Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Preparation of this chapter was supported by grants from the National Institute on Deafness and Other Communication Disorders (R01DC011555 and R01DC017924 [DJT], F32DC018195 [KLA], and K23DC013583 [KMU]).
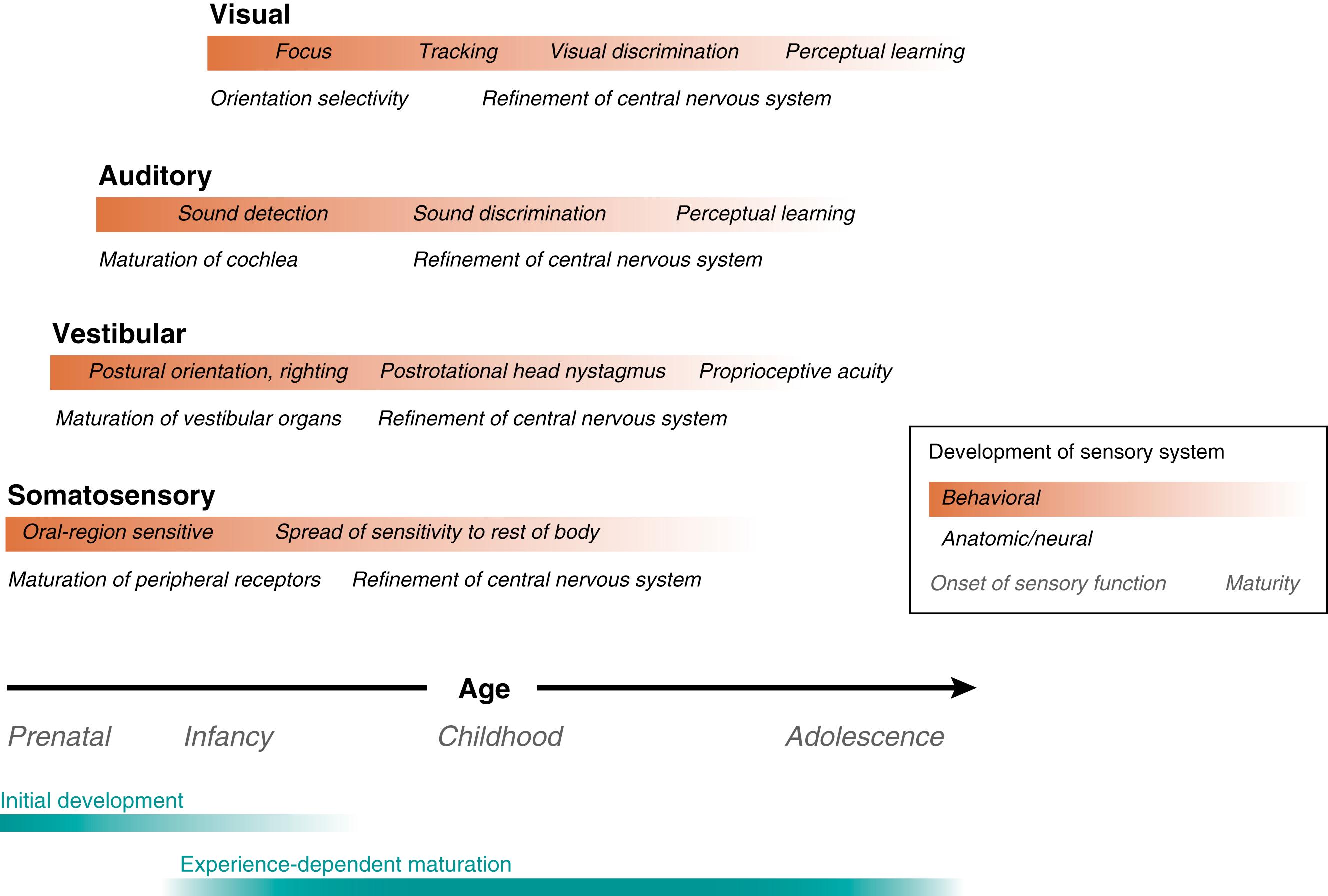
This section reviews the course of morphologic development of the auditory system and the physiologic correlates of these events in both humans and nonhumans. In studies of nonhumans, the function of the auditory system is primarily measured as electrical potentials recorded at the site of generation, such as single-neuron physiologic responses. Gross, scalp-recorded evoked potentials that result from auditory stimulation have also been used as an objective measure of auditory function. Many of the same auditory capacities—sensitivity, frequency resolution, and temporal resolution—have also been measured in human fetuses and neonates. In the case of humans, these measurements are largely based on noninvasive, scalp-recorded evoked potentials. Although the same capacities can be measured in this way, it is more difficult to fully characterize auditory maturation when evoked potentials are used; in addition, a satisfactory approach to assessing auditory function in fetuses in utero has yet to be developed. Fortunately, despite these difficulties, many of the developmental trends that have been observed in nonhumans have also been observed in human fetuses and neonates.
