Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Various non-neoplastic endometrial lesions, as well as normal and artifactual findings, can be problematic for the pathologist, especially their distinction from precancerous endometrial lesions and carcinomas.
Table 7.1 groups the findings according to the subtype of endometrial carcinoma most likely to be in the differential diagnosis.
| Benign entity | Carcinoma potentially mimicked |
|---|---|
|
|
|
Endometrioid |
|
Endometrioid |
|
Endometrioid |
|
Endometrioid |
|
Endometrioid |
|
Endometrioid |
|
Endometrioid |
|
Endometrioid or squamous cell |
|
Endometrioid |
|
Endometrioid |
|
|
|
Villoglandular endometrioid or serous |
|
Mucinous |
|
Mucinous |
|
Serous |
|
Serous or clear cell |
|
Serous or clear cell |
|
Clear cell |
|
Small cell |
|
Undifferentiated |
|
Undifferentiated |
|
Undifferentiated or signet-ring cell |
|
Undifferentiated |
|
Undifferentiated |
|
Squamous cell carcinoma or undifferentiated |
|
Undifferentiated or giant cell |
a Telescoping is more likely to be confused with hyperplasia than carcinoma.
b See Chapter 9 .
c Similar changes may be found within fragments of endocervical polyps procured during an endometrial sampling. Cervical microglandular hyperplasia or even crowded aggregates of endocervical glands with or without squamous metaplasia can also be associated with diagnostic problems if the fragments are not recognized as endocervical in origin.
d See Table 7.2
e See Chapter 10 .
The fragmentation and compaction of endometrial glands and surface epithelium associated with menses, often accentuated by curettage, may result in an appearance that can suggest hyperplasia or even neoplasia.
Compact, densely cellular nests of degenerating endometrial stromal cells with scanty cytoplasm and small, sometimes spindled, hyperchromatic nuclei are characteristic of menstrual endometrium, and have been mistaken for small cell carcinoma. Other typical findings include neutrophils, nuclear debris, syncytial papillary change (see corresponding heading below), and fibrin thrombi.
Features distinguishing menstrual changes from abnormalities include the fragmented and degenerative appearance of the epithelium and stroma, common residual secretory changes, and the absence of nuclear atypicality and mitotic activity other than that acceptable for a reactive process.
Menstrual endometrium can rarely be found within myometrial vessels, potentially mimicking intravascular carcinoma.
Gilmore et al. found that rare plasma cells are common in anovulatory/disordered proliferative endometria exhibiting menstrual changes, and accordingly this finding alone should not lead to a diagnosis of chronic endometritis (see Chronic Endometritis ).
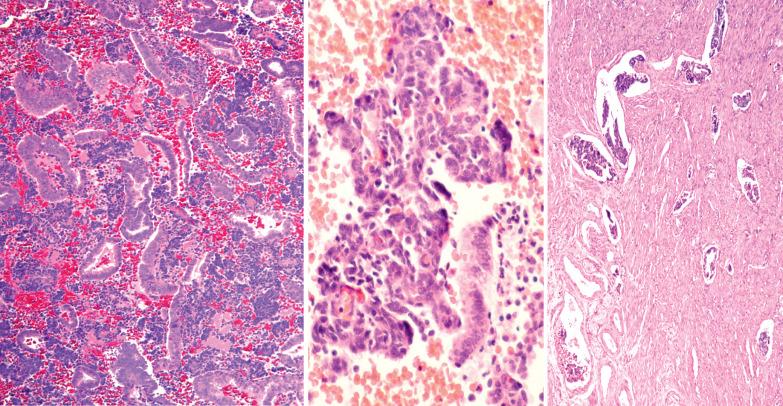
Atrophic endometria often have a cellular stroma with sparse or absent glands. This finding in biopsy or curettage fragments can suggest an endometrial stromal neoplasm or a small cell carcinoma. The scant tissue, atrophic appearance, and mitotic inactivity facilitate the diagnosis.
The surface epithelium in an atrophic endometrium may show nuclear atypia and/or enlargement that may cause concern for intraepithelial serous carcinoma but in the former severe nuclear atypia and mitotic activity are absent. There is often eosinophilic cytoplasm, a subtle distinction from most cases of minimal volume (intraepithelial) serous carcinoma. Furthermore, atypia in the setting of atrophy would demonstrate a wild-type p53 staining pattern and low proliferative index by Ki-67, whereas serous carcinoma would show an abnormal p53 pattern (diffuse overexpression or complete loss) with increased proliferation.
A sampling from an atrophic endometrium often yields only scanty strips of endometrial surface epithelium. Sakhdari et al. found that samples comprised of ≥10 strips had an almost 100% negative predictive value for an associated carcinoma, whereas a sample of <10 strips was associated with a 19% risk of unsampled malignancy.
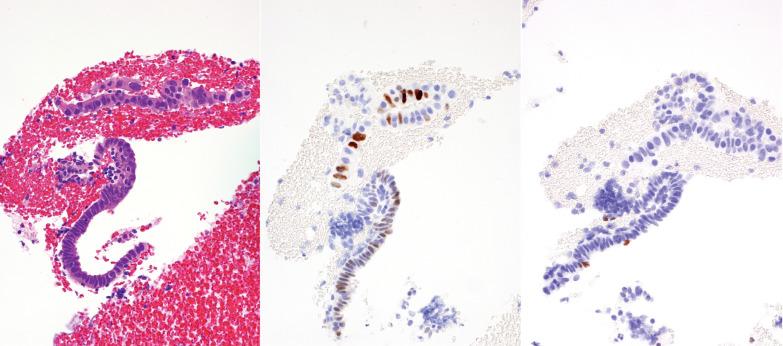
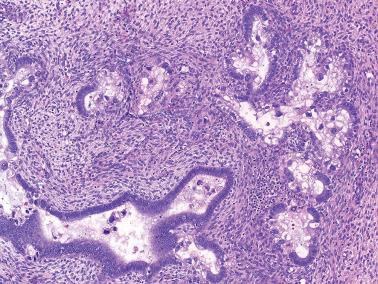
Curettage can result in artifactual glandular crowding, fragmentation, and telescoping (glands within glands). This appearance can be misinterpreted as a complex hyperplasia or carcinoma, especially if the glands are proliferative with mitotic activity.
Strips of endometrial surface epithelium can become coiled and compacted, producing a pseudopapillary pattern. This finding is often associated with an atrophic endometrium, but its appearance can be misconstrued as papillary hyperplasia or carcinoma.
