Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Endoscopic pituitary surgery highlights a confluence of anatomic insight, surgical technique, advancing technologies, and multidisciplinary collaboration. Starting with the ancient Egyptians, who used the nose as a route for embalming, the evolution of endonasal skull base surgery has extensively evolved. Pierre Marie (1886) set forth the pituitary as the master hormone driver and thus pushed pituitary surgery forward; however, first attempts at transcranial approaches were haunted by extreme morbidity.
Hermann Schloffer (1907) performed the first transsphenoidal pituitary surgery via a lateral rhinotomy approach, removing much of the endonasal structures. The patient suffered CSF rhinorrhea and died soon after. Over the next decades, surgeons such as Oscar Hirsch and Harvey Cushing attempted to refine the technique. The transsphenoidal surgical technique was mostly abandoned by neurosurgeons until the introduction of the microscope and fluoroscopy in the mid- to late 1900s. In 1965, Hardy was the first to use a microscope to approach the sella, pioneering the transsphenoidal approach. This approach was further refined with use of the endoscope, which was first described by Jho and Carrau in 1997. In this chapter, the relevant anatomy and surgical considerations will be reviewed.
It is important to thoroughly understand the surgical anatomy; here, we will highlight the most significant surgical aspects. Of note, it is important to prevent severe mucosal damage in order to prevent permanent ciliary dysfunction leading to nasal obstruction and sinusitis.
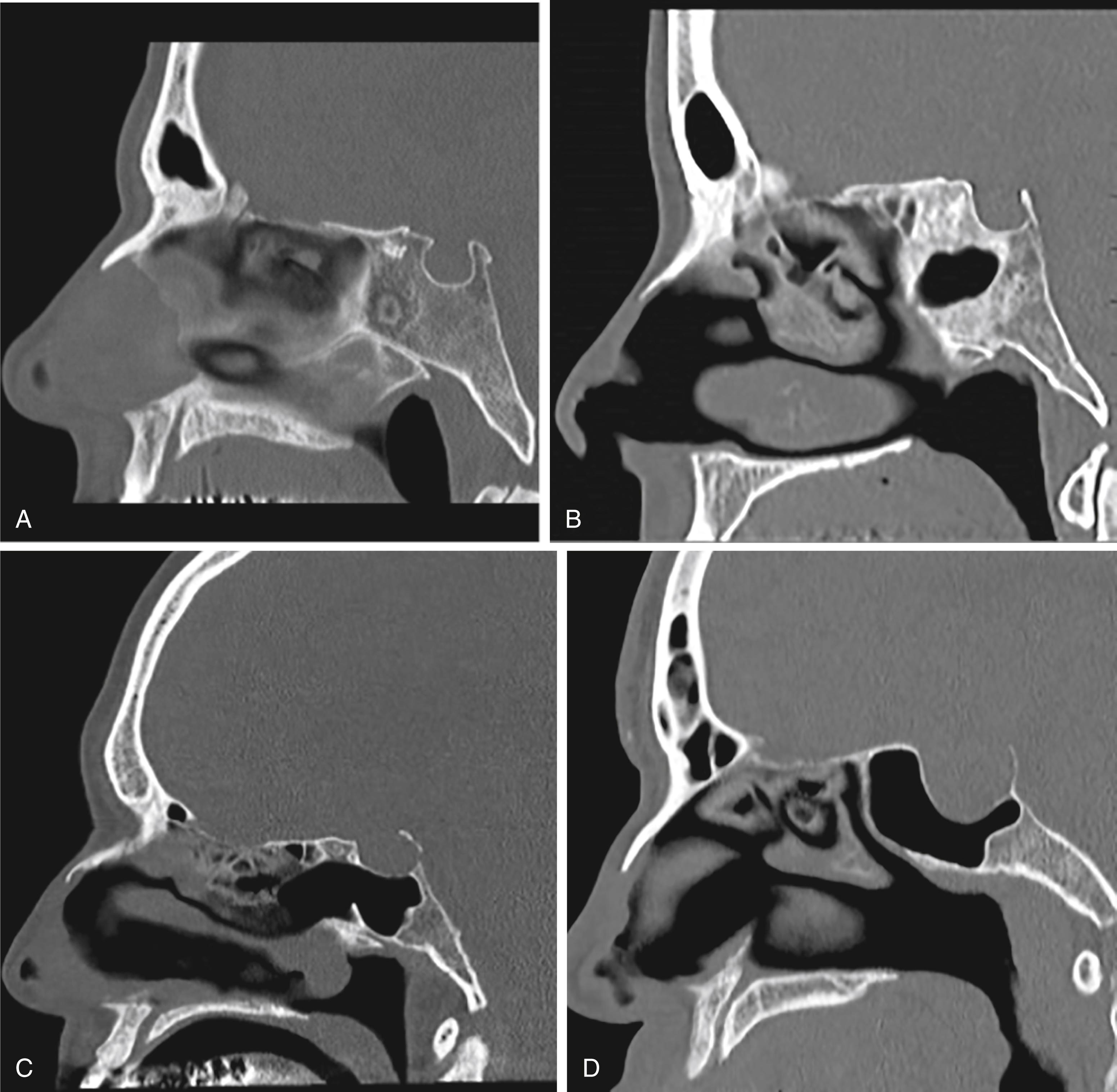
