Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Knee cartilage injuries present a great challenge for both clinicians in recent decades and may often lead to substantial morbidity, significant time away from work and sport, and possibly permanent disability. This chapter will review the current advanced and emerging strategies and techniques in the field of knee cartilage regeneration and restoration as well as the newest available and soon-to-be available techniques.
Common and inexpensive marrow stimulation techniques such as microfracture or subchondral drilling may provide some short-term symptomatic improvements, which tend to deteriorate over time and result in poor quality repair tissue; they are less successful in lager size lesions.
Due to the limitations of earlier cartilage regeneration/repair solutions as well as limitations of promising cartilage transplant techniques such as autologous chondrocyte implantation (ACI), osteochondral autograft transfer (OAT), and osteochondral allograft transplantation (OCA), new strategies and techniques have been developed in recent years.
Some of these strategies have focused on incorporating orthobiologic enhancement into the developed technique utilizing available biologic products such as growth factors (through platelet-rich plasma) and/or mesenchymal stem cells (MSCs).
Important recently emerged techniques include advanced marrow stimulation techniques, newer-generation ACI, or matrix-assisted autologous chondrocyte implantation (MACI), matrix with MSCs based treatments, particulated (also regarded as minced) autologous cartilage implantation (PACI), as well as cell-free and scaffold-free products.
While research involving currently available techniques has shown favorable results compared to conventional microfracture, literature is lacking with regards to comparisons of these techniques to other advanced techniques.
Despite exciting advances in the field, limitations of many existing and available techniques still revolve around production of hyaline-like cartilage, which is not similar enough to true hyaline cartilage, and other issues involve the complexity of a two-stage procedure in some techniques, associated costs of many procedures, and donor site morbidity.
In order to optimize knee cartilage repair techniques, the following aspects should be addressed:
Defining the optimal safe, effective, least invasive, and ethically permissible cell source for cell-based theories in cartilage regeneration.
Better understanding the molecular mechanism underlying cartilage regeneration by MSCs or MSCs-derivatives.
Defining the best cell derivatives (i.e., extracellular vesicles, exosomes, cytokines, and various RNAs) as a target for tissue-engineered articular cartilage to retain and augment the repair and regeneration function of MSCs.
Defining the optimal scaffold properties, structure, and components.
Finding the optimal cell (or cell derivatives) scaffold-binding methods (i.e., freeze drying, hydrogel loading, and 3D bioprinting).
Injuries to articular cartilage have presented a great challenge for musculoskeletal clinicians and for scientists over the last few decades. While relatively common, knee articular cartilage injuries often cause substantial morbidity for patients, with significant time lost from work and sport and even permanent disability. This is attributed to the limited ability of articular cartilage for spontaneous healing and recovery due to its limited vascularity and cellularity. Although the natural history of articular cartilage injuries is not yet fully understood, if left untreated, they may lead to the development of widespread degenerative joint disease. It has been shown that even knee joints with asymptomatic cartilage defects have substantially higher rates of further cartilage loss compared with intact knees, and the majority (over 80%) of cartilage defects will worsen at short term (2 years). These challenges have led researchers to investigate and develop durable regenerative treatment options to address the increasing disease burden.
Over the last couple of decades, various treatment strategies and techniques have been developed for cartilage regeneration, providing some promising results. However, while current treatment options may provide various degrees of symptom relief, the wider goal of regenerating or restoring a durable, smooth, hyaline tissue similar to native cartilage with its biomechanical properties has not been achieved, and each technique has its limitations. Common and inexpensive marrow stimulation techniques such as microfracture or subchondral drilling may provide some short-term symptomatic improvement. However, these improvements tend to deteriorate over time, produce poor quality repair tissue, and are less successful in lager size lesions and defects.
While the introduction of autologous chondrocyte implantation (ACI) initially brought great promise to the field of cartilage regeneration with improved results for larger cartilage defects, the resultant hyaline-like cartilage is of limited quality and not similar enough to true hyaline cartilage. Along with the complexity of a two-stage procedure and associated costs, there have been major limitations for promoting this technique as an ideal solution. Osteochondral grafting options, such as osteochondral autograft transfer (OAT) and osteochondral allograft transplantation (OCA), offer an immediate hyaline cartilage and subchondral bone construct; however, each technique has its limitations, with OAT requiring donor site morbidity and OCA carrying allograft-related issues, including limited availability, limited chondrocyte viability, and high costs.