Postcurettage epithelial atypia, which may be striking, is typically confined to the surface epithelium and superficial glands. The reactive cells may have enlarged hyperchromatic nuclei with occasionally prominent nucleoli and sometimes a hobnail appearance ( Table 7.2 ).
| Hobnail cell metaplasia (idiopathic) |
| isolated finding |
| within polyps and/or papillary proliferations |
| Arias-Stella reaction |
| Secondary to: |
| recent curettage |
| ischemia (within an infarcted polyp or adjacent to a thrombosed sinusoid) |
| chronic endometritis |
| intrauterine contraceptive device |
| radiation |
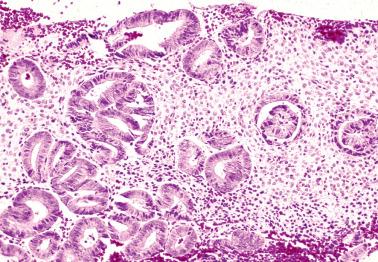
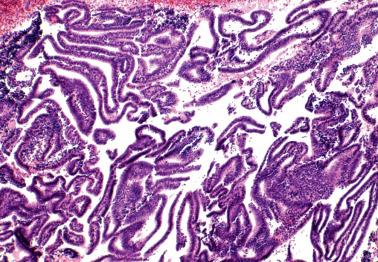
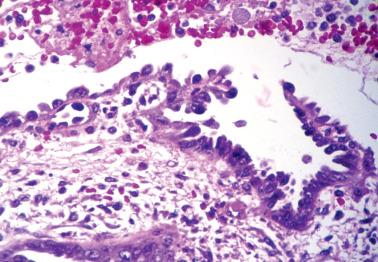
These findings may be misinterpreted as endometrioid adenocarcinoma with surface metaplastic changes or an unusual adenocarcinoma subtype ( Chapter 8 ).
The extent varies from a microscopic focus to most of the tissue. Glandular and surface epithelia, including those of polyps (especially those with papillary proliferations, see corresponding heading), may be involved. Two or more types of metaplasia commonly coexist.
As metaplasias often reflect unopposed estrogen stimulation, metaplastic glands may be synchronously hyperplastic or associated with a synchronous typical endometrial hyperplasia or adenocarcinoma. Other etiologic factors are considered under the specific types of metaplasia (including syncytial papillary change) in the following sections.
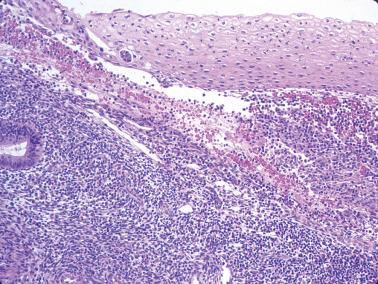
This common finding (SPC), previously referred to as papillary syncytial metaplasia, appears to be a reparative rather than metaplastic change and thus the former term is preferable. It is typically associated with postovulatory or anovulatory menstrual bleeding but may occur within or on the surface of an infarcted polyp. It may also overlie a carcinoma. The appearance varies with its extent, the degree of its syncytial and papillary features, and the prominence of the associated stromal breakdown.
The endometrial surface epithelium and less commonly the superficial glands are involved. Cells with eosinophilic cytoplasm and indistinct cell borders (sometimes with a squamoid appearance) are arranged in sheet-like syncytial aggregates, buds, and papillae lacking stromal cores.
The cells usually have bland nuclear features but occasionally show reactive atypia, a hobnail appearance, and rare mitoses.
Other menses-related changes (see separate heading) are often present, including neutrophils, nuclear debris, small nests of degenerating endometrial stromal cells, and thrombosed sinusoids.
The papillarity, occasional cytologic atypia, mitoses, and p16 positivity may suggest a papillary carcinoma, especially serous carcinoma.
This differential is complicated by endometrioid carcinomas with an SPC-like surface component that merges with the underlying, usually low grade, carcinoma ( Chapter 8 ). Scanty specimens with SPC-like changes may warrant additional sampling if there is a clinical suspicion for carcinoma.
Features favoring or indicating SPC vs carcinoma include its usual confinement to the endometrial surface, generally bland nuclear features, low MIB1 index, and associated menstrual-type changes.
Serous carcinomas (which may be exclusively on the surface), in contrast to SPC with reactive atypia, usually have diffusely higher-grade nuclear features, a higher mitotic rate, a higher Ki-67 index, mutational p53 staining (vs wild-type staining in SPC), and HMGA2 reactivity.
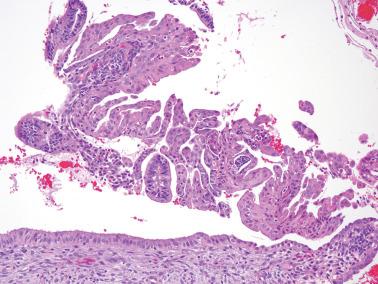
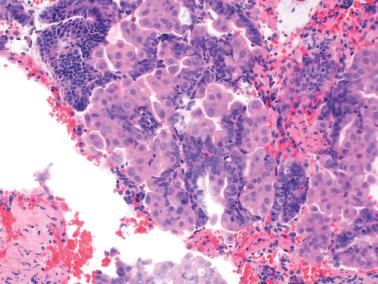
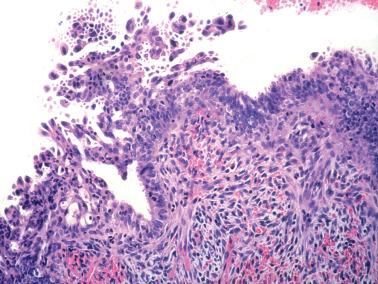
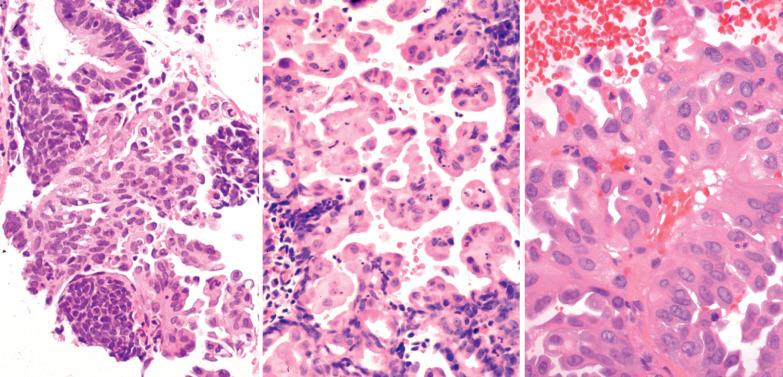
Squamous metaplasia in the endometrium has traditionally been considered to exist in two forms:
Surface (or less commonly glandular) mature squamous epithelium that is often keratinizing and/or glycogenated and usually related to chronic inflammation or irritation. It may extensively line the endometrial cavity (‘ichthyosis uteri’) and give rise to squamous cell carcinomas.
