DNA synthesis ( ). With such deficiency, the cells have both a prolonged intermitotic resting phase and a block early in mitosis. The number of mitotic figures is increased. Ribonucleic acid (RNA) synthesis is less impeded than DNA synthesis; hence, cytoplasmic maturation and growth continue, accounting for enlargement of the cells. The delicate chromatin and the prominent parachromatin result in a distinctly more “open” chromatin pattern than is seen in the erythroid precursors ( Figs. 31.28 and 33.6 ). The nuclei undergo karyorrhexis readily, and multiple Howell-Jolly bodies may be present. Usually, more cells analogous to the pronormoblast and basophilic normoblast are noted (i.e., promegaloblast and basophilic megaloblast) than are seen in normal erythropoiesis. This has sometimes been termed maturation arrest , or nuclear-cytoplasmic asynchrony . Giant polychromatic megaloblasts are especially distinctive. The same general features are seen in the other cell lines. In the granulocytic series, the cells are larger, with retarded nuclear maturation and large cytoplasmic mass. Often, the specific granules themselves are distinctly larger. The chromatin pattern is less condensed (more “open”). As a result, the nucleus appears to stain poorly. Abnormally contorted nuclear configurations are common. The giant metamyelocyte is the most characteristic of the abnormal granulocytes. Megakaryocytes, too, are large, polylobated, and have separated nuclear lobes or nuclear fragments ( ).
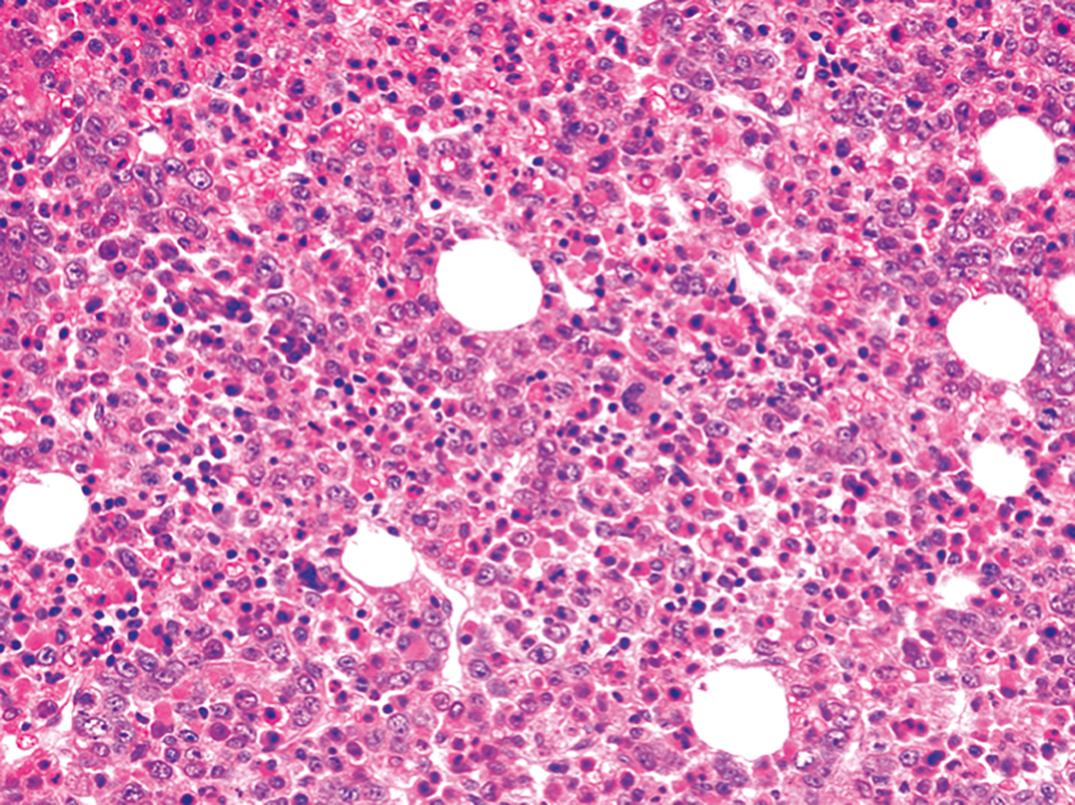
The bone marrow is hyperplastic ( Fig. 33.7 ). The fat is replaced, and red marrow extends into the long bones. The number of erythroid precursors (megaloblasts) is increased, and the myeloid/erythroid ratio is decreased. If the megaloblastic process is incompletely developed or if the patient has been inadequately treated, the findings may be only partial. Because they persist longer, granulocytic alterations are especially helpful in assessing recently treated megaloblastic anemia. Marrow findings result from the effects of impaired nucleic acid synthesis, leading to megaloblastosis and hypoxic stress, giving rise to increased numbers of erythroid cells. If the patient is transfused with packed red cells, the number of erythroid precursors diminishes but the cytologic abnormalities persist.
Erythrokinetics. In megaloblastic anemias, the mass of erythroid tissue is increased, plasma iron turnover is rapid, and urine and fecal urobilinogen are increased. These measures indicate an increase in total erythropoiesis that may be up to three times normal. The decreased rate of appearance of iron in the Hb of circulating erythrocytes and reticulocytopenia indicate ineffective erythropoiesis . In addition to increased destruction of defective erythroid precursors in the marrow, survival of circulating erythrocytes is short, indicating hemolysis. Indirect serum bilirubin is increased, serum iron is increased, endogenous carbon monoxide (CO) production is increased, and serum lactate dehydrogenase (LD) is usually greatly elevated. Serum muramidase may be elevated, implying ineffective granulocytopoiesis.
Megaloblastic anemia is nearly always due to cobalamin or folic acid deficiency. The aforementioned findings are similar for either.
Vitamin B 12 (cyanocobalamin) has a molecular weight of 1355 Da. The molecule’s two major parts are (1) a planar group (the corrin nucleus), a ring structure surrounding a cobalt atom; and (2) a nucleotide group , which consists of the base, 5,6-dimethylbenzimidazole, and a phosphorylated ribose esterified with 1-amino,2-propanol. A cyanide group is in coordinate linkage with the trivalent cobalt. Different forms of vitamin B 12 result from replacement of the cyanide by hydroxy, adenosyl, or methyl groups; generically, these are termed cobalamins .
Cobalamin is the only vitamin exclusively synthesized by microorganisms. It is found in practically all animal tissues and is stored primarily in the liver in the form of adenosylcobalamin. The human liver contains approximately 1 μg/g of liver. Cobalamin is released from food by gastric acid and peptic digestion and then becomes bound to R binders (cobalamin-binding proteins with R apid electrophoretic mobility compared with intrinsic factor) present in saliva. The R proteins belong to the same family of isoproteins as the plasma haptocorrin (HC) binder ( ). Salivary HC protects vitamin B 12 from the acidic environment in the stomach. On entering the duodenum, cobalamin is released from salivary HC by pancreatic enzymes to finally bind to gastric intrinsic factor (IF), a 44-kDa glycoprotein produced in the parietal cells of the stomach. The gene for IF is located on chromosome 11. This cobalamin–IF complex, which is highly resistant to digestion, then adheres to specific receptor sites on the epithelial cells of the ileum, at which site the cobalamin is absorbed. The cobalamin–IF receptor is a combination of two proteins: a 460-kDa protein called cubilin (CUBN) and a 45- to 50-kDa protein called amnionless (AMN). CUBN lacks both transmembrane and cytoplasmic domains and its internalization depends on other transmembrane proteins, particularly AMN. Both proteins are also present in renal tubules. Mutation of any of the two genes can produce megaloblastic anemia with proteinuria, also known as Imerslund-Gräsbeck syndrome ( ). The concentration of the receptor in the ileum increases progressively until it reaches its maximum near the terminal ileum. The cobalamin–IF complex is taken into the cell, where cobalamin is released and the IF is destroyed. The receptor recycles to the surface of the cell ( ). Cobalamin is exported from the enterocyte with multidrug resistance protein 1 ( ).
Cobalamin is transported in the plasma as methylcobalamin, with 10% to 30% bound to transcobalamin (TC), formerly known as transcobalamin II and 70% to 90% bound to HC (variously called TC I, TC III, R binder, cobalophilin; see later discussion). Ninety percent of newly absorbed cobalamin is bound to TC, which serves as the chief transport protein, rapidly delivering the vitamin to the liver, hematopoietic cells, and other dividing cells, which express transcobalamin receptors (CD320). The HC-bound cobalamine is not immediately available for body cells but it acts as a passive reservoir in equilibrium with body stores in the liver but not taken up by other body cells. The reference values for plasma cobalamin depend on the method of assay, but they commonly are 200 to 900 ng/L (150–670 pmol/L). The relative importance of the transcobalamins is illustrated by the clinical effects of congenital deficiency. Lack of TC results in severe megaloblastic anemia in infancy; yet, the serum cobalamin level is normal. Lack of HC is not accompanied by anemia or megaloblastosis; yet, the serum cobalamin level is decreased.
