Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
This chapter includes an accompanying lecture presentation that has been prepared by the authors: ![]() .
.
This chapter includes an accompanying lecture presentation that has been prepared by the authors: ![]() Video 77.1.
Video 77.1.
The most fundamental and clinically relevant electrophysiologic player in the nervous system is the neuron.
There are two approaches to study human brain electrophysiology: invasive and noninvasive.
Today’s neurosurgeon has at his or her disposal a myriad of techniques to explore the physiology of the human central nervous system at multiple spatiotemporal resolutions.
Using a combination of current techniques not only improves spatiotemporal resolution but also improves upon their inherent limitations.
To study the intricate orchestration of neural activity is exceedingly alluring. It is the synergy of excitations, inhibitions, and quiescence of cells that underlie homeostatic control, coordination of movement, and cognitive appraisal that establishes the vital executive role of the brain. It is for these reasons that electrophysiologic studies have attracted the foremost physiologists of the century. Considering their outstanding contributions, there are numerous fundamental questions in neuroscience that remain elusive. Traditionally, clinical electrophysiology has used a more pragmatic approach than nonclinical neurophysiology. Clinical insight into brain function (or dysfunction) is commonly achieved today by increasingly sophisticated imaging techniques, allowing real-time observations. The booming advancement of molecular biology, and its fundamental contribution to medicine in general and neuroscience in particular, has unveiled an incredible level of ordered complexity in neuronal function. Basic scientists are producing a large quantity of molecular data, spanning from investigations of the role of a single protein in the electrical behavior of neurons to genetic markers of neurological disease. Notwithstanding the immense popularity of these approaches, we must recall that the electrical properties of individual neurons, and their associated microenvironments, are the final arbiters of brain activity, consequently making diseases of the brain derive from abnormalities at the cellular level. Hence, it is reasonable to envision research directed toward developing innovative methods to investigate neurophysiologic phenomena.
Central nervous system (CNS) function is dependent on homeostatic mechanisms that accurately regulate the extracellular level/concentration of neurotransmitters, ions, pH, and other variables. The neuronal cell membrane is a complex biochemical entity that interfaces between the cell and its environment. Its functions include the directional transport of specific substances and the maintenance of electrochemical gradients across the plasma membrane. These ion gradients can be of high specificity (e.g., sodium vs. potassium ions) and of great functional significance (e.g., in the production of action potentials). In addition, CNS function is supported by numerous nonneuronal mechanisms responsible for the control of extracellular and intracellular homeostasis (glial cells, cerebral vasculature). It has become strongly evident that pathophysiologic alterations of ion channel function play a major role in the etiology of certain disorders of the nervous system.
The following introductory chapter on CNS electrophysiology does not intend to explain at length the complex biophysical properties essential to communication between individual neurons, nor does it seek to unravel the mechanisms behind signal transduction of environmental and sensory signals. There is a wealth of information from other resources pertaining to CNS function and electrophysiology along with recent publications that concisely yet comprehensively describe the complex properties of ion currents responsible for neuronal behavior. This chapter provides the reader with succinct background information on the electrical properties of neurons, and additionally focuses on other aspects of brain function relevant to modern understanding of the pathophysiologic changes occurring in the diseased brain. These include the description of some of the mechanisms involved in brain homeostasis, the genesis of synchronous activity by electrotonic and/ or ephaptic interactions, and molecular changes in ion channels underlying neurological diseases. Because complete referencing of such a broad topic would entail a bibliography of thousands of references, only relevant recent reviews, textbooks, and a nonexhaustive compilation of representative work are included.
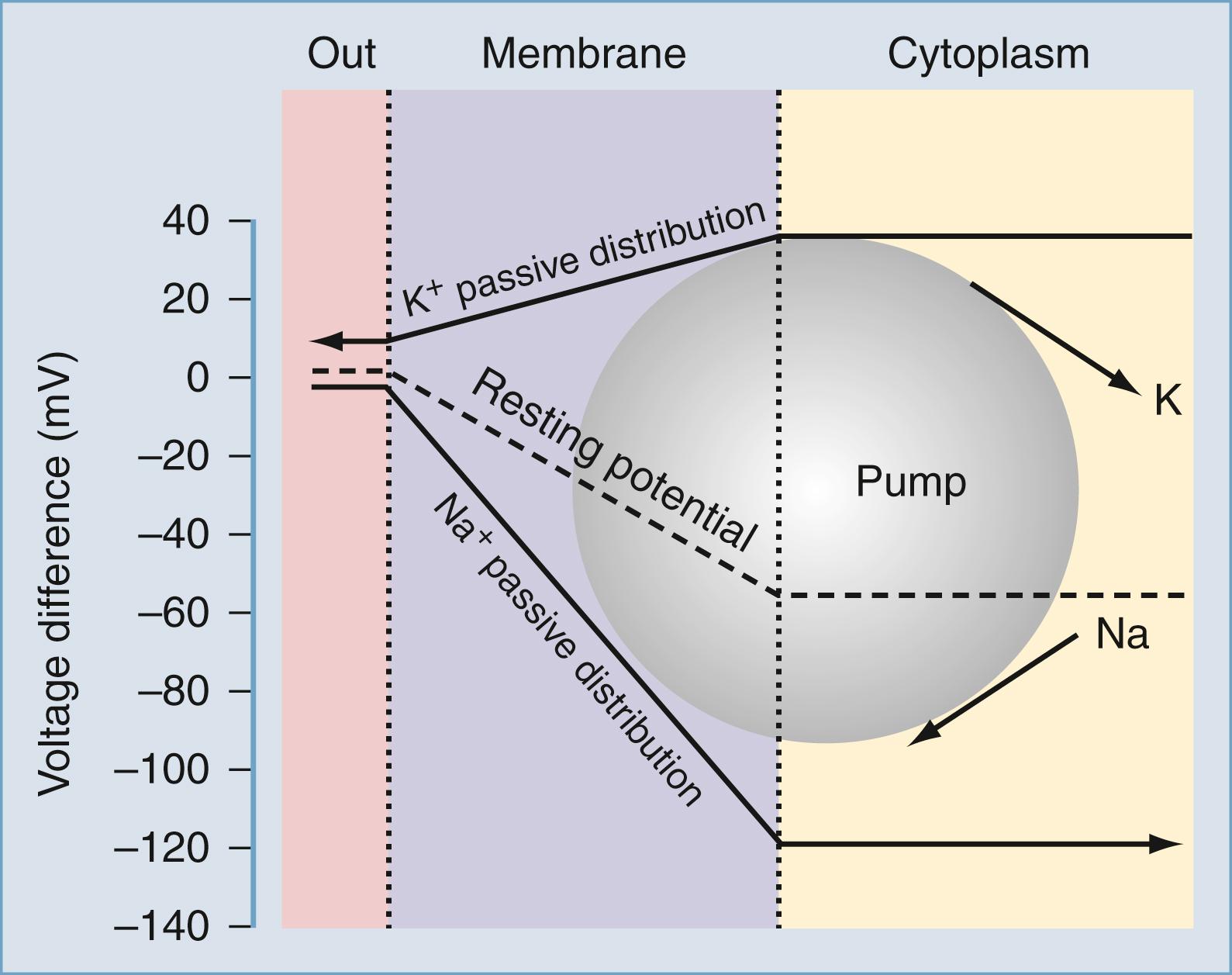
Matter is composed of atoms, which consist of positively charged nuclei and negatively charged electrons. Electrical phenomena occur whenever charges of opposite sign are separated or moved in a given direction: static electricity is the accumulation of electrical charge. An electrical current results when these charges flow across a permissive material, called a conductor. An ion current is a particular type of current carried by charges present on atoms or small molecules flowing in aqueous solution. Separation of charges in an aqueous solution can be achieved by inserting an impermeable membrane into the solution itself. In mammalian cells, these membranes coincide with the plasma membrane, and its lipophilic composition ensures a remarkable level of electrical isolation for cells and tissues. Excitable as well as most nonexcitable cells are characterized by an asymmetrical distribution of electrical charges across the plasma membrane. The biophysical bases for the maintenance of this electrical potential have been extensively investigated experimentally and modeled by mathematical simulations. Under normal resting conditions, mammalian cells allow transmembrane ion currents so that the internal portion of the cell is negatively charged; the presence of nonpermeant anions such as proteins also contributes to the maintenance of transmembrane potentials. This relatively stable state results in a net transmembrane potential of several millivolts (mV) and is commonly referred to as the resting membrane potential (RMP; Fig. 77.1 ).