Due to the limitations of earlier cartilage regeneration/repair solutions, new strategies and techniques have been developed in recent years, with several new techniques already commercially available or expected to become available in the very near future. Some of these strategies have focused on incorporating orthobiologic enhancement into the developed technique utilizing available biologic products, such as growth factors (through Platelet Rich Plasma) and/or mesenchymal stem cells (MSCs).
Important recently emerged techniques include advanced marrow stimulation techniques, newer-generation ACI, or matrix-assisted autologous chondrocyte implantation (MACI), matrix with MSCs based treatments, particulated (also regarded as minced) autologous cartilage implantation (PACI), as well as cell-free and scaffold-free products. This chapter will review the current advanced and emerging strategies in the field of knee cartilage regeneration and restoration as well as the newest available and soon to be available techniques.
Growth factors (GFs) have been long shown to have an important effect on chondrocytes’ function through regulation of cell growth, division, and differentiation as well as to regulate multiple catabolic and anabolic processes in cartilage tissue. Key GFs highlighted as important for cartilage homeostasis include GFs from the fibroblast growth factor family (FGF-2, FGF-18, and FGF-8), insulin-like growth factor 1 (IGF-1), and transforming growth factor β1 (TGFβ1), , although many others have been recently demonstrated to be involved in this process. Their role in cartilage regeneration strategies through enhancement of repair tissue quality has been highlighted in several in vitro and pre-clinical studies. The administration of these GF is aimed at optimizing the healing environment within the joint and in the cartilage defect as they increase glycosaminoglycan (GAG) synthesis as well as decrease catabolic cytokines, which have a role in the osteoarthritic process. However, despite the vast knowledge regarding the signal transduction pathways these factors activate and their net effect on cartilage tissue and related symptoms in in vitro studies, there is little clinical research regarding the application of growth factor-based therapy as part of cartilage regeneration strategies and techniques.
While use of targeted growth factors requires significant manipulation and therefore specific regulatory approval, autologous growth factors are easily available in the form of blood derivatives, which can be obtained and prepared with minimal manipulation in a minimally invasive and cost-effective process. The most studied blood derivative is undoubtedly platelet-rich plasma (PRP). Platelets play a vital role in tissue healing processes in which factors such as FGF, IGF-1, vascular endothelial GF (VEGF), and platelet-derived GF (PDGF)—which are released from alpha granules—are key factors, in addition to various microRNAs involved in tissue regeneration and MSCs differentiation into chondrocytes. , In vitro studies have shown that PRP administration has anti-inflammatory, antiapoptotic, and chemotactic effects on MSCs when applied to inflamed cartilage, resulting in a desirable outcome. ,
Mesenchymal stem cells (MSCs) have been studied widely over the years due to their broad-ranging clinical potential. They have drawn much interest in the field of cartilage regeneration due to their potential to differentiate into chondrocyte-like cells with appropriate stimulation. Recently, MSCs have been shown to have anti-inflammatory, immunomodulatory, trophic properties, which enhance the healing environment through secretion of bioactive molecules and are, therefore, thought to have the potential to promote the formation of a suitable environment for cartilage repair and regeneration. There is wide variability in sources for stem cells for cartilage regenerative strategies (i.e., bone marrow, adipose tissue, umbilical cord, synovial tissue, embryonic), as well as origin (autologous versus allogenic), manner of administration (injection versus surgical implantation), and the acquisition method (expanded versus concentrated).
To date, bone marrow–derived MSCs (BMSCs) are the most studied sources of MSCs. However, recently there has been a growing interest in exploring the use of adipose tissue–derived stem cells (ADSCs) due to their availability and higher immunomodulatory capacity compared to BMSCs. Moreover, their properties are less likely to be affected by patient age.
So far, the limited literature on ADSCs use for cartilage regeneration is heterogeneous and hard to interpret, , and further studies are needed to explore its efficacy and benefit compared to BMSCs.
Since the number of MSCs in the different tissues is limited, a strategy to obtain a higher and more homogeneous concentration of MSCs is the in vitro expansion. This allows researchers to accurately calculate the number of cells in each treatment, thus making it more reproducible. Nevertheless, expanded MSCs solutions involve a more expensive process, which includes culturing in specific media requiring a two procedure. In addition, expanded MSCs solutions are considered an advanced-therapy medical product (ATMP), which is therefore subjected to more rigorous regulatory requirements for clinical use, consequentially making the concentrated MSC option an easier and more practical alternative.
