Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
List the different types of ion channels and provide a description of how they function.
Describe the resting potential with respect to the major ions involved in its formation.
Be able to distinguish and describe the following terms: electrotonic potential, spatial summation, temporal summation, threshold, and trigger point.
Discuss the ion channel changes that accompany the development of an action potential and the concepts of all-or-none response and absolute and relative refractory period.
Contrast the mechanism or process of action potential development and propagation along an unmyelinated versus a myelinated axon.
Examples of clinical neurophysiology related to the peripheral nervous system await your attention in Chapter 12 .
In common with cells elsewhere, the plasma membrane of neurons is a double layer (bilayer) of phospholipids made up of phosphate heads facing the aqueous media of the extracellular and intracellular spaces and paired lipid tails forming a fatty membrane in between ( Fig. 7.1 ). The phosphate layer is water soluble ( hydrophilic , or polar ), and the double lipid layer is water insoluble ( hydrophobic , or nonpolar ).

Both the extracellular and the intracellular fluids (ECFs and ICFs) are aqueous salt solutions in which many soluble molecules dissociate into positively or negatively charged atoms or groups of atoms called ions. Ions and molecules in aqueous solutions are in a constant state of agitation, being subject to diffusion , whereby they tend to move from areas of higher concentration to areas of lower concentration. In addition to diffusing down their concentration gradients, ions are influenced by electrical gradients. Positively charged ions such as sodium (Na + ) and potassium (K + ) are called cations because, in an electrical field, they migrate to the cathode. Negatively charged ions such as chloride (Cl − ) are called anions because these migrate to the anode. Like charges (e.g. Na + and K + ) repel one another; unlike charges (e.g. Na + and Cl − ) attract one another.
The cell membrane can be regarded as an electric capacitor because it comprises outer and inner layers carrying ionic charges of opposite kind, with a (fatty) insulator in between. Away from the membrane the voltage in the tissue fluid is brought to zero (0 mV) by the neutralising effect of Cl − anions on Na + and other cations, and the voltage in the cytosol away from the membrane is brought to zero by the neutralising effect of anionic proteins on K + cations.
Ion channels are membrane-spanning proteins with a central pore that permits passage of ions across the cell membrane. Most channels are selective for a particular ion, such as Na + , K + , or Cl − .
Several channel categories are recognised, of which the first three are of immediate relevance.
Passive (non-gated) channels are open at all times, permitting ions to move across the membrane and helping to establish the resting membrane potential of neurons.
Voltage-gated channels contain a voltage-sensitive string of amino acids that cause the channel pore to open or close in response to changes in membrane voltage. Voltage-gated channels are essential to produce an action potential.
Channel pumps are energy-driven ion exporters and/or importers designed to maintain steady-state ion concentrations. The Na + –K + exchange pump is vital to maintain the resting membrane potential.
Chemically gated (or transmitter-gated) channels are used by the nervous system to temporarily alter the membrane potential and these channels abound in the postsynaptic membranes of a synapse. Some are activated directly by transmitter molecules, others indirectly (see Chapter 8 ).
Transduction channels are activated by physical stimuli, resulting in depolarisation and the subsequent creation of action potentials so the stimulus can be perceived by the nervous system. Each receptor can transduce a form of energy—for example, changes in length or tension in the muscle, tactile or thermal energy in the skin, chemical energy in the nasal and oral cavities, or electromagnetic energy in the retina. Fig. 7.2 depicts the three passive channels concerned with generating the resting potential.

The existence of distinct channels for Na + , K + , and Cl − ions would result in zero voltage difference across the membrane if passive diffusion of the three ions were equally free. However, the number of Na + channels is relatively small, the number of K + channels is relatively large, and the number of Cl − channels is roughly half the number of K + channels. In effect the membrane is many times more permeable to K + and Cl − than to Na + .
The membrane potential of the resting (inactive) neuron is generated primarily by differences in concentration of the Na + and K + ions dissolved in the aqueous environments of the ECF and the cytosol. In Table 7.1 , K + is 20 times more concentrated in the cytosol than in the ECF, while Na + and Cl − are 10 and 3.8 times more concentrated in the ECF than in the cytosol. Thus, the chemical driving force is outward for K + and inward for Na + and Cl − .
