Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Congenital anomalies affect 1% to 3% of newborns, and approximately 10% of these children have upper extremity abnormalities. , The incidence of congenital anomalies has not changed appreciably over the last decade. Congenital limb anomalies are second only to congenital heart disease in the incidence of birth malformations. Most anomalies occur spontaneously or are inherited. Few malformations are attributed to teratogens. Limb anomalies can be produced in animal models; this has increased our knowledge of and insight into limb development.
Recent advancements in understanding limb anomalies are directly related to our increased understanding of embryogenesis. Knowledge of hominoid limb development has been greatly expanded by the experimental embryologists. Animal models with limb patterning similar to that of humans have been used to dissect and manipulate crucial signaling centers that affect limb development and orientation. Research on gene misexpression and loss of function has enhanced our understanding of limb formation. , There is intense work focusing on genotype-phenotype correlations that may have substantial clinical implications. The hand surgeon is uniquely positioned between clinical care and genetic research. He or she must possess a basic comprehension of embryogenesis and limb formation to comprehend congenital limb anomalies and to communicate relevant knowledge to the family of an affected child. Parents may initially bear a tremendous amount of guilt, and the role of disseminating reliable information is the responsibility of the physician caring for the family.
Limb development begins during embryogenesis with events that affect the position and orientation of each limb and the number of limbs that appear ( Table 35.1 ). The limb bud is first visualized at 26 days after fertilization when the embryo is about 4 mm in length (crown-rump length), or about the size of a piece of rice. , The bud rapidly develops through 47 days of life until the embryo is close to 20 mm in length, or the size of a lima bean. Fifty-two to 53 days after gestation, the embryo is 22 to 24 mm long and the fingers are entirely separate. Eight weeks after fertilization, embryogenesis is complete and all limb structures are present. , , At this point, the joints form as condensation of the chondrogen creates dense plates between future bones. Joint cavitation further forms the articulation, although proper joint development requires motion for modeling of the ultimate joint surface. Most upper extremity congenital anomalies occur during this 4- to 8-week period of rapid and fragile limb development. After 8 weeks’ gestation, the fetal period commences, with differentiation, maturation, and enlargement of existing structures.
| Week | Day | Upper Limb | Lower Limb |
|---|---|---|---|
| 4 | 24 | Swelling appears in region of upper limb bud | |
| 28 | Scattered blood vessels | Appearance of lower limb bud | |
| 5 | 32 | Upper limb AER | |
| Early marginal vessel | |||
| Early brachial plexus development | |||
| 33 | Hand plate appears | Lower limb AER | |
| Humeral mesenchymal condensations | |||
| 6 | 37 | Humeral chondrification | Lumbosacral plexus |
| Radial and ulnar mesenchymal condensations | |||
| Brachial plexus with radial, median, and ulnar nerves to the elbow | |||
| Early muscle masses | |||
| 41 | Finger rays (webbed) | Mesenchymal condensations of femur, tibia, fibula, and tarsus | |
| Radial, ulnar, and metacarpal chondrification | |||
| 7 | 44 | Interdigital apoptosis | Chondrification of femur, tibia, and fibula |
| Scapular and humeral head chondrification | |||
| Chondrification of carpals and proximal phalanges | |||
| Trapezius innervated (accessory nerve) | |||
| Major muscles distinguishable | |||
| 48 | Chondrification of middle phalanges | ||
| Shoulder and elbow interzones (joint cavity formation) | |||
| 8 | 51 | Chondrification of distal phalanges | |
| 52 | Ossification of humerus | ||
| Ossification of radius | |||
| All muscles distinguishable | |||
| Wrist and carpal interzones | |||
| 54 | Ulnar ossification | Ossification of femur and tibia | |
| 57 | Scapular ossification | Ossification of fibula | |
| Intramembranous ossification of distal tip of distal phalanges | Chondrification of tarsus and digits |
a Selected milestones for the lower limb are provided for comparison.