HCs are found in milk, plasma, saliva, gastric juice, and other body fluids. HC may arise from granulocytes, salivary glands, and liver, as well as from other tissues. HC may help clear nonphysiologic cobalamin analogs. Elevation of HC accounts for the elevation of total cobalamin-binding proteins in myeloproliferative neoplasms. TC is also synthesized by a variety of cells, including renal cells, enterocytes, and hepatocytes, and acts as an acute-phase reactant; its levels are increased in inflammatory and infectious conditions ( ; ). In humans, cobalamins are involved in two important enzymatic reactions: a mitochondrial reaction involves the formation of succinyl coenzyme A (CoA) from methylmalonyl CoA utilizing adenosyl cobalamin; the second is a cytosolic reaction involving the acquisition of a methyl group from N 5 -methyl tetrahydrofolate through the conversion of homocysteine to methionine and, thus, forming tetrahydrofolate. The latter reaction allows tetrahydrofolate to change to 5,10-methylene tetrahydrofolate, which is the most important methyl donor for the formation of deoxythymidine from deoxyuridine. Therefore, to be useful to the cell, cyanocobalamin and hydroxocobalamin must be converted to adenosyl and methyl forms through reduction and alkylation ( ; ). In the absence of a sufficient amount of deoxythymidine, deoxyuridine will be incorporated in DNA, which will ultimately result in DNA strand breaks, fragmentation, and apoptotic cell death ( ). The daily requirement for cobalamin is 0.4 μg/day for infants and 2.4 μg/day for adults ( ). The body’s stores of 2 to 5 mg will last for several years if intake is cut off, as is the case when total gastrectomy is performed ( ).
Although the true prevalence of cobalamin deficiency in the general population is unknown, it increases with age. Approximately 15% of adults older than 65 years have laboratory findings of vitamin B 12 deficiency. This prevalence may be attributed to the high frequency of hypochlorhydria of 25% to 50%, which has been reported in the elderly population. The widespread use of proton pump inhibitors to control gastric secretion, inflammatory bowel disease, and gastric and small intestinal resections are contributing factors ( ). Cobalamin deficiency is produced by any of several mechanisms that are not always exclusive of each other and involve inadequate intake and reduced absorption.
A dietary deficiency is an extremely rare cause of megaloblastic anemia in the United States and is seen only in persons who completely abstain from animal food, including milk and eggs. Only strict vegetarians are known to develop this form of cobalamin deficiency.
This is the most common cause of cobalamin deficiency.
Pernicious anemia (PA) is a “conditioned” nutritional deficiency of cobalamin that is caused by failure of the gastric mucosa to secrete intrinsic factor. This abnormality is genetically determined but usually is not manifested until late in life; less than 10% of cases occur in persons younger than age 40 years. The annual incidence of PA is approximately 25 new cases per 100,000 individuals over the age of 40 years and the prevalence is higher in women ( ). Positive family history is obtained in approximately 30% of patients. Modern surveys indicate that PA is as common in blacks as in whites ( ).
Symptoms of anemia and the combination of skin pallor and jaundice giving a lemon-yellow appearance to the skin are often present. The tongue may be sore, smooth, and pale (atrophic glossitis) or red and raw (acute glossitis). GI symptoms may be prominent and include episodic abdominal pain, constipation, and diarrhea. Diffuse and irregular degeneration of the white matter of the central nervous system (CNS) characteristically involves the posterior and lateral columns of the spinal cord (subacute combined degeneration) and sometimes other sites. Symmetric sensations of “pins and needles” of the distal extremities, numbness and tingling, loss of position sensation (difficulty with balance and gait), and loss of vibratory sensation (perhaps the most constant sign) are indicative of peripheral neuropathy and posterior column lesions. Lateral column involvement gives rise to weakness, spasticity, and increased deep tendon reflexes. Sometimes, the brain may be affected, and the patient shows irritability, emotional instability, or a change in personality. Neuropsychiatric disorders may be associated with cobalamin deficiency even without accompanying hematologic manifestations ( ). Recent studies suggest that cobalamin is essential for the maintenance of certain levels of cytokines and growth factors in the CNS. Cobalamin deficiency has been reported to cause an increase in CSF levels of myelinotoxic cytokines, such as tumor necrosis factor-α, while decreasing myelinotrophic cytokines, such as interleukin (IL)-6 ( ).
Atrophic gastritis of varying degrees is found in most adults with PA, and gastric atrophy involving all coats of the wall in the remainder. IF and hydrochloric acid (HCl) are secreted by gastric parietal cells in the human. In adult PA, IF secretion is absent, and almost always histamine-refractory achylia and achlorhydria—decreased volume of gastric juice and lack of HCl secretion—are present.
Autoantibodies have been found in the serum of patients with pernicious anemia ( ). Anti–parietal cell antibodies react with gastric parietal cells, which secrete both HCl and IF, and are present in more than 90% of patients. These anti–parietal cell antibodies are also present in patients with chronic gastritis, such as that associated with iron deficiency, and in some patients with thyroiditis and myxedema; they are seen in 4%–5% of age-matched healthy individuals. When these antibodies are chronically injected into rats, they decrease gastric HCl, IF, and pepsin secretion and produce gastric atrophy. The major antigen to which these antibodies are produced is the acid-producing enzyme H + , K + -ATPase, a 92-kDa protein present on the luminal membrane of parietal cells, which is the target of proton pump inhibitors. A recent study suggests that antibodies to H. pylori are common in PA, particularly in young patients. Infection may be the first step toward initiation of an autoimmune response ( ).
Another type of autoantibody is directed against intrinsic factor. Anti–intrinsic factor antibodies occur in the serum, saliva, and gastric juice of about 75% of patients with PA. Two types of anti-IF antibodies occur: “blocking” antibodies, which block the binding of cobalamin to IF; and “binding” antibodies, which bind to the cobalamin–IF complex and prevent the complex from binding to receptors in the terminal ileum. Although these antibodies can cause some functional impairment in vivo, it is not clear whether the antibodies are the cause or an effect of the disease. IF antibodies in the absence of PA occur in a small percentage of individuals with hyperthyroidism (Graves disease) and similarly in persons with insulin-dependent diabetes. Therefore, IF antibodies are considered highly specific and confirmatory for PA although their absence does not exclude the diagnosis ( ).
Family studies in patients with PA have shown an increased incidence of the disease in relatives, and many relatives have achlorhydria and partial defects of cobalamin absorption. Relatives of patients with PA also have a higher incidence of gastric parietal cell antibodies and of thyroid antibodies than normal individuals.
It is possible that adult PA is a genetically determined autoimmune gastritis. However, the relationship of the gastric lesion to the antibodies remains unclear. Patients with PA have an annual incidence of gastric cancer at 0.1% to 0.5% ( ).
Two forms of PA are known to occur in children. Congenital or infantile pernicious anemia usually appears early in the second year of life. IF secretion may be lacking or the secreted IF may be functionally defective, but acid secretion and the appearance of the gastric mucosa are normal. Antibodies to parietal cells and to IF are absent. These cases are caused by mutations in the IF gene ( GIF ) mapped to chromosome 11q12. Juvenile pernicious anemia occurs usually in older children and is like that of adults, with gastric atrophy, achlorhydria, and serum antibody to IF and parietal cells, although the latter may be absent in a subset of patients (Whitehead, 2006).
Surgical removal of the stomach (total or even subtotal occasionally) will remove the source of IF. This will lead to megaloblastic anemia after the body’s stores of cobalamin have been exhausted—in 3 to 6 years—if cobalamin therapy has not been given. Frequently, the anemia is due in part to iron deficiency.
Malabsorption Syndromes. Celiac disease, tropical sprue, resection of the small bowel, or inflammatory disease of the small bowel may be associated with multiple defects of absorption, including other vitamins. Folic acid deficiency (absorbed principally in the upper small bowel) is more commonly seen than cobalamin deficiency (absorbed principally in the lower small bowel) in diseases leading to malabsorption. The reason for this is probably the lesser time necessary for depletion of body stores of folic acid.
Imerslund-Gräsbeck syndrome is an autosomally recessive inherited defect in the intestinal absorption of cobalamin that occurs in the presence of normal IF. In many patients, proteinuria of the tubular type is also found. Most reported cases are from Northern Europe. As mentioned earlier, the syndrome is caused by a defect in the CUBN/AMN receptor ( ).
Lack of Availability of Cobalamin. In certain countries, infestation with the fish tapeworm Diphyllobothrium latum is common enough that cobalamin deficiency may occur occasionally when it is present. The worm successfully competes with the host for the ingested cobalamin. Most common in Finland, it is rarely seen in the United States.
Bacteria in a blind loop of intestine may also preferentially utilize ingested cobalamin to the detriment of the host.
As mentioned earlier, inherited TC deficiency leads to megaloblastic anemia accompanied by neurologic symptoms in early childhood.
Another cause of inactivation of vitamin B 12 is the use of nitrous oxide (N 2 O) gas as an anesthetic and sometimes as a recreational drug. It affects mostly individuals who have low levels of vitamin B 12 ( ).
Vitamin B 12 or folate deficiency may exert indirect cardiovascular effects. Both deficiencies are associated with hyperhomocystinemia, which is an independent factor for atherosclerosis and vascular thrombosis ( ).
Recognition of megaloblastic anemia indicates the likelihood of cobalamin deficiency or folic acid deficiency. In addition, evidence of neurologic involvement favors cobalamin deficiency. This diagnosis can be established by one of four methods.