![Figure 77.1, Relationship between resting membrane potential (RMP) and extracellular potassium ions ([K + ] out ). Figure 77.1, Relationship between resting membrane potential (RMP) and extracellular potassium ions ([K + ] out ).](https://storage.googleapis.com/dl.dentistrykey.com/clinical/ElectrophysiologicPropertiesoftheMammalianCentralNervousSystem/0_3s20B978032366192800077X.jpg)
Most of what we know about the physiology of excitable cells is derived from electrical measurements ( Fig. 77.2 ). Our knowledge of the complex properties of the CNS is based on the application of simple physical rules governing movement of charged particles to neuronal cells (see Table 77.1 for explanations of commonly used electrophysiology terms). The physical principles of cell electrophysiology can be thus be compared to the biophysical rules governing electrical current flow through a so-called RC circuit (see Fig. 77.2 ), where electrons flow through a resistive component (represented by ion channels in the case of living cells) set in parallel to a capacitive component (represented by the poorly conductive phospholipid bilayer). Ohm’s law describes the relationship between current flow (I) and resulting voltage drop (E) across a resistor when no capacitive component is present. This is of course an abstract situation, in particular when dealing with ion fluxes across biologic membranes, but allows one to understand the basic principles governing electrical current flow.
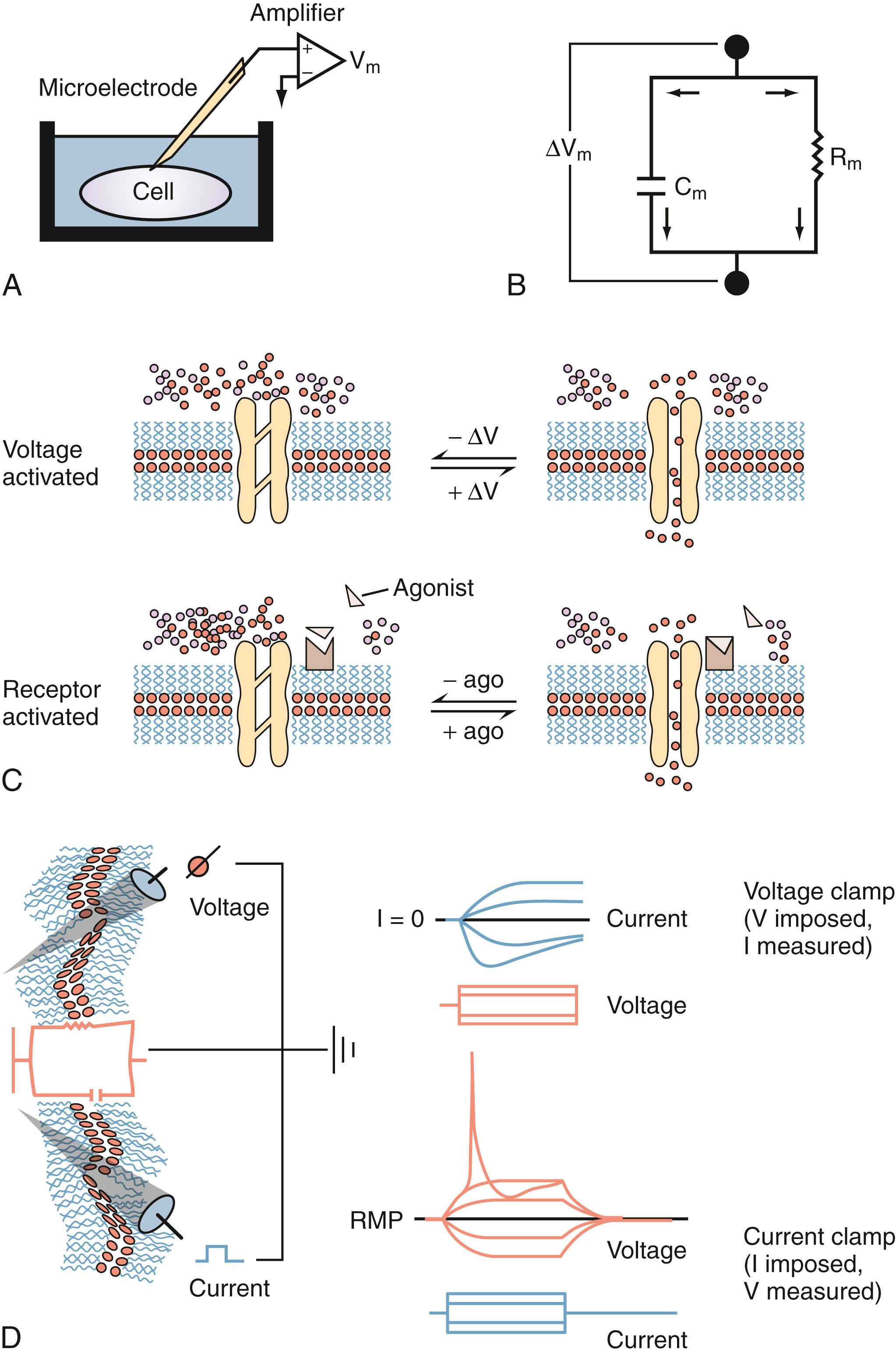
| Term | Definition |
|---|---|
| Inward current | Positive charges enter the cell (e.g., I Na responsible for action potential upstroke) |
| Outward current | Positive charges leave the cell (e.g., I K during action potential repolarization) |
| Depolarization | Change of RMP to less negative values (e.g., EPSP) |
| Hyperpolarization | Change of RMP to more negative values (e.g., IPSP) |
| Inward-going rectification | Tendency of some ionic currents to allow passage of inward-flowing but not outward-flowing ions (inward rectifier potassium currents) |
| Outward-going rectification | Tendency of some currents to allow passage of outward-flowing but not inward-flowing ions (most other potassium currents activated by depolarization) |
| Voltage clamp | Electrophysiologic technique allowing the study of ion currents and their modulation by voltage, or transmitters, or second messengers |
| Current clamp | Technique used during intracellular recordings to determine resting properties and excitability of cells |
| Single-channel recording | Modern variation (patch clamp) that allows study of the electrophysiologic properties of a single ion channel or protein |
| Multi–single-unit recording | Extracellular recording from a neuron or a cluster of neighboring neurons |
| Ephaptic transmission | Extracellular electric fields that affect neuronal activity, coding, and information processing |
The following relationship constitutes Ohm’s law (or principle):
where I represents the current flowing through a resistance (R) when a voltage (E) is applied. In a hypothetical cell this relationship can be written as follows:
where E m represents the voltage difference (in mV) between the inside and the outside of the cell, I m represents the net current flowing at that particular time across the cell membrane, and R m is the total membrane resistance. An important consequence of this relationship is that small changes in current will significantly affect cell RMP only when R m is large. This is particularly important for inhibitory synaptic currents: in the case of γ-aminobutyric acid (GABA) type A receptor activation, inhibition is achieved not only by hyperpolarizing cell RMP further away from the firing threshold but also by greatly increasing postsynaptic conductance, a
a Since the most common interest is in permeation (or flux) of charges across the membrane rather than its nonconductive properties, the term membrane conductance (G m ; equals 1/R m ) is used instead of R m .
thereby reducing the synaptic efficacy of concomitant excitatory signals.