Other recently explored autologous sources for MSCs include umbilical cord blood-derived mesenchymal stem cells (UCB-MSCs), peripheral blood progenitor cells (PBPCs) and peripheral blood-derived mesenchymal stem cells (PBMSCs), and synovium/synovial fluid-derived mesenchymal stem cells (SMSCs/SFMSCs). Interestingly, in a recent in vitro study, tissue specific MSCs from osteoarthritic articular cartilage were found with therapeutic potential for cartilage repair. This finding opens up new perspectives in terms of therapeutic strategies for cartilage regeneration since the cells are already present in the site to be treated.
While the vast majority of currently available cell-based treatments for cartilage regeneration utilize implantation of autologous cells, newly emerging technologies and studies are now exploring the possibility of using MSCs from allogenic sources. MSCs are nonimmunogenic due to low expression of antigen-presenting molecules. Despite this, limited number of clinical studies have been conducted on the possibility of allogenic transplant of these cells so far. Song et al. recently reported that surgically applied MSCs derived from human umbilical cords (hUCB-MSCs) were effective in treating OA of the knee. There are more studies currently conducted on the matter with different approaches to the MSCs source. The jury is still out on whether allogenic transplants are as effective as autologous ones.
Marrow stimulation is the most common and inexpensive arthroscopic procedure for cartilage defects. It involves perforation of the subchondral bone through microfracture (MF) or drilling to generate subchondral bleeding and allow bone marrow elements to accumulate in the defect. The resultant clot gradually matures to form a poor-quality and less durable fibrocartilaginous repair tissue. Marrow stimulation techniques have been shown to provide some short-term symptomatic improvement in up to 80% of patients. However, these improvements tend to deteriorate over time, they produce poor quality repair tissue, and they are less successful in lager size lesions and defects or those located in the patellofemoral joint.
One of the limitations of the standard marrow stimulation techniques lies in the unpredictable stability of the mechanically weak formed clot, which can easily detach from the defect and be released into the joint. Therefore, several advanced marrow stimulation techniques have been developed using mechanically stable scaffolds which incorporate specific cell sources and/or bioactive molecules to stabilize the marrow clot in the defect as well as improve mesenchymal stem cells (MSCs) differentiation into a more hyaline-like cartilage repair tissue.
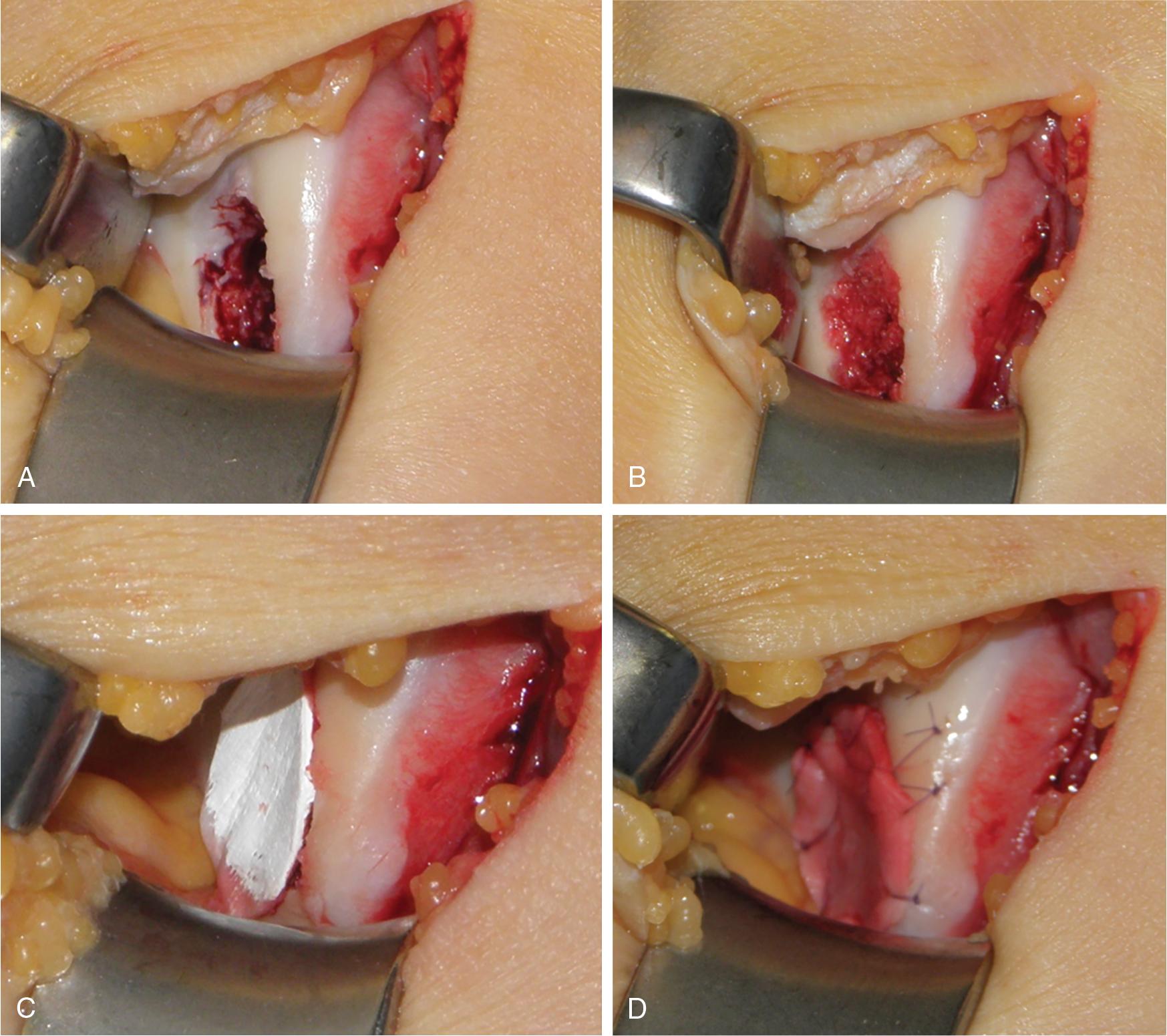