Intraglandular nests composed of immature cells (morular metaplasia). The immunoprofile of morular cells differs from that of typical squamous cells (see below), likely reflecting their immature nature. Morular metaplasia is often related to unopposed estrogen, or less commonly, progestin treatment, but may be idiopathic.
Morules are composed of immature, round to spindled, epithelial cells, with indistinct cell borders and bland nuclei that are occasionally optically clear.
Morules may undergo central necrosis and can fuse, resulting in large solid areas, findings that may cause concern but are of no significance.
Maturation of the morular cells into those with overt squamous features (copious eosinophilic cytoplasm, intercellular bridges, keratin) occurs in some cases.
Morular cells have a β-catenin+/CDX2+/CD10+/p16+/AE1/AE3+/EMA − /ER−/p63 − immunoprofile whereas mature squamous cells have the opposite findings. Morules enigmatically also occasionally stain for neuroendocrine markers.
As noted above, morular metaplasia most commonly reflects unopposed estrogen stimulation, being most frequent within endometrial hyperplasia and endometrioid adenocarcinoma ( Chapter 8 ) as well as atypical polypoid adenomyomas ( Chapter 9 ). Even rare morules, especially in a scanty specimen, should be noted, and warrant follow-up (depending on the clinical situation) to exclude a coexisting atypical glandular lesion.
Both morular and typical squamous metaplasia, if extensive, may be confused with well-differentiated squamous cell carcinoma or endometrioid adenocarcinoma with squamous differentiation ( Chapter 8 ), particularly in a curettage specimen. An absence of associated neoplastic glands and overtly malignant nuclear features facilitate the diagnosis, with the caveats that neoplastic squamous elements can be highly differentiated and abundant squamous elements should raise concern for carcinoma.
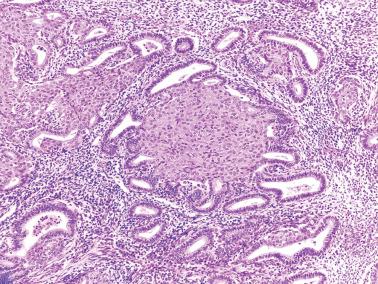
Mucinous metaplasia is uncommon but is seen occasionally, particularly within endometrial polyps, especially those with papillary proliferation (see corresponding heading).
Glandular or surface epithelium is replaced by columnar cells with mucin-rich cytoplasm, resembling endocervical epithelium.
Occasionally the cells are of intestinal type, including goblet or pyloric type cells, with positivity for CDX2, CK20, chromogranin, and villin. Pyloric-type glands may rarely exhibit lobular clustering and positivity for the pyloric gland markers MUC2 and HIK1083.
The metaplastic glands may show architectural complexity, with or without cytologic atypia, and/or coexist with an endometrial mucinous carcinoma (EMC). A microglandular pattern may occur within benign complex mucinous proliferations but should raise concern for mucinous carcinoma. Complex mucinous proliferations are considered further in the differential diagnosis of EMCs in Chapter 8 .
Yoo et al. found that papillary mucinous metaplasia was a possible precursor of EMC, showing decreased expression of PAX2 and PR and overexpression of p16. KRAS mutations were found in 89% of their cases in contrast to only 14% in simple mucinous metaplasia.
Synchronous metaplastic or neoplastic mucinous lesions elsewhere in the FGT (cervix, fallopian tube, ovary) may occur. A case of diffuse atypical mucinous metaplasia of the entire FGT was associated with cervical agenesis (Anjarwalla et al.).
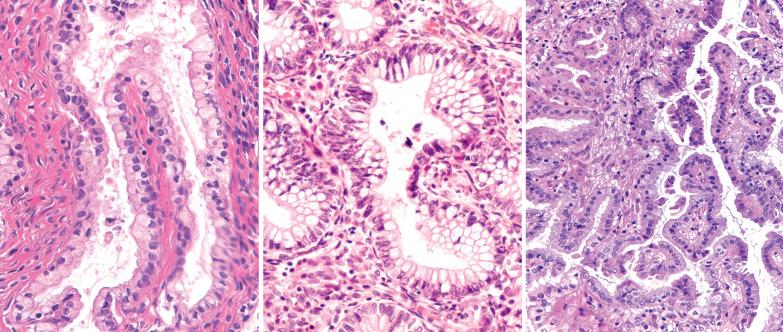
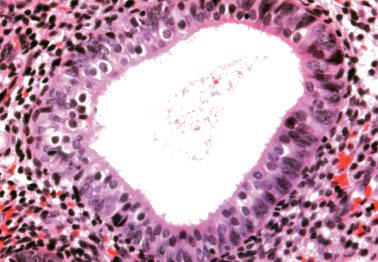
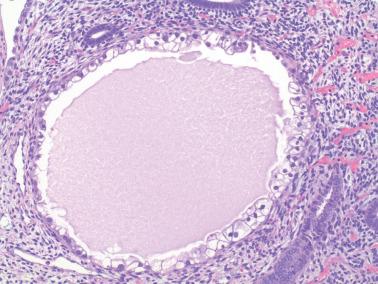
The lining of endometrial glands and/or the surface epithelium can consist predominantly of ciliated cells. Ciliated glands are often cystic and individually disposed amongst nonmetaplastic glands.
The ciliated cells usually have eosinophilic or occasionally clear cytoplasm and round uniform nuclei, often with a small nucleolus, and are disposed in a single or pseudostratified layer. Occasionally the cells stratify and may form a cribriform pattern.
Ciliated glands may show architectural and/or cytologic atypia (atypical hyperplasia with ciliated cells). The differential diagnosis in these cases is with ciliated adenocarcinoma ( Chapter 8 ).
Simon et al. (2011) found that ciliated metaplasia with cytologic atypia had low p53 and Ki-67 reactivity (similar to that of typical ciliated metaplasia) and was not associated with an increased risk of subsequent hyperplasia or carcinoma.