Therapeutic Trial. With the patient on a diet low in cobalamin and folate, a parenteral physiologic dose of cobalamin (10 μg/day) is given. Optimal hematologic response indicates deficiency and consists of reticulocytosis beginning on the third or fourth day, reaching a peak on the seventh day. Erythropoiesis becomes normoblastic by 2 days, and leukopoiesis becomes normal by 12 to 14 days. Within a week, leukocyte and platelet counts have returned to normal, and the Hb concentration begins to rise.
Serum Cobalamin Assay. This is the usual method of detecting a cobalamin-deficient state. The original microbiological assay of serum cobalamin employed an organism (e.g., Euglena gracilis ) that requires cobalamin for growth. Although the microbiological method is precise and reliable, it requires at least 48 hours of incubation and is subject to the inhibitory effects of antibiotics. These microbiological assays have been largely abandoned and replaced by immunochemical assays measuring serum vitamin B 12 . Reference values are 200 to 900 ng/L. In megaloblastic anemia due to cobalamin deficiency, serum cobalamin is usually less than 100 ng/L. Individuals with folate deficiency and mild cobalamin deficiency and who are pregnant have borderline values between 100 and 200 ng/L. Patients with human immunodeficiency virus (HIV) infection or multiple myeloma and those receiving megadose vitamin C therapy often have low serum cobalamin levels in the absence of clinical manifestations ( ; ). Spuriously normal cobalamin levels have been recorded in patients with cobalamin deficiency associated with overgrowth of intestinal bacteria, which produce biochemically inert B 12 analogs, and in patients with autoimmune disorders, myeloproliferative neoplasms, and active liver disease ( ). Measurement of TC–bound cobalamin (holotranscobalamin) may provide additional information in that its levels fall below the normal range long before total serum cobalamin does and probably represent a state of negative cobalamin balance ( ). This marker is considered to be more accurate in assessing the biologically active fraction of vitamin B 12 and has been proposed to be used also in assessing the absorption of vitamin B 12 when measured before and after an oral dose of nonradiolabeled cobalamin ( ). Individuals with TC polymorphism 67A>G have abnormally low levels of holoTC in the absence of vitamin B 12 deficiency ( ).
Methylmalonic Acid and Homocysteine Assays. Because a cobalamin coenzyme is essential for the isomerization of methylmalonate to succinate, urine excretion of increased amounts of methylmalonate is found in cobalamin deficiency. Provided that the rare inborn error of metabolism, methylmalonic aciduria, is not present, this is a sensitive test for cobalamin deficiency, but it is not usually necessary for the diagnosis. In addition, plasma levels of methylmalonic acid and homocysteine are increased in more than 90% of patients and their rise usually precedes the drop in serum vitamin B 12 level. Plasma levels of methylmalonate are normal in folate deficiency in the absence of cobalamin deficiency ( ). Following several weeks of therapy, their plasma concentration returns to normal. Plasma levels of these metabolites should be interpreted with caution in patients with chronic renal failure because of the tendency of these metabolites to accumulate ( ).
Deoxyuridine Suppression Test. This measures the ability of marrow cells in vitro to utilize deoxyuridine in DNA synthesis. Normally, in marrow cells, the major source of thymidine for DNA is de novo synthesis from deoxyuridine, which requires intact cobalamin and folate enzymes. Therefore, less than 10% of added tritium-labeled thymidine ( 3 H-Tdr) is incorporated into DNA. In megaloblastic marrows due to cobalamin or folate deficiency, deoxyuridine cannot be efficiently converted to thymidine, and more 3 H-Tdr is taken up into DNA. An abnormal deoxyuridine suppression test indicates cobalamin or folate deficiency. This test is very sensitive and produces abnormal results before anemia or macrocytosis is observed ( ). It currently is seldom used as a diagnostic test.
Clinical history is useful in suggesting whether cobalamin or folate deficiency is the cause of megaloblastic anemia. Clinical associations of PA include a family history of PA in one-third of patients, along with certain endocrine deficiencies (thyroid disease, diabetes mellitus, hypothyroidism, and Addison disease) and immune disorders (immune thrombocytopenic purpura, autoimmune hemolytic anemia, and acquired hypogammaglobulinemia). Cobalamin deficiency is likely to occur in strict vegetarians and in patients with paresthesias, neuropathy, or a previous gastrectomy.
In cobalamin-deficient patients, it is important to determine whether IF is lacking. To do so, the ability of the patient to absorb an oral dose of radioactive cobalamin may be measured. The Schilling test, which measures radioactivity in a 24-hour sample of urine, is now rarely used. Two hours after oral administration of 0.5 to 2.0 μg of radioactive cobalamin, a large “flushing” dose of nonlabeled cobalamin is given parenterally. Normal individuals will excrete more than 7% of a 1-μg dose of ingested cobalamin in the urine in 24 hours, whereas patients lacking IF will excrete less. If excretion is low, the test must be repeated using the same procedure, except that hog IF is given orally, along with labeled cobalamin. If 24-hour excretion is normal, the low value in the first part was due to IF deficiency. If excretion remains abnormal in the second part of the procedure, an explanation for malabsorption of cobalamin on the basis of intestinal disease must be sought. The test may be repeated after 7 to 10 days of antibiotic administration if bacterial overgrowth is suspected, and pancreatic extracts may be added to investigate the possibility of pancreatic dysfunction. The validity of the results depends on good renal function and accurate urine collection. The Schilling test will be abnormal in PA even after the patient is treated with cobalamin and is in remission. Some patients may absorb vitamin B 12 in water (as given in the original Schilling test) but fail to absorb vitamin B 12 bound to protein in food. A modification of the Schilling test is being introduced to include protein-bound B 12 using egg yolk or chicken serum ( ).
Other tests that will establish the diagnosis of PA include direct assay of IF in gastric juice. The combination of megaloblastic anemia, decreased serum cobalamin, and the presence of serum antibodies to IF is essentially diagnostic of PA, obviating the need for the Schilling test ( ). As mentioned earlier, the use of holoTC before and after oral dose of vitamin B 12 may be an alternative to test vitamin B 12 absorption defects. The combined assessment of antibodies to IF and parietal cells increase diagnostic sensitivity for PA to 73% and diagnostic specificity to almost 100% ( ).
Folic acid (or pteroylmonoglutamic acid) contains three parts: pteridine, p -aminobenzoate, and l -glutamic acid ( ). In nature, folic acid occurs mainly as less soluble polyglutamates, with multiple glutamic acid residues attached to one another by peptide bonds. Folic acid is present in a wide variety of foods, such as eggs, milk, leafy vegetables, yeast, liver, and fruits, and is formed by intestinal bacteria as well. Folates are extremely thermolabile; prolonged cooking (>15 minutes) in large quantities of water in the absence of reducing agents destroys folate.
Polyglutamates are hydrolyzed to monoglutamate by folate hydrolase, which is present in the brush border of proximal jejunum and has maximal exopeptidase activity at pH 6.5 ( ). Folate monoglutamates are transported across the intestinal membrane by a variety of carriers, including reduced folate carrier (Solute Carrier [SLC] 19A1) and PCFT (SLC46A1). The latter has been shown to be the most important folate transporter. It is highly expressed at the upper region of the small intestine and is most active at acidic pHs ( ). PCFT is also present in colonic mucosa but at a much lower concentration. In the plasma, one-third of the folate is free and two-thirds is nonspecifically and loosely bound to serum proteins. A small amount of folate is specifically bound to folate-binding proteins, the physiologic significance of which is unclear. Folate is rapidly removed from plasma to cells and tissues for utilization. The principal form of folate in serum, erythrocytes, and liver is 5-methyltetrahydrofolate (5-methyl-FH 4 ); the liver is the chief storage site. Uptake of folate by the cells is dependent on three folate receptors (FOLR1 to FOLR3), one of which has been shown to be deficient in a condition known as cerebral folate deficiency ( , ). Intracellular folates exist primarily as polyglutamates. A significant enterohepatic circulation is present, and bile contains 2 to 10 times the folate concentration of normal serum. Moreover, folates that are filtered in the renal glomeruli are extensively reabsorbed back into the renal circulation ( ). The minimal daily requirement is about 50 μg of pteroylmonoglutamate or 400 μg of total folate; a typical reference interval for serum folate is 5 to 21 μg/L (11–48 nmol/L), and for red cell folate, 150 to 600 μg/L (340–1360 nmol/L) of RBCs.
Anemia of cobalamin deficiency is partially corrected by folate even in the absence of cobalamin supplementation, but the reverse is not true. This is because folic acid will be reduced to dihydrofolate and tetrahydrofolate, partially and temporarily alleviating the anemia component. Therefore, some of the megaloblastic manifestations in cobalamin deficiency are actually caused by abnormalities in folate metabolism. The most accepted theory for their interrelationship is the methylfolate trap theory. This theory is based on the observation that the methyl form of tetrahydrofolate (FH 4 ) would leak out of cells unless conjugated to form polyglutamates. Methyl FH 4 is a poor substrate for the conjugating enzyme. Cobalamin is essential for the process of conversion of methyl FH 4 to FH 4 through the conversion of homocysteine to methionine. Absence of cobalamin causes accumulation of methyl FH 4 , which is followed by its leakage out of the cells ( ).