Autologous matrix-induced chondrogenesis (AMIC) , the first described advanced marrow stimulation technique (ChondroGide, Geistlich, Pharma AG), utilizes a scaffold membrane formed by a bilayer porcine type I/III collagen placed and fixated with fibrin glue, with or without sutures on to defects treated with marrow stimulation techniques. The AMIC technique can be performed arthroscopically or through a mini-arthrotomy ( Fig. 75.1 ) and has been shown to provide significant clinical and functional improvement with no deterioration of results for longer than 5 years post-surgery, including in patellar defects. Furthermore, follow-up with magnetic resonance imaging (MRI) has shown moderate-to-complete filling of all chondral defects. The AMIC technique has also been shown to provide improved results in larger size defects (>2 cm²) compared to standard marrow stimulation techniques, with evidence of high-quality repair tissue on MRI in two-thirds of patients. In a multicenter randomized controlled trial (RCT) in 47 patients with a mean defect size of 3.6 ± 1.6 cm 2 , AMIC was shown to have improved clinical results compared to standard microfracture at 5 years postoperatively. Patients in this study were randomized to receive either glued AMIC, sutured AMIC, or standard microfracture. While all three groups showed significant clinical improvement at 2-year follow-up, both AMIC-treated groups showed remaining improvement at 5 years postoperatively compared to the standard microfracture group, which outcomes had declined after the 2-year follow-up. Moreover, both AMIC groups presented better and improved defect fill on MRI evaluations compared to the standard microfracture group. Additionally, the use of the AMIC technique in combination with PRP has been shown to enhance chondrogenesis and hyaline-like tissue formation to fill the defect in several studies.
The BST-CarGel (Piramal Life Sciences, Laval, Quebec, Canada) technique uses a gel matrix bioscaffold composed of liquid chitosan and autologous blood, which is implanted into the cartilage defect following a marrow stimulation technique. This matrix has been shown to enhance early regenerative processes such as neovascularization, cell recruitment, and subchondral bone remodeling. A multicenter RCT comparing BSTCarGel with isolated microfracture in 80 patients with symptomatic grade III or IV articular cartilage lesions demonstrated superior repair features of the BST-CarGel technique at 1 and 5 years of follow-up. While there were no statistically significant clinical differences after 5 years of follow-up, improved histologic and MRI findings were evident. Histologic evaluation was performed following biopsies obtained during second-look arthroscopy at 1 year, demonstrating superior histologic features and improved collagen organization compared to the isolated microfracture group, as well as superior ICRS scores on Arthroscopic visualization. Superior defect filling in the BST-CarGel group was demonstrated using a 3D quantitative MRI evaluation performed at 5 years postoperatively.
