Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
acyl-CoA synthetase long chain
apolipoprotein AI
apolipoprotein AIV
apolipoprotein B48
apolipoprotein B mRNA editing enzyme, catalytic polypeptide-like
coatomer proteins
diacylglycerol
DAG acyltransferase
endoplasmic reticulum
fatty acid
FA-binding protein
fatty acid transport protein
sn-3-glycerol phosphate
glucagon-like peptide-2
G3P acyltransferase
high-density lipoprotein
heat-shock protein
low-density lipoprotein
acyl-glycerol-3-phosphate O -acyltransferase
monoacylglycerol
MAG acyltransferase
microsomal triglyceride transport protein
oleoylethanolamide
phosphatidic acid phosphatase
phosphatidylcholine
prechylomicron transport vesicle
protein kinase C
soluble N -ethylmaleimide-sensitive factor attachment protein receptor
scavenger receptor B1
triacylglycerol
vesicle-associated membrane protein
Dietary fats are the most concentrated caloric source consumed by humans. In addition to their caloric content, fats contain essential fatty acids (EFAs) and fat-soluble vitamins that are needed for health. As a consequence, humans and other animals have developed an efficient mechanism for fat absorption. In humans, 95% of a 500-g fat load can be absorbed each day, but the absorption process is quite intricate for several reasons. The majority of dietary fats are water insoluble triacylglycerols (TAGs) that must be made to interact with water in order to be absorbed by the intestine. As a corollary, lipids require specialized chaperones and enzymes with amphipathic properties in order to be processed and metabolized. In addition, potential toxicity from the fatty acids (FAs) produced during hydrolysis of dietary fat needs to be controlled. Thus, the processing of dietary fat from ingestion to output as chylomicrons is a complex story.
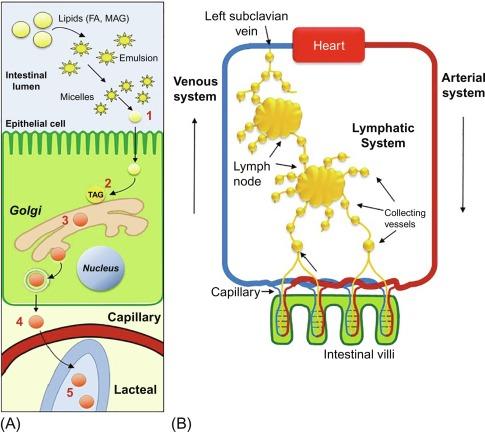
Dietary TAGs in the intestinal lumen are first hydrolyzed by pancreatic TAG lipase to 2 FA and one sn2-monoacylglycerol (MAG). These lipolytic products are absorbed through the apical (i.e., luminal) membrane of enterocytes, directed to the endoplasmic reticulum (ER) where they are converted back to TAG to be packaged into the intestine’s unique lipoprotein, the chylomicron. The TAG is first transported from the ER in a specialized vesicle, the prechylomicron transport vesicle (PCTV), which is directed to the Golgi where the lipoproteins of the chylomicron are processed. The mature chylomicron is then packaged into another vesicle for transport to the basolateral membrane where it is exocytosed into the intestinal lymphatic vessels called lacteals ( Fig. 48.1 A ). The chylomicron-rich lymph runs through mesenteric lymph nodes and collecting lymphatic vessels and ultimately into the thoracic duct that drains the transported lymph into the bloodstream at the left subclavian vein ( Fig. 48.1 B). The above processes will be described in detail in the following sections. This chapter is a revised updated version of the one previously coauthored with late Dr. Charles Mansbach, who made great contributions to our knowledge of chylomicron formation.
Our understanding of the mechanisms by which FAs, generated from TAG hydrolysis in the intestinal lumen, are delivered across the enterocyte membrane and then to the ER where they are reincorporated into TAG are incomplete. However, significant progress has been accomplished and will be summarized in the following sections.
Both diffusion and protein-facilitated membrane transfer mechanisms are likely to contribute in vivo in mediating FA uptake across the enterocyte membrane and are likely to coexist in vivo. In the circulation, FAs are carried quantitatively bound to albumin and the unbound FA concentration that is in equilibrium with albumin-bound FA (estimated to be in the low nanomolar determines the uptake by cells. In the intestine, the FA is presented to the enterocyte incorporated into bile salt micelles. Luminal micelles, like albumin in the serum, can solubilize millimolar concentrations of FA, and in both systems, the monomeric-free FA dissociated in the aqueous phase is extremely low relative to the total FA concentration. The free FA concentration in the intestinal lumen is estimated to be in the low micromolar (μM). Early studies showed that lipid constituents of micelles exhibit independent rates of cellular uptake arguing against the uptake of whole micelles. Uptake of FA from micelles appears to follow the FA monomer and in turn drives dissociation of more micellar FA.