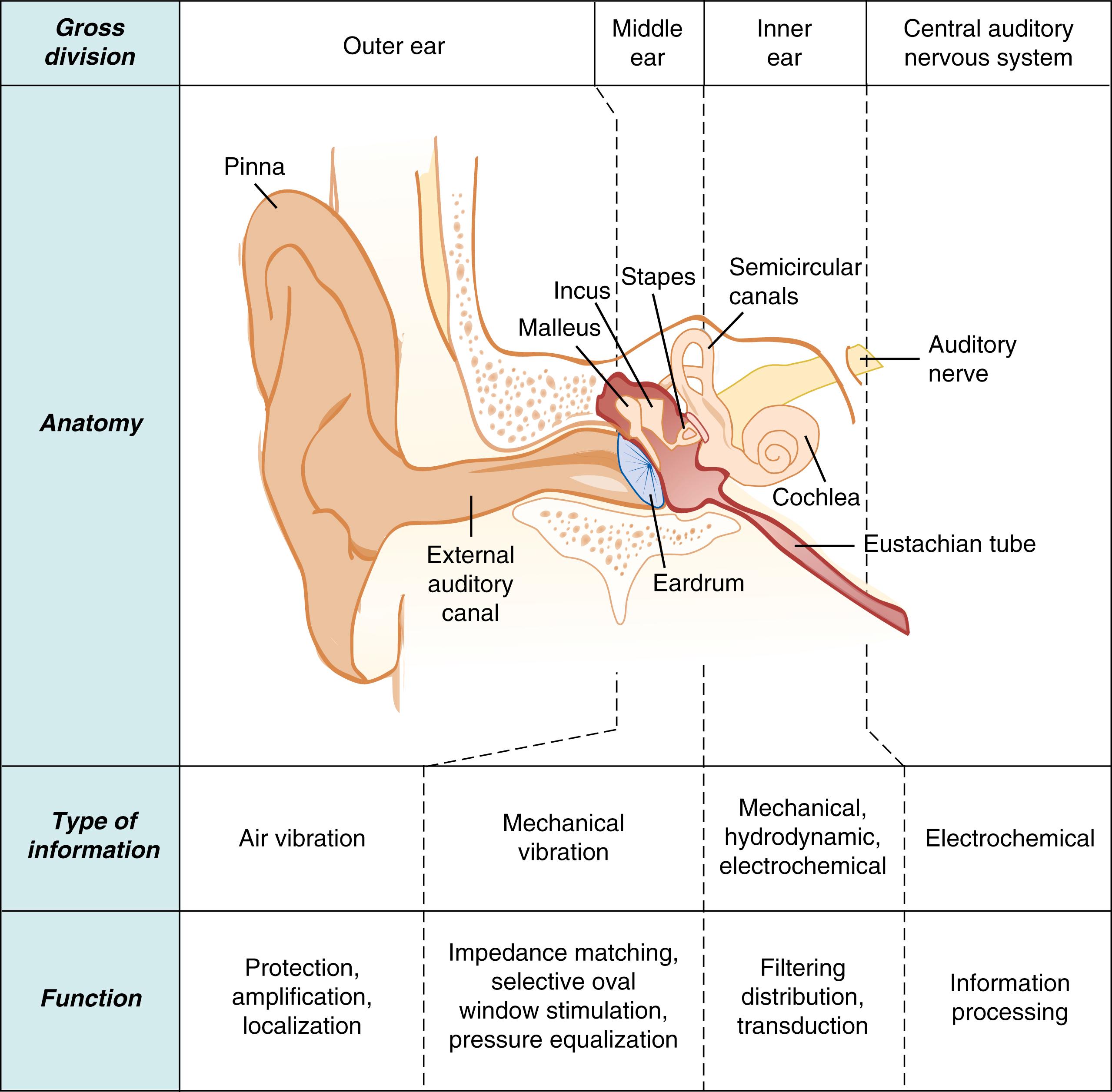
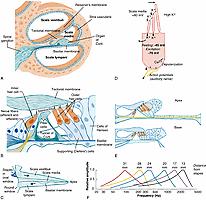
The ear is generally divided into three regional sections: the external ear, including the pinna and ear canal; the middle ear, including the tympanic membrane (eardrum) and ossicles (malleus, incus, stapes) suspended in the tympanic cavity; and the inner ear, including the semicircular canals, vestibule, and cochlea ( Figs. 135.2 and 135.3 ). Sound waves enter the external ear and travel through the ear canal, where they reach the middle ear. The tympanic membrane and middle ear ossicles vibrate and amplify the sound, transmitting the sound energy to the cochlea—a snail-shaped structure that contains the sensory organ of hearing. Within this organ are sensory cells known as auditory hair cells that are responsible for transforming the sounds into neural signals. This occurs when deflection of the hairlike bundles of the hair cells (known as stereocilia ) due to the incoming mechanical sound waves results in a pattern of electrical excitation that can be encoded by sensory neurons in the spiral ganglion for transmission to the brain ( Fig. 135.4 ). A detailed account of current understanding of sound conduction and transduction in the auditory system can be found in Musiek and Baran. Several classic texts describe the embryology and later morphologic development of the peripheral auditory system. , Texts on this topic are also widely accessible. See Fig. 135.3 for an overview of the developmental time course of the peripheral system.