The nasal cavity is bordered medially by the bony and cartilaginous septum ( Fig. 12.1 ) and laterally by the inferior, middle ( Fig. 12.2 ), and superior turbinates. The middle turbinate is a useful landmark for extended approaches and for finding the sphenopalatine foramen, posteriorly. The superior turbinate houses olfactory epithelium on its superior border and identifies the sphenoid ostia, which are found posteriorly and inferiorly ( Fig. 12.3 ).
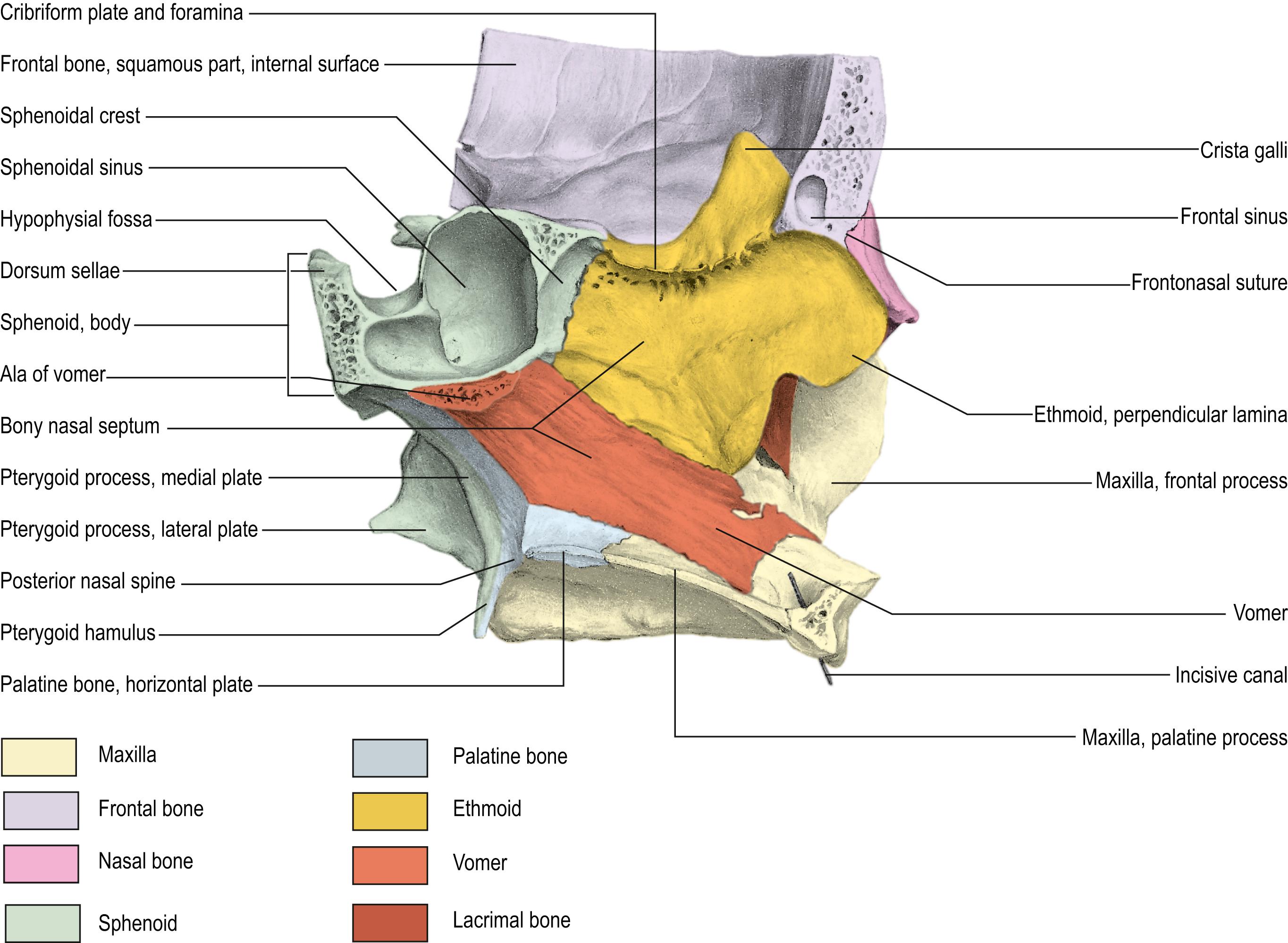
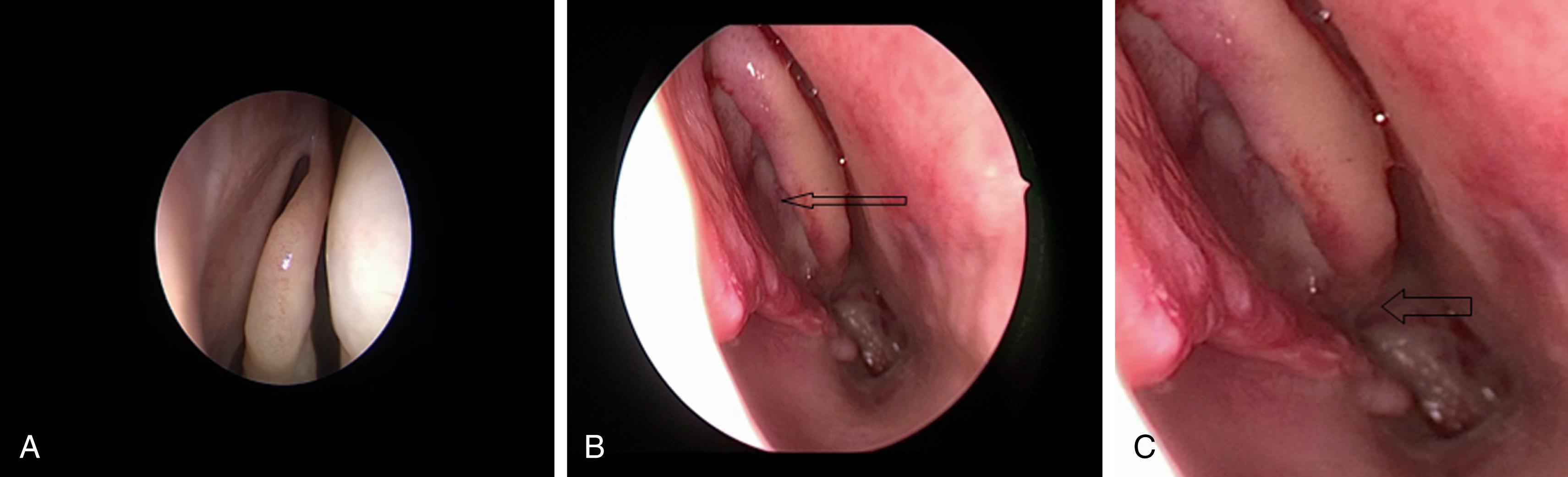
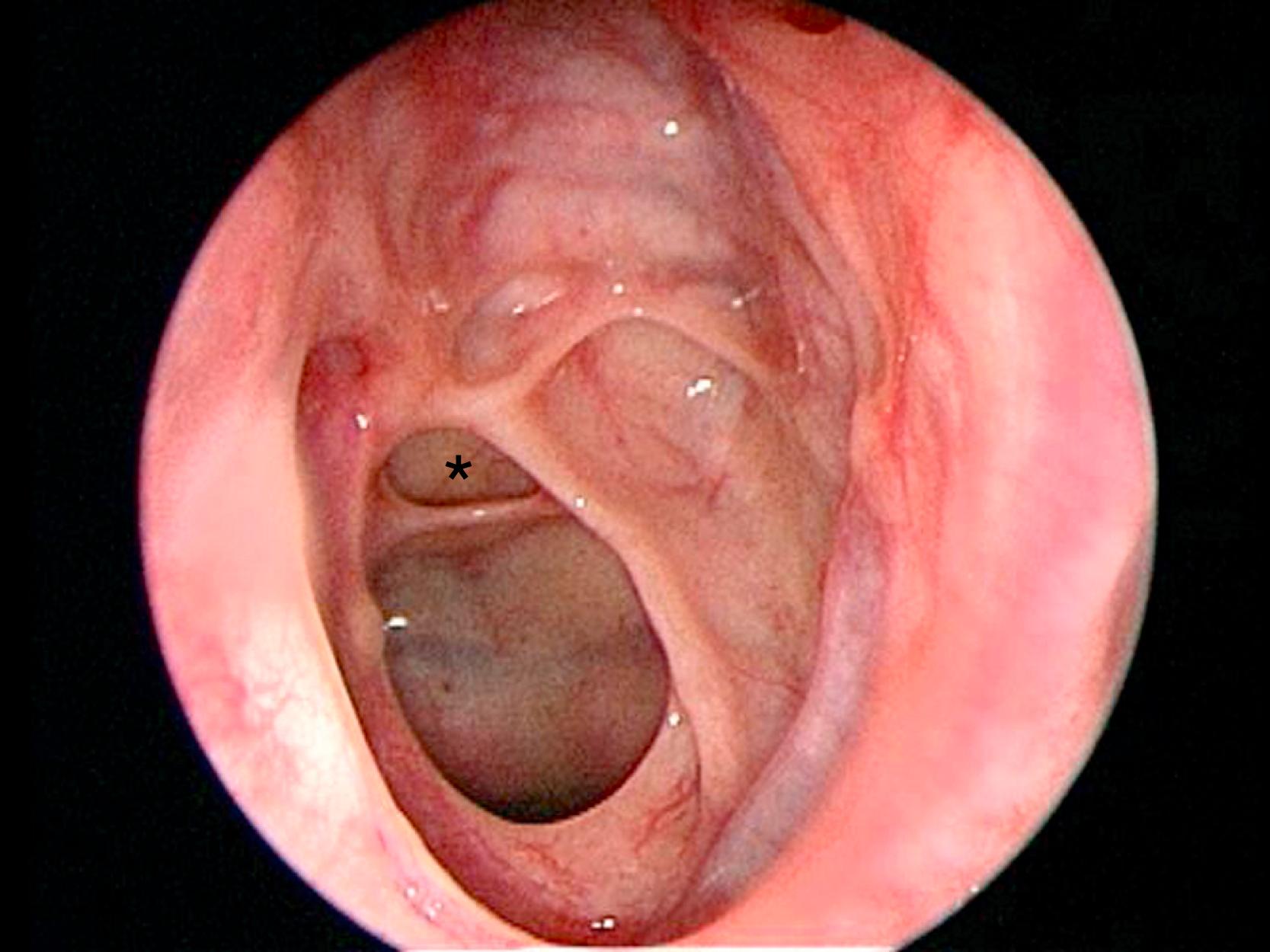
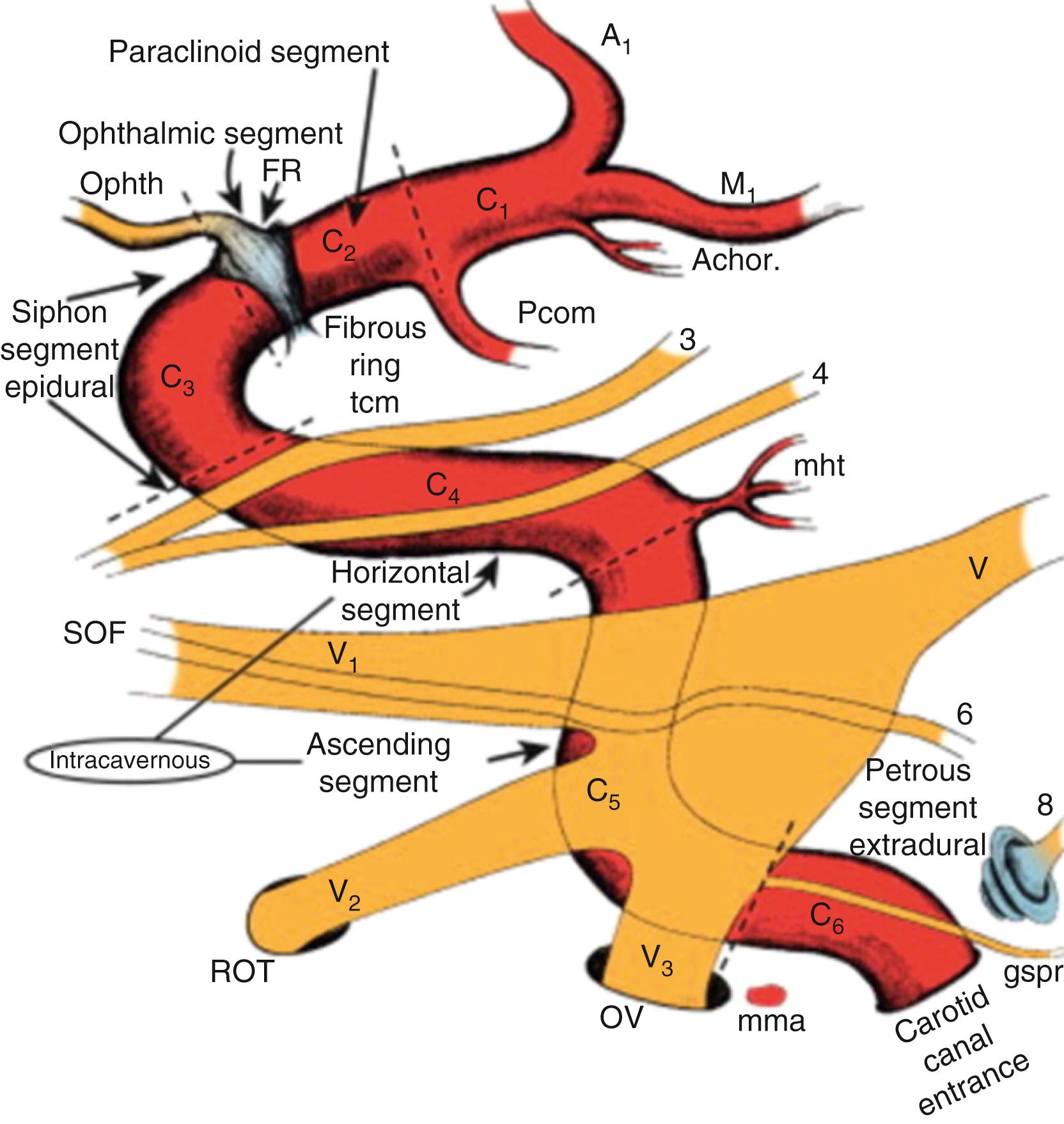
For extended approaches, one should be familiar with the maxillary sinus and the ethmoid cavity and their potential anatomical variations. One such variation is the sphenoethmoid cell or Onodi cell ( Fig. 12.4 ). The sphenoid sinus provides access to the sella and is classified based on its pneumatization as concha, presellar, sellar, or postsellar ( Fig. 12.5 ). Sellar anatomy and its boundaries provide important landmarks for surrounding anatomy, such as the cavernous sinus (CS) ( Fig. 12.6 ), which houses important neurovascular structures. Beyond the diaphragma sella is the suprasellar space.