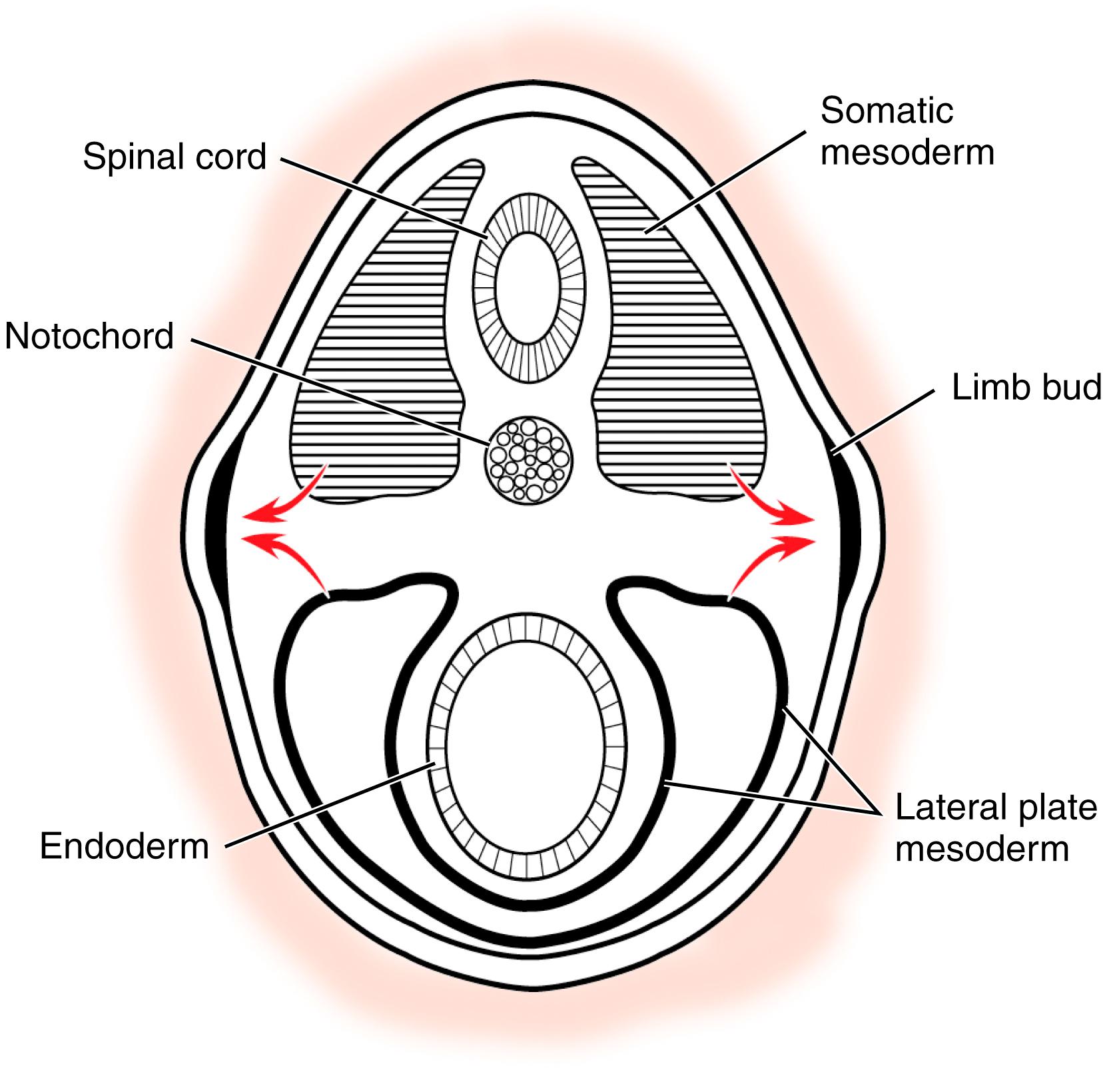
The limb bud represents an outgrowth of the mesoderm into the overlying ectoderm. Two sources of cells migrate from their origins into the limb bud. , The cells from the lateral plate mesoderm become bone, cartilage, and tendon. The cells from the somatic mesoderm form the muscular, nervous, and vascular elements of the limb bud ( Fig. 35.1 ).
In order to comprehend limb development and to explain it to families, the physician must appreciate the signaling centers that control the three spatial axes of limb development: proximodistal, anteroposterior, and dorsoventral. Each of these limb growth axes contains a signaling center (i.e., a group of cells) that is responsible for establishing the corresponding axes; they are the apical ectodermal ridge (AER), the zone of polarizing activity (ZPA), and the Wingless type (Wnt) signaling centers ( Table 35.2 ). , , A coordinated effort between the AER, ZPA, and Wnt pathways is necessary for proper limb patterning and axes development. The three signaling centers are interdependent so that the loss of one signal results in compromise of the entire system. There is considerable cross talk between these signaling pathways during limb development.