Evolution of Laboratory Abnormalities. Herbert delineated the sequence of events in the onset of folate-deficient megaloblastic anemia. After a folate-deficient diet was initiated, various abnormalities were established as follows: 3 weeks, low serum folate; 5 weeks, hypersegmented neutrophils in bone marrow; 7 weeks, hypersegmented neutrophils in peripheral blood, with bone marrow showing increased and abnormal mitoses and basophilic intermediate megaloblasts; 10 weeks, bone marrow showing some large metamyelocytes and polychromatophilic intermediate megaloblasts; 13 weeks, high excretion of formiminoglutamic acid (FIGLU) in urine; 17 weeks, low erythrocyte folate; 18 weeks, macroovalocytosis of erythrocytes with many large metamyelocytes in bone marrow; 19 weeks, overtly megaloblastic bone marrow; and 20 weeks, anemia ( ).
At this time, changes in the intestinal epithelium have not yet appeared. Therefore, in the human, with no dietary intake of folic acid, anemia will appear in 3 to 6 months. The peripheral blood and bone marrow features of megaloblastic anemia due to folic acid deficiency are similar to those of cobalamin deficiency; however, leukopenia and thrombocytopenia are less constant. Folic acid deficiency has usually been found in association with some complicating factor.
Nutritional Folate Deficiency. Megaloblastic anemia due to lack of folate is most commonly associated with insufficient dietary intake. The usual diet does not contain much above the minimal requirements, and body stores in the adult are sufficient for only about 3 months’ needs. Dietary folate deficiency is especially common in the tropics and in India and, even in those locations, it is usually associated with increased demand for folate in pregnancy, rapid growth in infancy, infection, or hemolytic anemia. Elderly persons on inadequate diets in the United States may develop folate-deficient megaloblastic anemia.
Folate deficiency in infancy is uncommon in the United States. Human milk or fresh cow’s milk contains sufficient folate; heated milk, powdered milk, and goat’s milk do not. If the infant’s milk lacks folate, if the diet is low in ascorbic acid, or if infection or diarrhea is a problem, megaloblastic anemia may occur.
Megaloblastic anemia in pregnancy is not uncommon because of the fetal requirements for folate. The mother’s plasma folate level gradually falls during pregnancy, and at birth the plasma level in the newborn averages five times that of the mother. Megaloblastic anemia is more frequent in multipara, may be precipitated by infection, and is usually due to folate deficiency rather than cobalamin deficiency. Pregnant women should receive, in addition to iron, folic acid supplements. Recent studies indicate that folate supplementation for pregnant women reduces the risk of giving birth to babies with neural tube defects, with anencephaly and spina bifida being the most common defects. It has been estimated that more than 50% of neural tube defects in babies are related to maternal folate deficiency in the periconceptual period ( ).
Liver Disease. Liver disease associated with alcoholism may be associated with folate-deficient megaloblastic anemia because of the grossly inadequate diet of the alcoholic and because the liver is the major site for folate storage and metabolism. With adequate dietary folic acid intake, however, the anemia that is found with liver disease is macrocytic and normoblastic—not megaloblastic.
Defective absorption of folic acid occurs in association with malabsorption syndromes discussed previously and in the blind loop syndrome, in which bacteria preferentially utilize folate.
Nontropical sprue, or adult celiac disease, is an important cause of malabsorption in adults or children that is related to dietary gluten (wheat protein). Included among the signs of malabsorption may be megaloblastic anemia due to folic acid deficiency ( ). Jejunal biopsy shows villous atrophy. The folate deficiency, as well as the malabsorption, responds to a gluten-free diet. Folic acid therapy (parenteral) corrects the folate deficiency but not the general malabsorption.
Tropical sprue is a poorly understood malabsorptive disorder that is common in the Caribbean, India, and Southeast Asia. It can be acquired by travelers to those regions and persists for many years after the travelers return. Evidence of malabsorption includes megaloblastic anemia due to folate deficiency. Treatment with folic acid brings considerable improvement in general malabsorption, as well as in the anemia, but additional antimicrobial treatment is recommended.
Megaloblastic anemia or decreased serum and red cell folate without anemia has been associated with the long-term use of anticonvulsant drugs such as phenytoin, phenobarbital, and primidone. The problem appears to be a drug-induced malabsorption of pteroylpolyglutamate. Oral contraceptives cause malabsorption of folate in a small proportion of women owing to impaired deconjugation of pteroylpolyglutamate ( ).
Hereditary folate malabsorption is a rare autosomal-recessive condition caused by mutation affecting the gene encoding the PCFT (SLC46A1) molecule ( ).
As mentioned earlier, cerebral folate deficiency is caused by a defective cerebral folate transport system, caused in most cases by mutation in FOLR1. Patients present in late infancy with severe developmental delay, motor dysfunction, and epilepsy. Oral folinic acid may improve the symptoms ( , ).
The increased need for folate in pregnancy and in infants has been mentioned. Increased cell turnover that occurs in neoplasia and in the markedly stimulated hematopoiesis of hemolytic anemias may result in megaloblastic erythropoiesis. The basis for this is the increase in need for a marginal supply of folate.
Inadequate utilization of folic acid is relatively rare. Folic acid antagonists such as methotrexate block folic acid metabolism—because of this, they are used in therapy of some malignant neoplasms. In addition to inhibiting growth of the tumor, they induce megaloblastic hematopoiesis.
In addition to the previously mentioned nutritional problem in alcoholics, alcohol may exert a direct effect in suppressing hematopoiesis by blocking the metabolism of folate. In addition, alcohol can interfere with folate absorption and folate enterohepatic circulation, usually resulting in increased loss of folate in urine. Animal studies suggest that alcohol may decrease the expression of PCFT ( ). Plasma homocysteine is usually elevated in alcoholics.
Folic acid deficiency or cobalamin deficiency is suspected when the blood and bone marrow show findings characteristic of megaloblastic anemia. Usually, serum folate and cobalamin levels are then determined. Some cases may be associated with marked red cell fragmentation simulating microangiopathic hemolytic anemia. Severe folate deficiency in pregnancy may mimic the syndrome of h emolysis, e levated l iver enzymes, and l ow p latelets (HELLP syndrome; see later discussion).
Serum and Red Cell Folate. A microbiological assay for folic acid activity employing Lactobacillus casei is a reliable method for definitive diagnosis ( ). However, these assays are laborious, have poor precision, and are affected by antibiotics. Radioisotopic and chemiluminescence methods employing different folate binders are widely used because of their rapidity and greater convenience. Commonly used assays utilize folate-binding protein to capture serum folate. Unlike serum folate (which is entirely 5-methyltetrahydrofolate), red cell folates are a heterogeneous mixture of different forms with varying polyglutamate chain lengths, which pose challenges to nonmicrobiological assays.
The serum folate level is decreased (<3 μg/L) in megaloblastic anemia due to folate deficiency but is usually normal or increased in cobalamin deficiency. A low serum folate level precedes a decrease in red cell or tissue folate. It indicates a negative folate balance but does not by itself indicate tissue folate deficiency. Serum folate is highly sensitive to folate intake and may normalize after a single adequate meal despite the presence of true underlying deficiency. In cobalamin deficiency, serum folate is decreased in 10% of cases, increased in 20%, and normal in the remainder ( ).
Red cell folate is a better test of body folate stores and is decreased in megaloblastic anemia due to folate deficiency. In cobalamin deficiency, however, red cell folate is low in almost two-thirds of cases; thus, this needs to be excluded before a low red cell folate is taken as proof of severe folate deficiency. Therefore, three measurements are often useful in distinguishing between deficiencies of folic acid and cobalamin ( Table 33.2 ).
| Clinical Situation | Serum Vitamin B 12 , pg/mL | Serum Folate, ng/mL | Red Cell Folate, ng/mL |
|---|---|---|---|
| Normal ∗ | Normal (200–900) | Normal (5–16), indeterminate (3–5), or low (<3) | Normal (>150) |
| Vitamin B 12 deficiency | Low (<100) | Normal (5–16) or high (>16) | Low (<150) |
| Folic acid deficiency | Normal | Low | Low |
| Deficiency of both | Low | Low | Low |
∗ Normal includes transient states of negative folate balance.
Physiologic doses of folic acid (parenteral, 50–200 μg/day) will allow an adequate reticulocyte response in patients with folic acid deficiency, but not with cobalamin deficiency. On the other hand, the usual therapeutic doses of folic acid (5–15 mg/day) or larger doses of cobalamin (500–1000 μg) may induce a partial response in a patient with megaloblastic anemia due to the other deficiency. Treatment of cobalamin deficiency with folic acid is hazardous in that, although a reticulocyte response may occur, neurologic damage will progress.
Folic acid coenzymes are required for the conversion of FIGLU to glutamic acid in the catabolism of histidine. When oral histidine is given, FIGLU will appear in increased amounts in the urine if folate deficiency is present. The test is useful in patients with megaloblastic anemia due to antifolate drugs; these patients have normal serum folate levels but greatly decreased tissue coenzyme levels ( ).
Deoxyuridine Suppression Test. See earlier discussion.
Plasma Homocysteine Assay. As with cobalamin deficiency, total plasma homocysteine is increased in approximately 75% of patients with folate deficiency. The level of methylmalonic acid is normal ( ).