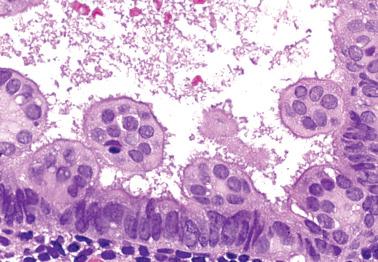
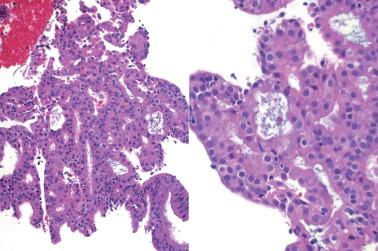
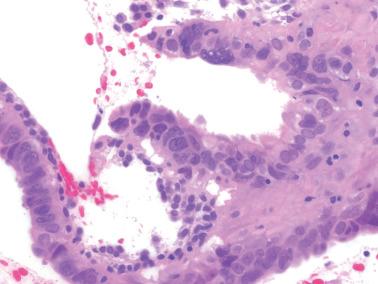
Occasionally endometrial glands are lined by, or the surface epithelium is replaced by, nonciliated cells with eosinophilic cytoplasm that can be abundant and sometimes granular. The nuclei are usually uniform, round, and typically central, and mitoses are rare, but occasionally reactive atypia may be seen. The cytoplasm of the eosinophilic cells is frequently MUC5AC+. Numerous mitochondria are found in the rare cases of oncocytic metaplasia.
Moritani et al. (2005) found that eosinophilic metaplasia frequently coexisted with mucinous metaplasia, and was more common in endometrial hyperplasia and carcinoma than in non-hyperplastic endometria.
The usual lack of atypical architectural and cytologic features excludes atypical hyperplasia (that often also has eosinophilic cytoplasm) and oxyphilic endometrioid adenocarcinoma.
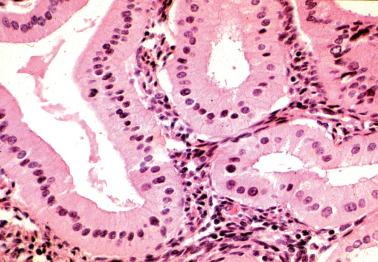
Although hobnail cells can be idiopathic, they are more commonly a reactive change, such as postcurettage or over or within an infarcted polyp. Hobnail cells are also commonly a progestational change as seen in pregnancy (with or without other findings of the Arias-Stella reaction, see below) or secondary to progestin treatment. Other rarer associations are listed in Table 7.2 .
The surface or glandular epithelium is replaced by a single layer of cells with scanty cytoplasm and enlarged hyperchromatic nuclei that project into gland lumina or off the surface.
In contrast to neoplastic hobnail cells in serous and clear cell carcinomas, metaplastic hobnail cells are typically an incidental microscopic finding, lack mitotic activity, and are not associated with a tumor.
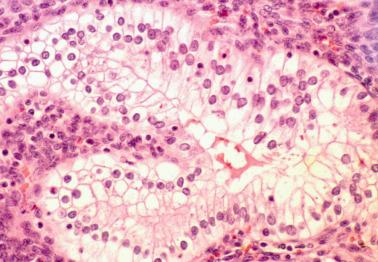
Clear cell change in endometrial glands is most often associated with pregnancy (see below) but can occasionally be an isolated finding with no apparent cause in a nonpregnant patient.
The cells have abundant clear glycogen-rich cytoplasm; a foamy appearance may suggest the presence of lipid.
Distinguishing features from clear cell carcinoma include the noninvasive microscopic size of the focus and the lack of other typical patterns of clear cell carcinoma and the usual absence of architectural and cytologic atypia.
Misinterpreting these changes as neoplastic or preneoplastic is more likely to occur if the pathologist is unaware that the patient is pregnant or receiving progestins. Rarely some of these changes have no apparent cause.
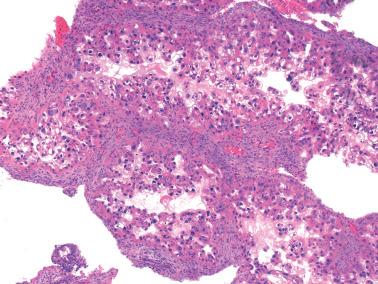
The Arias-Stella reaction (ASR) is a characteristic endometrial glandular change usually associated with an intrauterine or extrauterine pregnancy, trophoblastic disease, or hormone treatment (usually with a high-dose progestin). Although Arias-Stella described the ASR only in pregnancy and a purist could consider nonpregnant-related examples ‘Arias-Stella-like’, we refer here to all forms as ASR.
The involved glands range from few to many and are typically in the spongiosa but occasionally also in the basalis or surface epithelium. Intraglandular papillary tufts are lined by often stratified cells that have scanty to voluminous eosinophilic to clear glycogen-rich cytoplasm. A secretory appearance may be imparted by subnuclear and/or supranuclear vacuoles; sometimes the cytoplasm has a frothy appearance. Rarely, mucin-filled cytoplasm vacuoles can result in a signet-ring-like appearance.
The nuclei are often enlarged and irregular and range from vesicular to hyperchromatic. Some may be pyknotic, have smudged chromatin, contain intranuclear cytoplasmic pseudoinclusions, or appear optically clear (see below). Nuclear atypia is sometimes striking and hobnail-type cells may be seen.
Arias-Stella et al. encountered rare mitotic figures, which were occasionally abnormal, in about 10% of their cases. However, a diagnosis of ASR should be made with caution if there is more than a rare mitosis or an abnormal one.
Features distinguishing the ASR from clear cell and other adenocarcinomas include the typical association with pregnancy or hormonal treatment (although rare endometrial carcinomas occur in pregnancy), its focal microscopic nature, an absence of invasion, and the usual mitotic inactivity.
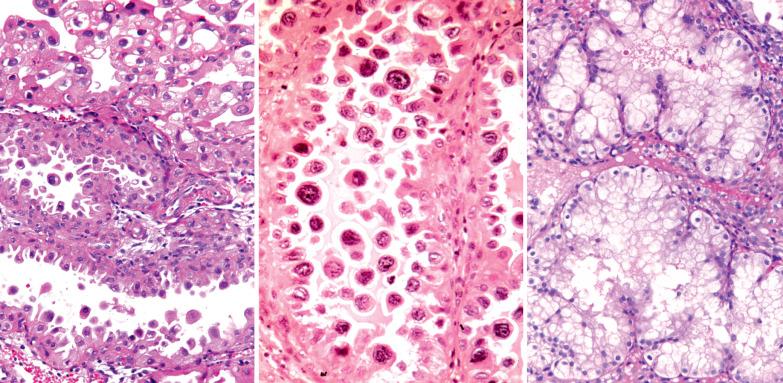
This alteration may accompany the ASR but it occasionally occurs in the absence of the latter's characteristic nuclear changes.