| Signaling Center | Responsible Substance | Action | Anomaly |
|---|---|---|---|
| Apical ectodermal ridge | Fibroblast growth factors | Proximal to distal limb development, interdigital necrosis | Transverse deficiency |
| Zone of polarizing activity | Sonic hedgehog protein | Radioulnar limb formation | Mirror hand |
| Wnt pathway | Transcription factor, Lmx-1 | Ventral and dorsal limb axis | Nail patella syndrome, abnormal nail and pulp arrangement |
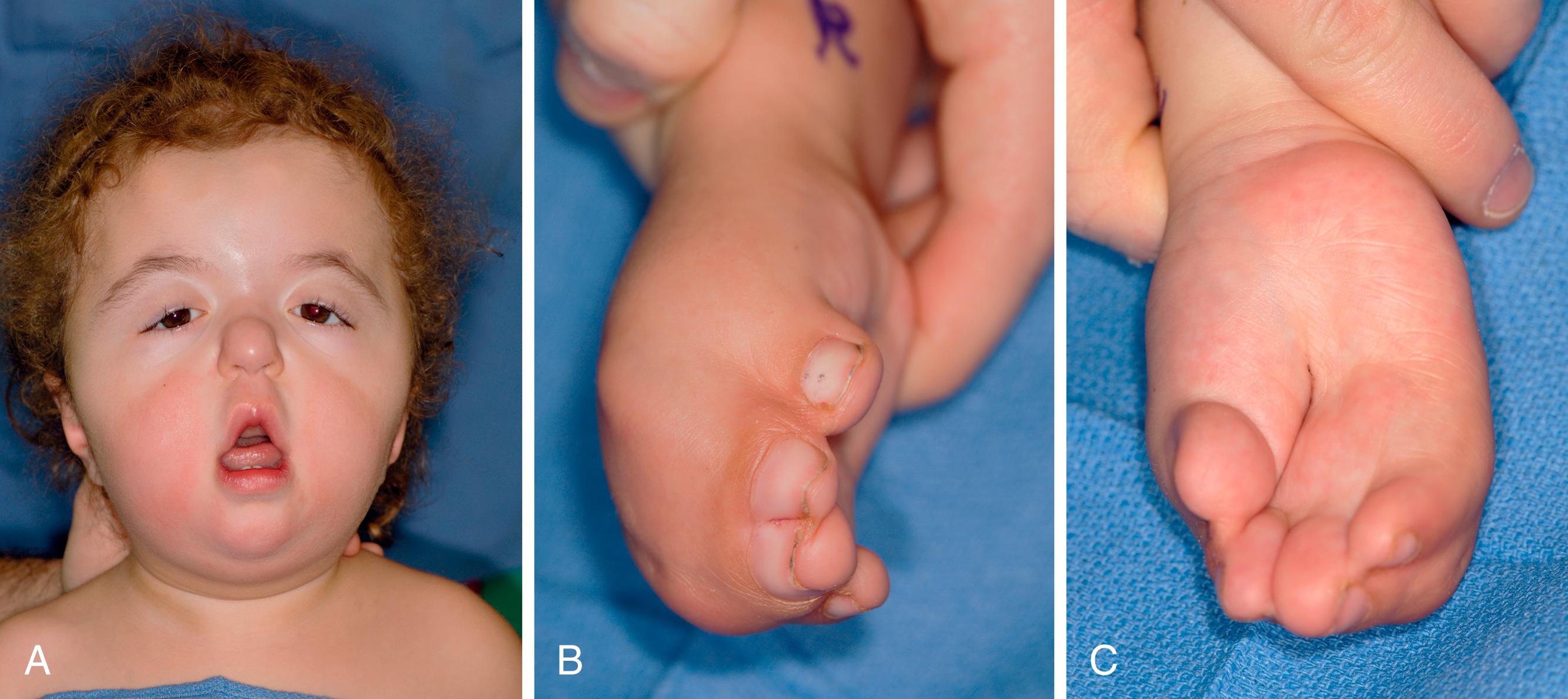
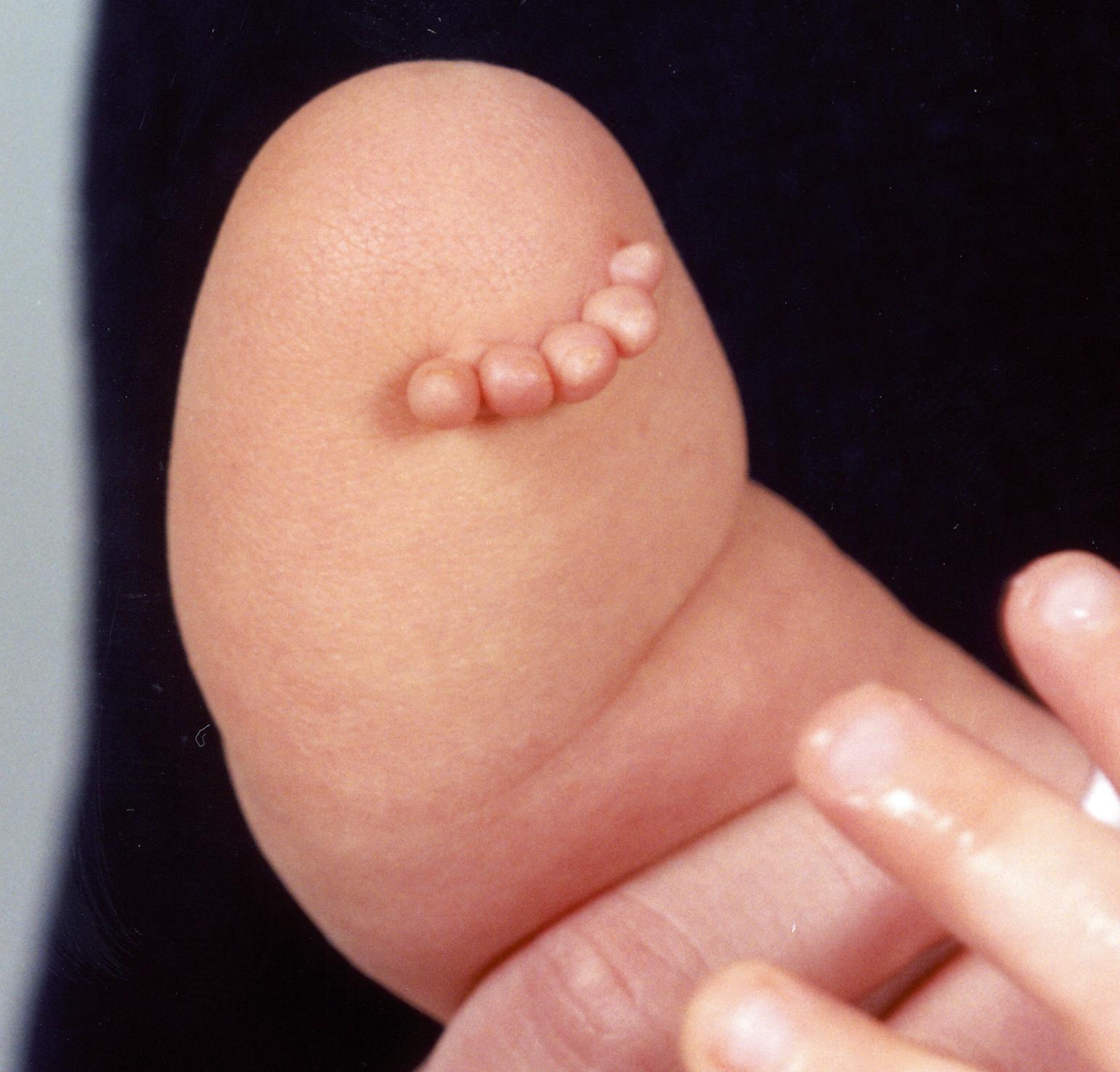
Programmed cell death plays an important part in limb development. Programmed cell death is an active process that is genetically