Preoperative assessment is initiated with a complete history and physical, paying particular attention to signs of underlying endocrine derangement such as dry skin, acne, body habitus, and facial structure. Formal endocrine testing is imperative to identify hormone-producing tumors as well as pituitary dysfunction. Laboratory examination should include a full endocrine panel: serum prolactin, AM cortisol, insulin-like growth factor (IGF-1), and adrenocorticotropic hormone (ACTH), thyroid stimulating hormone (TSH), free thyroxine (T4), serum testosterone level in males, follicle stimulating hormone (FSH), luteinizing hormone (LH), estradiol, and a serum AM cortisol level with cosyntropin stimulating hormone. The prevalence of preoperative partial hypopituitarism ranges from 37% to 85% in the published literature.
Patients with large lesions or visual symptoms should undergo an ophthalmologic exam including formal visual field examination and optical coherence tomography (OCT). Nasal examination should assess for past infections/bleeding, current smell capability, and any abnormal anatomy such as deviated or perforated septum, polyps, and turbinate health.
Magnetic resonance imaging (MRI) of the brain with and without contrast is the imaging modality of choice. CT scans are useful to assess the bony anatomy.
Surgical indications for sellar, suprasellar, and parasellar lesions are similar. In general, large lesions or lesions that demonstrate growth over time should be surgically resected. Surgical resection provides diagnosis, and control of symptoms that can include intractable headaches, hormone hypoproduction, and cranial nerve dysfunction, through relieving compression or involvement of surrounding structures. As for functional lesions, surgery aids in decreasing hormone levels and preventing secondary complications. The exception to this is a prolactinoma, which can be treated medically. While pituitary apoplexy is rare, when symptomatic, patients should undergo resection.
The endonasal route is becoming an increasingly common way to approach skull base lesions; however, there are some limitations that preclude its use or require a craniotomy in conjunction. While the endoscope provides enhanced visualization, certain anatomic locations render the use of a craniotomy more beneficial. Large lesions with significant extension into the suprasellar compartment, tumors above the optic nerves or invading the orbit, and those with CS involvement lateral to the internal carotid artery make a complete resection via an isolated endonasal corridor quite challenging. Lesions requiring complex dura or skull base reconstruction may need to utilize a transcranial route in order to minimize the risk of cerebrospinal fluid leak. Additionally, vascular tumors that provide a hemostatic challenge may be better approached via a transcranial route. With the ultimate goal of maximal resection and lowest morbidity, craniotomy remains a good tool for lesions in these locations.
The rate of meningitis after endoscopic transsphenoidal procedures ranges from 0.7% to 3.1% and is greatest when there is a postoperative CSF. There continues to be practice variability in the type and duration of perioperative antibiotics prescribed, commonly including a cephalosporin with or without a beta-lactamase inhibitor.