A mathematical derivation of Ohm’s law useful for studies of biologic membranes was first formulated by Nernst, who described the relationship between intracellular and extracellular ion concentrations and transmembrane potential changes attributable to permeation of an ion. In the case of potassium ions, the Nernst equation can be written as follows:
where E K is the potassium equilibrium potential; R and F are the gas and Faraday constants, respectively; T is the temperature at which the observation is performed; and z is the charge of the permeant ion. Note that if the charge sign (z in Eq. 77.3 ) of the permeant ion is changed (e.g., if we look at the Nernst equation for chloride [Cl − ]), the direction of the gradient is changed as well. The logarithm of the ratio between intracellular ([K + ] in ) and extracellular ([K + ] out ) potassium ions (K + ) dominates the right side of the equation since RT/zF is constant under most biologic conditions where temperature can be maintained within a few degrees. This equation predicts, as expected from Ohm’s law, that net potassium fluxes will approach zero when intracellular and extracellular potassium are iso-osmolar (i.e., when E K = 0 mV). Under physiologic conditions ([K + ] out = 3.5 mM; [K + ] in = 135 mM) the transmembrane potential at which potassium fluxes will be nil is around −90 mV. b
b The reader should bear in mind that ionic concentrations are not always constant. In reality, large changes occur during sustained neuronal activation or during pathologies when ion homeostasis is impaired (e.g., ischemia).
This value constitutes the potassium equilibrium potential at these concentrations. Note that small changes in external potassium concentration will cause relatively large changes in the fraction of total membrane currents attributable to potassium ions. Since permeability to potassium is essential in the maintenance of RMP, net increase in extracellular potassium will cause a significant departure from RMP (see Fig. 77.1 ).
In practical form, the Nernst equation for potassium can be rewritten converting to log 10 and by calculating RT/zF at 20 °C. These rearrangements lead to
It can be seen that a 10-fold change in the concentration gradient for potassium can produce a 58-mV change in membrane potential. Since under normal conditions there is an almost 40-fold outward gradient for potassium ions and a 12-fold inward gradient for sodium ions, the resulting equilibrium potentials are −92 mV and +65 mV, respectively. Since the membrane at rest is much more permeable to potassium than sodium (see below), the RMP is closer to E K than to the sodium equilibrium potential (E Na ).
Two additional considerations help to understand the genesis of cell RMP. First, if the membrane is exclusively permeant to potassium and no active, electrogenic transport of ions occurs, then the cell RMP will tend to equal E K and only small movements of potassium will be sufficient to maintain the RMP at −92 mV. Second, if the membrane potential is clamped at E K , then net potassium fluxes will be zero (as predicted by the fact that E K is the equilibrium potential for potassium). If the RMP is held at potentials positive to E K , outward potassium currents will develop; conversely, if the RMP is held at potentials negative to E K , inward potassium fluxes will develop. Interestingly, the notion that cell RMP is controlled by potassium ions was first derived independently from direct observations: Julius Bernstein in 1902 correctly predicted that in most mammalian cells potassium ions govern the transmembrane voltage difference. Direct experimental evidence was only achieved a half-century later when microelectrodes could be fitted into cells to directly measure RMP and the effects of changing [K + ] out . Bernstein also proposed that selective potassium permeability was lost during the process of excitation, during which numerous “pores” opened, allowing entry of other small ions (Cl − and Na + ). This theory explained several features of the regulation of RMP and the generation of action potentials, including the depolarizing effects of [K + ] out .
In fact, a prediction of the formalism presented previously is that RMP changes linearly with [K + ] out . As shown in Fig. 77.1 , experimental evidence contradicts this notion, at least in the case of mammalian neurons. c
c A linear dependence of RMP on [K + ] out is found in other CNS cells, such as glia and brain microvascular endothelium.
Most neurons depolarize significantly after large changes in extracellular potassium, whereas changes related to physiologic potassium concentrations do not significantly affect neuronal RMP. This can only be due to concomitant participation of other conductances. While the exact nature of the ionic conductances contributing to the regulation of neuronal RMP varies between different neurons, a generic set of equations predicts how these “parallel” conductances may affect E m . The most illustrious of these equations was provided by Goldman, who described the expected RMP in a cell endowed with more than one ion current mechanism as:
where G K, G Na , and G Cl represent the conductances for potassium, sodium, and chloride, respectively. Note that these conductance values are multiplied by the relative chemical concentration gradient for each ion, thus combining the “passive” electro-osmotic tendency for ion permeation with the average conductance of the membrane for a particular ion. Note also that if G Cl and G Na are close to zero, the transmembrane potential is governed almost exclusively by potassium ions and their conductance. This condition is common at resting potential in most neurons and glial cells, where the potassium current (I K ) and, to some extent, the current generated by the Na + ,K + -ATPase pump (I pump ) determine RMP ( Fig. 77.3 ).
In 1949 Alan Hodgkin and Bernard Katz first applied the Goldman equation systematically to changes in membrane potentials evoked by altering external ion concentrations in the squid giant axon. They measured changes in RMP induced by changes in [K + ] out , extracellular sodium [Na + ] out , and extracellular chlorine [Cl − ] out . They discovered that while changes in extracellular potassium dramatically changed RMP, comparable changes in [Na + ] out had little effect. Changing [Cl − ] out had an intermediate effect. The following ratios for permeability of potassium (P K ), sodium (P Na ), and chlorine (P Cl ) were obtained at rest:
When the measurements were performed at the peak of the action potential, however, these values changed dramatically:
Therefore when the predominant membrane conductance is P K , the Goldman equation ( Eq. 77.3a ) can be reduced to the Nernst equation for potassium:
whereas when P Na predominates, the following applies:
which is approximately the peak value of action potential overshoot. The direct measurement of changes in relative permeability for sodium and potassium contradicted one of the hypotheses formulated by Bernstein, who incorrectly predicted that neuronal excitation was due to loss of potassium permeability rather than activation of an inward sodium current. Had this hypothesis been correct, the maximal depolarizing value reached during the action potential would be around 0 mV, and not +30 mV as experimentally determined by Hodgkin and Katz.
More recently, it has become apparent that the sodium current (I Na ) is not the only ionic current that can generate action potentials: the current produced by calcium ions (I Ca ) also plays an important role in neuronal excitability. Similarly, I K is not the exclusive component of the electrical regulation of cell resting properties, as several other conductances are involved in the control of neuronal resting potential.
