Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The 2014 WHO/ISGyP simplified the classification of hyperplasias to:
Hyperplasia without atypia.
Atypical hyperplasia (AH). Atypia refers only to cellular atypia.
The traditional distinction between simple and complex endometrial hyperplasia is no longer included as it was not prognostic, although complex atypical hyperplasia (CAH) is used here when referring to studies preceding the current classification.
The term ‘endometrial intraepithelial neoplasia’ (EIN) has also been applied to endometrioid carcinoma precursors and is included in the 2014 WHO classification as an alternate designation for atypical hyperplasia.
EIN is defined by: (1) a gland to stroma ratio of >1:1; (2) epithelial cells that differ cytologically from those of the background glands; (3) a size of ≥1 mm.
Although some studies have found the EIN classification to be prognostically superior (and more reproducible) than the WHO/ISGyP classification, Lacey et al. in a recent large multi-institutional study found that both classifications similarly predicted the risk of progression to adenocarcinoma. We use hyperplasia with or without atypia in our practice and do so here.
The age of the patients generally is similar to that of those with endometrial carcinomas but given their precursor nature they may occur in younger women, including adolescents (Lee and Scully). Endometrial hyperplasias are usually a response to hyperestrinism, and accordingly the patients often have chronic anovulation (as in polycystic ovarian disease), a history of estrogen or tamoxifen treatment, or are obese.
Hyperplasia precedes an unknown proportion of endometrial carcinomas but being strongly linked only with endometrioid adenocarcinoma, which is usually well differentiated. Even these tumors, however, have synchronous hyperplasia at the time of hysterectomy in <50% of cases.
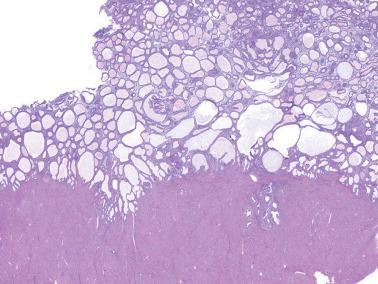
The gross appearance of the hyperplastic tissue varies with the extent and severity of the hyperplasia, ranging from no abnormality to focal thickening or diffuse polypoid foci. Endometrial polyps and foci of adenomyosis may also be involved by hyperplasia but usually without altering their gross appearance.
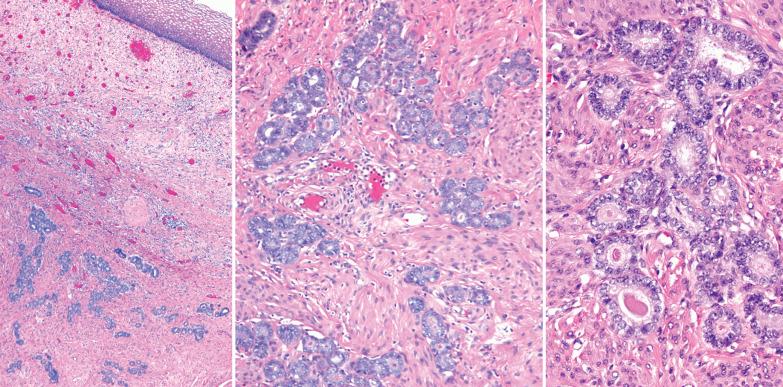
Hyperplasia without atypia.
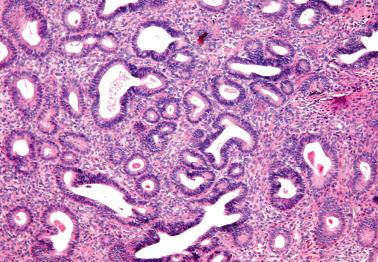
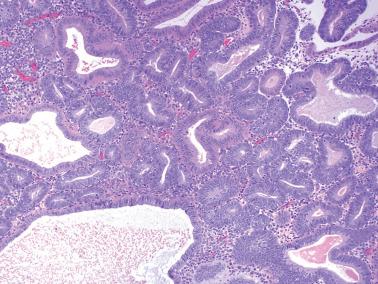
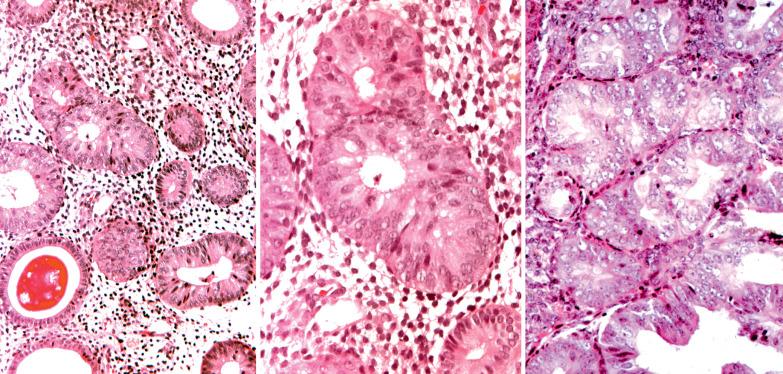
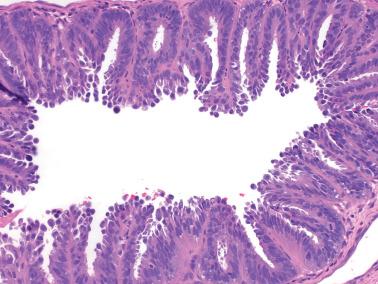
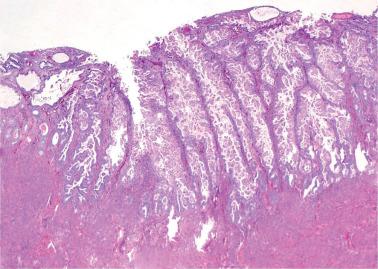
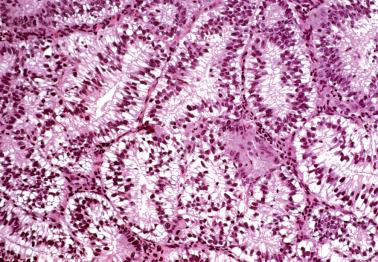
These lesions exhibit mild to severe degrees of architectural complexity due to gland crowding and irregularity in gland size and shape including cystic glands (cystic hyperplasia) and gland outpouching, branching, and papillary infolding. Care should be taken to avoid misinterpreting single highly coiled glands as well as artifactual or menses-related compaction of glands that are common in curettage specimens (see Differential Diagnosis ).
The cellular features usually resemble those of a normal proliferative endometrium, although some of the other features noted below may be present.
Atypical hyperplasia (AH).
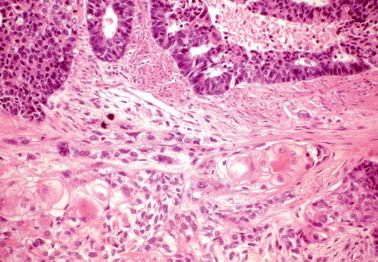
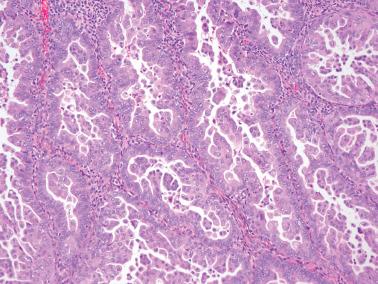
These lesions definitionally exhibit cellular atypia: loss of nuclear polarity, round rather than oblong nuclei, nuclear enlargement and pleomorphism, irregular nuclear borders, hyperchromasia, coarse chromatin, and nucleolar prominence. Chromatin condensation along the nuclear membrane may result in a vesicular nucleus. Mitoses are present but may not be conspicuous. The cytoplasm is usually increased in volume and may be eosinophilic.
Noting the degree of atypia (mild, moderate, severe) and its extent (focal or diffuse) may help clinicians determine further management.
Findings that can occur in any type of hyperplasia include:
Intraglandular papillae with fibrous cores (see Chapter 7 , Papillary Proliferation).
Cellular stratification, including cellular buds, intraglandular bridges, or even luminal obliteration; a cribriform pattern is uncommon (except in some cases of ciliated metaplasia) and favors adenocarcinoma.
Metaplastic changes ( Chapter 7 ) and surface epithelial changes as seen in endometrioid carcinoma (see corresponding heading). Florid morular metaplasia (‘adenoacanthosis’) can cause obliteration of gland lumina and a more complex histologic appearance, potentially leading to a misdiagnosis of carcinoma.
Focal menstrual-type breakdown resulting in compacted stroma, nuclear debris, regenerating surface epithelium, and syncytial papillary change ( Chapter 7 ).
Dilatation and thrombosis of endometrial sinusoids and focal infarct-like necrosis.
Stromal foam cells, although this finding is much more common in endometrioid adenocarcinoma.
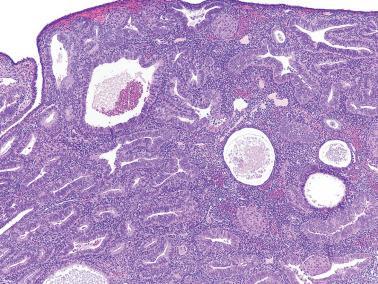
Hyperplasia within a secretory endometrium and/or a hyperplasia showing secretory changes can be diagnostically problematic and of uncertain significance.
Truskinovsky et al. evaluated the presence of hyperplasia (and carcinoma) in secretory endometria finding that hyperplastic foci contrasted with the normal secretory glands by their architectural disorder with greater gland crowding, dilated irregularly shaped glands (budding, branching, staghorn-shapes), glands with delayed or absent secretory development, and cytologic atypia.
Jeffus et al. reported similar findings as ‘secretory endometrial intraepithelial neoplasia’ (S-EIN). The lesional glands were larger, more complex and haphazardly disposed than those of the normal secretory endometrium, and exhibited voluminous vacuolated cytoplasm, nuclear overlap, and vesicular chromatin. Their study also included cases of EIN composed of proliferative-type glands within a secretory background. All EINs exhibited absent, decreased, or increased PAX2 staining compared to the background glands.
Gurda et al. found that the Ki-67 index was useful in the differential diagnosis of proliferative endometrial lesions with secretory change. The Ki-67 index was 2.6% in normal secretory endometrium, 17% in nonatypical hyperplasia, and 36% in AH (vs 60% in endometrial carcinoma). Similar results were found by Truskinovsky et al.
Wheeler et al. described changes induced by progestin treatment of hyperplasia:
A decrease in gland confluence and complexity often resulted in an inactive or secretory endometrium, although focal gland confluence was found in a third of cases. Metaplasias (eosinophilic, squamous, mucinous) were common.
Common cytologic changes included a decreased N:C ratio with increased cytoplasmic eosinophilia, secretory change (cytoplasmic vacuoles), nuclei with a smudged appearance, and small nuclei with fine chromatin.
Loss of PTEN staining may support a diagnosis of AH when PTEN− atypical glands are admixed with PTEN+ nonatypical glands, although otherwise normal glands may be PTEN−, and atypical glands may be PTEN+.
Ayhan et al. found that 70% of AHs had decreased or undetectable PTEN staining whereas Lee et al. found loss of PTEN staining in 24% of simple hyperplasias, 71% of CAHs, and 68% of endometrial endometrioid carcinomas (EECs).
Robbe et al. found loss of PTEN staining in 54% of CAH and 7% of patients with and without a coexistent endometrial adenocarcinoma, respectively.
Russo et al. found that all paired AH/EEC samples shared ≥1 identical somatic mutation (frequently in the PI3K pathway) and ≥1 large copy number alteration (CNA) (>10 genes in length) when CNAs were present. MMR protein expression matched in all paired samples as did Cancer Genome Atlas subtype (copy number low endometrioid or microsatellite instable (MSI)-hypermutated). They concluded that AH leads to EEC by a process of subclone evolution, not a linear accumulation of molecular events.
ARID1A , a tumor suppressor gene that encodes the BAF250a protein, plays a role in tumor progression in endometrial endometrioid carcinoma (EEC), being lost in some CAHs.
Mao et al. found clonal (but not complete) loss of ARID1A staining in 16% of CAHs (vs complete/clonal ARID1A loss in 25%/24% and 44%/9% in low-grade and high-grade EECs, respectively). Ayhan et al. had similar results in AHs and found that all specimens with ARID1 loss also had PTEN loss.