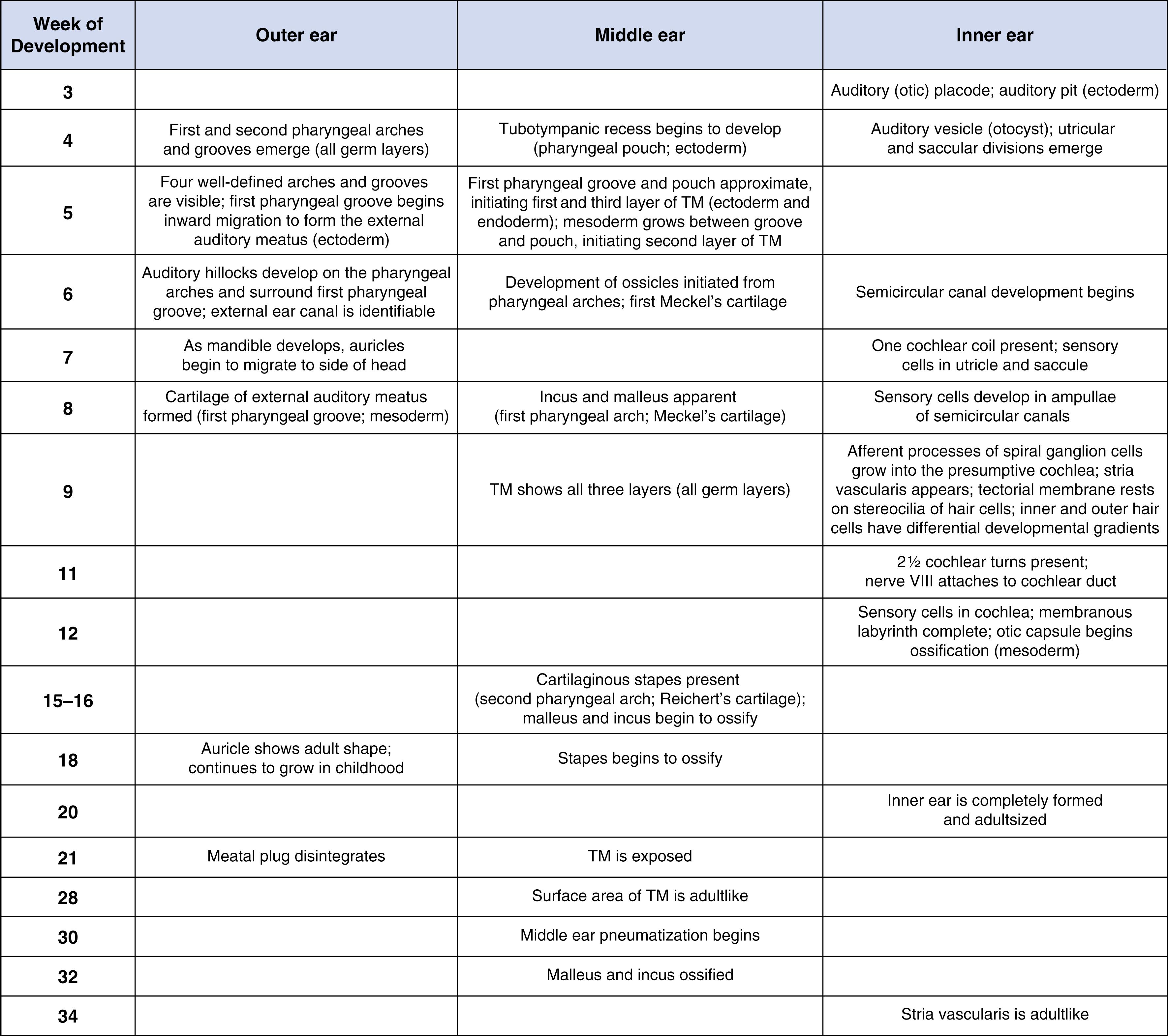
The external ear canal begins to form early in gestation, becoming easily identifiable by 6 weeks of gestation. The canal originates from the ectoderm that makes up the dorsal end of the first branchial groove . Initially, the ectoderm thickens and grows medially towards the tympanic cavity, resulting in the formation of a meatal plug or plate. The inner cells of the meatal plug subsequently degenerate, forming the ear canal. The distal end of the ear canal remains closed as it elongates and does not reopen until approximately 24 to 35 weeks of gestation. In addition, at around 6 weeks of gestation, six mesenchymal protuberances—the auricular hillocks—appear at the edges of the first branchial groove. The auricular hillocks fuse to form the pinnae, achieving adult form by approximately 18 weeks of gestation. Although the ear canal and the pinnae are well developed and fully functional at birth, both structures continue to grow in childhood with changes continuing into adult life. The tympanic membrane is also derived from the first branchial groove, where the most medial cells of the meatal plug eventually become the outer layer of the tympanic membrane. The mesenchyme, developing between the endoderm lining the presumptive tympanic cavity and the surface ectoderm, eventually differentiates into the collagenic fibers that give the tympanic membrane its elasticity. The developing tympanic membrane becomes thinner while expanding in surface area. The area of the membrane is largely adultlike by approximately 28 weeks of gestation. The ratio of the large tympanic membrane surface area to the small area of the stapes footplate (the portion of the stapes attached to the oval window of the cochlea) allows the middle ear to overcome the impedance mismatch between the air-filled ear canal to the fluid-filled cochlea, resulting in efficient transmission of sound. Although this ratio has not been followed during development in humans, it is known that in cats there is little change in the ratio following the onset of hearing function.
The mesenchyme that will form the middle ear ossicles is first identifiable at approximately 5 weeks of gestation. As the ossicles grow and begin ossification, the surrounding tympanic cavity expands and eventually suspends the small bones. The tympanic cavity continues to increase in size well into childhood. Between 21 and 40 weeks of gestation, the linear dimensions and mass of the ossicles are still increasing and continue to grow somewhat into the postnatal period.