Glandular epithelial cells contain abundant, clear, glycogen-rich cytoplasm. The clear cells may stratify as regular papillary tufts or as solid nests or sheets with luminal obliteration.
Distinguishing features from adenocarcinoma are the same as those for the ASR (see above).
This characteristic alteration of endometrial glandular nuclei has been found in 7% of first-trimester abortion specimens, and less commonly later in pregnancy or at term. The finding is often associated with the ASR.
The appearance is similar to that of herpetic inclusions, but ultrastructural examination shows a network of fine filamentous material rather than herpesvirus DNA. Biotin within the clear nuclei can result in false HSV immunoreactivity.
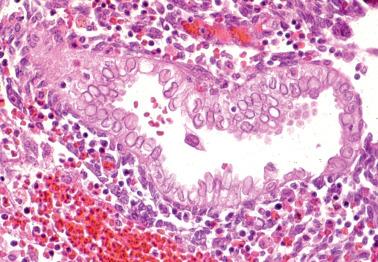
A decidual reaction is almost invariably confined to pregnant women and those being treated with progestins. However, rare examples of an idiopathic florid decidual reaction have occurred in pre- or postmenopausal women.
Polypoid decidual tissue (‘decidual pseudopolyp’) is common in pregnancy and is most frequently encountered in practice when it prolapses into the endocervix clinically mimicking an endocervical or prolapsed endometrial polyp.
Unusual features that may suggest a neoplasm include necrosis, nuclear pleomorphism and hyperchromasia, and spindled and signet-ring-like cells. In cases with signet-ring-like cells, the correct diagnosis is indicated by admixed typical decidual cells, acidic (rather than neutral) mucin within cytoplasmic vacuoles, and if doubt persists, negative staining for cytokeratin.
Estrogen-progesterone preparations are used widely for oral contraception, for hormone replacement in postmenopausal women, and for the treatment of endometrial hyperplasia.
Endometrial changes secondary to these agents vary with the regimen (combination or sequential), duration of use, dosage, and the estrogen–progesterone ratio of the drug.
Most women are treated with low-dose continuous combination preparations. The endometrium usually has a diminished gland–stromal ratio with sparse, small, inactive to weakly secretory glands. The endometrial stromal cells have an inactive, predecidual, or occasionally a fully decidualized appearance.
Oral progestins alone are used in the treatment of abnormal uterine bleeding, endometriosis, and tumors (endometrial and breast carcinomas and endometrial stromal sarcomas). Progestin-releasing intrauterine devices (IUDs) are used in the treatment of menorrhagia (see Intrauterine Device-related Changes ).
The endometrial glands are typically atrophic. Other findings may include morular metaplasia and the Arias-Stella reaction.
The endometrial stroma typically shows prominent decidual change, sometimes with focal myxoid areas, lymphocytes, necrosis, and as noted above, signet-ring-like decidual cells.
Changes related to progestin treatment of endometrial hyperplasia and endometrioid adenocarcinoma are considered in Chapter 8 .
Tamoxifen, a nonsteroidal estrogen and a selective estrogen receptor modulator (SERM), is now widely used for the treatment and prophylaxis of breast carcinoma.
Patients on long-term tamoxifen treatment have an increased risk of endometrial polyps (that may have unusual features; see Polyps), metaplasias, hyperplasia, and adenocarcinoma.
Bergman et al. found a relative risk of 1.5 for the development of endometrial adenocarcinoma, but this risk rose to 6.9 for women after ≥5 years of treatment.
Some studies have found a possible association between tamoxifen treatment and endometrial malignant müllerian mixed tumors, adenosarcomas, and pure uterine sarcomas.
Other SERMs, such as raloxifene, appear to have no estrogenic effect on the endometrium.
Progesterone receptor modulators (PRM) (mifepristone; ulipristal acetate [Fibristal®]) used to treat endometriosis and leiomyomas can induce endometrial thickening with a constellation of characteristic histologic changes (progesterone receptor modulator associated endometrial changes (PAECs) (Ioffe et al., Latta et al., Mutter et al.).
PAECs are observed in ~60% of patients after 3 months of ulipristal therapy and most are lost after treatment cessation. The frequency of the changes does not increase with repeated treatment.
Architectural alterations: variable gland architecture, often with a disordered proliferative pattern with large cystic glands (a key feature) and tortuous coiled glands with irregular luminal contours due to intraluminal projections. A normal gland–stroma ratio is usually maintained, although focal gland crowding can occur.
Glands vary from weakly proliferative (with a single layer of pseudostratified epithelium that may exhibit mitoses) to inactive. Apoptic bodies, secretory changes (subnuclear cytoplasmic vacuoles), and foci of ciliated or eosinophilic metaplasia may also be seen. Nuclear atypia is absent.
Nonspecific vascular changes including thick-walled arterioles, ectatic capillaries, and a complex chicken-wire-type capillary network.
Clomiphene citrate used to induce ovulation in infertile women affects the appearance of the secretory phase endometrium. Benda found a decreased gland–stromal ratio with smaller less tortuous glands, unusually distinct subnuclear vacuoles and luminal borders, and scanty inspissated luminal secretions.
Feeley and Rasbridge described four cases of striking endometrial epithelial atypia in women on HRT, tamoxifen, or norethisterone. Scattered surface epithelial and glandular cells had bizarre hyperchromatic nuclei with smudged chromatin but without visible nucleoli or mitotic figures.