Ayhan et al. also noted increased proliferative activity in areas of AH with concurrent loss of PTEN and ARID1A compared to adjacent AH areas with only PTEN loss, suggesting that ARID1A may prevent the inactivation of PTEN required for the progression of AH to EEC.
Zauber et al. found identical KRAS mutations and microsatellite instability in 95% of endometrial hyperplasias and synchronous EECs, suggesting that these molecular changes occur early in the neoplastic process.
Vierkoetter et al. found MMR deficiency in 4.5% of AHs, mostly related to sporadic methylation of MLH1.
Lee et al. found that a panel of four microRNAs (miR-182, 183, 200a, 200c) distinguished carcinoma from CAH with a 91% sensitivity and 94% specificity.
Buell-Gutbrod et al. found that Hand2 expression, which is normally expressed in the endometrial stroma, is lost or markedly reduced in the stroma of AH and EEC.
found progression to carcinoma in <3% of cases of hyperplasia without atypia (simple and complex), compared to ~25% of patients with atypia (simple and complex). Similar results have been obtained by Baak, Mutter et al. and .
Wheeler et al. found in progestin-treated CAHs that 67% had complete resolution, 11% regressed to complex hyperplasia without atypia, and 22% had persistent disease (median follow-up interval, 11 months).
The assessment of risk of progression of hyperplasia to carcinoma is complicated by the finding of EEC in a substantial proportion of uteri removed shortly after a diagnosis of CAH.
Trimble et al., after a consensus review diagnosis of CAH on an endometrial sampling, found that 39% of the removed uteri contained adenocarcinoma, a third of which were myoinvasive. Even after a review diagnosis of ‘less than CAH’, 19% of the hysterectomy specimens contained adenocarcinoma
Rakha et al. similarly found corresponding frequencies of 40–48% in recently published series; the risk was 13% in cases of mild to moderate atypia but 50% with severe atypia.
Artifactual changes due to the sampling procedure ( Chapter 7 ). These include artifactual crowding and fragmentation, telescoping (glands within glands), and compacted strips of surface epithelium often with a pseudopapillary pattern, a finding frequently associated with an atrophic endometrium.
Single highly coiled glands. As previously noted, when a highly coiled proliferative gland is sectioned longitudinally, the appearance can be misinterpreted as a focus of simple or complex hyperplasia.
Basalis glands. These glands in hysterectomy specimens can sometimes appear crowded, but normally merge with uncrowded glands in the functionalis layer.
Disordered proliferative endometrium (DPE).
DPE is a common finding in women with anovulatory cycles and reflects unopposed estrogen stimulation. It may precede and/or accompany hyperplasia.
The appearance is usually that of an otherwise typical proliferative endometrium except for occasional cystic glands and/or mild focal glandular crowding and budding. Vascular thromboses and menstrual changes may be present.
found that DPE regressed in almost 80% of cases and the remainder progressed to hyperplasia; no cases progressed to carcinoma.
Cystic atrophy (vs simple hyperplasia with cystic glands). Cystic atrophy, which is usually seen in elderly women, appears as cystic glands usually separated by fibrotic stroma. The glands may be crowded but are lined by simple, typically flattened, amitotic epithelium.
Epithelial metaplasias, pregnancy-related and other hormonal changes, and postmenstrual and postcurettage reparative changes ( Chapter 7 ).
Polyps ( Chapter 7 ). Glands within polyps may show some crowding and irregularity that may suggest hyperplasia but the latter should be diagnosed cautiously on the background of an unequivocal polyp. Polyps, however, may contain foci of bona fide hyperplasia or even carcinoma ( Chapter 7 ). A polypoid configuration and an abnormal stroma ranging from hypercellular to fibrotic and vascular with thick-walled blood vessels are helpful clues to the diagnosis.
Papillary proliferation ( Chapter 7 ).
Atypical polypoid adenomyoma ( Chapter 9 ).
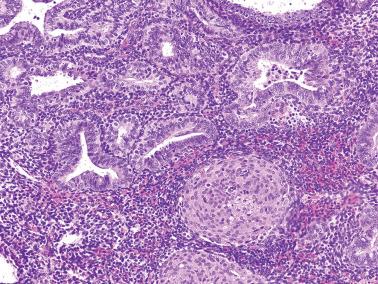
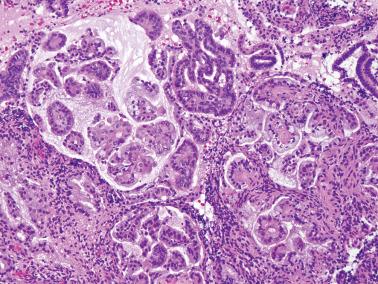
Endometrioid adenocarcinoma (EEC). The distinction of AH from grade 1 EEC is based on the evaluation of both architectural and nuclear changes that usually parallel each other, but occasionally the diagnosis is based mainly on only one feature.
EECs, unlike AH, typically exhibit a confluent, haphazard glandular pattern with merging of glands and interconnecting gland lumens; intraglandular complexity, including a cribriform pattern; an extensive or confluent villoglandular or micropapillary pattern (coarse or fine papillae with a branching or hierarchical growth), with the caveat that some papillary proliferations are noncarcinomatous (see Chapter 7 , Syncytial Papillary Change and Papillary Proliferation).
Even small foci of the above patterns in an endometrial sample can be associated with a myoinvasive EEC in the resected uterus. Mittal et al. found that extreme gland crowding (glands comprising ≥95% of a ≥3 mm area) and cribriform foci of any size had a high sensitivity and specificity for myoinvasive EEC in the hysterectomy specimen. While a cribriform pattern is strongly suspicious for EEC, we would not equate it with myoinvasion.
Grade 1 EECs almost always exhibit cytologic atypia (including macronucleoli) that exceeds that seen in AH, but at the upper end of AH the distinction is subtle.
A desmoplastic stroma, although uncommon in a biopsy specimen, strongly favors EEC. Myoinvasion is only rarely diagnosable in biopsy or curettage specimens but of course is diagnostically helpful if present.
Nonspecific findings that favor EEC over AH include florid squamous differentiation, a microglandular pattern, necrosis, numerous neutrophils, numerous mitotic figures, and atypical mitotic figures.
‘At least atypical hyperplasia, grade 1 EEC cannot be excluded’ is a reasonable diagnosis in cases in which a definite diagnosis cannot be made with confidence, as when dealing with a small sample.
McKenney and Longacre, using the presence or absence of the criteria of complex glandular architecture and marked cytologic atypia, delineated three categories: (a) AH, with a very low risk (<0.05%) of myoinvasion; (b) AH, cannot exclude grade 1 EEC, with an intermediate risk (5.5%) of myoinvasion; and (c) grade 1 EEC with a high risk (20%) of myoinvasion.
See Table 8.1 .
|
a See Chapter 10 .
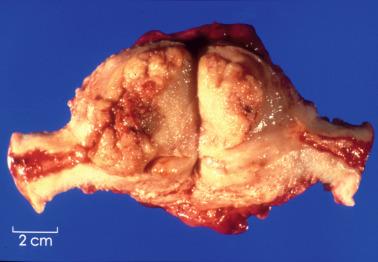
The various subtypes are usually indistinguishable on gross examination (rare exceptions are noted below). Grossly visible tumor may not be apparent, sometimes due to removal of most or all of it by curettage.
Visible tumors vary from polypoid masses that can fill the endometrial cavity to nodules or localized or diffuse areas of abnormality. Occasional tumors are confined to the lower uterine segment.
Some tumors with little or no mucosal abnormality may have extensive and deep myoinvasion whereas large polypoid tumors may lack myoinvasion. Synchronous adenomyosis may be misinterpreted as myoinvasion.
Thorough myometrial sampling is crucial to determine the maximal depth of myoinvasion unless deep invasion is obvious grossly. Additional blocks may disclose deeper myoinvasion, lymphovascular invasion, or more poorly differentiated tumor not present in the initial sections.
Although cervical involvement may be seen on gross inspection, it is more commonly discovered only on microscopic examination.
The neoplastic tissue is typically pale tan to white and soft and fleshy, but occasionally firm or gritty. Foci of hemorrhage, necrosis, or both are often visible, especially in poorly differentiated tumors.
This section focuses on endometrioid carcinoma (EEC), by far the most common subtype of endometrial carcinoma, although some aspects considered here, such as cervical involvement, patterns of myometrial invasion, carcinomas arising in unusual sites, carcinomas arising in patients with HNPCC (Lynch syndrome), and prognostic factors also apply to varying degrees to other subtypes.
EECs, which account for ~90% of endometrial carcinomas, typically arise in postmenopausal women (mean age, 60 years) but 5% occur in women <40 years of age and they rarely occur in adolescents (Lee and Scully). Tumors in younger women tend to be low stage and low grade and thus have a better prognosis.
The typical symptom is abnormal bleeding. The uterus may be enlarged. An elevated serum CA125 level may be found, especially in women with high-stage disease
Extrauterine disease (stage III or IV) is present in <10% of cases. Unlike serous carcinoma, peritoneal spread at presentation is uncommon. In 5% of cases, there is a synchronous endometrioid carcinoma of the ovary ( Chapter 14 ), which, if judged to be a separate primary tumor (as most are), does not affect the stage. Peritoneal keratin granulomas lacking tumor cells also do not alter the stage (see Squamous Differentiation, Clinical and Histologic Prognostic Factors, and Chapter 20 ).
There is often an association with unopposed estrogen stimulation, which may be due to obesity, chronic anovulation (polycystic ovarian disease), estrogen-secreting ovarian tumors, exogenous estrogens, or tamoxifen therapy.
These tumors are usually low grade and are often preceded and/or accompanied by endometrial hyperplasia. However, an appreciable proportion of low-grade EECs lack synchronous hyperplasia.
The cumulative dose/duration of tamoxifen treatment affects the relative risk of endometrial carcinoma; the risk persists after cessation of treatment. Although most tamoxifen-related endometrial carcinomas are low-grade EECs, some studies have found a higher proportion of high-risk carcinomas, especially in those with long-term treatment.
Prior pelvic radiation (usually for cervical carcinoma) increases the risk for endometrial carcinoma, some of which are EEC, although most are nonendometrioid.
HNPCC/Lynch syndrome-associated EECs are considered under a separate heading. Women with Cowden and Cowden-like syndromes are also at increased risk for endometrial adenocarcinoma due to germline PTEN, SDHB-D , and KLLN alterations (Mahdi H et al.).
Zighelboim et al. found an excess frequency of early onset multiple myeloma in women with endometrial cancer and their relatives, suggesting common susceptibility.
A rare complication is paraneoplastic cerebellar degeneration, most commonly related to Anti-Yo autoantibodies (Karpathiou et al.)
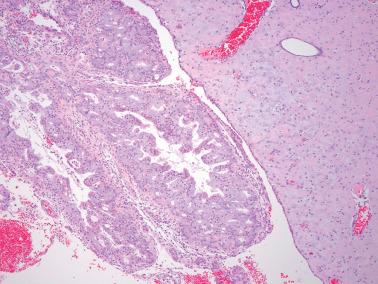
The appearance of EEC is quite variable according to the nature of the endometrioid glandular component, various associated findings (such as squamous differentiation, see corresponding heading), and the features of any associated stroma. Tumor within the endometrium may differ in appearance from myoinvasive tumor in the same specimen.