Ion channels are protein channels in cell membranes that allow ions to move from the extracellular solution to the intracellular solution and vice versa. Similarly, transporters are specialized enzymes that carry specific ions or molecules across otherwise impermeant membranes, or against electro-osmotic gradients. Not surprisingly, from a purely thermodynamic (or energetic) point of view, ion channels are less “expensive” to operate, whereas pumps or exchangers require considerable consumption of energy. Most ion channels and pumps are selective, allowing only certain ions to pass. Additionally, an individual cell has ion channels with various ion selectivities. In the context of studies of biologic cell membranes, the term ion selectivity refers to the ability of all cell membranes to distinguish between various ions such as Na + , K + , Ca 2+ , and Cl − . We will focus on Na + , K + , and Ca 2+ channels. All of these voltage-gated channels are made up of one or more pore-forming α subunits and variable numbers of accessory subunits, denoted β, γ, and so on. The α subunits determine the ion selectivity and mediate the voltage-sensing functions of the channel. The voltage-gated mechanism of ion-selective channels, which is highly characteristic of neurons and other excitable cells, consists of the electrostatic translocation of positively charged amino acids within their transmembrane domains (upon membrane potential shifts), which in turn allows the flow of solvated ions between fluid compartments. Ion selectivity involves specific pores or channels in the cell membrane, with certain channels specific for certain ions, and the channels capable of opening or closing (gated) depending on conditions and various interactions with ligands binding to receptors. These receptors are in some cases part of the channel itself and in other cases neighboring entities that control channel dynamics. The selectivity of an ion channel can be “gated”—the channel effectively opened or closed—and ion channels are said to be voltage-gated or ligand-gated depending on how the change in selectivity is provoked. A summary of the most studied CNS ion channels is shown in Table 77.2 .
| Ion Channel a | Localization | Physiologic Significance | |
|---|---|---|---|
| Na + | Fast Na current (I Na ) | All neurons | Generation of action potential |
| Slow Na current | Astrocytes b Pyramidal neurons |
Depolarizing afterpotentials; firing rate | |
| K + | Delayed rectifier (I DR ) | All neurons | AP repolarization |
| Inward rectifier (K IR ) | Most glial cells, some neurons | K + homeostasis (glia) | |
| I hERG | Astrocytes | GABA type B inhibition (neurons) | |
| M channel (I M ) | Neurons | Presynaptic effects of adenosine | |
| K ATP channel | Neurons, glia, BBB | K + homeostasis Firing threshold, RMP (modulated by Ach) Couple intracellular metabolism to electrical activity |
|
| Cl − | I GABA | Neurons, glia | GABA type B inhibition (neurons) Unknown in glia |
| Ca 2+ | I Ca T-type | Neurons | Firing threshold |
| I Ca L-type | Neurons | Neurotransmission | |
| I Ca N-type | Neurons | Synaptic release | |
| I Ca not found in glia, BBB endothelium | |||
| Mixed (K + , Na + ) | Anomalous rectifier (I h ) | Neurons, glia, BBB endothelium | Neuronal firing rate; firing threshold |
| I AMPA | Most neurons, glia | Homeostasis (?) Synaptic transmission |
|
| Mixed (K + , Na + , Ca 2+ ) | I NMDA | Most neurons | Synaptic transmission, LTP, and LTD |
Neuronal cells use a single type of signaling based on all-or-nothing action potentials. Sodium action potentials such as those recorded in axons or cell bodies are relatively invariant in normal tissue, and thus the shape and duration of these electrical signals does not change significantly within neuronal subtypes in the nervous system. Calcium action potentials are similarly predictable, but the underlying ionic mechanism can be rather complex, depending on the cell type and on the topographic location within the cell (see later). Calcium action potentials are more commonly seen in pacemaker-type cells (e.g., cardiac myocytes of the sinoatrial and atrioventricular nodes for cardiac muscle contraction and the enteric interstitial cells of Cajal for peristalsis). The terms sodium action potential and calcium action potential refer to the initial (depolarizing) phase of these rapid membrane polarity changes. While genetic or molecular alteration of I Na and I Ca can significantly affect neuronal firing and, ultimately, CNS and peripheral nervous system neurophysiology, it must be remembered that gross changes in neuronal excitability may also result by altering the repolarization phase of individual action potentials (i.e., either lengthening or shortening the repolarization period by modulating K + permeability).
Action potentials have a characteristic shape once a certain threshold is reached. In normal tissue, stereotyped electrical events follow the initial depolarization ( Fig. 77.4 ). The sequence can be described as follows:
The RMP moves from an initial negative value (−65 to −80 mV for most neurons) toward the so-called threshold for activation of sodium channels (around −40 mV). This change can be slow and may occur spontaneously due to fluctuations in RMP. Alternatively, the threshold is rapidly attained when the initial depolarization is triggered by a synaptic potential (or a summation of synaptic potentials).
After reaching the threshold value, an extremely rapid (1- to 2-ms) depolarization occurs, due to opening of sodium channels and massive influx of sodium ions into the cell.
Termination of the “upstroke” phase depends on a combination of factors: voltage- and time-dependent inactivation of I Na and concomitant decrease of the driving force for sodium, occurring in parallel to voltage-dependent activation of I K .
Further increases in the permeability to potassium ions, and the resultant K + efflux, restore the pre–action potential RMP values, forcing the membrane toward E K .
Under most circumstances, an undershoot of cell resting voltage occurs (a few millivolts from RMP); this is due to residual activation of I K and to the contribution of the electrogenic Na + ,K + -ATPase, extruding 3 Na + ions in exchange for 2 K + . The return to pre–action potential voltage favors the so-called removal of inactivation, a necessary step allowing a subsequent cycle of depolarization-induced action potential firing.