JointRep (Oligomedic, Laval, Quebec, Canada) is another chitosan-based gel matrix injectable bioscaffold composed of a polyglucosamine/glucosamine carbonate (PG/GC) based thermogelling system. Pipino et al. recently published the outcomes of their 2-year follow-up study in which 46 patients treated with MF + JointRep were compared to a subsequent, consecutive, matched, control group of 23 patients treated with MF alone. Authors reported improved WOMAC scores in the JointRep group compared to baseline scores and statistically significant improvement compared to the control group as early as 6 months post-surgery, a difference which was maintained at 2 years post-surgery. In a parallel and separate in vitro histological study, adipose derived mesenchymal stem cells (ADMSCs) were encapsulated in the hydrogel scaffold, induced to differentiation into chondrocytes, and observed for a 3-week period. The in vitro analysis revealed a histological characterization typical of hyaline cartilage in the study group.
As previously described, PRP has been suggested to possess a potentially significant augmentation value in cartilage restoration and regeneration techniques due to the anti-catabolic and pro-anabolic properties of the growth factors, potentially generating a pro–hyaline tissue environment. , For this reason, different techniques exploit this PRP capability in order to enhance the structural integrity of the repair tissue, hopefully translating to more reliable long-term clinical outcomes for patients following marrow stimulation.
Bio-Cartilage (Arthrex, Naples, Florida) is a recently developed advanced marrow stimulation technique that utilizes a bioactive scaffold composed of micronized allogenic cartilage in combination with PRP to be populated by MSCs from the microfractured defect. The scaffold contains components of hyaline cartilage extracellular matrix, including type II collagen and proteoglycans aimed at drawing MSCs, and fill the defect with higher quality cartilage. Fortier et al. evaluated the BioCartilage technique in an equine model, demonstrating significantly better histologic features and MRI features at 13 months postoperatively compared to isolated microfracture. A recent case series (six cases) evaluated clinically and used modified clinical Magnetic Resonance Observation of Cartilage Repair Tissue (2D MOCART), reporting the BioCartilage technique promotes regeneration of more robust hyaline-like cartilage compared to the fibrocartilage formed following conventional microfracture. The authors reported T2 mapping evaluation showed the repair tissue had very similar features to that of the adjacent native cartilage. In a recent prospective multicenter clinical study of 48 cases treated with the Biocartilage technique, with 24 months postoperative follow-up, authors reported improvement in functional outcomes, high rates of achieving clinically significant outcomes (CSO), and low failure and complication.
Chondrotissue (BioTissue AG, Zurich, Switzerland) is another augmented marrow stimulation technique offering to utilize the potential additive effect of PRP for cartilage repair. The technique involves applying a scaffold composed of highly porous textile polyglycolic acid (PGA) and hyaluronic acid (HA). The scaffold is hydrated with either PRP, physiologic saline, or autologous human serum right before implantation to provide it with the desired elasticity for implantation after marrow stimulation. Siclari et al. reported a 5-year clinical and MRI follow-up in a series of 52 patients with focal chondral lesions in the knee treated with Chondrotissue following subchondral drilling. Clinical assessment was evaluated via the KOOS score, showing meaningful and significant improvement in all subcategories compared to baseline. Cartilage repair was complete in 20 out of 21 patients at 4-year follow-up as shown by magnetic resonance observation of cartilage repair tissue (MOCART) scoring. Significant short-term improvements were reported in this cohort via KOOS scores and histological features obtained from second biopsies at less than 12 months (four cases), showing hyaline-like repair tissue with increased proteoglycan content and type II collagen. Becher et al. reported good to excellent MRI features following Chondrotissue implantation in retropatellar cartilage defects in five patients, with a mean MOCART score of 61 and clinical improvement measured by the KOOS score in four out of five patients. Glasbrenner et al. recently published the results from their multicenter randomized controlled trial comparing Chondrotissue augmentation (saturated in autologous blood serum) following MF to MF alone in patients with femoral cartilage defect of 0.5 to 3 cm 2 in the weightbearing area. Twenty-four patients completed the study and were assessed by MRI as well as clinically at 12, 54, and 108 weeks postoperatively. MRI scans showed no significant difference between the Chondrotissue and MF groups in the percentage of defect filling at 12, 54, and 108 weeks postoperatively. No significant differences were found in terms of patient-reported clinical scores.