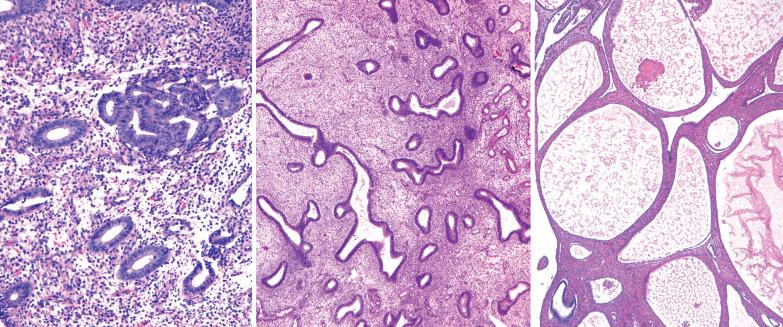
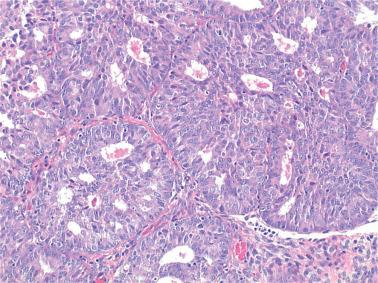
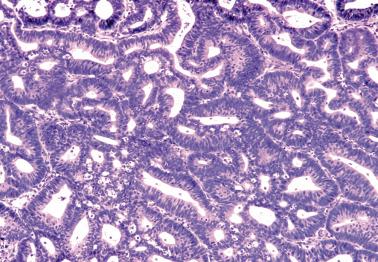
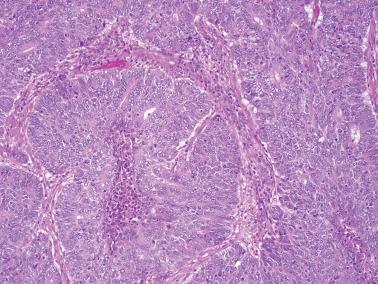
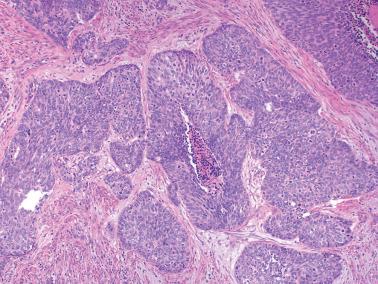
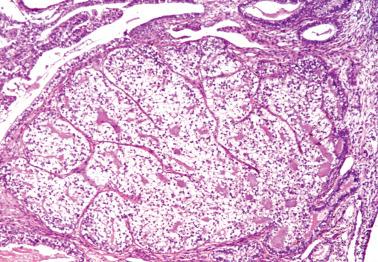
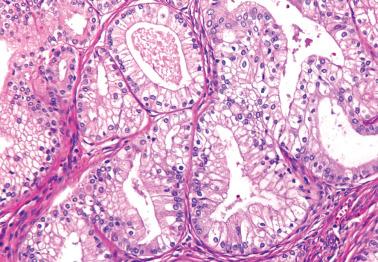
EECs, whether intracavitary or myoinvasive, are typically composed of tubular glands, most of which are of medium size, but can range from small to large and cystic. The glands are generally round to oval, but may be angulated or branched.
Nonmyoinvasive tumor is usually comprised of confluent or anastomosing glands, often with a cribriform pattern; fusion of glands can result in long labyrinthine gland lumina. A similar picture may be seen in myoinvasive tumor but in these foci the glands can be widely separated. The adjacent myometrium may appear normal or exhibit a variety of reactive changes (see below).
The glands are lined by stratified or pseudostratified columnar cells with usually only modest amounts of pale to lightly eosinophilic cytoplasm with rounded nuclei and variably prominent nucleoli. Nuclear pleomorphism is variable but is usually only mild to moderate; grade 3 nuclear features may affect the grade (see grading ). Mitotic figures are usually more numerous than in endometrial hyperplasia.
EECs with distinctive papillary patterns are considered under separate headings (see Villoglandular EECs; EECs with Small Nonvillous Papillae).
Additionally, some EECs have a papillary surface resembling syncytial papillary change ( Chapter 7 ) in which the nuclear atypia is usually less than in the underlying tumor, suggesting a maturational change. When only foci of this type are present in a scanty sample in a postmenopausal woman, additional sampling to exclude carcinoma may be required.
We have also seen what we refer to as a ‘sloughing’ phenomenon that gives rise to micropapillae that can be confused with serous differentiation, but without the high-grade nuclear features of the latter.
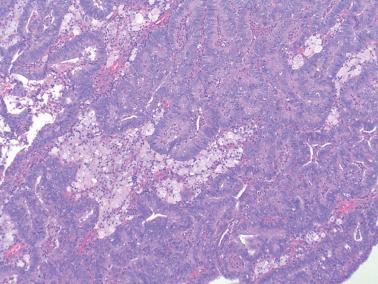
Luminal mucin is often present and can be prominent. When luminal mucin is associated with a neutrophilic infiltrate and occasionally a microglandular pattern, the findings may resemble cervical microglandular hyperplasia (MGH). Cytoplasmic mucin is usually absent or confined to luminal tips of the cells, but cells with abundant intracellular mucin of variable prominence are present in 20–40% of tumors (Worley et al., Abdulfatah et al.).
Stromal changes:
EECs confined to the endometrium usually do not elicit a typical desmoplastic stroma. Ali et al., however, found that most EECs exhibited focal ‘fibroblastic metaplasia’ of the endometrial stroma in which fibroblasts were haphazardly arranged or in poorly formed streams and that often merged with the normal endometrial stroma.
Ali et al. also found that stromal smooth muscle metaplasia was common in EECs (and usually associated with fibroblastic metaplasia) appearing as poorly formed fascicles with a vaguely fibrillar appearance. They noted that this finding within the endometrium or adenomyosis can be misinterpreted as myoinvasion.
Foamy histiocytes are seen in 15% of tumors that are usually low grade. Their presence alone in a small sample should raise concern for EEC.
Focal or confluent necrosis or necrotic luminal debris (particularly in poorly differentiated tumors) and inflammatory cells, most often neutrophils, may be conspicuous.
EECs are usually vimentin+/ER+/PR+ , although ER/PR staining can be low or absent in grade 3 tumors. Peevey et al. found that PR glandular staining is stronger in women with BMI of ≥40. Zadeh et al. found AR in 60% and 70% of low-grade and high-grade EECs respectively; only high-grade tumors showed AR in the absence of PR.
CEA is usually negative but some EECs may show weak luminal staining and squamous elements may be strongly positive. p16 staining, if present, is unrelated to HPV and is typically weak and focal and/or is confined to squamous elements.
In contrast to serous carcinomas, most EECs exhibit wild-type p53 staining (absence of ‘all or nothing’ staining). However, Schultheis et al. found TP53 mutations in 15% of EECs, usually those harboring PTEN mutations or frameshift or nonsense mutations, suggesting that TP53 / p53 status alone may be insufficient for the separation of the two tumors.
Reddy et al. found immunohistochemical expression of PD-L1 in 27% of EECs, suggesting a possible role for anti-PDL1/PD1 immunotherapy. Li et al. found tumoral PDL1 expression to be significantly associated with loss of MMR.
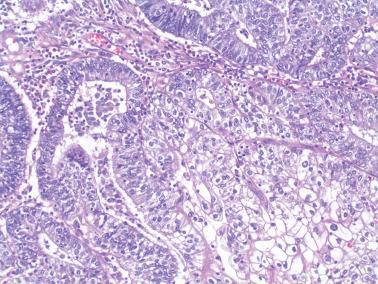
Squamous differentiation is present in ~25% of EECs and can take a variety of forms that can strikingly alter the appearance of the tumor.
‘Adenoacanthoma’ and ‘adenosquamous carcinoma’ were once widely used terms to refer to EECs with benign and malignant appearing squamous differentiation respectively.
Both are now designated collectively ‘EECs with squamous differentiation’ in the WHO classification, although the other terms are allowable alternate designations and are used here for brevity and because they may have merit.
Adenoacanthomas contain morules ( Chapter 7 ) that can be extensive and confluent, potentially obscuring the glandular component, which is usually grade 1. The morules are composed of polygonal to spindled immature squamous cells with bland nuclear features and that stain for CD10, β-catenin, and CDX2. The cells, especially in the centers of the nests, may undergo maturation and even keratinization. Morules can also exhibit central necrosis.
The squamous epithelium of adenosquamous carcinomas may be focally intraglandular, but is typically independently invasive of the endometrium or myometrium and usually of higher grade than that of adenoacanthomas. Spindled squamous cells may impart a sarcomatoid appearance.
Occasionally the squamous cells in adenoacanthomas and adenosquamous carcinomas have clear glycogen-rich cytoplasm.
Other forms of squamous metaplasia include:
Plaque-like squamous metaplasia that is often a surface phenomenon. It usually occurs as isolated small patches but may be striking and may occur with other surface metaplastic changes.
Masses of well-differentiated, sometimes glycogenated squamous epithelium.
Small nonvillous papillae (see corresponding heading) may exhibit squamous change or individual cell keratinization, typically to a limited degree.
The behavior of EECs with squamous differentiation is similar to that of typical EECs when matched for grade and depth of myoinvasion.
Peritoneal keratin granulomas rarely occur in EECs with squamous differentiation, and do not upstage the tumor (see staging section and Chapter 20 ).
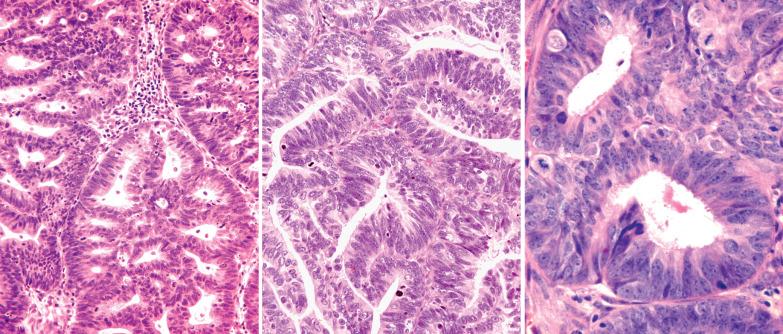
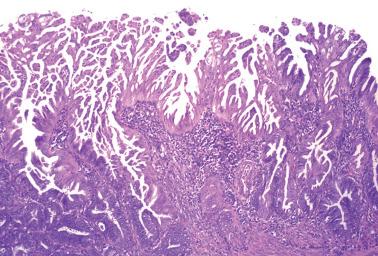
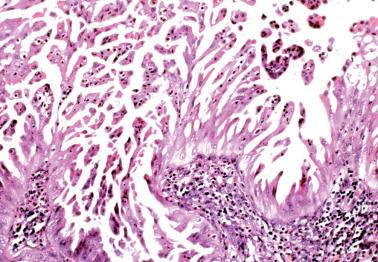
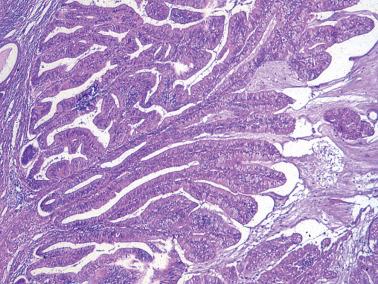
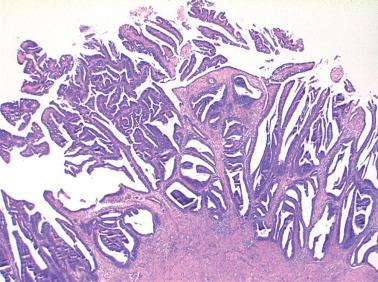
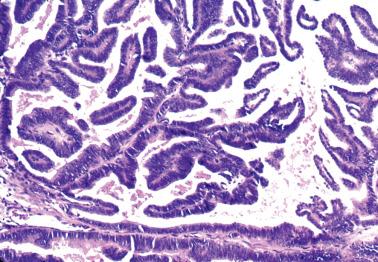
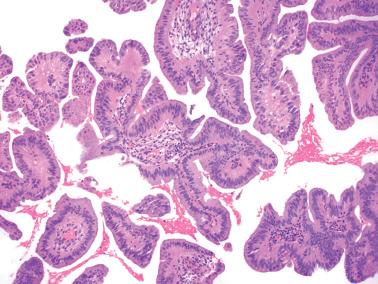
Villoglandular endometrioid carcinoma (VGEC) is the most common EEC with a papillary pattern, accounting for 13% and 31% of EECs in two different studies. Our experience is closer to the lower figure as we reserve the use of VGEC only for EECs that are predominantly villoglandular.
VGECs are characterized by often long, slender, villous papillae with thin fibrovascular cores, usually admixed with a variable proportion of endometrioid glands, although rare tumors are purely or almost purely villous. The villous pattern can occur in myoinvasive foci, but is usually most conspicuous in intracavitary tumor.
The cells lining the villi and glands resemble those of typical EEC. With rare exceptions the tumors are grade 1 or 2; squamous differentiation may be present.
The differential is with other papillary endometrial lesions (see Table 8.3 ).
Serous carcinomas. Unlike VGECs, these tumors exhibit complex papillae, highly stratified cells and epithelial buds (resulting in a serrated luminal profile), high-grade nuclei, and p53 mutations. Squamous differentiation and the typical immunoprofile of EEC support VGEC.