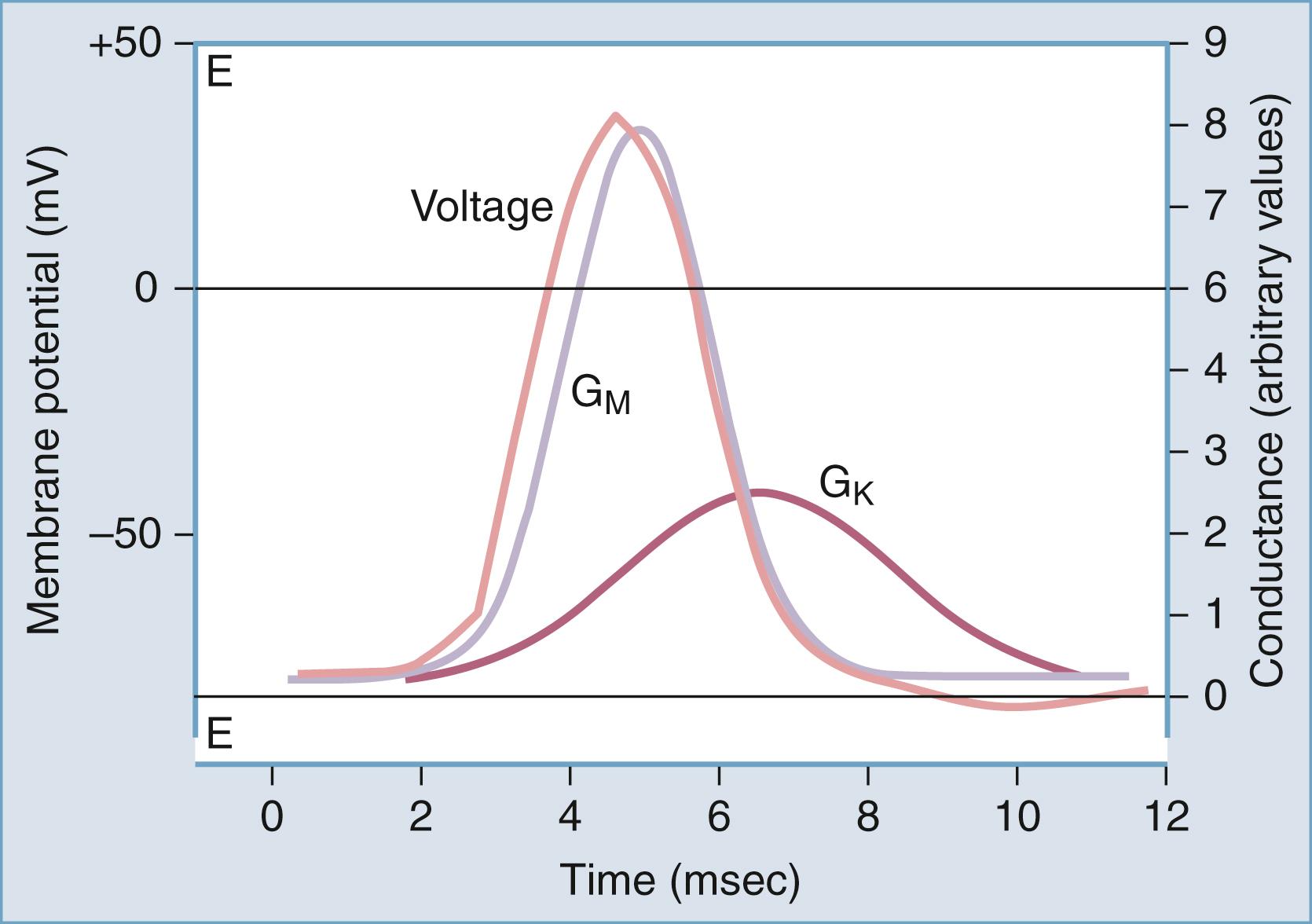
This stereotyped and relatively simple sequence of events is typically recorded in axons; other more complex interactions of various I Na and I K may lead to slightly different voltage profiles.
From a functional standpoint, it is important to remember that genesis of fast sodium action potentials is a hallmark of neuronal function, to the degree that during neurophysiologic recordings, presence or absence of Na + spikes is frequently used to determine the neuronal or glial cell type. Recently this notion has been challenged, and glial “action potentials” have been reported with increasing frequency. These responses, however, appear to be usually associated with pathologic conditions (e.g., brain tumors, epilepsy) and the old perception that neuronal cells are the exclusive tenants of sufficient I Na density to promote active responses is still generally accepted. Within the same neuronal cell, Na + channels involved in the generation of action potentials can be located heterogeneously, and it is common that clusters of channels are located at specific and crucial membrane segments. The most commonly encountered clustering of Na + channels occurs at the nodes of Ranvier of myelinated axons, but clustering also occurs at synaptic contacts, dendrites, and cell bodies, in proximity of the initial segment of axons.
Early pharmacologic studies attempting to elucidate the ionic components of the action potential greatly benefited from the availability of naturally occurring toxins that specifically and powerfully block I Na . The magic bullet for sodium channels was tetrodotoxin (TTX), a lethal poison produced by a specific genus of pufferfish ( Takifugu ). Interestingly, the testes of these hermaphroditic fish are true culinary delicacies but must be carefully separated from the TTX-containing liver and female genital organs to ensure consumer survival. Sea anemone toxin and α-scorpion toxin are also powerful blockers of I Na . TTX as well as other natural toxins block the external portion of the ion channel. Interestingly, while most sodium channels are blocked by micromolar concentrations of TTX, genetic ablation of one amino acid (Tyr or Phe) in the sequence of the ion channel protein confers relative resistance to TTX; this is due to the fact that binding of the toxin is restricted to this region. The amazing specificity of TTX binding suggested that normally occurring variation in the amino acid sequence of ion channels may prove an extremely important determinant of not only the pharmacologic properties of the channels, but also of their inactivation, activation, and voltage dependency. In fact, while the poisonous actions of TTX are primarily due to direct blockade of Na + fluxes through the channel’s pore, sea anemone toxin and α-scorpion toxin bind to a portion responsible for channel inactivation. Mutations of these regions cause faulty inactivation, a condition linked to neuropathogenesis.
The biophysical correlates of Na + channel function are well understood today. The general scheme of
explains the properties of whole-cell I Na recorded from neurons. The voltage dependency of each process justifies the initial depolarization required to promote opening of channels; the consequent depolarization induced by sodium current promotes further opening of channels, the process being terminated by time- and voltage-dependent closure of the channels. The passage from closed to open (and vice versa) is referred to as activation ( deactivation ), while the passage from open to inactivated is called inactivation. Removal of inactivation occurs when the channel returns to its closed state.
From a structural point of view, Na + channels are constituted by α, β 1 , and β 2 heterotrimers, often with four repeated domains each with six membrane-spanning subunits. The voltage sensor is located on the fourth transmembrane domain. Different subunits are represented differently in the CNS and peripheral nervous system. According to the literature the following tissue-specific localization and pharmacology can be derived. With regard to α subunits, SCN1A subunits are primarily expressed in brain tissue and are blocked by TTX and saxitoxin (SXT). The most abundant brain α subunit is encoded by SCN2A1; this subunit is also found in peripheral nerves, in the initial axonal segment, and in nodes of Ranvier (TTX and SXT sensitive). SCN2A2 and SC2NA3 are predominantly expressed in the brain while SCN4A encodes channels found in muscle. In addition to TTX, these channels are sensitive to ω-conotoxins (GIIIA, GIIIB, and GIIIC). Mutations of these channels are responsible for hyperkalemic periodic paralysis, paramyotonia, and myotonia. The SCN5A subunit is expressed in the heart and in denervated skeletal muscle; in the heart, these channels are responsible for the upstroke of the action potential. These subunits are resistant to blockade by TTX and SXT; mutations of these genes are involved in the subtypes of the long QT syndrome, a cardiac condition that is sometimes associated with epileptic seizures. SCN6A (uterus), SCN10A, SCN11A, and SCN9A (dorsal root ganglion) are all sensitive to TTX. β Subunits are bound covalently to α subunits and provide inactivation kinetics to Na + channels. Mutations of the SCN1B and SCN1A subunits have been linked to generalized epilepsy with febrile seizures. ,
The molecular nature and role of glial sodium channels are yet to be fully determined. However, recent literature implicates voltage-gated sodium channels, and SCN5A in particular, in astrocyte excitability and homeostasis. Specifically, voltage-gated sodium channels may mediate Ca 2+ influx in vitro, which in turn modulates astrocyte response to injury and precipitates astrogliosis. Given the prevalence of gliotic scar formation in numerous CNS pathologies, including epilepsy and demyelinating diseases such as multiple sclerosis, the inhibition of astrogliosis is a burgeoning interest in therapeutic pharmacology research. (For a review of sodium homeostasis in glial cells, see Rose and Karus. )
The fact that even slight mutations cause profound deficits in sodium channel function and that these mutations lead to neurological diseases provides evidence to hypothesize that the replacement of defective channels by gene therapy may repristinate the loss of function caused by the initial genetic deficit. If a single gene mutation is responsible (as in cystic fibrosis and in a number of neurological disorders), and if the tissue to be transfected with the repair mechanism is accessible (such as skeletal muscle), it is possible that viral delivery of genetic products may alleviate the consequences of faulty ion channels. A positive outcome of this approach is somewhat dependent on the pathogenesis of the disease itself. If the observed deficit is the consequence solely of the inherited mutation, replacement by a normal genotype is likely to be successful. However, if the initial deficit has led to extensive rearrangement of neuronal connections, or if the deficit causes extensive neuronal cell death (such as in epilepsy), it is unclear whether restoring normal function by simple gene replacement may be beneficial. It is also worth noting that while a small fraction of neurological disorders are clearly imputable to a single gene mutation affecting a particular ion channel, the most common forms of disease result from a complex interaction of initial genotypic changes followed by adaptive responses, including apoptosis or necrosis. Finally, given that mutations of crucial ionic mechanisms such as I Na affect cardiac, neuronal, and muscular function, it is possible that a large number of mutations are nonvital, and that the vital mutations are possible because of redundancy of gene expression, or compensatory overexpression of similar ion channel proteins.
