Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The American College of Cardiology Foundation (ACCF) and American Heart Association (AHA) define heart failure with preserved ejection fraction (HFpEF) as the clinical syndrome of heart failure (HF) with evidence of a left ventricular ejection fraction (LVEF) 50% or greater. An estimated 6.5 million adult Americans have HF, and over the past 30 years, there has been a rise in the prevalence of HFpEF from 41% to 56%, surpassing the prevalence of HF with reduced ejection fraction (EF). , The diagnosis of HFpEF remains challenging because it involves the exclusion of other dyspnea-causing disease processes and the demonstration of increased left ventricular (LV) filling pressures. Because echocardiography provides important information regarding LV and left atrial (LA) structure, function, and hemodynamics, the ACCF/AHA make a class I recommendation to perform two-dimensional (2D) echocardiography with Doppler during the initial evaluation of all patients with HF ( Fig. 179.1 ).
Echocardiography detects and quantifies the hallmarks of HFpEF, namely preserved LVEF, diastolic dysfunction, and elevated LV filling pressures at rest or with exertion. Importantly, although more than 80% of patients with HFpEF have diastolic dysfunction, the majority of patients with critical diastolic dysfunction do not have symptomatic HFpEF ; indeed, one prospective study reported a HF incidence of only 12% over the course of 6 years for patients with moderate or severe diastolic dysfunction.
Conceptually, normal diastolic function implies an active (i.e., energy-requiring) LV relaxation in early diastole that, in addition to restoring forces ( Fig. 179.2 ), contributes importantly to the gradient for flow from the left atrium to the left ventricle at normal levels of LA pressure (i.e., filling vis-a-tergo) and that becomes quantitatively more critical during exercise. In addition, a normally compliant passive diastolic pressure–volume relation maintains normal ventricular diastolic pressures over a wide range of ventricular filling. In contrast, in diastolic dysfunction, the rate of LV relaxation is slowed, restoring forces are decreased, and the gradient for filling at rest and especially with exercise (when the increased heart rate preferentially shortens diastole) is at the expense of an increase in LA pressure (i.e., filling vis-a-fronte). Thus, in patients with HFpEF, when delayed LV relaxation is coupled with an increase in passive ventricular stiffness, an abnormal increase in LA pressure is required to achieve the same degree of LV filling. The development of LA hypertension not only contributes to the symptoms of dyspnea but also predisposes the patient to the development of LA remodeling, pulmonary hypertension, right ventricular (RV) dysfunction, and atrial fibrillation.
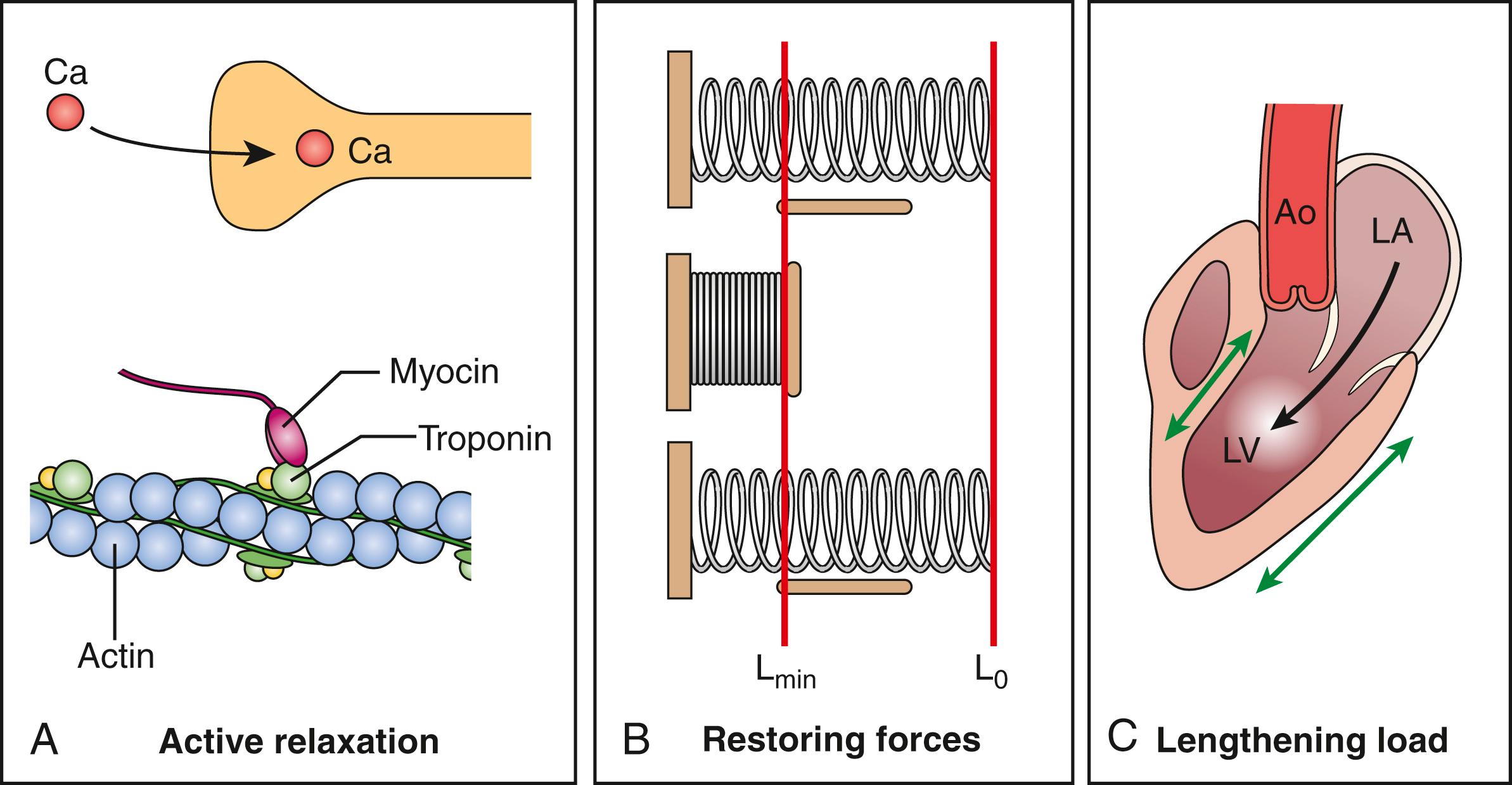