ECs with small nonvillous papillae (see next heading).
Endometrial mucinous, clear cell, and TCCs. These tumors are often at least focally papillary but have other features that distinguish them from VGECs.
Non-neoplastic papillary endometrial proliferations ( Chapter 7 ).
found that the behavior of VGECs is similar to that of typical EECs of similar grade, whereas Ambros et al. found that myoinvasive VGECs had a higher frequency of vascular invasion and nodal involvement and a worse outcome than usual myoinvasive EECs.
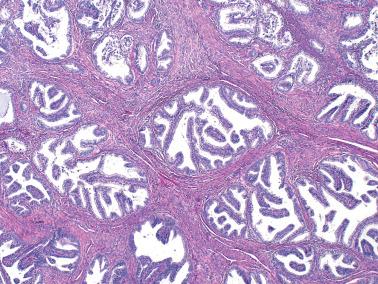
found this pattern in 8% of EECs. The mean patient age was intermediate between that of usual EEC and serous carcinoma. The tumors have a prognosis identical to that of other EECs of similar grade.
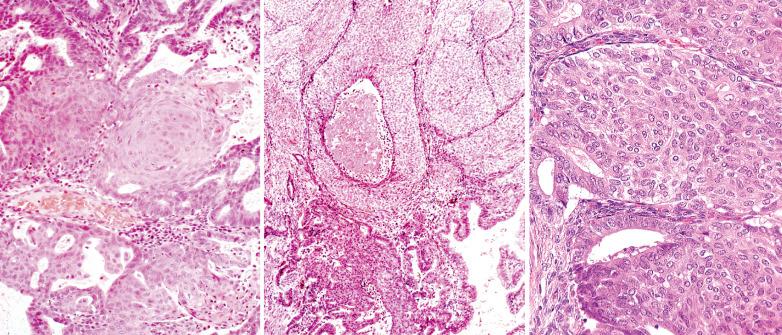
These are usually otherwise typical grade 1 or 2 EECs that contain small papillae that are intraglandular or on the tumor's surface.
Most papillae are in the form of buds of rounded cells with ample eosinophilic cytoplasm and a low N:C ratio. Longer sometime filiform papillae may extend across gland lumens resulting in a more complex pattern.
Although the eosinophilic cells in the buds may reflect abortive squamous differentiation, overt squamous differentiation occurs in half the tumors,
Distinction from serous carcinoma rests on the presence of:
A background of an otherwise typical EEC or VGEC. Squamous differentiation, if present, excludes serous carcinoma.
The papillae composed of large rounded cells with eosinophilic cytoplasm and usually low-grade nuclei contrast with the high-grade nuclei of the cells in the papillae of serous carcinomas. Rare high-grade EECs, however, can have small nonvillous papillae, but this is usually only a focal finding.
Absence of the typical immunoprofile of serous carcinoma.
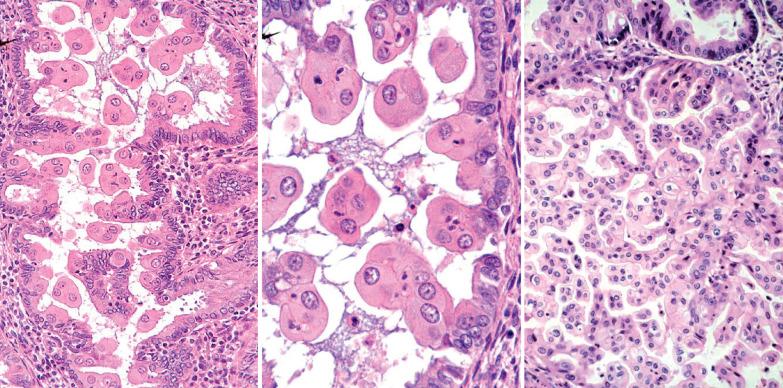
Microglandular pattern . Otherwise typical EECs (particularly those with an appreciable mucinous component) may have conspicuous microglandular patterns with luminal eosinophilic secretion and luminal and stromal neutrophils. As bona fide endometrial MGH does not occur in our opinion, a diagnosis of MGH should be made with great caution if the tissue is unquestionably from the endometrium. Usually the differential is with endocervical MGH procured during an endometrial sampling procedure.
The distinction rests on the merging of the microglandular pattern with typical EEC and nuclear atypia and mitotic activity exceeding those in MGH. found that a p16+/PAX2− profile favors MGH; cyclin D1, vimentin, and Ki67 were not helpful.
Hong et al. found KRAS mutations in 60% of microglandular EECs but in none of their cervical MGHs.
Qiu and Mittal found that subnuclear vacuoles favor microglandular hyperplasia, whereas luminal squamous metaplasia, stromal foam cells, mitoses, and a vimentin+/MIB1+ profile favor carcinoma. Chekmareva et al., however, found considerable overlap in the expression of vimentin and MIB1 in the two lesions.
Sertoliform pattern . Occasional, usually low-grade, EECs have a focal to predominant sertoliform pattern of small hollow or solid tubules and short slender cords lined by or composed of columnar cells. This variant is much less common than in the ovary.
Merging of the sertoliform elements with typical EEC and/or squamous elements and an absence of a neoplastic mesenchymal component excludes endometrial stromal sarcoma (ESS) with sex cord-like elements and uterine tumor resembling ovarian sex cord tumor (UTROSCT) ( Chapter 9 ).
Corded and hyalinized pattern . Rare EECs are characterized by cords of epithelioid cells and fusiform to spindled tumor cells that are typically small and with scant cytoplasm, within a hyalinized stroma (corded and hyalinized EEC; ‘CHEC’). Osteoid has been more common than in conventional EECs; rarely the stroma is myxoid.
The cells in the cords are variably CK+, but negative for muscle markers, CD10, and inhibin.
The differential diagnosis is mainly with a MMMT (carcinosarcoma). Unlike the latter, CHECs typically have a grade 1 or 2 glandular component, are of low stage with a high survival rate, and occur at a young age (even slightly younger than those with typical EEC).
The differential diagnosis also includes other uterine tumors with sex cord-like patterns such as ESS, adenosarcoma, UTROSCTs, and occasional epithelioid smooth muscle tumors. All of these tumors have their own distinctive features and lack the definitional components of CHECs.
Occasional epithelioid trophoblastic tumors have a hyalinized stroma that can result in a vague resemblance to CHEC, but a premenopausal age, an absence of typical EEC, and staining for trophoblastic markers aid diagnosis.
Mesonephric-like pattern . Rare endometrial adenocarcinomas histologically resembling cervical mesonephric adenocarcinomas ( Chapter 6 ) have been referred to as ‘mesonephric-like adenocarcinomas’ (Howitt et al., Kenny et al., Kim et al., McFarland et al.). Similar tumors have been described in the ovary.
None of the seven tumors reported by McFarland et al. were contiguous with mesonephric remnants, although cervical mesonephric remnants were present in one case. Two patients had extrauterine spread at presentation.
The tumors contained an admixture of small glands or tubules, often with colloid-like luminal material, and less commonly, solid and papillary patterns. The tumor cells had angulated, overlapping, vesicular nuclei. The typical immunoprofile was TTF1+/ER−/PR− with variable staining for CD10, calretinin, and GATA3. Some tumors are PAX8+ (Kenny et al.).
Some tumors may be a variant pattern of EEC as we have seen typical EECs with cervical involvement in which the latter was histologically indistinguishable from mesonephric hyperplasia (Tambouret et al.). However, Howitt et al. have found molecular alterations in mesonephric-like carcinomas that are similar to bona fide mesonephric carcinomas.
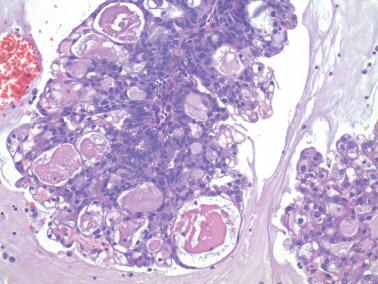
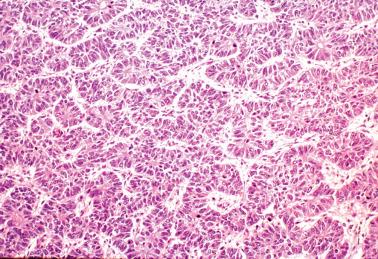
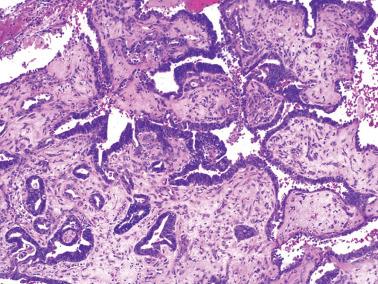
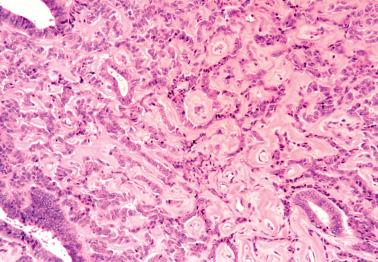
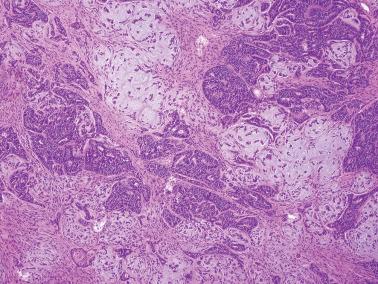
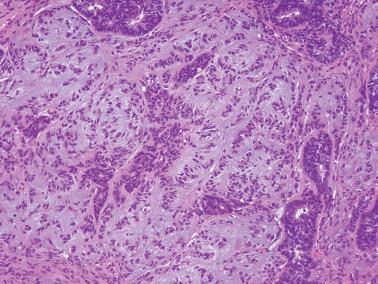
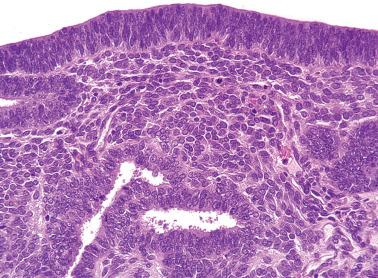
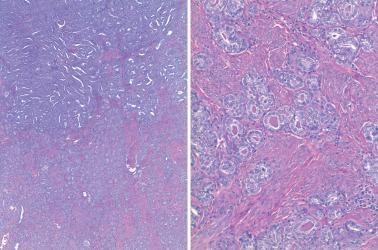
Ciliated cells . Most of these rare carcinomas resemble typical well-differentiated EECs except that the glands are lined predominantly by ciliated cells.
A rare type of ciliated carcinoma (Hendrickson and Kempson), of which we have not seen an example, has distinctive features. Sheets of cells are punctured by small extracellular lumina (imparting a cribriform pattern) that are lined by low-grade cells with cilia that project into extracellular and intracellular lumina. Two of their seven patients had extrauterine spread at presentation.
The behavior of ciliated carcinomas appears similar to that of typical EECs but experience is largely limited to the one report just noted.
Secretory changes . Minor foci of secretory morphology are common in otherwise typical EECs and merit no comment whereas the term ‘secretory carcinoma’ is warranted when the secretory changes are extensive. These tumors are rare in pure form and usually grade 1 with cells containing subnuclear and/or supranuclear glycogen vacuoles. Their behavior is similar to that of other EECs of similar grade.
Rare solid foci of tumor may be seen, but differ from clear cell carcinoma (CCC) by their low-grade nuclear features, a merging with typical secretory carcinoma, the occasional presence of squamous differentiation, and absence of all of the following: tubulocystic and papillary patterns; hobnail cells; high-grade nuclear features; and the immunoprofile of CCC.
There is usually no obvious hormonal cause, but some cases are attributable to an endogenous or exogenous progestational stimulus; in such cases the secretory appearance may be transitory.
Other ECs with clear cells (see below).
Clear cells . Some ECs contain cells with clear glycogen-rich, foamy lipid-rich, or hydropic cytoplasm (Silva and Young) but lack the typical supra- and subnuclear clarity of the cells in secretory carcinoma. Typical EECs rarely recur as clear cell-rich tumors ( ).
Oxyphilic including histiocytoid cells . These cells have abundant eosinophilic cytoplasm, some due to the presence of abundant mitochondria. These cells are particularly common in EECs with a MELF pattern and may have a bland histiocytoid appearance (see Patterns of Myometrial Invasion ).