Acute megaloblastic anemia may develop over the course of only a few days. The most common cause has been related to N 2 O anesthesia. N 2 O rapidly destroys methylcobalamin, causing a rapidly progressive megaloblastic anemia. Acute folate deficiency may occur in some patients in intensive care units because of a combination of factors (decreased intake, total parenteral nutrition, dialysis, surgery, sepsis, medications). These patients may present with rapidly progressive thrombocytopenia in the absence of hypersegmented neutrophils. Serum folate may be normal ( ).
Although it may be necessary to treat severely anemic patients with both vitamins, it is usually possible to determine which deficiency is the cause and treat only for that.
The maximal reticulocyte response occurs in 5 to 7 days. Within 4 to 6 hours after the initial therapy (if parenteral), the marrow shows decreased early megaloblasts and the appearance of pronormoblasts. Within 2 to 4 days, the marrow is predominantly normoblastic. Granulocytic abnormalities return to normal more slowly, and hypersegmented neutrophils disappear from the blood only after 12 to 14 days.
PA is treated parenterally with 1000 μg of cyanocobalamin daily for 1 week, once weekly for 1 month, then monthly for the lifetime of the patient ( ). High concentrations of oral cobalamin (e.g., 1000 μg per day) force absorption of approximately 1% through an alternate system. Some reports recommend the use of oral cobalamin therapy instead of injections ( ).
In folate deficiency, oral therapy is generally used at a dosage of 1 to 2 mg/day. Cobalamin deficiency must be excluded and corrected if present to avoid the occurrence of neuropathies of cobalamin deficiency. Supplemental dietary folic acid during pregnancy is reported to reduce the incidence of neural tube defects in infants.
Other defects of nucleoprotein synthesis may lead to megaloblastic anemias that do not respond to cobalamin or folic acid.
Orotic aciduria is a very rare autosomal-recessive condition in which certain enzymes required for pyrimidine synthesis are absent. Findings include excessive urinary excretion of orotic acid, failure of normal growth and development, and megaloblastic anemia that is refractory to cobalamin and folate but that responds to uridine.
Inborn defects in enzymes involved in folate metabolism, including methyltetrahydrofolate reductase and glutamate formiminotransferase deficiencies, have been described. Similarly, disorders of enzymes responsible for converting cobalamin to its methyl or adenosyl forms causing methylmalonic aciduria, homocystinuria, or both have also been described ( ).
Synthetic inhibitors of purine synthesis (6-mercaptopurine, thioguanine, azathioprine), of pyrimidine synthesis (5-fluorouracil), or of deoxyribonucleotide synthesis (cytosine arabinoside or hydroxyurea) are used in chemotherapy for neoplasia and may concomitantly produce megaloblastosis.
Some macrocytic anemias are caused by myelodysplastic syndromes (see Chapter 34 ). They are associated with certain dysplastic features in the marrow or blood and will show no response to cobalamin or folate therapy.
Anemia of inflammation (AI) ( ) is an anemia syndrome typically found in patients with chronic infections or inflammatory or neoplastic disorders. It is characterized by reduced reticulocyte response accompanied by low serum iron despite adequate iron stores. It is also termed anemia of chronic disease , or cytokine response , although the term anemia of inflammation is now a more common term. Estimates suggest that up to 40% of all anemias worldwide can be considered AI or combined anemia with a significant AI component. The frequency is higher in the elderly population. AI has also been observed in acute trauma and critical care patients.
The anemia is mild to moderate, with Hb level rarely below 8 g/dL. Erythrocytes are usually normocytic and normochromic, although in 20% to 50% of patients, the anemia is microcytic and hypochromic. Anisocytosis and poikilocytosis are slight. The reticulocyte count usually is not elevated. Leukocytes and platelets are not distinctively altered, except by the causative disease.
The marrow is normocellular or minimally hypocellular or hypercellular, and the cell distribution is not greatly disturbed. The normoblasts may have frayed hypochromic cytoplasm, and the appearance of Hb in the cells may be delayed (as in IDA). Sideroblasts are decreased, but storage of iron is normal or increased.
The serum iron concentration is characteristically decreased, the TIBC is decreased or normal (in contrast to IDA, in which the TIBC is elevated), and the percent saturation is decreased. Erythrocyte protoporphyrin and serum ferritin are elevated.
The most important pathogenetic mechanism of AI is the presence of high levels of cytokines, which may result in decreased red cell survival, altered iron metabolism, direct inhibition of hematopoiesis, and decreased EPO secretion. Tumor necrosis factor-α (TNF-α) plays a significant role in inflammation and immune response. TNF-α levels are increased in patients with cancer, rheumatoid arthritis, infection, and the acquired immunodeficiency syndrome. In vitro inhibition of human erythroid colony formation, burst forming units-erythroid (BFU-E), and colony forming units-erythroid (CFU-E) by TNF-α has been reported. Similarly, an inhibitory action of IL-1 and interferon-γ (IFN-γ) on erythropoiesis has been implicated. Ceramide, a product of cytokine-induced enzymatic hydrolysis of cell membrane sphingomyelin, plays a role as a messenger in the inhibitory effects of IFN-γ ( ).
EPO levels, although above normal, have been disproportionate to the degree of anemia, indicating relative EPO deficiency in AI. Inhibitory effects of cytokines on EPO synthesis sites such as renal and liver cells have been suggested. The relative deficiency of EPO induces neocytolysis (i.e., selective hemolysis of the youngest RBCs). Thus, a mild hemolytic event usually accompanies AI although shortened red cell survival may be more significant in acute conditions, such as acute infections and severe sepsis. In addition, macrophage activation by inflammatory cytokines may play an additional role in shortened red cell survival ( ).
Recently, hepcidin has been shown to be elevated in AI through induction by IL-6 and is considered an acute-phase reactant. As mentioned earlier, hepcidin interferes with the release of intracellular iron. Recent evidence also suggests that hepcidin may exert an inhibitory effect on erythroid colony formation at certain levels of EPO and, thus. may provide a bone marrow inhibitory effect.
The anemia usually fails to respond to iron therapy. However, patients treated with EPO have shown improvement.
A normocytic normochromic anemia is commonly encountered in patients with chronic renal failure (CRF). The correlation between severity of anemia and degree of elevation of blood urea nitrogen (BUN) is positive but not strictly linear. When creatinine clearance falls below 20 mL/min, the Hct is usually below 0.30 ( ).
Several factors are often involved in the anemia of CRF. Decreased production of EPO by the damaged kidney is probably the important factor in most cases in which the BUN exceeds 100 mg/dL. Both ineffective erythropoiesis and impaired ability of the marrow to respond to EPO appear to be present to some degree.
Inhibitors of erythropoiesis have been demonstrated in the plasma of patients with CRF. The nature of these inhibitory factors is not known; however, parathyroid hormone and spermine have been implicated as inhibitors of erythropoiesis. Recent studies indicate the presence of a high level of inflammatory cytokines, such as IL-1, IL-4, IL-6, and TNF-α, in patients with CRF. As discussed under Anemia of Inflammation, these cytokines exert a bone marrow suppressive effect and probably contribute to the development of anemia ( ). The role of hepcidin in this type of anemia is also being investigated.
Hemolysis is a significant feature in many cases of CRF. There appears to be an extracorpuscular factor in uremic plasma that has a detrimental effect on red cell metabolism and results in morphologically deformed cells (echinocytes and spiculated red cells). Numerous irregularly contracted and fragmented cells are seen in the hemolytic-uremic syndrome (HUS) and in malignant hypertension as a result of traumatic damage incurred by the red cells in traversing damaged small blood vessels. Changes in red cell membrane adenosine triphosphatase (ATPase) and transketolase may render the red cells more sensitive to oxidant drugs or chemicals.
In addition, bleeding is a common problem in chronic renal disease, probably owing to thrombocytopenia in some patients, or to platelet functional defects, which are present in most patients. Anemia due to iron deficiency from blood loss should always be suspected. Folic acid deficiency may be a problem in patients in a dialysis program in that folic acid is readily moved into the dialysis bath. Aluminum in dialysis water may interfere with iron incorporation in erythroid cells producing microcytic anemia ( ). A novel therapeutic approach is the potential use of prolyl hydroxylase inhibitors, which stabilize hypoxia-inducible factors and increase the level of endogenous EPO ( ).
Anemia of liver disease is multifactorial and is very common, affecting approximately 75% of patients with chronic liver disease ( ). Associated pathologies include IDA, congestive splenomegaly, chronic posthemorrhagic anemia, and folate-deficient megaloblastic anemia due to poor nutrition in alcoholic cirrhosis. Hypoplastic anemia secondary to viral-induced marrow suppression, acquired hemolytic anemia associated with Coombs-positive red cells and anemia due to lipid disturbances or drug interactions also occur ( ). Iron deficiency is most common, occurring in approximately 50% of patients, followed by hypersplenism in approximately 25% ( ).
IFN-α and ribavirin (Rbv) are common therapies for hepatitis C infection. Anemia (hemoglobin <11 g/dL) is seen in 30% to 40% of patients. Rbv induces suppression of erythropoiesis, possibly through downregulation of EPO receptors, and can also cause dose-dependent hemolytic anemia, particularly in patients with impaired renal function. IFN-α can produce anemia through suppression of hematopoietic progenitor cell proliferation and induction of apoptosis of erythroid progenitor cells ( ).