The existence of a FA transport system at the apical surface of enterocytes was suggested by findings that metabolic processing of the FA that enters the enterocyte apically is different compared to FA that enters via the basolateral route. In addition, intestinal FA uptake exhibits variability between mice strains suggesting potential genetic determinants and shows adaptive changes in response to challenges. Over the last two decades, evidence accumulated to support protein involvement in membrane FA uptake. Saturability of the process, its sensitivity to protein reactive agents, specificity for long-chain FA and a transport Km in the low nanomolar range were demonstrated in many cell types (for detailed reviews refer to Refs. ). Most studies of FA uptake by intestinal epithelial cells used immortalized cell lines such as Caco-2 and IEC-6 as enterocyte models. Existence of saturable and nonsaturable FA uptake components was reported using either albumin:FA or micellar FA delivery systems. An interesting finding was that FA uptake could be inhibited by 2-MAG, a TAG digestion product along with FA, while triolein, glycerol, diacylglycerol (DAG), and monooctanoate had no effect. This observation suggested that enterocytes may coordinate the transport of FA and 2-MAG to optimize resynthesis of TAG inside the cell. Studies with isolated primary enterocytes from the proximal intestine of mice confirmed existence of a saturable and specific FA uptake component in these cells. However, the need to use a FA:albumin delivery assay with isolated primary enterocytes provided kinetic measures at levels of unbound FA that may be orders of magnitudes lower than those present in the intestinal lumen. Thus, the relative contribution of the protein-mediated component to total uptake by enterocytes in vivo is unclear and likely to be small as compared to cell types such as muscle or adipose cells where the saturable process may predominate. One function of the saturable FA component in enterocytes would be to serve in a regulatory capacity, transducing signals that coordinate enterocyte FA processing as discussed in following sections.
Together, the above observations supported existence of membrane proteins on the apical surface of enterocytes that might influence the uptake by recruiting the FA or contributing to regulate its intracellular trafficking to specific metabolic sites. Membrane proteins that have been proposed as potential intestinal FA transporters include the scavenger receptor CD36, the fatty acid transport protein 4 (FATP4), and the plasma membrane FA-binding protein FABPpm. Other proteins that may have relevance to FA absorption include the scavenger receptor B1 (SR-B1), which is a member of the CD36 family. FABPpm, a membrane associated form of the mitochondrial enzyme aspartate aminotransferase, was isolated from intestinal as well as liver membranes, and early studies showed that an antibody against the protein reduced FA uptake into isolated enterocytes. However, there is little additional evidence to support its role in intestinal FA uptake.
CD36/FAT (FA translocase) is a 75–88-kD scavenger receptor abundant in muscle, adipose tissue, the intestine, and the capillary endothelium that recognizes a range of ligands. This includes long-chain FA, native or modified lipoproteins, thrombospondin-1, collagen, amyloid B, and malaria-infected erythrocytes. Extensive evidence, both in rodents and humans, also shows a role of CD36 in facilitating tissue FA uptake. Studies in CD36-deficient mice and humans have documented disturbances in tissue FA uptake and in overall FA metabolism. Polymorphisms in the CD36 gene have also been linked to abnormal lipid metabolism and to susceptibility to the metabolic syndrome. For reviews refer to Refs. .
Significant insight into the potential mechanism for the CD36-mediated FA transport was obtained from the recently reported crystal structure of the CD36 family member lysosomal LIMP-2 and by homology that of CD36 and SR-B1. A large cavity traversing the entire length of these proteins was identified that is proposed to serve for lipid transfer to the membrane vicinity. The crystal structure of CD36 has been reported more recently, and the crystallized protein has been found to be in complex with long-chain FA with preponderance of palmitic and stearic acids. The FAs are proposed to dock within a hydrophobic pocket on the surface of the CD36 ectodomain leading toward the internal tunnel within the protein. In the surface pocket, the FA carboxylic tail is in close proximity to lysine 164 (K164), previously shown to bind the oleate derivative sulfo- N -succinimidyl oleate (SSO). Interaction of K164 with the carboxylic group of the FA could be important for its correct positioning allowing it to slide into the tunnel. Similar results were reported in the Drosophila melanogaster CD36 homologue named sensory neuron membrane protein (SNMP). Drosophila SNMP1, as well as its orthologues in other insects, is expressed in the olfactory sensory neurons that detect lipid-derived pheromones. The SNMP1 ectodomain is required for detection and binding of pheromones and the tunnel is required to funnel these molecules to their cognate receptors. K164 in the CD36 ectodomain is also involved in the binding of oxidized low-density lipoprotein (LDL). Interaction between FA and LDL for CD36 binding has been reported using surface plasmon resonance (SRP) that shows binding of the CD36 ectodomain to different long-chain FAs, including oleic acid (OA), docosahexaenoic acid (DHA), elaidic acid (EA), and palmitic acid (PA), could affect CD36 protein conformation in a manner that enhances binding of oxLDL to the receptor.