Phenotypic changes caused by relatively minor alterations in ion channel gating sometimes become clinically relevant only when concomitant deficits not necessarily associated with action potentials are present. This is the case, for example, in SCN4A mutations leading to hyperkalemic periodic paralysis. For the paralytic symptoms to occur, the patient must concomitantly experience variations in plasma potassium (by either K + intake or exercise followed by rest). This leads to opening of Na + channels that switch into a non-inactivating mode, leading to the development of a persistent inward Na + current. The ensuing depolarization of muscle membrane will further increase [K + ] out via loss through voltage-dependent K + channels, aggravating the initial trigger. Furthermore, the persistent depolarization causes inactivation of normal Na + channels, leading to rapid loss of tissue excitability and paralysis. This example accentuates the complex interactions between normal and abnormal ion channels expressed in a certain cell type, the importance of the extracellular milieu in biophysical signaling via ion channels, and the difficulties associated with the diagnosis of altered ion channel phenotypes.
The mechanism of calcium action potentials is somewhat different but follows the general principles of threshold for activation and rapid gating mechanisms. As is the case with sodium channels, Ca 2+ channels are distributed heterogeneously in the CNS and even within the same cells. Different subspecies of Ca 2+ channels may even be found within the same cell. This inhomogeneous expression is functionally significant in that it allows that Ca 2+ influx to perform several different cellular tasks, including depolarization of dendrites and propagation of signals to the cell body, synaptic release of neurotransmitter, contraction, and second messenger function. Similar to sodium channels, membrane depolarization is the most common trigger for Ca 2+ channel opening; despite this, the kinetic properties of Ca 2+ channels are characterized by longer time constants. This kinetic behavior underlies the different durations of neuronal/axonal action potentials (I Na , 1–3 ms) versus cardiac action potentials (several hundred milliseconds; large inward currents carried by I Ca) . d
d The overall duration of action potentials is also determined by other factors, such as duration of repolarizing potentials.
An additional difference between Na + and Ca 2+ action potentials (or underlying ion channel mechanisms) is constituted by the fact that the threshold for I Ca activation varies greatly between different Ca 2+ channel families. Low -threshold (or low-voltage-activated) Ca 2+ channels are also characterized by relatively rapid opening and closing and are also referred to as T-type (transient). High-threshold (N or high-voltage-activated) Ca 2+ channels can be further subdivided into L P, R, and Q types. The pharmacologic properties of the calcium channel families are equally complex ( Table 77.3 ). , Whereas sodium action potentials are typically triggered and terminated by depolarization (inactivation of I Na and voltage-dependent activation of I K ), an additional mechanism is involved in the repolarization; the activation by intracellular Ca 2+ channels acts as a powerful terminator of cell depolarization. Ca 2+ channels are modulated by important intracellular signals such as cyclic adenosine monophosphate (cAMP; cAMP-dependent protein kinases) and G proteins. These modulatory signals arise from receptor stimulation, thus coupling the activity of postsynaptic (or presynaptic, in the case of presynaptic receptors) Ca 2+ channels to the activity of neighboring cells.
| Low-Voltage-Activated I Ca | High-Voltage-Activated I Ca | ||||
|---|---|---|---|---|---|
| Additional names | T | N | L | P | R, Q |
| Threshold (mV) | −60 | −40 | −40 | ||
| Pharmacologic antagonists | Nickel Mibefradil a Kurtoxin |
ω-Conotoxin MVIIC | Calcium channel blockers (nimodipine) Phenylalkylamines (verapamil) Benzodiazepines (diltiazem) |
ω-AGA IVA ω-Conotoxin GIVA |
ω-Conotoxin MVIIC (Q) SNX-482 |
| Second messengers | cAMP/PK-G s | ||||
| Neurotransmitters | Somatostatin, Ach, ATP, ADO | ||||
| Cell type and subcellular localization | Neuronal and cardiac | Presynaptic | Skeletal muscle: α 1S Brain (neuronal soma and proximal dendrites): α 1B Cardiac muscle: α 1C Neuroendocrine: α 1D Retina: α 1F |
High concentration of α subunit in cerebellum, Purkinje cells Neuromuscular junctions |
Cerebellar granule cells; hippocampal pyramidal neurons (Q) Neuronal presynaptic |
| Function | “Pacemaker” potentials in cardiac muscle and neurons | Transmitter release | Excitation-contraction coupling Dendritic action potentials |
Transmitter release | |
Ca 2+ channels contain four or five distinct subunits. The α subunits display different tissue and peptide specificity. They consist of transmembrane-spanning proteins, acting in both voltage sensor and selectivity filter capacities. The calcium channel blockers verapamil and nifedipine bind to α 1 subunits. In the Lambert-Eaton myasthenic syndrome (LEMS), the autoimmune component of the disease is due to immunoglobulin G binding to α 1A subunits of presynaptic Ca 2+ channels. Most commonly, the P/Q-type channels are involved; in a small percentage of cases the α 1B subunit constituting N-type channels mediates the autoimmune response. Other subunits increase the amplitude of Ca 2+ currents and bind the antiepileptic drug gabapentin (α 2δ subunit). The β subunit is localized in the cytoplasm, associates with α subunits that are membrane bound, has regulatory functions, and mediates the effects of cAMP: cAMP-dependent protein kinase phosphorylation modifies current, voltage dependence, and activation-inactivation. The γ subunits are exclusively localized within the membrane and lack a cytoplasmic component. Similar to subunits in other channels, γ subunits modulate channel voltage dependency.
Not all calcium channels are involved in transmembrane fluxes of calcium. Ca 2+ release channels are located ubiquitously in intracellular organelles and regulate the cytoplasmic Ca 2+ content of virtually every mammalian cell type. These channels belong to two separate families, those sensitive to the alkaloid ryanodine and those activated by inositol 1,4,5-triphosphate (IP 3 ). Ryanodine-sensitive Ca 2+ release is triggered by activity of calcium channel blocker–sensitive Ca 2+ channels and therefore acts as a signal amplifier. These channels are mainly located in skeletal and cardiac muscle. Disorders resulting from changes in these channels include malignant hyperthermia and central core disease. The IP 3 receptors are primarily located in glial and neuronal endoplasmic reticular membranes. In response to stimulation of cell surface receptors, intracellularly generated IP 3 binds to these receptors, releasing intracellular Ca 2+ stores. Thus while IP 3 receptors link intracellular calcium changes to chemical transmission and act as second messengers, the ryanodine-sensitive mechanism is optimized to link electrical activity promoted by Ca 2+ action potentials to calcium mobilization.