controlled to eliminate unwanted cells during embryogenesis. Apoptotic cells undergo a degeneration process with DNA fragmentation and are eventually engulfed by phagocytic cells. Genetic control of cell death is necessary during limb bud formation. For example, the digits initially develop as webbed fingers. Extraneous tissue between fingers must undergo apoptosis and interdigital necrosis for finger separation. Failure of interdigital apoptosis results in syndactyly. Interestingly, bone morphogenetic proteins (BMPs), widely recognized for their role in chondrogenesis and osteogenesis, trigger apoptotic pathways of the interdigital mesenchyme to produce separated fingers. BMP antagonist can block BMP signaling and prevent apoptosis and interdigital necrosis. For example, bats have web limbs, and BMP is blocked during their limb embryogenesis. Similarly, altered signaling of fibroblast growth factors, such as occurs in Apert syndrome, can negate BMP-mediated apoptosis, resulting in syndactyly ( Fig. 35.2 ). Apert syndrome is inherited in an autosomal dominant pattern and can be passed on to future generations ( Fig. 35.3 ).
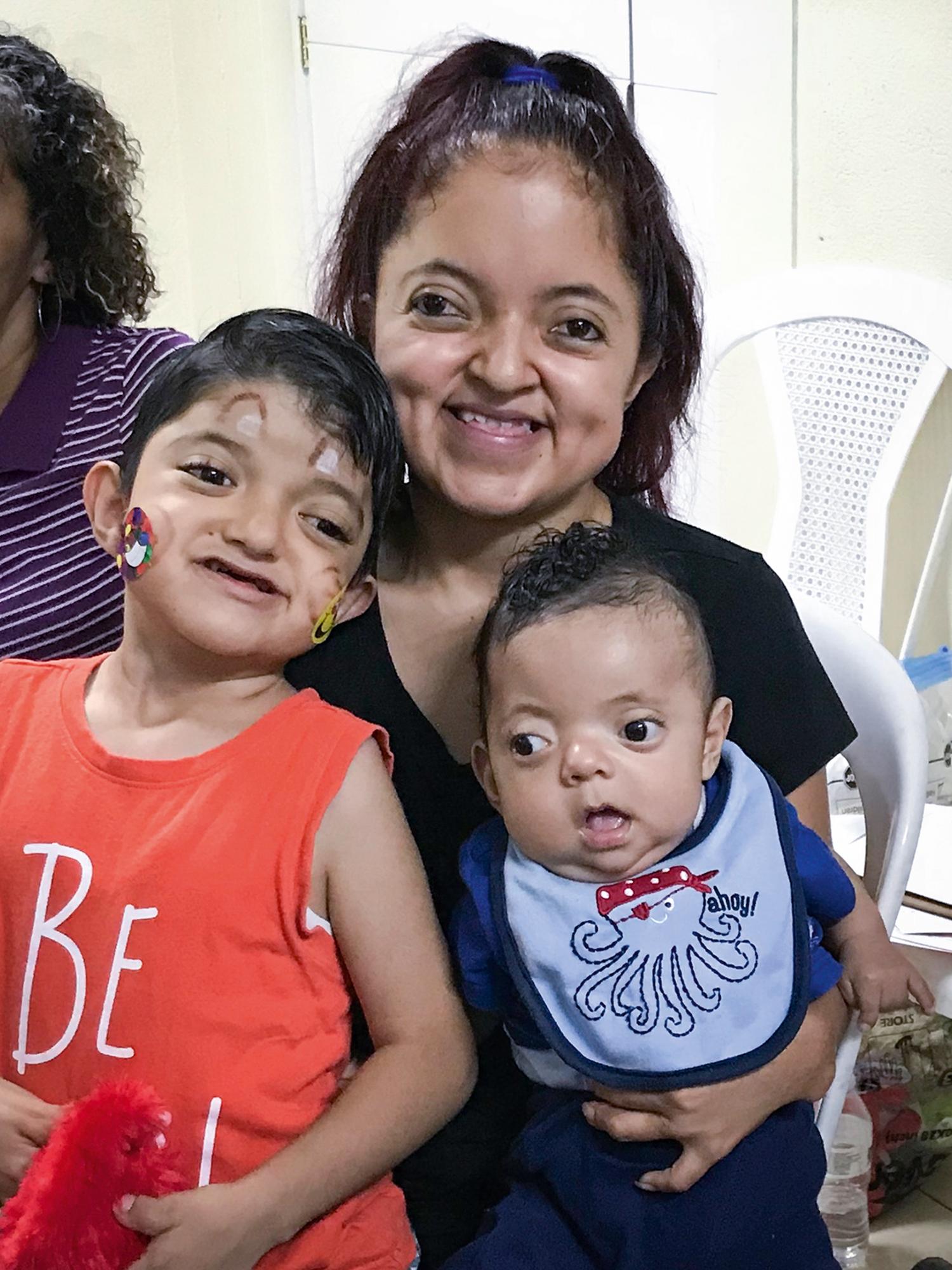
The limb develops in a proximal to distal direction, with the shoulder forming before the elbow and the elbow before the wrist (shoulder → arm → forearm → hand). This progression is controlled by the AER, a thickened layer of ectoderm that condenses over the limb bud. This signaling center guides the underlying mesoderm to differentiate into appropriate structures. , Removal of the AER results in limb truncation ( Figs. 35.4 and 35.5 ). Ectopic implantation of the AER causes additional limbs to form ( Fig. 35.6 ). , , , The secreted proteins within the AER that yield this effect are fibroblast growth factors. , In fact, removal of the AER can be overcome by application of fibroblast growth factors ( Fig. 35.7 ). Mice deficient in various fibroblast growth factors have complete transverse limb defects.
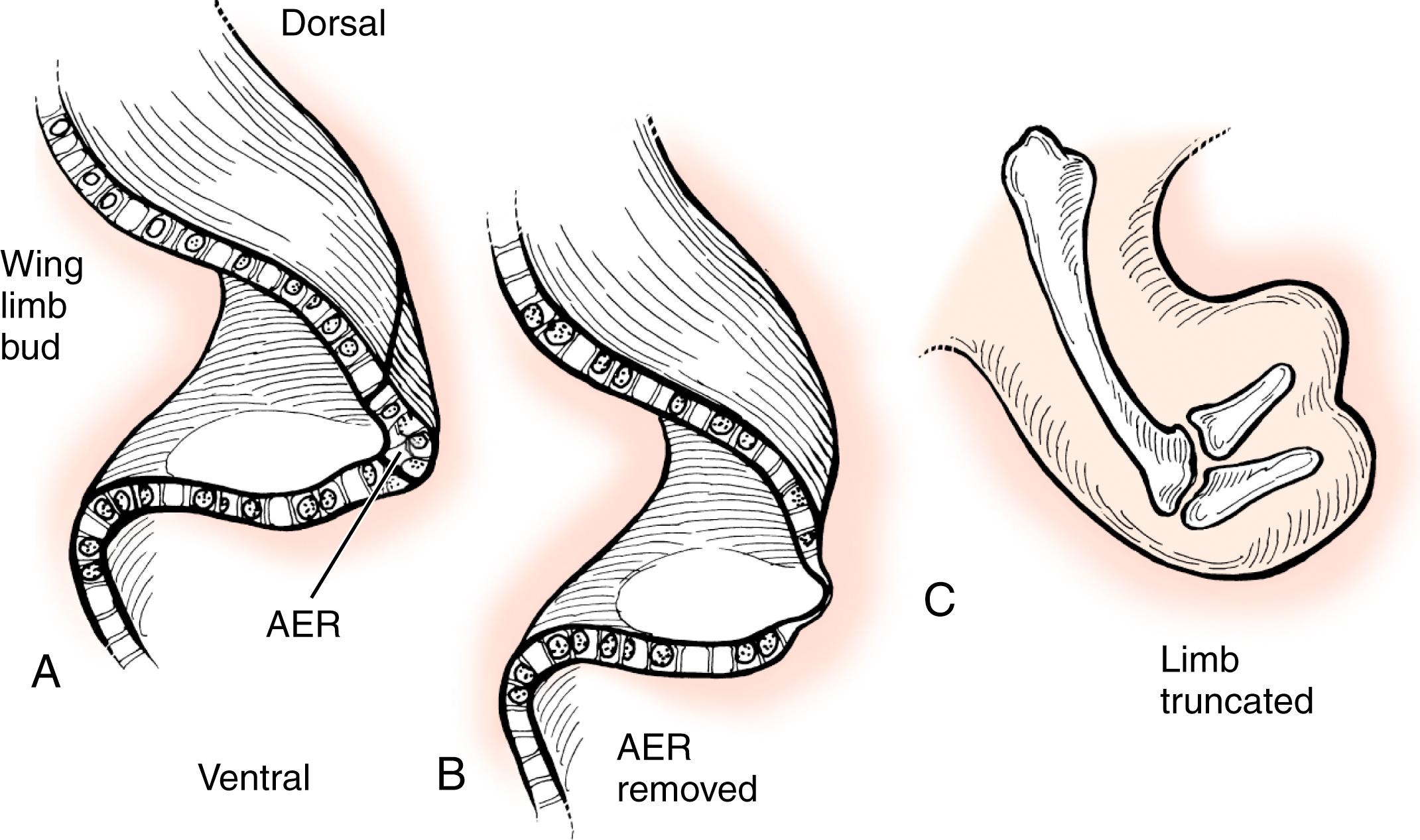
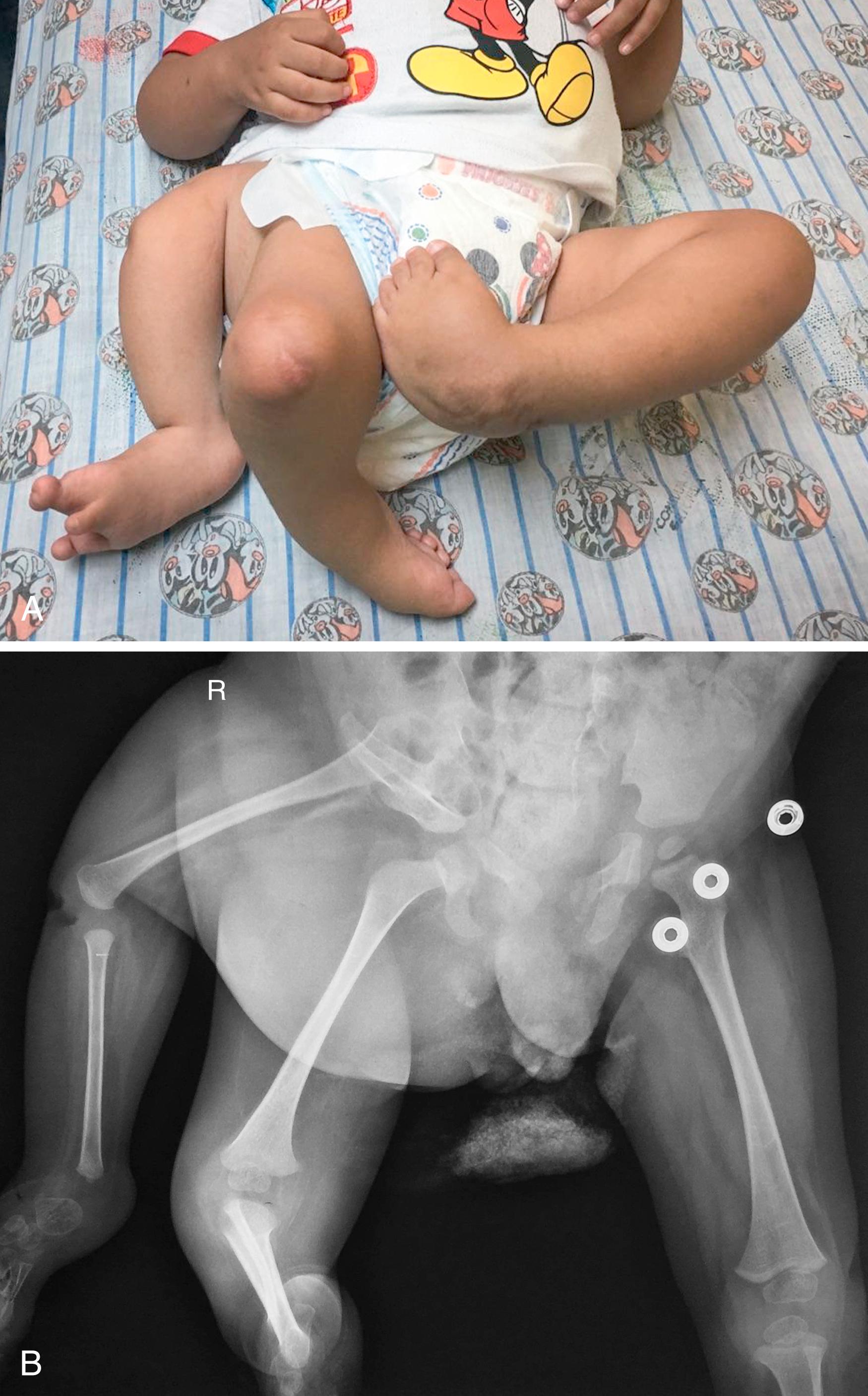
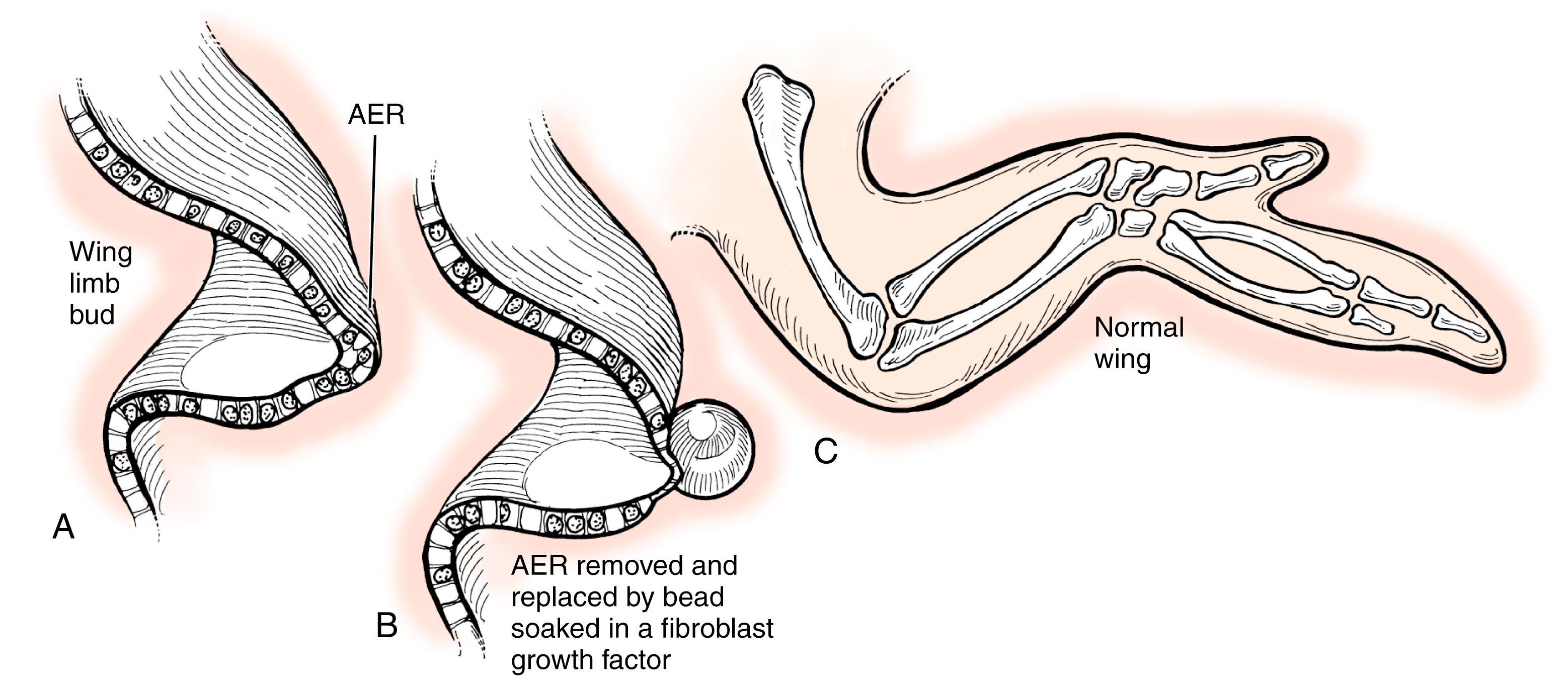
When explaining limb truncation or transverse deficiencies to a parent, we explain that the AER was not physically removed. However, we do have evidence to suggest that bleeding or ischemia within the AER resulted in its failure to work properly.
Transverse deficiencies are usually sporadic without any underlying reason. Concern for teratogen exposure is raised when multiple limbs are involved, suggesting a widespread insult to all developing limb buds. Transverse differences are not inheritable, and future children are unlikely to be affected.