The endonasal approach to the sella has evolved extensively over the past centuries. From renditions including transglabellar, sublabial, infranasal, and multiple transnasal routes, the submucosal transseptal-transsphenoidal corridor is now considered the main route to access the sella and parasellar lesions. For ease, this can be broken down into steps: nasal approach, sphenoidotomy, sella exposure, lesion resection. Traditionally, neurosurgeons have used the microscopic approach to reach the sella. While the procedure is quite similar, endoscopy is becoming more commonly used, which can be performed by a neurosurgeon alone or in conjunction with a rhinologist. For the purposes of this chapter, we focus on the endoscopic approach.
The right nostril is typically used to access the sphenoid sinus. The alternative nostril may be used when abnormalities in the nasal anatomy, such as septal deviation, are present. If an S-shaped deflection of the septum is present, collaboration with an otolaryngologist is recommended to reduce the risk of mucosal damage during the surgical procedure. Topical anesthetic is then applied to the sphenoethmoid recess and septum. After appropriate time for hemostasis, the septal mucosa is incised, which allows access to the sphenoid rostrum. The septum is then fractured off of the maxillary crest to the contralateral side. The sphenoid ostium is then identified at its location at the lower one-third of the superior turbinate. The sphenoid ostium is opened with a sphenoid mushroom punch and the floor of the sphenoid is identified. The sphenoid rostrum and the sphenoid floor are drilled down until the sella, carotid prominence, optico-cartoid recess, and clivus are identified. Once tumor resection is complete, a Freer elevator is used to carefully place the septum back into midline.
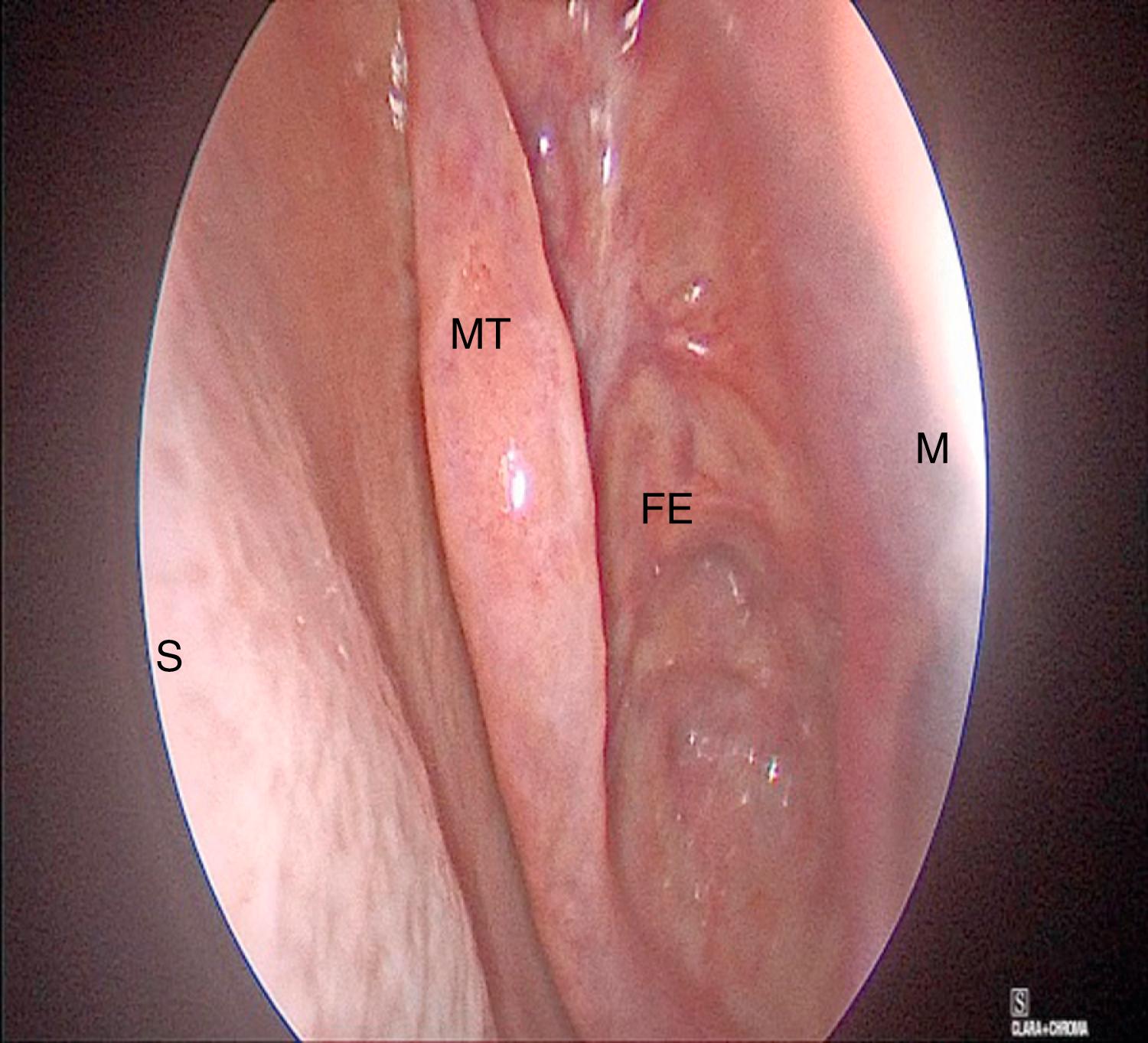
Neuropatties soaked with nasal decongestant, such as Oxymetazoline, are placed in the nasal cavity during the surgical preparation time. This allows the nasal mucosa and inferior turbinates to become decongested before endonasal instrumentation begins. One percent lidocaine with 1:100,000 epinephrine is then injected into the anterior vertical portion of the middle turbinate, anterior wall of the sphenoid sinus just inferior to the superior turbinate, and the posterior portion of the septum. If a nasoseptal flap is not being harvested, injection is placed at the sphenopalatine foramen, which