Given the large number of Ca 2+ channel families, and their widespread distribution in the nervous system, it is not surprising that numerous neurological disorders have been linked to mutation, autoimmune inactivation, or altered expression of I Ca . Disorders affecting the presynaptic terminal of the motor axons cause the aforementioned LEMS, and mutations of the α 1A subunit are responsible for a form of episodic ataxia (type 2). LEMS is an autoimmune disorder associated with an immunologically abnormal response to neoplasms, while type 2 episodic ataxia is caused by a defective production of ion channels. Familial hemiplegic migraine is associated with missense mutations in transmembrane segments, and progressive ataxia is caused by either trinucleotide repeat expansion in an intracellular region near the carboxy terminus or missense mutation.
There are many subtypes and functions of potassium channels in the mammalian CNS, with whole textbooks devoted to them. The different families of K + channels in the mammalian CNS include: (1) K v channels, which are activated by a change in transmembrane voltage; and (2) inward rectifier channels (K IR channels), which have a high conductance for K + ions moving into a hyperpolarized cell (in contrast to K + ions moving out of a depolarized cell) and are often regulated by intracellular messengers. K v channels are typically closed at RMP and rapidly open following membrane depolarization. They are variably spliced tetramers composed of four homologous α subunits that each contain a voltage sensor and a sequence that provides cation selectivity for potassium. K v channels are variably composed from alternative RNA splicing and subunit duplication of gene products from four subfamilies, K v1 to K v4 , which correspond to the murine Shaker, Shab, Shaw, and Shal. The rates of channel inactivation vary dramatically between the different subtypes.
Potassium channels are the major contributor to both neuronal and glial RMP. As described earlier, neuronal membranes are predominantly permeable to potassium at rest, resulting in cell RMP approaching the potassium reversal potential, E K . Even more so than neurons, the majority of glial resting channels are K + permeable, resulting in a relatively hyperpolarized RMP. Because of their lack of electrical excitability, a hyperpolarized RMP was previously a defining criterion during physiologic recordings from glia in situ. However, it has been demonstrated over the last several years that glial cells can have a wide range of RMPs, depending on the glial cell subtype, ion channel endowment, location within the nervous system, and developmental stage. ,
Hodgkin and Huxley initially characterized the important role of what are now termed delayed rectifier K v potassium channels in action potential repolarization. These channels, which are blocked by tetraethylammonium (TEA), are similar to sodium channels in that the probability of channel opening is increased by membrane depolarization, with increased opening in proportion to the amount of membrane depolarization. Delayed rectifier potassium channels nonetheless differ from sodium channels in that they open more slowly and they do not inactivate in the presence of persistently maintained depolarization. As discussed earlier, it is this sodium channel inactivation together with persistent activation of outward potassium channels (and inward chloride channels, by allowing negatively charged chloride ions to enter the cell) that results in action potential repolarization. Following action potential repolarization, there is a refractory period during which the threshold for initiation of another action potential is elevated. The absolute refractory period, during which an action potential cannot be generated, is followed by the relative refractory period, during which an increased stimulus is necessary to generate an action potential. The refractory period results from residual sodium channel inactivation and potassium channel activation; it limits the maximum firing frequency of different classes of neurons.
The M-type potassium channel has distinctly different properties from the K v potassium channels that are responsible for action potential repolarization. While activated by membrane depolarizations, these channels are inhibited by muscarinic acetylcholine receptor binding, as well as by a variety of other neurotransmitters and neuroactive compounds. The rates of channel opening and closing are approximately 100 times slower than delayed rectifier channels. Based on these different characteristics, M-type channels are thought to have divergent functions in the CNS. On the one hand, by means of their slow kinetics, they prevent repetitive neuronal discharges and hyperexcitability; on the other hand, their inhibition by modulatory neurotransmitters results in local increases in excitation. Inhibition of these channels is thus a double-edged sword, promoting local increases in excitation important to such processes as learning and memory while also potentially rendering areas of the brain proepileptic. The M-type channel KCNQ1 gene is mutated in a subtype of the long QT syndrome, while KCNQ2 and KCNQ3 are mutated in two different forms of benign familial neonatal convulsions.
Several other potassium currents present in CNS neurons are beyond the scope of this chapter. These include (1) channels that are opened by intracellular calcium (I K(Ca) ) that function in action potential repolarization and determining the interspike interval between action potentials; (2) slowly afterhyperpolarizing currents (I AHP ), the blockage of which promotes neuronal excitation; (3) mixed cation currents (I ha ) that are permeant to K + and Na + and are involved in rhythmic burst firing (see “Expression of Ion Currents in Different Neuronal Populations” later); (4) the transiently inactivating current (I A ) that lengthens the interspike interval between action potentials; and (5) K ATP channels, an inward-rectifying subtype that opens in response to lowered intracellular adenosine triphosphate (ATP) and has been implicated in protection from ischemia, insulin-mediated hypothalamic neuronal glucose homeostasis, and glial potassium homeostasis.
By considering the clinical manifestation of diseases caused by altered expression of I Na , I K , and I Ca , it is surprising that permanent changes in the normal endowment of ion channels can cause episodic diseases. This is true for a variety of inheritable cardiac conditions (arrhythmias), as well as neurological disorders such as episodic ataxia and epilepsy. How it is possible that most of the time the patients affected by these disorders are lacking symptoms and what precipitates the clinical manifestations remain largely unknown. The modern techniques used to map and pinpoint the molecular mechanisms of diseases have so far failed to determine the cofactors that transform a small ion channel deficit into a full-blown neurological disease. Understanding these coexisting conditions will perhaps provide information sufficient to chart an effective therapy. Channelopathies are overviewed in Table 77.4 . ,
| Disorder | Ion Channel | Mutation(s) |
|---|---|---|
| M uscular | ||
| Myotonia congenita, autosomal recessive (Becker disease) | CLCN1 (skeletal muscle chloride channel) | Point mutations: reduced Cl − conductance; increased membrane resistance; less current required for action potential, slower repolarization; more sensitive to K + concentration |
| Myotonia congenita, autosomal dominant (Thomsen disease) | CLCN1 (skeletal muscle chloride channel) | Missense mutations and deletions: reduced Cl − conductance; increased membrane resistance; less current required for action potential, slower repolarization; more sensitive to K + concentration |
| Sodium channel myotonia Paramyotonia congenita Periodic paralysis, type II (hyperkalemic) |
SCN4A (skeletal muscle sodium channel) | Point mutations in regions involved in channel inactivation: impaired inactivation resulting in slowed decay of transient sodium current |
| N eurological | ||
| Familial hemiplegic migraine | CACNL1A (α 1 voltage-sensitive calcium channel subunit) | Assorted mutations (α 1 voltage-sensitive calcium channel subunit) |
| Episodic ataxia type 2 | CACNL1A | Assorted mutations (α 1A calcium channel subunit) |
| Spinocerebellar ataxia | CACNL1A | Expansion of trinucleotide repeat |
| Episodic ataxia type 1/partial complex seizures | Kv1.1/KCNA1 (delayed rectifier K + channel) | Multiple missense and stop codon mutations; rare human, severe mouse seizures |
| Benign neonatal convulsions | KCNQ2 and KCNQ3 (M-type potassium channel) | Multiple mutations, altering regulation of rapid neuronal firing |
| Generalized epilepsy with febrile seizures | SCNA1 and SCNB1 | Multiple mutations |
| A cquired C hannelopathies | ||
| Long QT syndrome Neurological effects prevented by BBB |
HERG K + channel | Antiarrhythmic medications, class IA or III HERG channel blockade |
| Myasthenia gravis | AChR | Autoimmune |
| Acquired myotonia | Voltage-gated K + channel | Autoimmune |
| LEMS | Voltage-gated Ca 2+ channel | Autoimmune |
Glial potassium channels are the most common electrophysiologic feature of both cultured and in situ astrocytes and can be categorized as follows: channels that allow inward but not outward current flow ( inward rectifiers, K IR ); channels that allow outward but not inward current flow ( delayed rectifier, I DR ; transient outward current, I A ); and channels that are opened by intracellular calcium (I K(Ca) ). Glial potassium channels differ in their sensitivity to blockers: inward rectifiers are blocked by submillimolar concentrations of external cesium and barium; outward I DR and I A are both sensitive to TEA and 4-amino-pyridine (4-AP) but I A blockade by TEA requires high concentrations. Recently, a mixed cation channel (I ha ) permeant to K + and Na + and human ether-a-go-go currents (HERG1) has been described in both cultured and in situ astrocytes and may assist in potassium homeostasis in the CNS.