Spindle-shaped epithelial cells .
Unlike the sarcomatous component of MMMTs, the spindle cells merge with obvious glandular or squamous epithelial cells (or foci of keratin), are usually less atypical than the sarcomatous cells of an MMMT, and lack a heterogeneous glandular component present in most MMMTs. Heterologous elements (excluding osteoid) are not seen.
Negative or weak staining for p53 and WT1 and positive staining for cytokeratin, ER, and PR favor EEC.
Arias-Stella-like reaction (ASR ). The ASR-like reaction may occur in an EEC secondary to progestational treatment. Hobnail-type cells may raise concern for a CCC component, but the history and the presence of ASR in benign glands aid diagnosis (Yamani and Fadare).
Signet-ring cells . These have been found in rare otherwise typical EECs; foci of the latter exclude metastatic signet-ring cell carcinoma ( Chapter 10 ).
High-grade component , such as undifferentiated carcinoma, giant cell carcinoma, small cell carcinoma, hepatoid carcinoma, trophoblastic elements, and foci of MMMT (see separate headings later in this chapter).
Gastric-type differentiation . Rare endometrial endometrioid carcinomas have exhibited focal gastric-type mucinous differentiation resembling that in gastric-type endocervical adenocarcinomas, including positivity for MUC6 and HIK1083 (McCarthy et al.).
Psammoma bodies . In one study (Parkash and Carcangiu) these correlated with inflammation (including pyometra), necrosis, deep myoinvasion, and lymphovascular invasion.
Benign heterologous elements . Osteoid is one of the more common and may lead to a misdiagnosis of MMMT but the latter diagnosis requires a definite sarcomatous component.
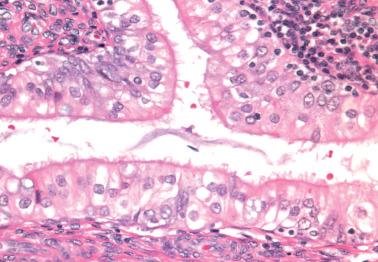
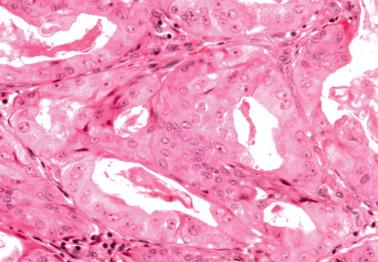
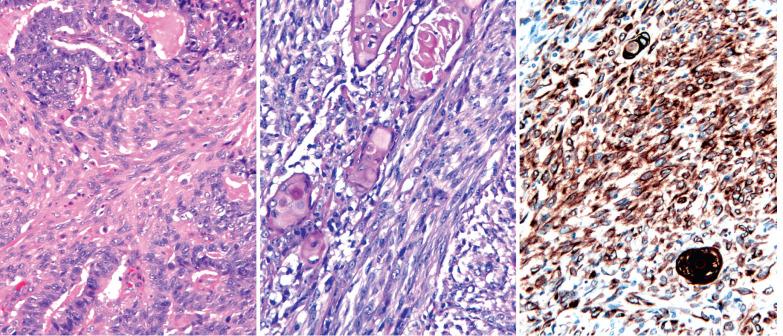
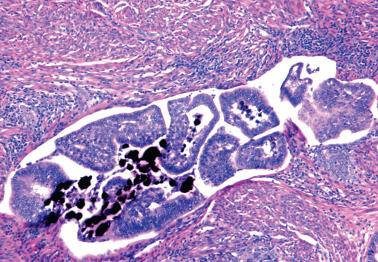
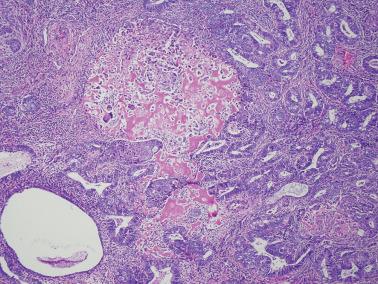
Lower uterine segment (LUS) .
Westin et al. found that only 1.8% of endometrial cancers in the general endometrial cancer population arise in the LUS vs ~10% in those <50 years of age and ~30% of Lynch-related endometrial carcinomas (see Carcinomas Related to HNPCC/Lynch Syndrome).
Some but not all studies have found that LUS tumors are more likely to be high stage, of aggressive subtype (high-grade EEC, serous, clear cell, undifferentiated), and deeply invasive, with a worse prognosis than tumors arising elsewhere in the corpus.
The differential includes isthmic involvement by non-isthmic endometrial carcinomas. Lavie et al. found that ~25% of their stage I endometrial carcinomas involved the isthmus. These tumors were disproportionately grade 3 and associated with myometrial and lymphovascular invasion.
Endometrial polyps .
About 1% of endometrial carcinomas arise in a polyp. The carcinoma may be confined to the polyp or associated with similar tumor elsewhere in the endometrium although in such cases the involvement of the polyp may be secondary.
The frequency of the various histologic subtypes of carcinomas arising in polyps is similar to that arising in the endometrium in general, EECs being the most common, followed by serous, or, rarely, clear cell, mucinous, and MMMTs. However, a disproportionate number of serous carcinomas arise in polyps.
Adenomyosis and adenomyomas.
Rare EECs arise in adenomyosis as evidenced by a lack of endometrial involvement and a transition between the carcinomatous and adenomyotic glands. In the absence of such a transition and the presence of a normal endometrium, the rare possibility of a metastasis should be considered.
EECs also occasionally arise in adenomyomas, most commonly atypical polypoid adenomyomas but occasionally adenomyomas of usual type ( Chapter 9 ).
Uterine serosa.
We have seen a case in which a grade 1 endometrioid adenocarcinoma arose from uterine serosal endometriosis in a patient with a similar endometrial tumor.
Quick, May et al. found myoinvasion and deep myoinvasion (>50% of myometrium) in 30% and 6% of grade 1 EECs respectively; Nofech-Mozes, Ghorab et al. found deep invasion in 26% of low-grade tumors and 59% of high-grade tumors. Winham et al. found that ~80% of grade 2 EECs were myoinvasive.
Myoinvasion is obvious when carcinomatous glands, nests, cords, or single tumor cells have an irregular border with the myometrium often accompanied by an inflammatory, myxoid, or desmoplastic stromal response.
Other myoinvasive tumors have a well-circumscribed, expansile, or pushing border with the myometrium, with little or no stromal response. In such cases, myoinvasion may not be recognized (and its depth not measurable) if the normal endomyometrial junction is not in the tissue section. This pattern of myoinvasion may be confused with carcinomatous involvement of adenomyosis but it lacks the adenomyotic stroma and the typical surrounding myometrial hyperplasia of the latter.
described a distinctive pattern of myoinvasion denoted by the acronym ‘MELF’ (microcystic, elongated, and fragmented).
This pattern was first recognized when one of us (RHY) noted that some EECs had microcystic glands that were often elongated, fragmented, and embedded within a prominent fibromyxoid stroma. The ‘F’ of the acronym denoted the fragmentation but it can also refer to the fibromyxoid stroma that is often the most striking feature on low-power examination and should suggest MELF when dealing with an EEC.
The MELF pattern is found in 10–15% of myoinvasive EECs that are usually grade 1 or 2 and myoinvasive into the outer half. The often predominant fibromyxoid stroma and fragmentation of the tumor can lead to its lack of recognition, and as MELF often occurs within the deepest myoinvasive tumor, underestimation of the depth of myoinvasion.
Microcystic or slit-like glands are often lined by flattened (endothelial-like) cells or cells that may appear histiocytoid with abundant eosinophilic or clear vacuolated cytoplasm with bland nuclear features. Detached single cells or clusters of the latter can be found within myometrial lymphatics and lymph nodes (see below).
The MELF pattern correlates with an increased frequency of lymphovascular invasion (LVI) ( ) and lymph node involvement (LNI), the latter being present in ~50% of cases studied by Espinosa, Serrat et al. and Pavlakis et al. More strikingly, among myoinvasive well-differentiated EECs, Hertel et al. found LNI in 19% of non-MELF tumors, in 67% of mixed MELF–non-MELF tumors, and in 100% of pure MELF tumors; among EECs with LVI, LNI was more common in MELF than in non-MELF tumors.
LNI typically occurs as isolated, often histiocytoid, isolated tumor cells (ITCs) within subcapsular lymphatics that are difficult or impossible to distinguish from histiocytes without cytokeratin staining (Espinosa, Serrat et al., Pelletier et al.).
Tumor cells within MELF foci are CK7+ and CK19+, and have decreased ER, PR, and E-cadherin expression, and a lower proliferation index compared to the non-MELF areas. Kojiro-Sanada et al. found that MELF cells stained immunohistochemically for CXCL14 and CXCL12 (ligands of the metastasis progression gene CXCR4).
The rare ‘diffusely infiltrating’ or ‘single gland pattern’ of invasion appears as single often widely separated myoinvasive glands. The neoplastic glands can be very well differentiated and associated with little or no stromal response (‘adenoma malignum pattern’) and thus can be misdiagnosed as a benign process, such as atrophic adenomyosis.
All patterns of myoinvasion may be associated with LVI, the recognition of which often can be complicated by retraction artifact and vascular pseudoinvasion (see Effects of Treatment ). Perivascular lymphocytes can be a clue to the presence of vascular invasion. Immunostaining with endothelial markers may be helpful.
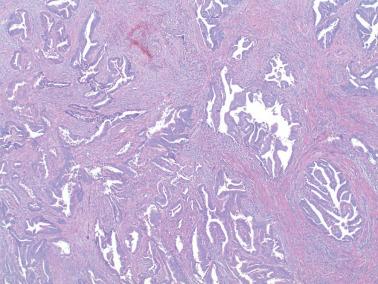
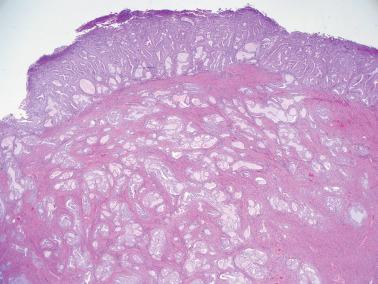
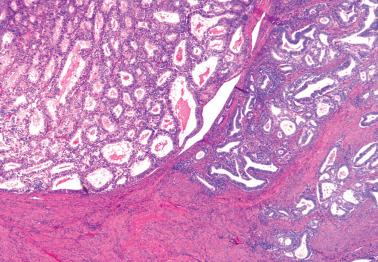
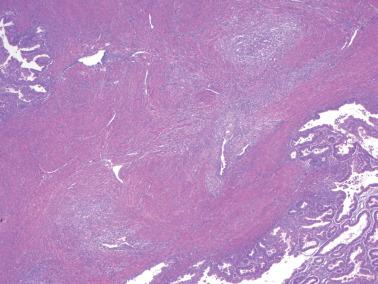
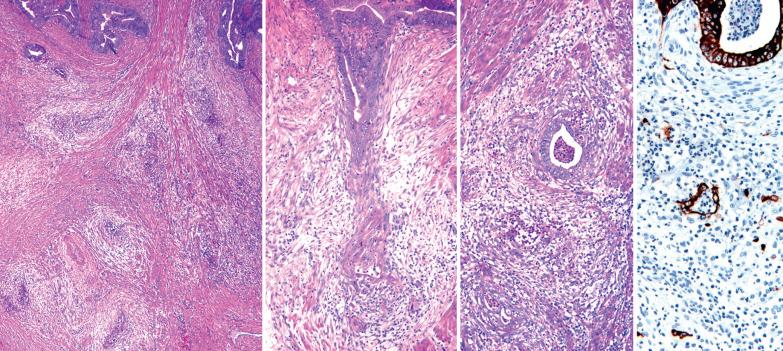
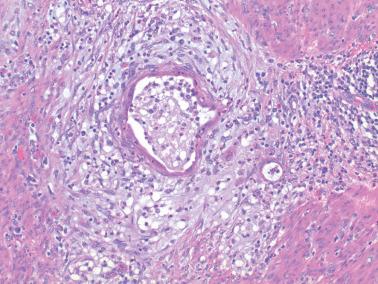
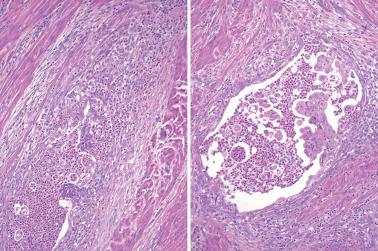
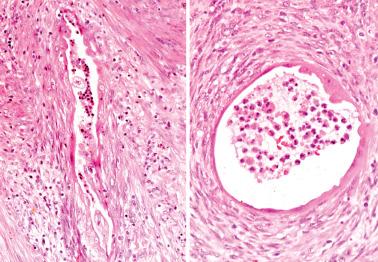
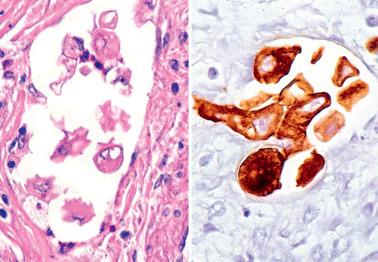
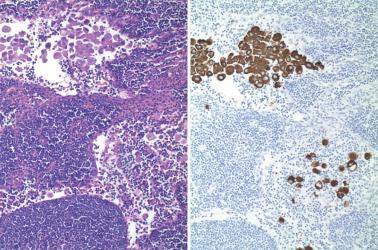
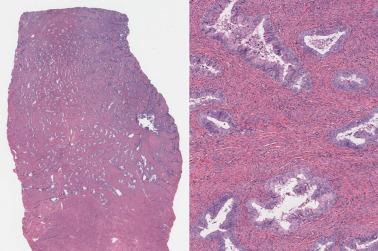
Myoinvasion, especially superficial myoinvasion, is frequently over-diagnosed in EECs because of a variety of findings.