is immediately anterior inferior to the attachment of the horizontal portion of the basal lamella of the middle turbinate ( Fig. 12.7 ). Some surgeons elect to perform a transoral injection of the sphenopalatine foramen, placing a 25 g spinal needle with a 45-degree up angle in the greater palatine foramen. The greater palatine foramen is located approximately 1 cm medial to the space between the first and second molar, in a small groove. The needle is aspirated before injection of approximately 1 cc of local anesthetic into the foramen. Some surgeons continue to use oxymetazoline throughout the case until the sella is opened, while others prefer the use of a mixture of reconstituted thrombin 5000 mg with 1:1000 adrenaline. This mixture can also be used until the sella is opened. If arterial bleeding occurs while opening the sphenoid sinus due to the posterior nasal artery, a Bovie suction coagulator or bipolar cautery can be used to quickly control the bleeding.
Video 12.1
Video 12.2
Minimal to moderate inferior turbinate hypertrophy can be treated with outfracturing using a butter knife. Under endoscopic visualization a butter knife is placed along the medial side of the inferior turbinate and gentle steady pressure is placed to fracture the bone laterally. Care is taken to not traumatize the mucosa with this maneuver, as the inferior turbinate can be very vascular and may lead to undue oozing during the procedure. If there is significant hypertrophy, an otolaryngologist may be needed to perform a submucosal partial resection of the inferior turbinate using a microdebrider. This removes a portion of soft tissue and bone from the turbinate while maintaining the overlying mucosa.
The middle and superior turbinates are then lateralized using a Freer instrument, in order to visualize the sphenoid ostium. The Freer is placed on the medial surface of the middle turbinate at the lower one-third of the turbinate. With the tip of instrument placed parallel to the middle turbinate at its turn into the basal lamella, it is then gently lateralized, which allows a better view of the superior turbinate. Care is taken not to traumatize the mucosa on the lateral side of the vertical portion of the middle turbinate, as this can lead to synechiae formation and subsequent sinusitis.
The superior turbinate is then lateralized at its lower one-third, which puts the sphenoid ostium in view.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here