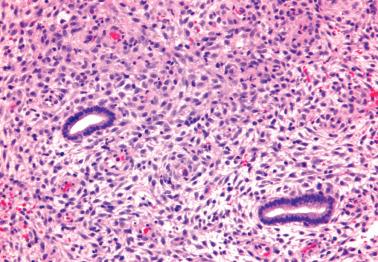
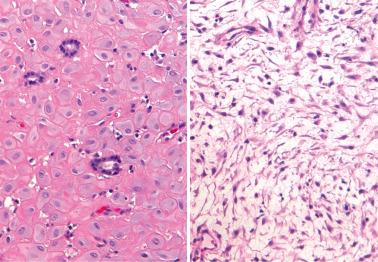
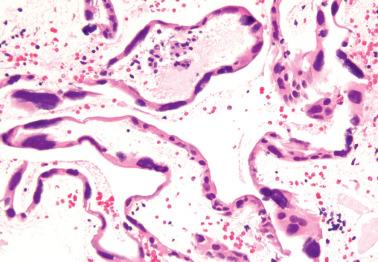
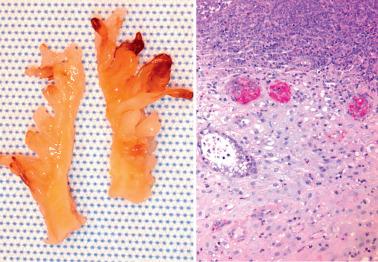
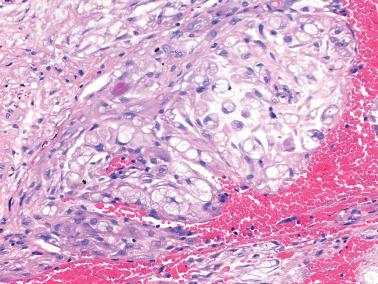
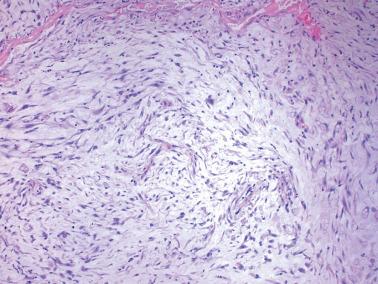
Heterotopic tissues are considered here, although they are not always pregnancy-related. They are usually an incidental microscopic finding in women of reproductive age. In some cases, the heterotopic tissue may have an IUD-like effect, resulting in infertility.
The tissues are most commonly cartilage, bone, glia, and fat and are usually within the endometrium (including endometrial polyps), less commonly the cervix or myometrium.
Implantation of fetal tissue during therapeutic or spontaneous abortion is the presumed (and in some case proven) pathogenetic mechanism. Some heterotopic tissues are likely (or proven by genetic testing) to be metaplastic, such as nodules of endometrial smooth muscle, fat, and bone. Some tissues may be a result of true heterotopia or are dystrophic.
Lomme et al. have described an unusual form of smooth muscle differentiation in the endometrial stroma that they designated ‘pseudorosette-like proliferations’. These are discrete ≤2 mm foci of spindle cells in short fascicles arranged radially around an eosinophilic acellular zone.
Fragments of fat in a curettage specimen may also be due to uterine perforation or be derived from a submucosal lipoleiomyoma or lipoma. Fat should be distinguished from ‘pseudolipomatosis’ (see corresponding heading).
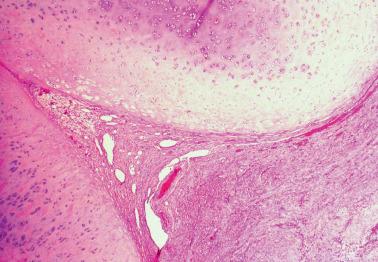
Luteal phase defect (LPD) is a term that has been traditionally used to denote a deficient secretory phase due to inadequate progesterone production by the corpus luteum or deficient local endometrial response to normal levels of progesterone.
Although LPD has been claimed to be responsible for up to 5% of cases of infertility/recurrent miscarriage, its clinical significance has been questioned and possibly rendered moot by recent artificial reproductive techniques.
Microscopic findings, which should be found in two secretory phase biopsy specimens, have included a secretory endometrium with a variable appearance from one area to another; a secretory endometrium with dyssynchronous glands and stroma (e.g. early secretory glands admixed with late secretory stroma); and a normal-appearing secretory endometrium but one that lags behind that expected by the date of the LMP by >2 days.
More recently, Russell et al. reported the presence of asynchronous endometrial glands in 2% of women with recurrent reproductive failure. The finding can occur in sequential specimens from the same patient.
Solitary or clustered proliferative-type glands (MIB1+/ER+/usually PR+) are disposed within the functionalis layer of an otherwise typical late secretory endometrium.
This finding was deemed to differ from those of typical LPD as it occurs within an endometrium appearing to reflect an otherwise adequate progesterone effect. Supplemental first trimester progesterone therapy appeared to have a beneficial therapeutic effect in some patients.
Some of the findings already considered, such as some menstrual-related changes and hobnail cells, are reparative. This section deals with other reparative as well as inflammatory lesions.
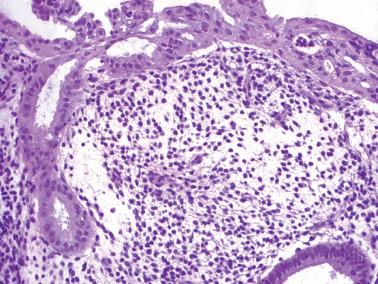
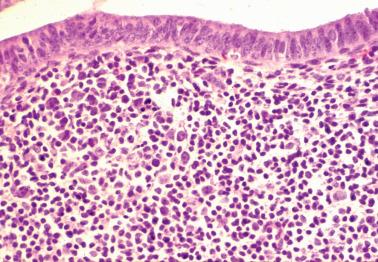
The term ‘chronic’ endometritis is used here and elsewhere to denote the presence of plasma cells within the endometrium. However, in most cases of endometritis related to clinically significant pelvic inflammatory disease (PID), acute and other chronic inflammatory cells and other findings noted below are present.
The diagnosis of chronic endometritis rests primarily on the presence of plasma cells within the endometrial stroma and usually the other features noted below.
The plasma cells are most common beneath the surface epithelium, around glands, at the periphery of lymphoid follicles, or around distended sinusoids and deeper stromal vessels; the basalis and even the superficial myometrium may be infiltrated in severe cases. Identification of plasma cells can be improved by syndecan-1 (CD138) positivity, but the majority of clinically significant lesions should be identifiable on routine H&E examination.
Other inflammatory cells often present, include neutrophils (usually within the surface epithelium and gland lumens, sometimes with microabscesses), subepithelial lymphocytic infiltrates, hemosiderin-laden histiocytes, and occasional eosinophils.
A positive correlation has been found between the numbers of endometrial plasma cells and the numbers of lymphoid follicles (with or without germinal centers), lymphocytes, and eosinophils.
Superficial stromal edema, increased stromal density, periglandular palisading of the stromal cells, spindle cell (fibroblastic) or predecidual-like alteration of the stromal cells, stromal necrosis and breakdown, sinusoidal fibrin thrombi, and tiny polyps (‘micropolyps’) may also be seen.
The normal glandular and stromal response to the hormonal milieu of the menstrual cycle is usually diminished, often resulting in an inactive appearance or gland-to-gland or gland-to-stroma dyssynchrony. Histologic dating is thus not usually reliable, although otherwise normal-appearing proliferative or secretory endometrium is seen in some cases.