Contribution of CD36 to net intestinal FA absorption is likely to be small. CD36 can bind nanomolar concentrations of FA and would function in high-affinity uptake of FA in enterocytes of the proximal intestine, similar to its role in other cell types. At the concentrations of free FA present during absorption in the intestinal lumen CD36 will be rapidly saturated and its function would be important only in very early stages of digestion such as during the cephalic phase (see below). Consistent with this is the observation that dietary FAs downregulate levels of enterocyte CD36 protein by promoting its ubiquitination and degradation.
Two main classes of taste receptors for FAs have been proposed, CD36 and the G-protein-coupled receptors (GPRs). The FAs released by lingual lipase interact with the FA receptors CD36 and GPR120 expressed on the surface of taste receptor cells. CD36 is abundantly expressed on the apical surface of taste bud cells in the tongue of rodents, pigs, and humans and deletion of CD36 reduces FA preference. Consequent to long-chain FA binding, a CD36-mediated signaling cascade is triggered in taste bud cells resulting in the release of neurotransmitters and signals to the central nervous system. These events are thought to mediate fat perception, as well as the cephalic phase of digestion that primes the organism for fat absorption. With respect to the GPRs involved in fat taste perception, GPR120 is detected in gustatory and nongustatory epithelia, whereas GPR40 is not expressed in gustatory tissues, suggesting that its role in fat perception might be indirect. CD36 functions as fat taste receptor at low FA concentrations, whereas GPR120 appears to be poorly responsive to long-chain FA and might function in amplifying the response to high concentrations of dietary FA and other tastants, consistent with its expression in a variety of taste cells responsive to various stimuli.
In humans, less is known about the role of CD36 as a fat taste receptor. Several studies have investigated the role of a common CD36 polymorphism (SNP) (rs1761667 involving A/G substitution) on oral sensory fat perception. Obese African American individuals carrying the A allele of the SPN have decreased fat orosensory detection thresholds compared to noncarriers. The effect of the SNP on sensory fat perception has been replicated in other populations. A study conducted by Keller and colleagues found that subjects homozygous for the A allele, while they perceived increased creaminess in a salad dressing, were insensitive discriminators of fat content. It is possible that carriers of the CD36 SNP, which display lower FA sensitivity, reach taste “saturation” at higher FA concentrations and might be prone to increase fat intake. However, although proposed this hypothesis has not been fully tested yet.
The links between fat taste, food intake, and disease are complex, and causality has not been established. In the small intestine, CD36 is also important for fat-induced satiety via production of the messenger oleoylethanolamide (OEA), which prolongs the intermeal interval. OEA, in turn, increases intestinal CD36 expression and FA uptake. Although the above findings might suggest that CD36 absence would enhance food intake, it is actually decreased in CD36-deficient mice. This likely reflects a postingestive effect consequent to slower intestinal lipid processing as more of fat absorption is shifted to distal segments (see following sections).
In small intestines of humans and rodents, the CD36 expression pattern is higher in proximal as compared to more distal segments but one study found that levels in the human ileum exceeded those in the duodenum. CD36 localizes on the apical membrane of villi (as opposed to crypt) enterocytes. The exact role of CD36 in lipid absorption is not completely clear but most evidence suggests that it functions during the early phase of the digestive process and plays a regulatory role in intestinal lipid processing. Studies in Cd36- null mice support the interpretation that the quantitative contribution of CD36 to net lipid absorption is small. Using 24-h fecal lipid recovery measurements there is no evidence of lipid malabsorption except for very long-chain FA and administration of a lipid load results in similar blood appearance of intestinally derived TAGs. These findings support the efficiency and redundancy of intestinal transport systems which easily compensate for absorption defects consequent to CD36 deficiency. CD36 expression being most abundant in the proximal intestine, a proximal defect in absorption would be expected and indeed could be demonstrated. Primary enterocytes isolated from the proximal intestine of Cd36- null mice show a 50% reduction in FA uptake as compared to enterocytes from wild type mice and in vivo intragastric administration of triolein associates with reduced OA enrichment of mucosal lipids in the proximal intestine of Cd36 -null mice. CD36 deletion also suppresses OA uptake into the duodenum and reduces it in the jejunum of mice fed after 6 h fast and then refed for 30 min. In contrast to proximal segments, a defect in FA uptake is not apparent in enterocytes isolated from the distal small intestine of Cd36 -null mice where CD36 is less abundant. Feeding of a high-fat diet results in lipid accumulation in the proximal segment with evidence for an absorption delay and for more of the lipid being absorbed distally.