Another type of anemia uniquely associated with liver disease is characterized by shortened red cell survival and relatively inadequate red cell production. It is exaggerated by an increased blood volume that appears to correlate with the degree of portal hypertension. The red cells are normocytic or macrocytic (thin macrocytes). Frequently, target cells are present, especially in obstructive jaundice. These cells have increased surface membrane with increased cholesterol and lecithin content. However, phospholipid/cholesterol ratio is normal. Reticulocytes may be slightly increased and platelets may be normal or decreased depending on the level of thrombopoietin production. The bone marrow may be slightly hypercellular, and erythropoiesis is macronormoblastic rather than megaloblastic. Changes in leukocytes such as those seen in megaloblastic anemias are not seen, and this type of anemia does not respond to cobalamin (vitamin B 12 ) or folic acid. The anemia is of unknown origin. However, abnormal erythrocyte oxidative metabolism, including decreased pentose phosphate shunt and reduced ATP, may contribute to decreased red cell survival ( ).
Approximately 10% to 30% of patients with severe cirrhosis have a hemolytic anemia associated with “spur cells,” which are red cells with thorny projections similar to acanthocytes in more than 5% of red cells. As with target cells, the spur cells occur secondary to lipid abnormalities in the plasma. They have increased surface membrane with increased cholesterol, but normal phospholipid content in the membrane. The increased membrane cholesterol tends to associate with the outer membrane leaflet, making it more rigid. The spleen attempts to remodel the membrane, resulting in characteristic membrane projections ( ).
Uncomplicated anemia in hypothyroidism is mild to moderate, with Hb concentration rarely less than 8 to 9 g/dL; it is normochromic and normocytic without reticulocytosis and with normal red cell survival. Anemia is observed in 21% to 60% of hypothyroid patients ( ). It reflects decreased marrow production due to a smaller tissue O 2 requirement and subsequent reduced EPO secretion. Because plasma volume is decreased in hypothyroidism, the apparent degree of anemia may not be proportional to the decrease in red cell mass.
Hypothyroidism may be complicated by IDA, producing microcytic anemia. This is most often seen in females as a result of menorrhagia, although in males it may occur due to achlorhydria and diminished iron absorption.
Macrocytosis is frequently observed and, although the incidence of PA in patients with hypothyroidism may be increased, the exact etiology of macrocytosis remains unknown. A mild anemia occurs in 10% to 25% of patients with hyperthyroidism. The anemia may be slightly microcytic. Plasma volume may be increased, suggesting dilution.
In adrenal cortical hormone deficiency, a mild normochromic normocytic anemia is present although the decreased plasma volume may mask the degree of anemia. The cause is unclear, but the condition is corrected by hormone replacement.
Deficient testosterone secretion in men results in a decrease in red cell production of 1 to 2 g Hb/dL (to a value comparable with that of women). This appears to be due to a stimulatory effect of androgens on EPO secretion, occurring through enhanced EPO action on the marrow and possibly as a direct effect on a subset of marrow cells, such as stromal cells, macrophages, and myeloid precursors.
Pituitary deficiency also causes moderately severe normocytic anemia, which is associated with marrow hypoplasia. The mechanism is probably complex and related to loss of function of multiple endocrine glands and possibly loss of growth hormone effect.
A small number of patients with hyperparathyroidism have a normocytic, normochromic anemia. Earlier studies suggested that the parathyroid hormone may suppress normal erythropoiesis but more recent studies failed to support this theory. The cause of the anemia is unknown but is likely related to marrow fibrosis ( ).
This anemia is associated with marrow replacement by (or involvement with) abnormal cells or tissue component, such as metastatic carcinoma, multiple myeloma, leukemia, lymphoma, lipidoses or storage diseases, and certain other conditions.
Normochromic and normocytic (occasionally macrocytic) anemia of varying severity is present. Reticulocytes are often increased, and normoblasts are present in a number that is usually out of proportion to the severity of the anemia. The leukocyte count is normal or reduced (occasionally elevated), and immature neutrophils and even myeloblasts may be found. Platelets are normal or decreased, and bizarre, atypical platelets can sometimes be seen.
Examination of the marrow will usually reveal the condition responsible for this reaction. Mechanical crowding out of the hematopoietic tissue by the pathologic process has been assumed but not proved and probably is not the usual cause. Often, the amount of erythropoietic tissue in the marrow as determined by morphologic and kinetic studies is normal or increased. The mechanism described earlier under Anemia of Inflammation may often play a role, but the reason for the outpouring of immature cells into the blood is not clear.
In addition to myelophthisic anemias, circulating normoblasts and immature neutrophils can be seen in hemolytic anemias, severe anemias due to other causes, severe infections, recovering marrow, and congestive heart failure, but usually the normoblasts are not as numerous.
The leukoerythroblastotic (or leukoerythroblastic) reaction associated with myelophthisic anemias cannot always be distinguished from the blood picture of primary myelofibrosis, which is one of the myeloproliferative neoplasms. In primary myelofibrosis, particularly the fibrotic phase, enlargement of the spleen and liver is almost always found. In the blood film, more severe red cell abnormalities, such as tear-drop shapes (dacrocytes), leukocytosis, myeloblasts and immature granulocytes of all varieties (not just neutrophils), increased basophils, more atypical platelets, more numerous megakaryocyte fragments, and dwarf megakaryocytes are all findings more characteristic of primary myelofibrosis than of a leukoerythroblastotic reaction of some other cause. Examination of the bone marrow biopsy is necessary for the final diagnosis.
AA usually refers to pancytopenia associated with a severe reduction in the amount of hematopoietic tissue that results in deficient production of blood cells in the absence of a bone marrow infiltrative process or increased reticulin ( ). Usually two of the following three blood parameters are needed to diagnose AA: Hgb less than 100 g/L, granulocyte count less than 1.5 × 10 9 /L, and platelet count less than 50 × 10 9 /L. The marrow, although hypocellular, may have patchy areas of normocellularity, or even hypercellularity. The diagnosis of severe AA is made in pancytopenic patients when at least two of the following three peripheral blood values—granulocyte count less than 0.5 × 10 9 /L, platelet count less than 20 × 10 9 /L, or reticulocyte count less than 20 × 10 9 /L, or 1% (corrected for Hct)—are present and the bone marrow is less than 25% cellular. With the pervasive use of automated reticulocyte counts, the threshold for severe AA stands at 60 × 10 9 /L using automated counts ( ). The term very severe AA is used when the granulocyte count is less than 0.2 × 10 9 /L ( ). Otherwise, the AA would be classified as moderate or nonsevere. AA is usually classified as inherited (see later discussion) or acquired. The incidence of acquired AA is 2 per million per year based on retrospective studies in Europe and North America, and is about 2 to 3 times higher in East Asia, with the highest being in Japan and estimated at 14 per million per year ( ). There is a bimodal age of peak presentation, with the first occurring in patients aged 15 to 25 years and the second in patients over 60 years old.
The clinical course may be acute and fulminating, with profound pancytopenia and a rapid progression to death, or the disorder may have an insidious onset and a chronic course. The symptoms and signs depend on the degree of the deficiencies: bleeding from thrombocytopenia, infection from neutropenia, and signs and symptoms of anemia. As a rule, splenomegaly and lymphadenopathy are absent. Bleeding is the most common presentation of acquired AA, occurring in approximately 40% of patients ( ). Bleeding usually manifests as easy bruisability, gum bleeds, and episodic nosebleeds. Less than 5% of patients present with infection.
Acquired aplastic anemias are of diverse origin. Since 1980, in approximately 70% of cases, no specific etiologic agent could be correlated with the disease; such cases are considered idiopathic. Drug- and chemical-related AAs account for 11% to 20%, and those associated with infectious hepatitis account for 2% to 9% of cases ( ).
Hematopoietic failure may occur at any level in the differentiation of bone marrow precursor cells, whether pluripotential or committed stem (progenitor) cells. The CD34+ cells in the bone marrow, which contain most of the stem cells and committed progenitor cells, are markedly decreased in patients with AA, with a percentage of marrow CD34+ cells of <0.3% ( ).
The mechanism of acquired AA is suggested to fall under two main categories: direct damage and immune-mediated destruction of marrow cells. Direct damage to the hematopoietic stem and progenitor cells could be caused by a known agent, such as cytotoxic drugs, irradiation, and viruses, or an unknown agent that in some way alters the ability of the cell to proliferate or differentiate. Immune-mediated destruction of stem and progenitor cell compartments results from cytotoxic lymphocyte activation, cytokine production, and specific cell elimination. Most studies indicate that stem cell destruction results from T-cell autoimmunity. An unidentified antigenic exposure leads to polyclonal expansion of CD4+ T cells with overproduction of proinflammatory cytokines, such as INF-γ and TNF-α. There is also oligoclonal expansion of a CD8+ cytotoxic cell population. AA patients have increased T helper 17 (Th17) cells secreting IL-17. The stem cell destructive process is augmented by deficits in regulatory T cells (Tregs), which normally suppress autoreactivity ( ). Recent studies favor immune-mediated mechanisms for AA and suggest that chemical and viral antigens may initiate the destructive immune process ( ). A role for intrinsic stem cell defects and abnormal bone marrow microenvironment may be contributory. For example, approximately 35% of AA patients are found to have leukocytes with short telomeres, suggesting a stem cell defect ( ). In addition, the role of thrombopoietin and the thrombopoietin signaling pathway in stem cell homeostasis and survival is also being investigated to the extent of using thrombopoietin agonists as potential therapeutic agents. However, the immune destruction of stem cells is the most important factor and most patients with acquired AA are now viewed as suffering from an immune-mediated disease.