Other epithelial findings in some cases include squamous metaplasia and reactive changes such as cellular stratification, more abundant eosinophilic cytoplasm, prominent nucleoli, and mitotic figures.
Paukku et al. identified Chlamydia by immunohistochemistry or polymerase chain reaction in 24% and 4% of cases with and without chronic endometritis, respectively.
Actinomyces infection of the endometrium is considered under Intrauterine Device-related Changes.
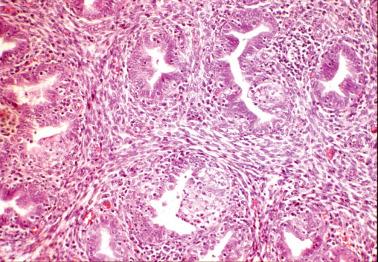
Smith et al. found no correlation between the presence of plasma cells or severity of the inflammation with any clinical findings; specifically, the presence of plasma cells was not associated with a clinical diagnosis of PID in any of their cases. These and other studies suggest that the presence of occasional endometrial plasma cells, when it is the only abnormal finding, usually has little or no clinical significance.
In contrast, Kiviat et al. found that endometritis with superficial neutrophils (including intraepithelial neutrophils and microabscesses) was associated with PID/salpingitis. Correlation of the histology with the clinical findings (including laparoscopic and culture results) facilitates the diagnosis.
Aside from an endometrial infection, endometrial plasma cells may be see in anovulatory/disordered proliferative endometria with menstrual changes (Gilmore et al.), polyps, leiomyomas, an intrauterine device, vaginosis or cervicitis, and urinary tract infection.
When fragments of endocervical tissue containing plasma cells are present in an endometrial curettage specimen, misdiagnosis of chronic endometritis can occur if the endocervical origin of the tissue is not appreciated.
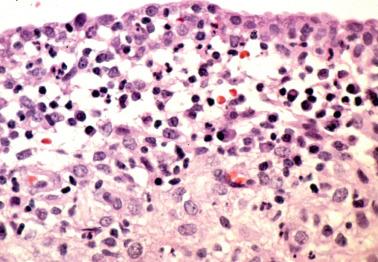
This uncommon finding, which is of unknown clinical significance, is typically encountered in premenopausal women who present with abnormal vaginal bleeding.
The lesion consists of a focal periglandular infiltrate of lymphocytes and neutrophils; plasma cells are absent. Usually only rare glands are involved. The infiltrate typically extends into the gland lumen with disruption or partial or subtotal necrosis of the glandular epithelium, the appearance resembling a crypt abscess.
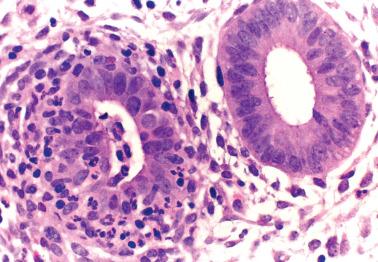
This rare lesion typically occurs in women of reproductive age who present with abnormal bleeding, although occasionally it is an incidental microscopic finding. There is almost always a background of typical chronic endometritis.
The microscopic findings are generally similar to those in the uterine cervix ( Chapter 4 ). Differences from the latter include an absence of a band-like distribution and more numerous ill-defined aggregates of large lymphoid cells, the appearance resembling reactive germinal centers but usually without a mantle of mature lymphocytes.
Distinguishing features from lymphoma include an absence of a mass, the presence of reactive germinal centers within the lymphoid aggregates, a mixed inflammatory infiltrate within and/or at the periphery of the aggregates, and chronic endometritis elsewhere in the specimen.
A unique lesion consisted of intravascular blast cells within an endometrial polyp in a woman with chronic endometritis (Bryant et al.). The cells had a polyclonal pattern for T-cell receptor β and γ but clonal rearrangement for IgH. The findings raised concern for an intravascular lymphoma, but hematologic assessment and follow-up were negative.
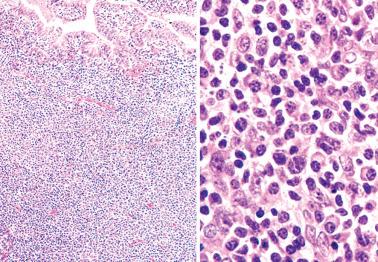
Endometrial and myometrial granulomas are rare, being found in only 0.15% of endometrial specimens in one study.
Granulomas are usually related to a prior operation or are idiopathic, less commonly to infection (tuberculous, fungal, parasitic), foreign material, tumor-derived keratin, or sarcoidosis. Endometrial tuberculous granulomas are often noncaseating.
Kelly and McCluggage have described idiopathic uterine granulomas within the myometrium or cervical stroma that were typically multiple and related to thin-walled vascular channels, although there was no evidence of vasculitis.
Necrotic granulomas, in which palisaded histiocytes (including giant cells) surround necrotic zones, can occur after diathermy/laser endometrial ablation or with the Mirena coil (see Intrauterine Device-related changes ). Ablation-related granulomas are typically associated with necrotic tissue and refractile brown hematoidin-like pigment and/or black (carbon) pigment.
Simon et al. studied uteri in cases of endometrial ablation failures, finding necrotic tissue in the endometrium or endometrial cavity, endometrial fibrosis or hyalinization, large congested blood vessels with atherosis, residual cornual endometrium, and ablation changes confined to the lower uterine segment.
Xanthogranulomatous inflammation is considered below.
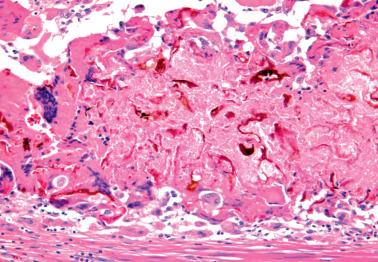
Xanthogranulomatous endometritis typically occurs in postmenopausal women who present with vaginal bleeding or discharge. Some have had radiation treatment for endometrial or cervical carcinoma.
Pelvic examination often reveals cervical stenosis, pyometra, or both. Necrotic, friable, yellow-brown tissue is obtained by D&C or lines the endometrial cavity of a hysterectomy specimen.
The endometrium is infiltrated by numerous histiocytes with abundant, eosinophilic, granular or foamy cytoplasm without Michaelis–Gutmann bodies (see Malacoplakia ). The cytoplasm is typically rich in lipid and in some cases, ceroid pigment. Rarely the histiocytes have a signet-ring-like appearance.
Neutrophils, lymphocytes, plasma cells, hemosiderin-laden histiocytes, and foreign-body giant cells are often admixed. Other findings may include cholesterol crystals, focal calcification, necrosis, and radiation-induced changes. Bacteria have been cultured in rare cases.