EECs confined to the endometrium can accentuate the normally irregular endomyometrial junction, so that well-circumscribed, rounded nests of tumor appear to bulge into the superficial myometrium. Tumor filling a tubal recess can mimic deep myoinvasion. Awareness of these findings, the presence of benign endometrial glands or endometrial stroma between the tumor and the myometrium, the absence of a reactive stroma, and when the tubal recess is involved, proximity to the intramural fallopian tube, facilitate interpretation.
Nonmyoinvasive tumors that are entirely exophytic can be misinterpreted as invasive if the tissue section does not include the normal endometrial-myometrial junction.
As noted earlier (see Typical Microscopic Features, Stromal Changes), smooth muscle metaplasia of the endometrial stroma should not be misinterpreted as myoinvasion.
When an EEC and adenomyosis coexist, the latter process is involved by the EEC in ~25% of cases, a finding without adverse prognostic significance on its own.
Findings indicating involvement of adenomyosis include a smooth contour with the surrounding myometrium (which is often hyperplastic), non-neoplastic glands or adenomyotic stroma within the focus, no inflammatory or desmoplastic response, and the presence of adjacent uninvolved islands of adenomyosis. Additionally, unlike myoinvasive cancer, foci of involved adenomyosis are usually more evenly spaced.
Determining if malignant glands abut myometrial smooth muscle (and thus myoinvasive) or are surrounded by atrophic adenomyotic stroma is difficult. As the latter and reactive fibroblasts around myoinvasive tumor are both CD10+, only negative CD10 staining (indicating an absence of adenomyosis) is helpful. However, Busca et al. found that myoinvasive foci were negative for the endometrial stroma marker IFITM1 whereas ~70% of nonmyoinvasive foci were IFITM1+.
The differential of EECs involving adenomyosis includes rare EECs arising within adenomyosis or an adenomyoma (see below).
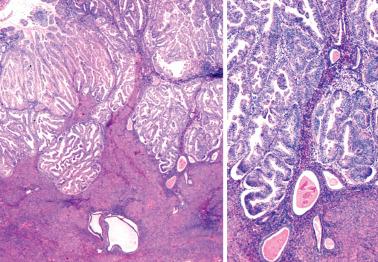
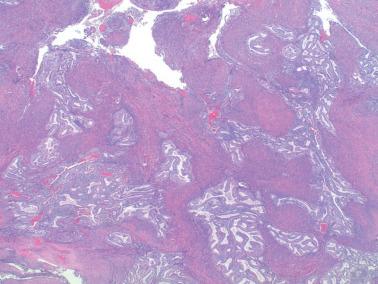
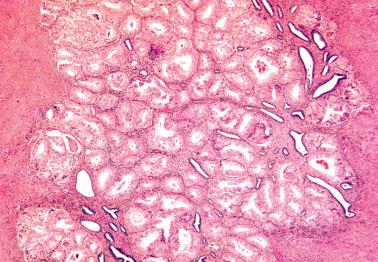
The prognostic value of grading of EECs is well established. The 1988 FIGO/ISGyP grading system (with suggested modifications in 1995) is by pattern and nuclear features. Tumors that are <5% solid, 5–50% solid, or >50% solid are grades 1, 2, and 3, respectively. Solid growth is assessed only on the glandular (not squamous) component. If >50% of the tumor has high-grade nuclear features the grade is increased from 1 to 2 or 2 to 3.
Winham et al., however, found that even tiny foci (>a 20× field) of marked nuclear atypia warrant upgrading architecturally grade 1 tumors to grade 2 based on their high risk of myoinvasion. Similarly, Takeshima et al. found that architectural grade 1 or 2 tumors with >25% grade 3 nuclei had a similar behavior to those with >50% grade 3 nuclei.
The three-tier FIGO grading scheme stratifies 5-year survival for stage I tumors (92%, 88%, and 75%), combined stage I and II tumors (89%, 84%, 63%), and stage III tumors (70%, 63%, and 40%) (Gayar et al., Zaino).
Grade 3 EECs have 5-year survival rates similar to that of serous or clear cell carcinomas in some studies (Ayeni et al., ).
Proposed binary grading schemes for EECs:
Taylor et al. proposed a two-tier variation of the FIGO system in which low-grade and high-grade tumors have, respectively, ≤20% or >20% nonsquamous solid areas. This system was found less cumbersome, had less interobserver variation, and had the same or better prognostic significance as the three-tier system.
adapted the Nottingham system for breast carcinoma into a binary scheme: high-grade tumors had at least two of: predominantly papillary or solid growth, >5 mf/10 hpf, and severe nuclear atypia. They found this system was prognostically significant and highly reproducible, whereas Nastic et al. found that it conferred no significant advantage to FIGO grading.
Lax, Kurman et al. used three features (>50% solid growth without distinction of nonsquamous vs squamous; a diffusely infiltrative myoinvasive pattern; tumor cell necrosis) defined low-grade tumors as having only one feature and high-grade tumors as having two or three features (non-myoinvasive tumors with >50% solid growth and tumor cell necrosis were considered high-grade). 1988 FIGO stage IA or IB low-grade tumors had 100% 5-year survival; stage IC, III, and IV low-grade tumors and stage IB and IC high-grade tumors had a 67–76% survival; and high-stage high-grade tumors had a 26% survival.
Scholten et al. subsequently simplified the grading scheme of Lax et al. by grading solely on the proportion of solid growth: low-grade tumors had ≤50% solid growth and high-grade tumors had >50% solid growth. This change resulted in superior prognostic power and greater reproducibility.
Conlon et al. argued for a two-tier system in which G1 and G2 EECs are considered low-grade and those with >50% solid growth or >50% of cells with high-grade nuclei are considered high-grade.
Cervical involvement occurs in ~20% of EECs and is usually due to direct surface or stromal extension but occasionally a result of implantation or lymphatic spread.
Significant interobserver variation exists in its assessment, including the determination of the isthmic-endocervical junction and the distinction of cervical glandular involvement from superficial stromal invasion.
In the 1988 FIGO staging system ( Table 8.2 ), tumors confined to the endocervical mucosa were stage IIA and those invading the endocervical stroma were stage IIB. However, in the most recent FIGO staging system, only the latter tumors (those with cervical stromal invasion) are stage II, and the substaging designation has been removed.
|
a From Int Gynecol Obstet 2009;105;103–104.
b Endocervical glandular involvement only is stage I.
c Positive cytology is reported separately without changing the stage.
Histologic diagnosis of cervical mucosal involvement in an ECC specimen can be complicated by detached endometrial tumor fragments admixed with normal endocervical tissue. A diagnosis of ‘detached fragments of adenocarcinoma, definite cervical involvement by tumor is not identified’ is appropriate in such cases.
Occasional EECs deeply invade the endocervical stroma such that the volume and depth of the endocervical tumor can exceed that in the myometrium, findings that can lead to a misdiagnosis of an independent primary endocervical adenocarcinoma, especially if superficial invasive glands are mistaken for endocervical AIS.
In such cases, the cervical component can be highly differentiated with a diffusely infiltrating or adenoma malignum invasive pattern. In some such cases, small tubular glands lined by cuboidal cells with bland nuclear features and an eosinophilic luminal secretion can mimic mesonephric hyperplasia (Tambouret et al.; Chapter 4 ).
Awareness of these findings, demonstration of continuity between the tumor in the cervix and corpus, and an endometrioid immunoprofile of the tumor in each site facilitate the correct interpretation.
The differential diagnosis of tumor spread to the endocervical mucosa includes reactive atypia of the endocervical epithelium ( Chapter 4 ), which can include cellular stratification and a micropapillary pattern. The differential of stage II disease also includes synchronous endometrial and endocervical endometrioid carcinomas ( Chapter 6 ); such cases are very rare in our experience.
Molecular alterations in EECs, which differ from those of non-EECs, can be grouped as follows:
MSI-hypermutated group characterized by MSI due to deficient DNA mismatch repair enzyme resulting in high mutation rates, few copy number alterations, and recurrent mutations in RPI22 , KRAS , and PTEN (with loss of PTEN staining). Lee et al. found that MSI EECs exhibited twice as many mutations as EECs without MSI, the most commonly mutated genes being PTEN , PIC3CA , ATM , and FLT3 .
Copy-number low subgroup characterized by microsatellite-stable tumors with low mutation rates, few copy number alterations, and frequent CTNNB1 mutations (resulting in β-catenin nuclear expression).
Copy-number high subgroup.
The POLE (ultramutated) subgroup (see Carcinomas with Ambiguous Differentiation ).
Mutations in the tumor suppressor gene ARID1A play an important role in the carcinogenesis of EECs via MSI.
Guan et al. found that unlike nonendometrioid carcinomas, 26% and 40% of low-grade EECs showed loss of ARID1A expression and harbored ARID1A mutations, respectively.
Mao et al. found complete loss of ARID1A staining in 25% of low-grade and 40% of high-grade EECs respectively. Allo et al. found ARID1A (BAF250a) loss in 46% of high-grade EECs (vs 9% in serous carcinomas) and that it was associated with MMR protein loss and normal p53 expression.
Nelson et al. found that 45% of high-grade EECs were MMR-deficient, a finding associated with loss of ARID1A , loss of PTEN , and wild-type TP53 expression.
found a strong association between ARID1A loss and MSI in sporadic but not Lynch-associated EECs and concluded that ARID1A is the causative gene of MSI via epigenic silencing of the MLH1 gene.
found that high levels of MSI and loss of ARID1A or PTEN expression were more common in EECs than in their ovarian counterparts, implying different tumorigenetic pathways.
Suemori et al. found that the number of TILs, which were increased by MSI and decreased by COX-2 expression, was associated with a poorer prognosis.
found KRAS mutations in 67% of EECs with prominent mucinous differentiation compared to only 25% of typical EECs (see Mucinous Carcinoma ). Hong et al. found KRAS mutations in 60% of microglandular EECs.
Kurnit et al. found that although CTNNB1 (β-catenin) mutations or TP53 mutations in EECs were associated with low-grade tumors and lower rates of deep myoinvasion and LVI, these mutations were associated with a high risk of recurrence.
found that EECs arising from atrophic endometria show reduced expression of E-cadherin and fewer KRAS mutations than EECs arising from hyperplastic endometria.
found that L1CAM expression of >50% in high-risk (high-grade, deep myoinvasion) EECs and other endometrial carcinomas was associated with mutant p53 and a high risk for distant metastases. Similarly, Dellinger et al. found that L1CAM expression was an independent predictor of poor survival in endometrial cancer and associated with high stage.
Lynch-related findings are considered under a separate heading below.
Occasional EECs (particularly those arising in polyps) are entirely removed by curettage without evidence of residual tumor in a subsequent hysterectomy specimen.
Progestational treatment is often used, particularly in younger patients with well-differentiated tumors.