Complications of AA are infection, bleeding, and problems of iron overload from repeated transfusions. The prognosis appears to depend on the severity of marrow damage; however, the initial blood count and response to therapy during the first few months of treatment are important prognostic factors ( ). Usually, the age of the patient influences the decision regarding which therapy the patient should receive. In some survivors, partial recovery is common. With the introduction of modern treatment protocols utilizing immunosuppressive therapy and bone marrow transplantation, long-term survival rates greater than 60% are reported. Survivors have a much higher risk of developing myelodysplastic syndrome, paroxysmal nocturnal hemoglobinuria (PNH), acute leukemia, and solid tumors. Clonal evolution to myelodysplastic syndromes and acute myeloid leukemia occurs in 10% to 20% of patients with acquired AA. Cytogenetic abnormalities, such as monosomy 7 and trisomy 8, occur in 13% and 7% of acquired AA, respectively. Uniparental disomy in the short arm of chromosome 6 (6pUPD) occurs in approximately 13% ( ). Genetic mutations in myeloid malignancy-associated genes, such as ASXL1 , DNMT3A , and BCOR are reported in up to half of acquired AA patients and may be related to progression ( ). PNH clones occur in 50% to 70% of acquired AA patients, representing somatic mutation in the PIGA gene. PNH clone size is usually small and of uncertain clinical significance, although some studies suggest that it may indicate a favorable response to immunosuppressive therapy ( , ).
Treatment of acquired AA includes bone marrow transplantation for patients younger than 40 years of age with severe disease if there is a human leukocyte antigen (HLA)–matched sibling. Children and young adults have a much higher survival probability compared with older adults ( ). Immunosuppression using horse antilymphocyte globulin (ATG) and cyclosporin A produces hematologic remission in 60% to 80% of patients. Rabbit ATG produces slightly lower remission rates in some studies ( ). Androgens appear to be least helpful in the stimulation of any residual marrow. Supportive care must be used with caution. Certainly, appropriate antibiotics should be used to combat infection; however, the risk for sensitization should be considered with the administration of blood products. Single-donor platelets or platelets from HLA-matched donors are preferred ( ).
In patients with pancytopenia and a hypocellular marrow, a search should be made for evidence of exposure to radiation, drugs, and chemicals of known or possible propensity to injure the marrow so that further exposure can be eliminated. Nevertheless, in approximately 70% of cases of AA, no suspected causal relationship to toxic agents can be found. These cases are designated as idiopathic with a presumed immunologic mechanism, as mentioned earlier. The symptoms and signs do not differ, but the onset is commonly more insidious than in toxic or hypersensitive AAs.
The red cells are usually normal to increased in size with a varying degree of anisocytosis and poikilocytosis, particularly oval macrocytes. Macrocytosis may be a prominent feature, particularly in response to high levels of EPO. The RDW may be increased, even without transfusion ( ). Polychromasia, stippling, and normoblasts are most often conspicuously absent. Leukopenia with marked decrease in granulocytes and a relative lymphocytosis are observed. In severe leukopenia, there is often absolute lymphocytopenia. Neutrophil granules may be larger than normal and may stain dark red (unlike the “toxic” granules found in infections), and the neutrophil alkaline phosphatase may be elevated. However, neutrophil left shift is not seen in the absence of infection ( ). Thrombocytopenia is part of the picture. Monocytopenia may be present. The serum iron is usually increased, and serum cobalamin and folate levels are usually normal. Although an occasional patient is hypogammaglobulinemic, most patients have normal levels of serum immunoglobulins.
In most cases, the aspirate consists of red cells, lymphocytes, some plasma cells, mast cells, and fatty particles. Marrow sections will show fatty tissue with inconspicuous fibrosis and islands of lymphocytes and plasma cells ( Fig. 33.8 ). Although focal areas of predominantly erythroid normocellularity or hypercellularity (hot spots) may sometimes be present, the overall cellularity is decreased. The hot spots usually show erythroid precursors with delayed nuclear maturation at a high proliferative rate. Vascularity may be prominent with occasional ectatic sinuses. Storage iron is increased. Rare cases may show lymphoid aggregates.
An increased serum iron concentration is a valuable early sign of erythroid hypoplasia and reflects decreased plasma iron turnover. In addition, erythrocyte utilization of iron is decreased. Therefore, both effective and total erythropoiesis are decreased in AA.
Toxic Aplastic Anemias. A number of physical and chemical agents produce marrow damage in all humans and animals exposed to a sufficient dose. Examples include ionizing radiation, mustard compounds, benzene, and antineoplastic agents such as busulfan, urethane, and antimetabolites. Benzene, although strongly linked to AA, is now considered less of a problem than in the past because of reduction in exposure.
Ionizing Radiation. Effects depend on the radiosensitivity of the cells and the capacity of the cells to regenerate, as well as the survival rate of cells in the blood. Erythroid cells are most sensitive, granulocytes have intermediate sensitivity, and megakaryocytes are the least sensitive of the three. Stromal cells are relatively insensitive.
After acute exposure to radiation, the reticulocyte count falls, but the red cells decline slowly because of their long survival. Within the first few hours, neutrophilic leukocytosis occurs as the result of a shift from marginal and probably marrow storage pools. A fall in lymphocytes occurs after the first day and is responsible for the early leukopenia, because lymphocytes are sensitive to irradiation and are directly killed. After 5 days or so, granulocytes begin to fall. The platelets decrease later. Platelets are often the last to return to normal in the recovery phase. Quiescent marrow stem cells are remarkably resistant to a single dose of irradiation. However, chronic exposure to low-dose irradiation, including localized radiation for ankylosing spondylitis, is associated with delayed increased risk for AA because of its impact on the stem cell replicating pool ( ).
Hypersensitive Aplastic Anemias. A large number of drugs produce marrow damage in some individuals after single or repeated exposures. Effects are not dose related as they are in the previously discussed toxic aplasia. Agents include antimicrobial drugs (salvarsan, chloramphenicol, sulfonamides, chlortetracycline, streptomycin), anticonvulsants (mephenytoin, trimethadione), analgesics (phenylbutazone, indomethacin), antithyroid drugs (carbimazole), antihistaminics (tripelennamine), H 2 -histamine receptor antagonists (cimetidine), insecticides (dichlorophenyltrichloroethane [DDT]), and other chemicals—some known (gold compounds, quinacrine, chlorpromazine, hair dyes, bismuth, mercury) and others unknown. The nonsteroidal antiinflammatory medications are becoming more linked to an increased risk for developing AA following marked reduction in the use of chloramphenicol.
Chloramphenicol is an important drug in this category. This antibiotic was considered to be the most common cause of AA at the peak of its use, which began in 1949. Reactions of the marrow to chloramphenicol are of two types, which are possibly unrelated ( ).
In about half of patients who receive chloramphenicol, increased serum iron, reticulocytopenia with anemia, neutropenia, and thrombocytopenia are found. The marrow may show decreased erythroid cells and vacuolization of primitive erythroid and granulocyte precursors. These changes are dose related, time dependent, and reversible.
In a very small proportion of persons receiving chloramphenicol, an irreversible AA develops that may be fatal. Pancytopenia occurs 3 weeks to 5 months from the last dosage. No relationship has been established between the reversible erythropoietic lesion and the development of AA; it may be that individual susceptibility is responsible for the latter. For this reason, it is essential that restraint be employed in using the drug; monitoring its administration with blood cell counts is not an effective preventive measure.
Infection. Viral infections are frequently associated with limited marrow suppression, typically neutropenia and less commonly thrombocytopenia. Marrow aplasia has been described as a rare sequela to viral hepatitis, occurring two to three months after an episode of acute hepatitis. As mentioned earlier, hepatitis-associated AA represents approximately 5% of cases of acquired AA ( ). Almost all cases are seronegative for hepatitis A, B, C, and G. These patients are usually males younger than age 20 years. The prognosis is usually grave, with severe AA; bone marrow transplantation is considered early in the course of the disease ( ). After orthotopic liver transplantation for fulminant seronegative hepatitis, up to 30% of patients develop hepatitis-associated AA ( ). Parvovirus has been associated with transient erythroid aplastic crisis in patients with chronic hemolytic disorders although some cases of AA have been reported with such infection. Human immunodeficiency virus, dengue virus, cytomegalovirus, and Epstein-Barr virus may also cause hematopoietic depression. Several immunologic abnormalities have been documented in patients with hepatitis-associated AA, including increased soluble IL-2 receptor, low ratios of CD4+/CD8+ cells, high percentages of CD8+ cells, and reduced proportions of CD4+CD25+ Tregs. Clonal expansion of T cells with conserved antigen specificity has been found in these patients, suggesting that abnormal immune responses underlie the disease and viral antigens may elicit T-cell responses that cross-react with antigens expressed by the stem cells ( ).