The pathogenesis is likely related to cervical obstruction resulting in pyometra, hematometra, endometrial necrosis, or combinations thereof. Radiation-induced tumor necrosis and bacterial infection may be additional factors in some cases.
The differential diagnosis includes malacoplakia (see below) and endometrial stromal foam cells occurring in some cases of endometrial hyperplasia and carcinoma ( Chapter 8 ).
Xanthogranulomatous myometritis is also present in some cases. Myometrial infiltration by foamy histiocytes (‘myometrial xanthomatosis’) has also been described in women after cesarean section or therapeutic abortion.
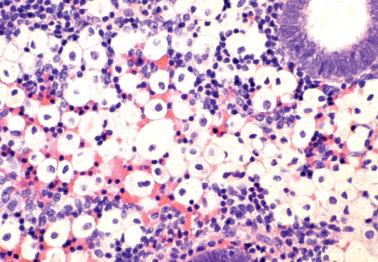
Endometrial malacoplakia is rare and typically presents with postmenopausal vaginal bleeding or spotting. Other sites (cervix, broad ligament) or the inguinal region may also be involved.
The endometrium may be grossly thickened, nodular or polypoid, soft, yellow to brown, and focally hemorrhagic.
The typical microscopic features are sheets of histiocytes with copious granular cytoplasm (von Hansemann cells), Michaelis–Gutmann bodies, and intracellular bacilli. Other inflammatory cells are also typically present.
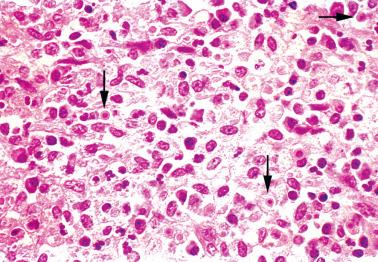
Kim et al. (2002) described nodular endometrial aggregates of histiocytes resembling Langerhans’ interdigitating cells. The nodules, which were an incidental microscopic finding in curettage specimens in women of reproductive age, were solitary, free-floating, and up to 1.5 cm in size.
The round to polygonal histiocytes, which may be focally dyscohesive, have distinct cytoplasmic borders and pale amphophilic or eosinophilic granular cytoplasm. Small cytoplasmic vacuoles may impart a signet-ring-like or plasmacytoid appearance.
The ovoid to reniform nuclei have occasional grooves, fine chromatin, and inconspicuous nucleoli. Mitotic figures may be found and occasionally are focally numerous (up to 4 mf/hpf).
The histiocytes are CD68+/lysozyme+/S100−/CK−.
Parkash et al. found the nodules were associated with endometrial polyps and/or prior endometrial sampling and suggested that the histiocytes may be a response to debris introduced into the endometrial cavity.
Uterine involvement by Langerhans’ cell histiocytosis (eosinophilic granuloma) ( Chapter 10 ) can be excluded by the absence of both eosinophils and S100 staining. The distinctive appearance of the histiocytes, their nodular arrangement, and the absence of lipid and/or pigment differ from those of usual histiocytic or xanthogranulomatous endometritis.
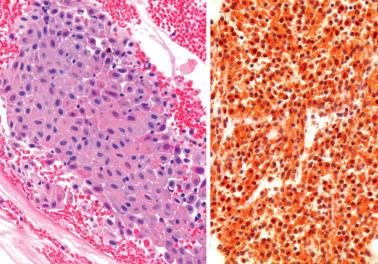
Eosinophils in the endometrium and/or myometrium in a hysterectomy specimen are usually an incidental microscopic finding days to weeks after a curettage.
As noted previously, eosinophils may be part of a mixed inflammatory infiltrate in endometritis.
Occasional endometrial and myometrial mast cells are considered a normal finding.
Mast cells may occur in striking numbers as a response to IUDs, within the stroma of endometrial polyps, in areas of hyperplastic glands, and within leiomyomas. They appear to have no clinical significance.
None of the few documented cases of this lesion were associated with conjunctival disease at the time of reporting. Myometrial, tubal, and peritoneal involvement may also be present. The histology is similar to that of ligneous cervicitis ( Chapter 4 ).
One example of this lesion ( Chapter 3 ) was found in a hysterectomy specimen 2.5 weeks after a curettage in a 75-year-old woman. A 1.0 cm nodule in the endometrium and superficial myometrium was composed of a densely cellular proliferation of mitotically active spindle cells, small blood vessels, and a few inflammatory cells.
IUD-related changes vary with the type of device and duration of use, and include reactive epithelial changes, interglandular or gland-stromal dyssynchrony, glandular metaplasias (squamous, morular, hobnail), chronic endometritis (including granulomatous inflammation), fibrosis, and microcalcification.
Progestin-releasing IUDs (e.g., Mirena coil), can result in polypoid endometrial thickening due to pronounced progestational changes (decidua, stromal mucin, atrophic glands, Arias-Stella-like changes).
Other IUD-related findings may include necrotic granulomas, stromal collagenous nodules, surface micropapillary change, and a synovial-like membrane comprised of palisaded vimentin+/CD68+ cells .
Endometrial actinomycosis may complicate the presence of an IUD, and can lead to tubal and ovarian involvement, including a tubo-ovarian abscess.
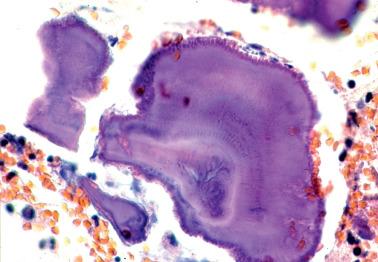
Bacterial colonies (sulfur granules) are composed of thin radiating filaments at their periphery, a dense finely granular core, and positive staining with Gram and methenamine silver stains.
Sulfur granules can rarely be combined with PAMRAGs (see next point).
Pseudoactinomycotic radiate granules (PAMRAGs) are a tissue response to IUDs and may also form around sulfur granules, as noted above. They may also be seen in tubo-ovarian and other abscesses, and in the cervix.
PAMRAGs are composed of neutral glycoproteins, lipid, and calcium. In contrast to sulfur granules, they lack radiating filaments and a dense core, are refractile (but nonbirefringent), and often exhibit laminations (‘tide-water’ marks) and club-like peripheral projections.
Unlike sulfur granules, PAMRAGs are Gram negative (or exhibit nonspecific Gram staining) and are negative with silver stains, findings helpful in the recognition of combined sulfur-PAMRAG granules.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here