Architectural abnormalities generally persist but the low-power picture often differs due to the presence of more copious eosinophilic cytoplasm than present in most untreated carcinomas.
Follow-up in a study by Wheeler et al. revealed that 58% of patients had persistent disease, with tumor resolution in the remainder. Cytologic atypia and/or a crowded or confluent gland pattern in samples at 7–9 months after initiation of treatment predicted treatment failure warranting hysterectomy. Similar findings were also reported by Mentrikoski et al.
Gunderson et al. studied 46 patients (37% with CAH, 63% with EEC) treated with progestins (median duration, 6 months); 65% had a complete response (CR), 28% had persistent or progressive tumor, and 23% had a CR followed by a recurrence. Only ‘decreased gland cellularity’ was significantly associated with a CR.
A GOG study ( ) found that while only one of 59 patients had a complete response, 63% were partial responders. Tumors in the latter group, unlike those of nonresponders, had pale eosinophilic cytoplasm and luminal secretion. Response correlated with lower pretreatment Ki-67 levels but not pretreatment ER and PR positivity.
Radiation-induced changes are variable, probably due to different responses between tumors, radiation dosage levels, and interval between treatment and hysterectomy.
The changes may include absence of tumor; necrotic tumor; an increased cytoplasmic volume and vacuolation; bizarre, pyknotic, or karyorrhectic nuclei; and enlarged nucleoli.
The tumor may be more differentiated and squamous elements absent in the pretreatment specimen may be prominent.
Artifactual changes related to robotic-assisted hysterectomy. Several studies have described the artifactual presence of tumor within tissue spaces, myometrial vessels (vascular pseudoinvasion), and tubal lumina secondary to laparoscopic or robotically assisted hysterectomy for endometrial carcinoma.
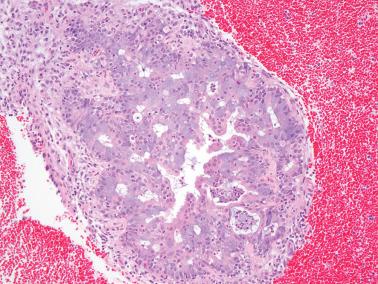
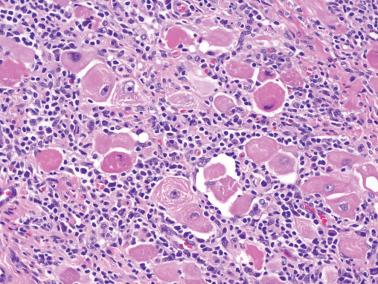
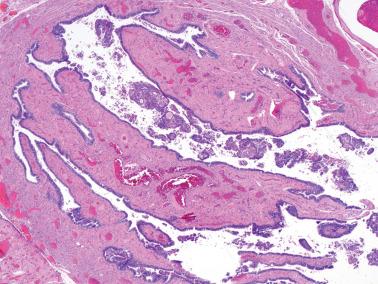
20–25% of endometrial carcinomas show mismatch repair deficiency, but only a small subset of them are related to HNPCC/Lynch syndrome (hereafter referred to as LS) (Segura et al.).
Women with the LS account for 2–6% of endometrial cancers for which they have a 40–70% lifetime risk; it is often the sentinel cancer.
LS is associated with mutations in mismatch repair (MMR) genes ( MLH1 , MLH2 , MSH6 , PMS2 ) resulting in a high level of MSI and DNA MMR protein defects.
Downes et al., in a literature review of LS-related endometrial carcinomas, found that 56% of the mutations involved MSH2 , 26% involved MLH1, 7% MSH6 , and 3.5% PMS2 .
Up to 50% of patients with MMR-deficient endometrial carcinomas lack a germline mutation (Lynch-like cancers) or are associated with sporadic MLH1 promoter hypermethylation ( MLH1 hm) (Mas-Moya et al.; ; Sloan, Ring et al.) (see below). As isolated loss of PMS2 expression can be due to MLH1 hm, analysis for the latter is required before further PMS2 genetic testing (Dudley et al., Kato et al.).
Segura et al. found that approximately two-thirds of MLH1 hm EECs are grade 1 or 2 (the rest grade 3) and occurred in women older than those with LS-related EECs; ~6% had false-positive MLH1 immunoreactivity. Rare patients had a synchronous ovarian carcinoma; 22% were AWD or DOD at last follow-up.
found discrete subclonal loss of MMR expression in 7.2% of endometrial carcinomas (all EECs) without evidence of a germline mutation and likely related to methylation and somatic events.
Al-Nourhji et al. found PD-L1, which suppresses T-cell activation, in most MMR-deficient endometrial carcinomas, including all LS-related cases and 55% of MLH1hm cases. PD-L1 was found in 100% of CCCs, 49% of EECs, and 33% of serous carcinomas.
Similarly, Sloan, Ring et al. found that PDL1 expression was more common in LS-associated endometrial carcinomas (histotypes not specified) than in MLH1hm and MMR-intact tumors and that MMR deficiency may be a better predictor of response to PD-1/PD-L1 inhibitor therapy than tumor grade.
In another study, Sloan, Moskaluk et al. compared the features of sporadic MLH1 hm endometrial carcinomas with LS/LS-like endometrial carcinomas. The former occurred at a mean of ~8 years later than the latter and were more likely to be EEC, exhibit mucinous differentiation, and have TILs.
Most LS-related endometrial carcinomas occur in the 45–55 years age group (a decade younger than those with sporadic endometrial carcinoma); some are even younger.
Presumptive LS has been identified in 18% of women <50 years of age presenting with an endometrial carcinoma and in another study in 16% of women with endometrial carcinoma <40 years of age. Chu et al. found that a third of all endometrial carcinomas in those ≤45 years of age had loss of expression of at least one MMR protein, most commonly MSH6. In an unselected population of endometrial cancer patients, Ring et al. found that 5.8% of women had deleterious mutations in LS genes.
EEC is found in ~8% of prophylactic hysterectomy specimens in LS patients; atypical hyperplasia has been found in 10–25% of cases.
In the absence of a visible mass in prophylactic hysterectomy specimens, Downes et al. submit the endometrium and the lower uterine segment in toto , a block from each cervical quadrant, and the tubes and ovaries as per the SEE-FIM protocol ( Chapter 11 ).
Most studies indicate that the EEC:non-EEC ratio of LS/LS-like endometrial carcinomas is similar to that of sporadic tumors, but the EECs may have more aggressive features that include higher grade, deep myoinvasion, more frequent LVI and nodal metastases, a component of UC (see corresponding heading), higher stage, and a worse outcome.
Other studies have found that a higher proportion of LS-related carcinomas are serous, clear cell, have ambiguous or overlapping serous-clear cell or endometrioid-clear cell features, or are rarely MMMT.
Other more common features include LUS origin, peritumoral and tumor-infiltrating lymphocytes (defined by as ≥40/10 hpfs), diminished ER/PR expression, and synchronous ovarian carcinoma (usually endometrioid or clear cell carcinoma).
Ruiz et al. found no association between deficient MMR expression and prognosis in EECs. Chu et al., however, found that defective MLH1 and MSH2 expressions were associated with, respectively, a higher incidence of nodal involvement and a higher frequency of LVI; there were no differences between MSI-stable and MSI-high tumors.
found that clinical and pathologic features failed to detect 41% of carcinomas with MMR protein defects, indicating a need for reflex screening of all newly diagnosed endometrial carcinomas (and MMMTs) for MLH1, MLH2, MSH6, PMS2. Loss of MLH1 and PMS2 expression can be due to heterogenous methylation of the MLH1 promoter requiring hypermethylation analysis of MLH1 promotor locus for dual MLH1/PMS2-negative tumors (Najdawai et al., Pai et al.).
Kobayashi et al. found loss of at least one MMR protein in 53% of EECs and in 31% of synchronous ovarian endometrioid carcinomas, but in only 12.5% of cases was the MMR protein loss of the same type. They concluded that most synchronous endometrial and ovarian cancers are sporadic and not caused by germ-line mutations.
Complex atypical hyperplasia (see corresponding heading).
Atypical polypoid adenomyoma ( Chapter 9 ).
Microglandular hyperplasia vs EECs with microglandular patterns (see latter heading).
Serous carcinoma vs typical EEC, villoglandular EEC, and EEC with small nonvillous papillae (see corresponding headings).
Clear cell carcinoma vs EECs with clear cells) (see Clear Cell Carcinoma ).
Undifferentiated carcinoma (pure or admixed with an EEC vs grade 3 EEC) (see Undifferentiated Carcinoma).
MMMTs vs EEC with spindle cells. A scanty sample containing a high-grade adenocarcinoma with endometrioid or mixed endometrioid–serous features, particularly in an elderly patient, should prompt thorough sampling of the resected uterus to exclude a sarcomatous component and thus a diagnosis of at least focal MMMT.
Endocervical adenocarcinoma.
The rarity of endocervical endometrioid carcinomas and the different histology of usual endocervical adenocarcinomas ( Chapter 6 ) and EECs are helpful. In architecturally well-differentiated tumors, usual endocervical adenocarcinomas typically have more nuclear pleomorphism, mitotic activity, and apoptotic nuclear debris than do EECs.
The distribution of tumor in a fractional D&C specimen and the presence of atypical endometrial hyperplasia or endocervical AIS, can be helpful. Morular metaplasia within the tumor and stromal foam cells favor an endometrial primary.
Usual endocervical adenocarcinomas, unlike EECs, are p16+/CEA+/vimentin −/ ER − (some have weak ER staining); the usual strong/diffuse p16 staining denotes the presence of HRHPV. These differing immunoprofiles may also aid classifying tumors in the lower uterine segment/upper endocervix. A caveat to the foregoing is the absence of both HRHPV and p16 staining in many of the unusual subtypes of cervical adenocarcinomas ( Chapter 6 ).
Jiang et al. found that tumors involving the endocervix and endometrium may be separate primaries, especially if they differ histologically and/or immunohistochemically.
Metastatic adenocarcinomas ( Chapter 10 ).
found that 54% of EECs had a morphologically discordant recurrence that included clear cell features, diminished PR expression, and loss of expression of MLH1 and/or PMS2. Sampling of recurrent tumor may be helpful if hormonal therapy is planned.
EECs that recur only in the vagina tend to be low-grade, only superficially myoinvasive, and without the high-risk factors (deep myoinvasion, LVI, cervical involvement) associated with extravaginal recurrences (Roma et al.).
Stage is the most important prognostic parameter (see Table 8.2 , FIGO staging system).
The 1988 FIGO substages are IA (no myoinvasion), IB (inner half invasion), and IC (outer half invasion). The 2008 FIGO substages ( Table 8.2 ) are IA (no invasion or inner half invasion) and IB (outer half invasion). Abu-Rustum et al. found that 1988 FIGO stages IA, IB, and IC identify three subgroups with different survivals, whereas Wen et al. found prognostic differences between 2008 stages IA and IB but not between 1988 stages IA and IB. found that myoinvasion of >4 mm correlated better with recurrence and survival than a depth of >50%.
Barlin et al. proposed substages within stage I tumors that are prognostically superior to 2008 FIGO. In this proposal, low-grade tumors are grade 1 and 2 EECs and high-grade tumors are grade 3 EECs, serous and clear cell carcinomas, and carcinosarcomas.
IA: low-grade tumors with <50% myoinvasion; IB: high-grade tumors with no myoinvasion; IC low-grade tumors with ≥50% myoinvasion; ID: high-grade tumors with any myoinvasion. Each substage was further divided into those with negative nodes (e.g. IA1) and those with no nodes removed (e.g. IA2).
The 5-year survival differences were statistically significant: IA1 96.7%; IA2 92.2%; IB1 92.2%; IB2 76.4%; IC1 83.9%; IC2 78.6%; ID1 81.1%; and ID2 68.8%.
Cervical stromal invasion (stage II). Cervical stromal invasion has been an adverse prognostic factor in some studies (Euscher et al.), whereas cervical mucosal involvement has not. Contrarily, found no difference in survival between patients with 1988 FIGO stage IIA (cervical mucosal involvement) tumor or IIB (cervical stromal involvement) tumors and those with stage I tumors. The worse prognosis of stage II tumors in some studies may be due to the more frequent presence of other poor prognostic features.