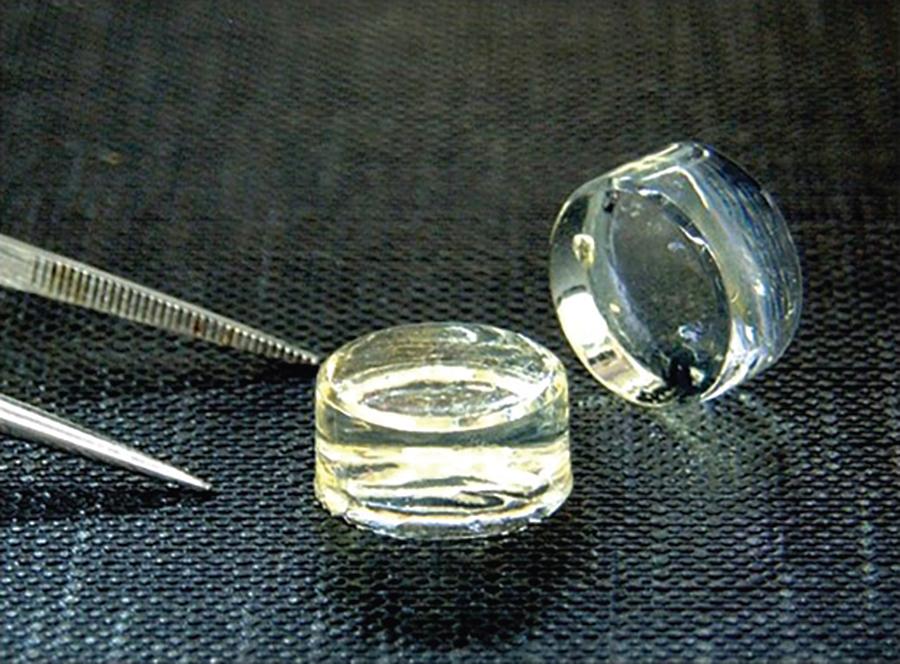
Another advanced marrow stimulation technique is the GelrinC technique (Regentis Biomaterials, Or Akiva, Israel), which uses an acellular biodegradable hydrogel scaffold composed of polyethylene glycol diacrylate (PEG-DA) and denatured fibrinogen injected into the defect following microfracture and crosslinked with UVA light in-situ ( Fig. 75.2 ) to form a semi-solid dense hydrogel matrix for MSCs ( Fig. 75.3 ). Trattnig et al. reported healthy cartilage quality in 81% of cases following GelrinC augmentation after microfracture in 21 patients as evaluated by MRI (global T2 index) after 24 months. The average MOCART score was reported to improve at each follow-up assessment from 6 to 24 months. In a recently published prospective single-arm, open label, multicenter study in 56 patients treated with the GelrinC technique for chondral and osteochondral lesions, cartilage evaluations were performed via morphological (MOCART) and quantitative (MRI T2-mapping) assessments. The mean MOCART score significantly increased from baseline to the 24-month follow-up for all lesions combined as well as for chondral lesions and for osteochondral lesions assessed separately. Furthermore, T2 mapping assessments revealed significant zonal variation of the repair tissue at 24 months, which did not differ significantly from healthy reference cartilage.