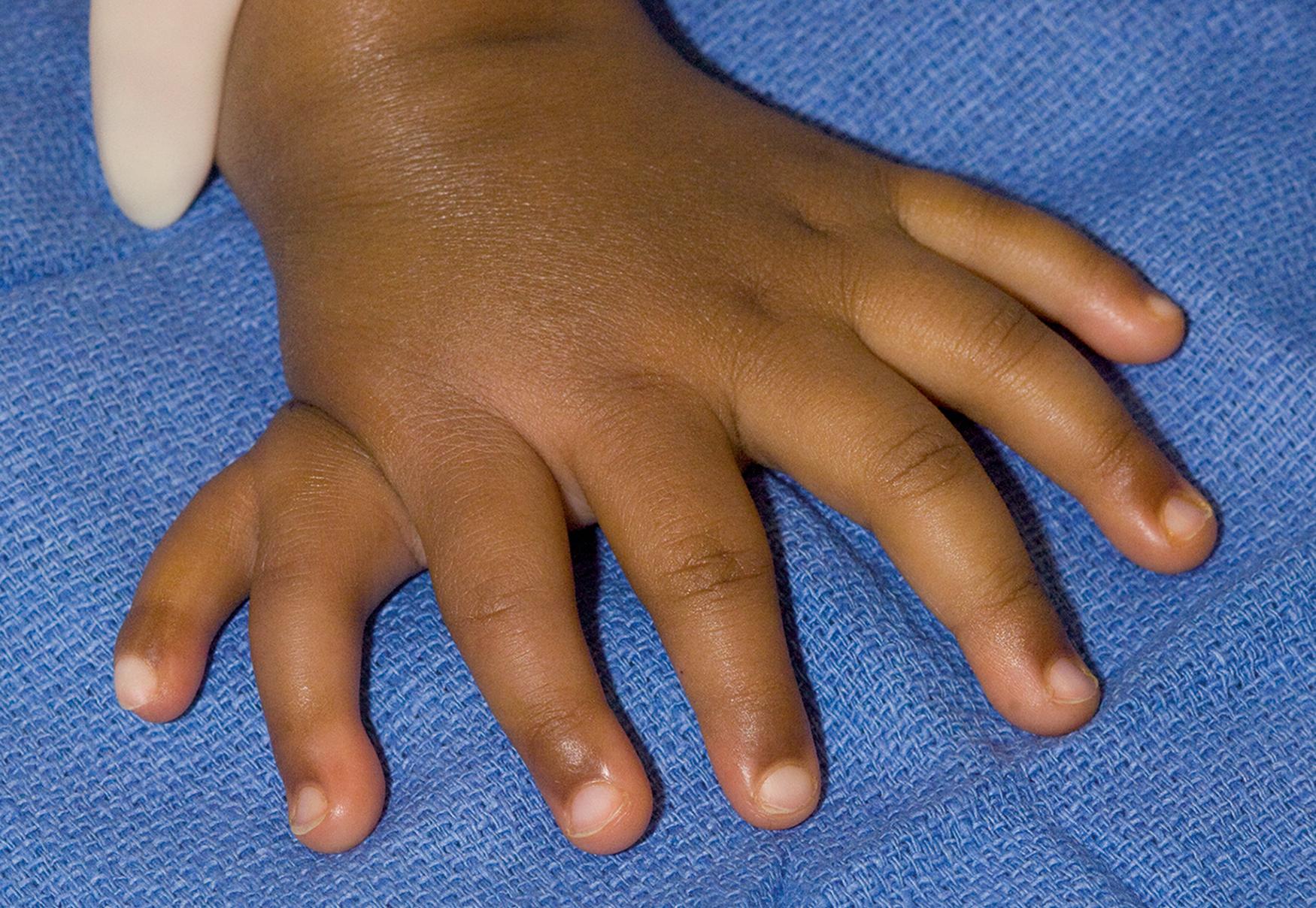
The limb also develops in an anteroposterior (i.e., radioulnar or preaxial-postaxial) direction. The ZPA resides within the posterior margin of the limb bud and functions as a signaling center for anterior to posterior limb development. , This signaling pathway polarizes the limb into a radial margin and an ulnar border. The signaling molecule within this pathway is the sonic hedgehog compound. Transplantation of the ZPA or sonic hedgehog protein causes mirror duplication of the ulnar aspect of the limb ( Fig. 35.8 ). The extent of duplication is dose dependent, and greater transference results in more replication ( Fig. 35.9 ). This explains the variable numbers of fingers and different phenotypes of mirror hands ( Table 35.3 ). Mutant mice that express sonic hedgehog protein in the anterior limb bud are polydactylous, with duplication of their ulnar digits. Triphalangeal thumbs (thumbs with three phalanges, instead of the usual two) arise secondary to point mutations, resulting in ectopic sonic hedgehog compound at the anterior margin of the limb bud.

| Type | Name | Clinical Features |
|---|---|---|
| 1 | Ulnar dimelia | Multiple fingers with two ulnas Type A: Each ulna well formed Type B: Preaxial ulna hypoplastic |
| 2 | Intermediate form | Multiple fingers with two ulnas and one radius |
| 3 | Intermediate form | Multiple fingers with one ulna and one radius Type A: Radius well formed Type B: Hypoplastic radius |
| 4 | Syndromic form | Bilateral, mirror feet, and nasal defects characteristic Type A: Laurin-Sandrow syndrome: two ulnas Type B: Martin syndrome: one ulna and one radius |
| 5 | Multiple hand | Complete duplication of the hand, including the thumb; forearm normal |
Sonic hedgehog compound is also expressed in a gradient fashion, and the duration of exposure of a digit to it determines the identity of the digit. The anterior digits (index and long fingers) are exposed for the shortest time, and the small digit is exposed for the longest time. ,
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here