Voltage-dependent, TTX-sensitive, and insensitive sodium channels are also expressed in both cultured and in situ glial cells. While astrocytes are incapable of producing action potential–like responses, possibly due to the relatively low Na + current densities in these cells, a role of Na + channels in extracellular buffering of potassium has been proposed. According to this hypothesis, Na + influx sustains the Na + ,K + -ATPase pump, resulting in net K + uptake. Finally, calcium channels are represented sparingly in glial cells and require either neuronal or otherwise differentiating factors for expression; whether I Ca can be recorded from in situ hippocampal astrocytes is still unknown, but release of calcium from intracellular stores in response to neurotransmitters acting on astrocytes has been clearly demonstrated. Relevant to potassium homeostasis, micromolar intracellular calcium ([Ca 2+ ] in ) can cause opening of I K(Ca) and may thus participate in the generation of outward potassium fluxes.
Astrocytes can release the excitatory transmitter glutamate, which acts on at least three families of receptors. This astrocytic glutamate release can occur through several mechanisms some of which are specific to glia (e.g., reversal of glutamate uptake) or via Ca 2+ -dependent exocytosis, which shares similarities to neurotransmission. In addition to glutamate, astrocytes can release a variety of neurotransmitters such as taurine or adenosine. Unlike synaptic transmission, which is specific for a postsynaptic site, single astrocyte release of glutamate affects several adjacent neurons, thereby controlling simultaneously the excitability of several neighboring pyramidal cells. This may constitute one of the mechanisms of neuronal synchronization in epilepsy (being asynchrony favored for normal physiologic function).
If astrocytes release glutamate and have neurotransmitter receptors, what differentiates neurons from glia? Are these phenomena operating in vivo, or are these findings limited to slice preparations? For example, glial cells display intrinsic activity in the absence of neuronal stimulation, but this finding was only observed in vitro. Astrocytes greatly outnumber neurons, and the astrocyte-to-neuron ratio is larger in the more evolved brain. There is no question regarding the importance of nonneuronal cells to CNS function and human disease; however, the laboratory findings need to be confirmed in preparations in which artifacts are controlled and known. An important physiologic aspect of the astrocyte in situ is its proximity to the capillary and perivascular space of arterioles. Astrocytes influence blood-brain barrier (BBB) maintenance, which in turn regulates brain ion homeostasis. It is noteworthy to recognize the foundational role of astrocytes not only in controlling BBB permeability, but also in modulating K + levels in brain extracellular fluid (BECF) via spatial buffering; which further promotes the state of population asynchrony needed for normal neurophysiologic function. In addition, markers of inflammation (i.e., cytokines and chemokines) have been shown to be determinant regulators of BBB permeability and have been implicated in illnesses such as multiple sclerosis. Therefore we must also consider the electrophysiologic consequences brought by neuroinflammatory states and further investigate if neural electrical activity can be used as a biomarker for assessing neuroimmunologic diseases. , Whether astrocytes also release significant levels of angiogenic modulators (e.g., angiopoietins) remains to be proven in vivo in preparations in which the surrounding tissue is intact. But a clear effect of astrocytes on small-diameter, capillary-like structures has been demonstrated. This effect may be important when capillary perfusion pressure is altered by brain edema or other disruptors of brain homeostasis.
Morphologically distinct neuronal populations in different areas of the CNS are endowed with distinct mixtures of ion channels, resulting in widely differing electrophysiologic properties such as RMP, intrinsic ionic conductances, and rhythmicity. Several different patterns of neuronal activity can be classified. Brainstem and spinal cord motor neurons generate single spikes of action potentials that form trains of activity in direct correlation to the degree of depolarization. In contrast to this nearly linear firing pattern is that exhibited by many hippocampal and cortical pyramidal cells that display spike frequency adaptation, in which trains of action potentials decrease in frequency over time. Other neuronal populations such as thalamic relay neurons, inferior olivary neurons, and some pyramidal cells have intrinsic rhythmicity that allows the generation of bursts of activity without afferent stimulation. Other cells, such as many GABAergic inhibitory interneurons, are specialized to generate a high firing frequency (>300 Hz) of short-duration (<1 ms) action potentials. A final type of pattern is that exhibited by cholinergic, serotoninergic, noradrenergic, and histaminergic cells that innervate large areas of the brain. These discrete cell populations carry out their modulatory function by spontaneously generating low firing frequencies (1–10 Hz).
Electrophysiologic heterogeneity impacts the function of particular cell populations in the brain. Individual cell types possess particular combinations of the previously described Na + , K + , and Ca 2+ currents that merge to result in the patterns of neuronal activity that are found in different areas of the CNS. These patterns can be investigated by in vitro isolated brain slice recordings as well as computer modeling simulations to dissect the individual channel components. For example, relay neurons in the lateral geniculate nucleus that project to the visual cortex are characterized by an RMP of −70 mV during sleep in contrast to −55 mV during the awake state, thus decreasing the transmission of information to the visual cortex during sleep. This sleep-wake variability is modulated by the low-threshold calcium current (I T ), which together with the mixed cationic current I ha , generates rhythmic bursts of action potentials in thalamic relay neurons. During slow wave sleep, thalamic relay neurons have a relatively hyperpolarized RMP that de-inactivates low-threshold calcium currents, resulting in I T generated slow spikes of activity. Between these spikes, a slowly depolarizing potential is generated by activation of I ha . Together these two currents result in spontaneous synchronized bursts of low frequency action potentials. In contrast, the transition from slow wave sleep to either wakefulness or rapid eye movement sleep is characterized by relative depolarization of thalamic relay neurons. This depolarization inactivates I T and I ha , resulting in conversion to a tonic action potential mode in which single spikes are produced one at a time in response to stimulation.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here