| Ion | Concentration (MMOL/L) | Equilibrium Potential (mV) | |
|---|---|---|---|
| Cytosol | Extracellular Fluid | ||
| K + | 100 | 5 | –90 |
| Na + | 15 | 150 | +60 |
| Cl − | 13 | 50 | –70 |
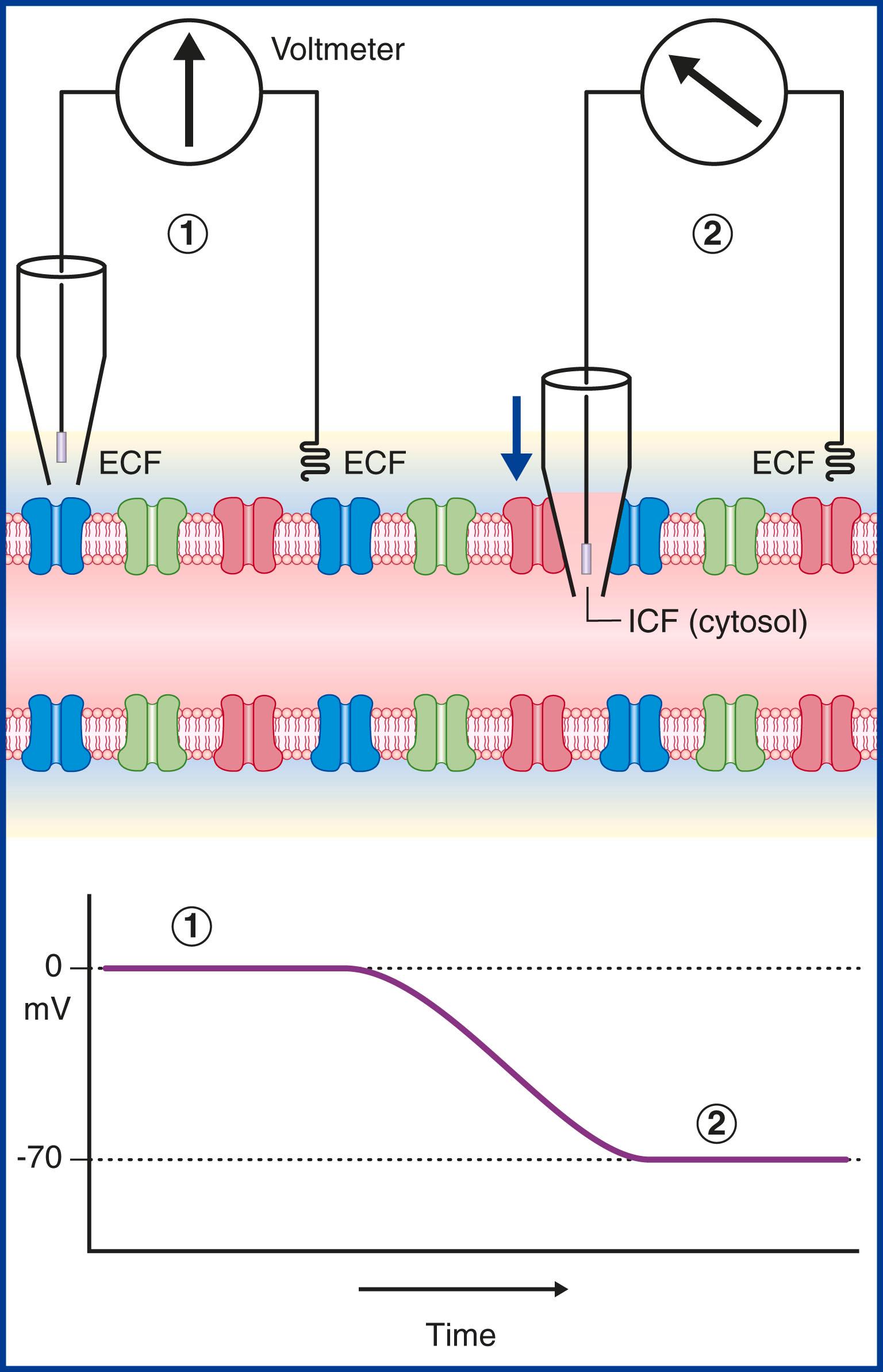
In Fig. 7.3 a voltmeter is connected to electrodes inserted into the ECF surrounding an axon. One of the electrodes has been inserted into a glass pipette with a minute tip. On the left side of the figure both electrode tips are in the ECF and there is no voltage difference; a zero value is recorded. On the right side the pipette has pierced the plasma membrane of the axon, allowing the measurement of the ICF of the cytosol. The electrical charge now reveals a potential (voltage) difference of −70 mV. In practice the membrane potential ranges from −60 mV to −80 mV in different neurons. These values represent the resting membrane potential (i.e. when impulses are not being conducted).
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here