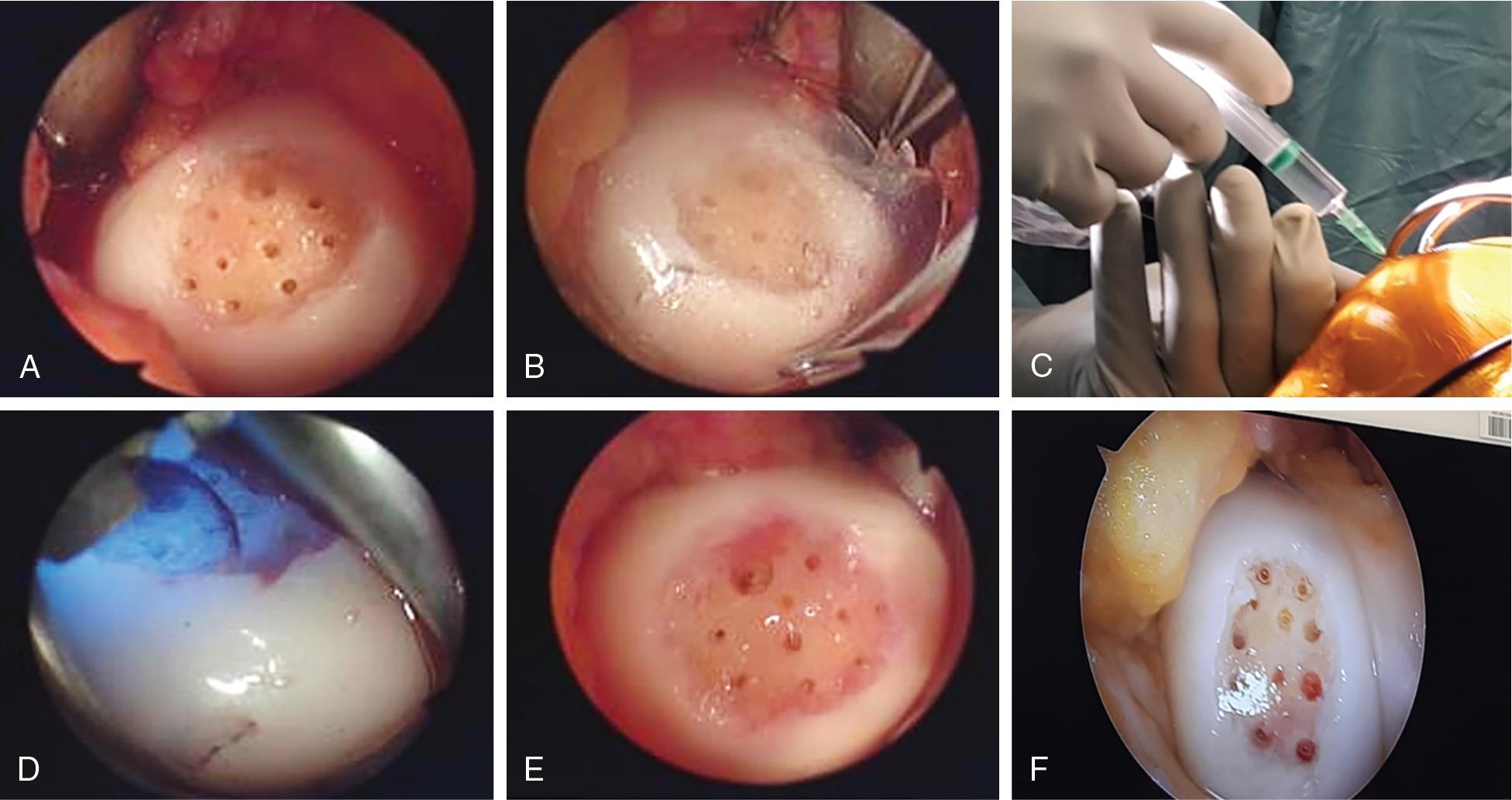
Hyalofast (Anika Therapeutics, Bedford, Massachusetts, USA) is a non-woven biodegradable hyaluronic acid-based scaffold used for the entrapment of mesenchymal stem cells (MSCs). Hyalofast is composed of a single 3D fibrous layer of HYAFF-11 (Fidia Advanced Polymers, Abano Terme, Italy), a benzyl ester biopolymer of hyaluronic acid (HA). While originally designed to be combined with MSCs concentrates such as BMAC (Bone Marrow Aspirate Concentrate), it can also be used to augment marrow stimulation techniques and provide a matrix for the MSCs arriving from the subchondral area. A recent study in 46 patients with grade 4 defects treated with microfracture + Hyalofast has shown statistically significant improvement in all categories of the KOOS (symptoms, pain, daily living, sports and quality of life) at 1, 2, and 3 years post-surgery compared to the preoperative assessment. MRI evaluations with T2 mapping in two patients revealed satisfying hyline-like cartilage filling of the defects. A histological assessment of samples obtained from one of the patients in a second look arthroscopy due to a meniscal tear 18 months following the procedure revealed the cartilage to be mainly fibrocartilage with islands of hyaline cartilage.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here