Adnexal involvement (stage IIIA). This finding is associated with deceased survival, but it is often associated with other adverse prognostic factors. Connell et al. found a 5-year DFS of 37% for those with adnexal spread but 71% if no other extrauterine sites were involved. The important distinction between metastatic EEC to the ovary and an independent primary ovarian EC is considered in Chapter 14 .
Uterine serosal involvement (stage IIIA). Ashman et al. found that this finding was associated with a high rate of relapse and a 30% 5-year survival.
Vaginal involvement (stage IIIB). Although vaginal recurrences are common, stage IIIB disease at presentation is rare and in most cases is likely due to lymphatic rather than direct spread. The 5-year survival rate is ~25%.
Lymph node involvement (LNI) (stage IIIC):
Chi et al. found that 9% of patients with EEC undergoing lymphadenectomy had LNI. No grade 1 tumors had LNI regardless of depth of myoinvasion, whereas the frequency of LNI in stage IA, IB, and IC grade 2 tumors was 4%, 10%, and 17% and for grade 3 tumors was 0, 7%, and 28%.
Cox Buer et al. found that tumor diameter of >5 cm and myometrial invasion of >33% correlated with LNI.
Some studies have found that nodal micrometastases identified with CK staining were an adverse prognostic factor whereas others have not (Amezcua et al., Gonzalez Bosquet et al., McCoy et al., Todo et al.). Similarly, Plante et al. found that isolated tumor cells (ITCs) in LNs had no adverse prognostic significance. found that LNI by ITCs did not affect prognosis in grade 1–2 EECs but was prognostically adverse in grade 3 tumors.
An increasing number of positive nodes, an increased ratio of positive nodes to total number of nodes, desmoplasia within the positive lymph nodes, and extension into perinodal fat have been adverse prognostic features in some studies.
Patients with pelvic LNI have a 70–80% 5-year survival whereas those with para-aortic LNI have a 30–40% survival.
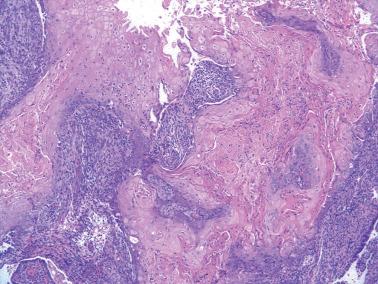
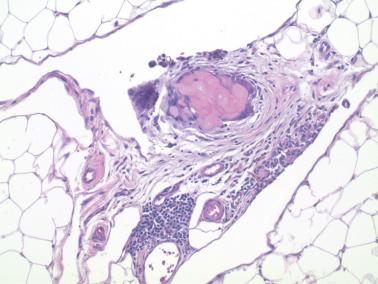
As noted earlier, the presence of peritoneal keratin granulomas does not alter stage ( Fig. 8.68 ).
Grade . See section on grading (Microscopic Features).
Lymphovascular invasion (LVI) .
This usually refers to lymphatic invasion (LI) and/or blood vessel invasion (BVI), which are difficult to distinguish on routinely stained slides. The differential diagnosis of LVI includes vascular pseudoinvasion, which is more common in laparoscopic hysterectomy specimens ( Chapter 7 ).
Nofech-Mozes, Ackerman et al. found LVI in 22% of EECs; it was the only independent predictor for distant recurrence. Narayan et al. found the LVI was associated with a five-fold increased risk of recurrence, which increased to 8.8 when associated with nodal spread. Simpkins et al. found a 23% recurrence rate in stage IB–IIA tumors (1988 FIGO stages) with LVI; adjuvant radiation did not improve overall survival.
In a study of stage I and II EECs with one or more of: grade 2 or 3 histology, LVI, and outer-half myoinvasion, Weinberg et al. found that LVI was the only significant independent risk factor for total and distant recurrences and that it was a significant risk factor for PFS, OS, and DFS.
Han, Lim et al. found that LVI was the only factor that was significantly associated with nodal metastases on multivariate analysis.
Using the National Cancer Database, Jorge et al. found the LVI was independently associated with nodal spread in stage I EECs and independently predicted survival even after adjusting for the presence of nodal spread.
Weber et al. found that D2-40 immunostaining increased the detection of LVI from ~18% to ~30%. LVI was found in 81% of carcinomas with LNI, significantly predicted LNI, and was an independent prognostic factor.
Two studies distinguished between lymphatic invasion (LI) and blood vessel invasion (BVI). Alexander-Sefre et al. found that only LI was prognostic: the 5-year recurrence-free survival rates were 53% and 93% for those with and without LI. Mannelqvist et al. found decreasing survival among these groups: no BVI or LI; LI only; and BVI (with or without LI).
Pattern of myoinvasion .
MELF. Kojiro-Sanada et al. and Kihara et al. found that MELF and non-MELF tumors had similar survivals, Kihara et al. suggesting that MELF cells undergo growth arrest or become senescent. Sanci et al., however, found that MELF was significantly associated with decreased OS.
Quick, May et al. found that grade 1 tumors with an infiltrative pattern of invasion (vs a pushing or broad front pattern) were more commonly associated with stage IB or II disease, LVI, and recurrence.
Similarly, Winham et al. found that grade 2 tumors with infiltrative invasion were associated with LVI, cervical stromal invasion, lymph node involvement, and recurrence.
Euscher et al. found that single-cell/cell-cluster myoinvasion was a significant predictor of nodal metastasis or extrauterine disease in low-grade EEC.
Positive peritoneal cytology (PPC) . Whereas found PPC to be associated with a significant reduction in survival in endometrioid, serous, and clear cell carcinomas, Han, Lim et al. found it was only a risk factor for recurrence (regardless of stage) in nonendometrioid carcinomas. Milgrom et al. found that PPC in stage III carcinomas correlated with higher recurrence rates in para-aortic nodes and peritoneum and increased hazard for relapse and death. Immunostaining with epithelial markers can improve the detection rate of malignant cells (Benevolo et al.).
Other histologic prognostic factors .
Mucinous differentiation. Abdulfatah et al. found that mucinous differentiation (≥10%) in low-grade EECs was a favorable prognostic finding despite the presence of potentially poor prognostic factors including old age, papillarity, and MELF pattern.
Lower uterine segment involvement. This finding tends to be associated with LVI, deep myometrial invasion, grade 2 histology, and decreased DFS.
Desmoplastic stromal response. Wei et al. found that this was an adverse prognostic factor on multivariate analysis.
Co-existent endometrial hyperplasia. This is a favorable prognostic factor due to the lower grade of hyperplasia-related EECs.
Distance of tumor to serosa. Lindauer et al. found that a tumor-free distance (from the deepest tumor to the serosa) of <1 cm is an adverse prognostic factor.
Aneuploidy . Several studies have found that this feature identifies high-risk tumors among what would otherwise be considered low-risk tumors based on stage and grade.
Vaginal vs nonvaginal recurrences . Krystel-Whittemore and Oliva found that vaginal (vs nonvaginal) recurrences were more common in EECs with nondestructive myoinvasion, suggesting that the former are less aggressive and may have a different molecular landscape than EECs with destructive myoinvasion.
Mutations.
p53 . found that grade 3 EECs with a p53 mutational pattern have a worse prognosis, similar to that of serous carcinomas, compared to p53 wild-type grade 3 EECs. Alvarez et al. found that EECs that overexpressed p53 were often high stage. Kurnit et al. found p53 mutations in 13% of low-grade EECs, a finding associated with a high risk of recurrence.
PIK3CA . Using whole exome sequencing, Matrai et al. found that recurrence of low-stage low-grade EECs was associated with broad copy number gain in multiple genes and higher rates of PIK3CA mutations. McIntyre et al. found that PIK3CA missense mutation is associated with unfavorable outcome in grade 3 EEC but not in serous carcinoma.
CTNNB1 ( β-catenin ) . Kurnit et al. found that even though CTNNB1 (β-catenin) mutant EECs were more likely to be grade 1 or 2 and have lower rates of deep myoinvasion and LVI, they were associated with an increased risk of recurrence.
PTEN-PI3K . found that pathologic scoring of PTEN immunoreactivity in EECs (and nonendometrioid carcinomas) may predict response to targeted therapy.
were able to group grade 3 EECs into the following molecular subtypes: ~36% MMR deficient, ~30% no specific molecular profile, ~20% p53 mutational, and ~13% POLE. POLE and MMR were independent prognostic factors for better prognosis and p53 status for worse recurrence-free survival.
Proliferation markers . found that high mitotic index and MIB1 index were significant independent adverse prognostic factors in low-grade EECs; both correlated strongly with p53 expression.
Hormonal markers .
found that PR expression in high-grade EECs was associated with favorable OS. Similarly, Huvila et al. found that lack of PR expression is a strong risk factor for tumor recurrence in stage I–II EECs.
found that an ERα/ERβ1 or ERα/ERβ2 ratio of ≤1 is an adverse prognostic factor. Backes et al. found the ERα− EECs were more often high grade and high stage.
Mahdi et al. found that AR expression significantly correlated with absence of LVI, decreased LNI, increased DFS, and late disease recurrence.
Microsatellite instability . This is a poor prognostic factor and is associated with other poor prognostic factors (high grade, deep myoinvasion, LVI, high stage). Crumley et al. found that positive PD-L1 expression in microsatellite stable grade 2 EECs was associated with LVI and/or myoinvasion, advanced stage, and loss of PTEN expression.
Other markers .
L1 cell adhesion molecule (L1CAM) and Her2. Dellinger et al. found that high expression of L1CAM was a significant predictor of advanced stage, high grade, and nodal metastases. Azim et al. found that both L1CAM and Her-2 expression (also see next point) correlated with recurrence, especially when both markers were present.
Alvarez et al. found that high-grade EECs showed strong diffuse expression of p16, cyclin D1, and Her-2 in 30%, 36%, and 12% respectively. Tumors that overexpressed p16 or Her2 were frequently high stage, whereas tumors with cyclin D1 overexpression or the rare tumors with loss of MLH1/MSH2 were often stage I.
Li et al. found immunohistochemical expression of programmed death 1-ligand (PD-L1) in a minority of endometrial carcinomas (most of their cases were EECs); the finding was strongly associated with MMR protein loss, LVI, and LN metastases.
Steinbakk et al. found that low p21 and high survivin expression are poor prognostic factors in stage I tumors, especially if associated with high MSI. Immunoreactivity for bcl-2 and cyclin A are also potential adverse prognostic factors.
Ervine et al. found TTF-1 immunoreactivity in 2% of low-grade and 11% of grade 3 EECs and that this finding was an adverse prognostic factor in both groups.
Miyamoto et al. found that immunohistochemical expression of core 2 β1,6-N-acetylglucosaminyl transferase 1 (C2GnT1) was an adverse prognostic factor on multivariate analysis.
Serous carcinomas account for ~10% of endometrial carcinomas; some studies report a recent increase in frequency. Patients are on average a decade older than those with EEC; tumors associated with predisposing factors (see below) may occur in younger women.
Estrogen use and obesity are less common than in women with EEC. A history of long-term tamoxifen treatment or pelvic radiation is occasionally present; Pothuri et al found that 40% of radiation-associated endometrial carcinomas were serous.
There is an increased frequency of breast carcinoma and BRCA1 germline mutations in patients with serous carcinomas, especially in those ≤55 years of age. Serous carcinomas in younger women may be related to HNPCC/Lynch syndrome.
The usual symptom is postmenopausal bleeding, or occasionally a serosanguineous vaginal discharge. Patients with high-stage disease may present with abdominal symptoms including ascites. An elevated serum CA125 level and malignant cells in Pap smears at presentation may be found. Unusual presentations include paraneoplastic hypercalcemia, paraneoplastic cerebellar degeneration, and axillary or cervical node metastases.
Some patients are asymptomatic or have no symptoms referable to the carcinoma, such as symptoms related to an endometrial polyp, the serous carcinoma being found on microscopic examination of the polyp.
A synchronous serous carcinoma may be found in the fallopian tubes, ovaries, or peritoneum. Clonality studies (although rarely done in practice) may determine if these are multifocal primaries or one primary tumor with metastases. However, the primary site is usually apparent based on the distribution of tumor and the pattern of involvement at each site.
Extrauterine spread at presentation is more common than in the other major subtypes of endometrial carcinoma, with surgical stage of ≥III in up to 60% of patients, with frequent involvement of the adnexa, pelvic and para-aortic lymph nodes, and peritoneum, including upper abdomen. Nonmyoinvasive (even microscopic) tumors can spread beyond the uterus via the fallopian tubes.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here