During the postprandial period, CD36 undergoes rapid proteolysis involving the ubiquitin-proteasome pathway, causing disappearance of the protein from the luminal side of intestinal villi. This event is lipid-mediated and triggered by long-chain fatty acid (LCFA) and/or diglycerides derived from early digestion of dietary TAG. In a mouse model of diet-induced metabolic syndrome, abnormal chylomicron production and impaired clearance efficiency are observed. These events correlate with dysfunctional lipid trafficking and lipid sensing by intestinal CD36 most likely as a result of higher insulin. The defect in CD36 function during chronic high fat feeding in rodents was proposed to explain the secretion of a substantial fraction of smaller intestinal triglyceride-rich particle (TRL).
There is clear evidence for a role of CD36 FA uptake and signaling in chylomicron production. Intestinal lipid secretion into the lymph measured by cannulating the mesenteric duct is 50% lower in Cd36 -null mice. The lipoproteins secreted by CD36 deficient intestines are 35% smaller and exhibit delayed blood clearance. CD36 mediates the effect of glucagon-like peptide-2 (GLP-2) to increase intestinal FA absorption and chylomicron production in hamsters and mice as the GLP-2 effect is absent in Cd36 -null mice. In addition to its facilitation of proximal FA uptake, CD36-mediated signaling contributes to other steps related to chylomicron production including enhanced expression of key chylomicron proteins such as apolipoprotein B48 (apoB48) and microsomal triglyceride transfer protein. Using an in vitro system that allows measurement of ER production of PCTVs, CD36 was identified as part of the multiprotein complex required to generate the PCTV by the enterocyte ER. In vitro PCTV budding from ER isolated from CD36 or liver fatty acid-binding protein (L-FABP) deficient enterocytes was found to be markedly defective. L-FABP binding to the ER is essential for budding of the PCTV as will be detailed in later sections.
In addition to FA, CD36 may also facilitate intestinal cholesterol absorption, which is critical for optimal chylomicron production. Enterocytes isolated from Cd36 -null mice exhibit reduced cholesterol uptake and in vivo cholesterol output into the lymph is reduced by 50%. Other findings are also consistent with CD36 functioning in intestinal cholesterol absorption. Ezetemibe treatment in mice and phytosterol (plant sterol) intake in humans, which inhibit cholesterol absorption decrease CD36 expression. Ezetimibe also inhibits CD36 facilitated cholesterol uptake in COS-7 cells. It is still unclear whether the effects of CD36 on cholesterol uptake are direct. For example, CD36 deficiency increases cellular efflux of cholesterol and phospholipids, effects which may be due at least in part to influencing membrane localization of the ATP cassette cholesterol transporter ABCA1.
CD36 is also expressed in enteroendocrine cells (ECCs), which are specialized secretory cells that release a number of peptides, including cholecystokinin (CCK) and secretin in response to FA. CCK helps optimize fat digestion by regulating gastric emptying, pancreatic secretion, intestinal motility, and gallbladder contraction. Secretin inhibits gastric emptying and synergizes with CCK to induce pancreatic secretions. The Cd36 -null mouse displays a 50% reduction in basal release of CCK and secretin and in response to gastric administration of oil. Diminished release of CCK and secretin in response to FA is also observed with CD36 deficient intestinal segments in vitro. In EEC expressing CD36 release of CCK and secretin involves FA-induced and CD36-mediated increases in calcium and the second messenger cAMP. A dysfunction in CD36 may affect secretions of these hormones resulting in a reduction of fat digestion capacity, which could contribute to more overflow of dietary lipids reaching the distal intestine.
In summary, the findings suggest that CD36 plays an important role in chylomicron production due in part to its high-affinity uptake of FA and cholesterol by proximal enterocytes. CD36-mediated signaling in response to digestion products appears to play an important role in coordinating FA and possibly cholesterol metabolic targeting for efficient lipoprotein formation. There is extensive evidence in cells other than enterocytes that documents regulated trafficking of CD36 between the plasma membrane and intracellular sites, and this may contribute to the intracellular transfer of FA and cholesterol to sites important for their packaging into lipoproteins. The possibility that CD36-mediated signaling triggered by binding FA early during digestion is important for initiating assembly of the multiprotein complex needed for formation and budding of the PCTV from the ER still needs to be tested.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here