With these consequences in mind, in the initial evaluation of patients with normal LVEF, the American Society of Echocardiography (ASE) recommends assessment of diastolic function using an algorithm comprised of four indices: average E/e′ ratio greater than 14, mitral septal e′ velocity less than 7 cm/s or lateral e′ velocity less than 10 cm/s, tricuspid regurgitation (TR) velocity greater than 2.8 m/s, and LA volume index greater than 34 mL/m 2 . If a patient meets zero or one criterion, diastolic function is normal, and if a patient meets three or more criteria, diastolic function is abnormal. Meeting two criteria would yield an indeterminate assessment and often requires incorporation of other variables such as E/A ratio, pulmonary vein Doppler systolic/diastolic ratio, and atrial systolic reversal duration, the ratio of the isovolumic relaxation time (IVRT) divided by the time (t) from the early diastolic transmitral flow velocity (E) to the early diastolic annular tissue velocity (e′), LV mass index, and LV or LA strain. Developed by expert consensus, the algorithm yielded a sensitivity and specificity of 69% and 81%, respectively, for the estimation of LV filling pressures when validated against invasive left heart catheterization. Limitations of this approach in the HFpEF population (which often includes older hypertensive women with atrial fibrillation and obesity) include an overlap between delayed LV relaxation and normal aging, the effect of atrial fibrillation on transmitral velocities, and an underestimation of LA volume when indexed for increased total body size. However, indices such as E/e′ and estimated pulmonary artery systolic pressure (PASP) are relatively age independent and play an important diagnostic role. Nevertheless, the accurate diagnosis of HFpEF remains challenging.
It has become widely accepted that the HFpEF syndrome consists of a very heterogenous group of patients; as such, pharmacologic treatment strategies have yielded mixed results. Identifying specific phenotypes of the HFpEF syndrome is important for prognostication and may help to guide future therapies. Of note, at present, published phenotypes are not universally accepted. Furthermore, infiltrative cardiomyopathies (e.g., amyloidosis), restrictive pericarditis, and valvular disease (e.g., aortic stenosis) are considered by some as specific types of “HFpEF”; however, these entities have disease-specific prognoses and treatment strategies and for our purposes are not included as HFpEF phenotypes. This definition is aligned with the prospective comparison of angiotensin receptor neprilysin inhibitors with angiotensin receptor blockers Global Outcomes in HF With Preserved Ejection Fraction (PARAGON-HF) trial definition of HFpEF, which excludes patients with isolated right-sided HF, pericardial constriction, genetic hypertrophic cardiomyopathy, infiltrative cardiomyopathy, and hemodynamically significant valvular heart disease. Furthermore, the phenotypes presented here are in line with contemporary published views.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here