Paroxysmal Nocturnal Hemoglobinuria. This rare clonal disorder (see later discussion) may be followed by AA. Usually, in PNH, a variable degree of marrow hypofunction coexists. Alternatively, in some patients who present with AA, the red cell defect of PNH may be present or may appear during the course of the disease. As mentioned earlier, PNH defect may be detected in as many as 50% to 70% of patients with AA. However, hemolytic disease occurs in approximately one-third of these patients.
Pregnancy. Pregnancy occurring in a patient with acquired AA may make the pancytopenia more severe. Occasionally, however, AA occurs during pregnancy and remits following delivery. In some such cases, aplasia recurs during a second pregnancy. Infants may be anemic, thrombocytopenic, or leukopenic. Survival rates for AA in pregnancy have been relatively high for the mother (83%) and the baby (75%). Hemorrhage is the most common cause of death in these patients ( ).
Thymoma. Although thymomas are usually associated with pure red cell aplasias, other bone marrow elements may also become depressed. Pancytopenia, often with hypoplastic bone marrow, occurs in rare cases with thymoma. AA may occur as a late complication after the treatment of thymoma ( ).
Immunologic Diseases. AA occurs in approximately 10% of cases of eosinophilic fasciitis. The prognosis of AA in this setting is usually poor. AA may be associated with systemic lupus erythematosus (SLE) and rheumatoid arthritis (RA), although the role of drug therapy in the pathogenesis of AA has been considered ( ). AA may also complicate multiple sclerosis, congenital immunodeficiency syndromes, and immune thyroid disease.
The term inherited bone marrow failure syndromes ( IBMFS ) designates individuals with a genetic predisposition to chronic bone marrow failure, which may be associated with other congenital anomalies. IBMFS may involve a single cell line, such as Diamond-Blackfan anemia, or multiple cell lines, such as Fanconi anemia. Several of these syndromes are associated with increased risk for hematologic and solid organ malignancies. Between 30% and 35% of childhood AA cases are inherited. In a study of 134 children with AA, 40 patients were diagnosed with inherited AA. Twenty-six had Fanconi anemia (FA), 10 patients had familial AA without classic signs of FA, and 4 patients presented with amegakaryocytic thrombocytopenia that later developed into complete aplasia ( ). The current literature and large-center studies suggest an annual incidence of IBMFS of approximately 65 per million live births ( ).
Fanconi Anemia. FA is an autosomal-recessive disorder with a carrier frequency of 1/300 in the United States and Europe and is higher in individuals of Ashkenazi Jewish descent ( ). Rare cases are X linked or autosomal dominant. Often, more than one member of a family is affected. The pancytopenia becomes obvious after infancy and is usually significant in early childhood, with median age at diagnosis of 6.5 years. The anemia is usually normochromic and may be macrocytic; increased levels of HbF and i antigen may be observed; the marrow is generally hypocellular. Developmental anomalies are present in approximately 60% of patients and may include hyperpigmentation, short stature, hypogonadism, malformations of the extremities (e.g., aplasia of the radius, frequent abnormalities of the thumbs), microcephaly, and malformation of other organs (e.g., heart, kidneys). Chromosomal defects consisting of random breaks and rearrangements characteristically are present in blood lymphocytes as well as in marrow cells. Chromosomal breakage becomes more evident when the cultured cells are challenged with alkylating and DNA cross-linking agents such as mitomycin C and diepoxybutane. Some mosaic cases may be difficult to diagnose; studying skin fibroblasts may be better than blood cells. A breakthrough in investigating FA evolved from the observation that hybrid cells from FA and normal cells resulted in correction of the abnormal chromosome fragility, a process known as complementation . At least 21 complementation groups have been distinguished to date. The most common is complementation group A (FANCA), which occurs in approximately 70% of FA patients. Several FA proteins form a nuclear complex that is involved in DNA repair. The FA/BRCA DNA repair pathway repairs covalently cross-linked strands of DNA that will not separate and will interfere with DNA replication. The DNA cross-linking may result from endogenous reactive aldehydes or exogenous cross-linking agents. The predicted median survival in FA is 39 years. Patients with FA are at higher risk of developing myelodysplastic syndromes, acute leukemia, and nonhematologic tumors, particularly in the head and neck, liver, lower esophagus, vulva, and anus, with an overall cumulative incidence of neoplasia of 15% ( ). The median age for all cancers in patients with FA was 30 years ( ).
Other Inherited Aplastic Anemias. A few children present with bleeding manifestations during their neonatal period secondary to amegakaryocytic thrombocytopenia. As their disease progresses, they develop a pancytopenia and a hypocellular marrow. Chromosome breakage studies are usually normal. The mode of inheritance is autosomal recessive. The molecular defect is related to a mutation in the gene for thrombopoietin receptor, c-MPL , which maps to 1p35 ( ).
Pancytopenic anaplastic anemia may develop in a subset of patients with other familial disorders. Some patients with dyskeratosis congenita (DC; reticulated hyperpigmentation of skin, dystrophic nails, and mucous membrane leukoplakia) and Shwachman-Diamond syndrome (SDS; exocrine pancreatic insufficiency and neutropenia) develop AA during the course of their disease (Alter, 2002). Recent studies indicate that DC results from abnormalities in telomere maintenance system and is now considered one of the telomere biology disorders. SDS results from biallelic mutation of the SBDS gene, which interacts with molecules responsible for ribosomal subunit assembly, resulting in abnormal ribosome biogenesis ( ). Both DC and SDS are associated with increased risk for hematologic malignancies and solid tumors ( ).
This may occur during the course of a hemolytic anemia (often preceded by an infection). The combination of aplasia and hemolysis becomes a life-threatening situation due to rapidly progressing anemia. Red cell production may occasionally cease during or following rather minor infections in normal children or adults, at which time the marrow will show absence of all but a few of the most immature erythroid precursors. Aplastic episodes in chronic hemolytic anemias often appear to be due to parvovirus B19 infection. This virus inhibits erythropoiesis by infecting mature CFU-Es. The morphologic hallmark of the disease is the presence of scattered giant pronormoblasts in the bone marrow aspirate with marked reduction in the more mature erythroid precursors. Nuclear inclusion may be difficult to identify with Wright’s stain. The aplastic crises resulting from parvovirus infection are transient, with erythroid marrow recovery in 1 to 2 weeks after onset ( ).
Transient erythroblastopenia of childhood (TEC) occurs in previously healthy children, usually younger than 8 years of age, with most affected children between 1 and 3 years of age. It is characterized by a moderate to severe normocytic anemia that develops gradually, severe reticulocytopenia, transient neutropenia (20%), and increased platelet counts (60% of patients). Macrocytosis is usually observed during recovery because of the effects of reticulocytes. A history of a viral infection within the previous 3 months is frequently elicited. The bone marrow is generally normocellular and shows virtual absence of erythroid precursors, except for a few early forms. Granulocytic maturation arrest may occur in some neutropenic patients. Patients recover within 1 to 2 months without therapy except in rare occasions in which RBC transfusions may be needed for symptomatic anemia. TEC can occur in siblings simultaneously and in seasonal clusters. Transient neurologic manifestations, such as hemiparesis and seizures, may accompany TEC. In most cases, the pathogenesis involves humoral inhibition of erythropoiesis; cell-mediated immunosuppression was identified in about 25% of cases, but parvovirus has not been proven to be the cause of TEC ( ).
Diamond-Blackfan anemia (DBA) is a rare, congenital red cell aplasia that usually becomes obvious during the first year of life, with the median age of presentation being 2 months and more than 90% of cases being identified during the first year of life ( ). Abnormal physical findings, such as short stature, craniofacial abnormalities, and thumb anomalies, are seen in more than 50% of patients. Severe anemia is usually macrocytic, reticulocyte level is low, leukocytes are normal or slightly decreased, platelets are normal or increased, and the marrow usually shows a reduction in all developing erythroid cells, except normal granulocytic and megakaryocytic cell lines. In a small number of cases, residual erythroid precursors are detected. These precursors are mostly pronormoblasts. HbF is elevated (5%–25%) to a degree not expected for the patient’s age, and the antigen i is often present. Red cell adenosine deaminase (ADA) is usually increased. These findings contrast with TEC, in which the red cells are normocytic, the HbF is normal, the antigen i is absent, and red cell enzymes are at a lower level (characteristic of an older cell population) ( ).
DBA has an autosomal-dominant pattern of inheritance with variable penetrance. The defect appears to be in the erythroid-committed progenitor cells. CFU-Es and BFU-Es are decreased in the marrow, and BFU-Es, which normally circulate, are absent or decreased in the blood. In addition, these progenitor cells exhibit acceleration in programmed cell death (apoptosis) and fail to respond in in vitro culture systems to normal T cells and to usual levels of EPO, suggesting a qualitative defect. DBA results from defects in ribosomal proteins, whether small (S) or large (L) units. The most common is a mutation in the RPS19 gene, which was detected in 25% of patients ( ). Rare cases have been caused by mutations in the GATA1 gene. About 75% of patients respond at least partially to corticosteroids. Approximately 20% of patients experience spontaneous remission by the age of 25 years. The overall long-term survival is about 65%, although many patients require long-term steroid use. DBA is also associated with a higher risk of developing solid tumors, acute myeloid leukemia, and myelodysplastic syndrome ( ; ).
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here