The conductive mechanism has several characteristics that appear to be common to all mammals through the development of the neonate, particularly at the point in development when responses to sound can first be recorded. In general, the ear canal and pinna are relatively small; at birth, the human ear canal is only 14 to 15 mm long, whereas the adult canal length is approximately 23 mm long. In some mammals, the ear canal may not be completely open at birth, and the walls of the canal of young animals are generally more compliant than those of adults. The middle ear cavity is also small, with partial ossification of the ossicles resulting in middle ear bones that are somewhat smaller and lighter than in the adult. In addition, the tympanic membrane of young animals tends to be less rigid than their adult counterparts.
Unlike humans, there are several species of mammals that have altricial hearing, where auditory function develops only after birth. The functional implications of conductive development are quite different for animals with altricial hearing than for those that begin to hear in utero. Perhaps the greatest difference in the development of the conductive ear is the environment in which the peripheral auditory system matures. In the case for neonatal animals that begin to hear after birth, the external and middle ears transform the spectrum of sounds reaching the inner ear in air, whereas for animals that begin to hear in utero, the external and middle ears are surrounded in a fluid-filled environment. Not surprisingly, this fluid-filled environment alters the normal conductive route to the inner ear. It is likely that sounds reach the inner ear via bone conduction through the skull instead of traditional conductive mechanisms (although how much sound reaches the fetus is a separate issue considered in a later section). Nonetheless, the cochlea responds similarly whether a sound arrives via air conduction through the middle ear or bone conduction through the skull. However, it is important to note that the spectrum of sounds is shaped differently with the two conduction mechanisms. When sounds are transmitted to the adult inner ear by bone conduction (this can be done by placing a vibration device on the mastoid process), much greater sound energy is required to elicit a response, on the order of 40 dB at 1000 Hz. Furthermore, bone conduction through the skull has a “high-frequency emphasis,” where higher-frequency sounds travel much more efficiently through bone than low-frequency sounds. For example, in adults, the detection threshold at 1000 Hz is approximately 15 dB lower than that at 250 Hz for air-conducted tones but approximately 22 dB lower for bone-conducted tones. Of course, the fetal skull is not as well ossified as the adult skull, and in utero sound is transmitted to the skull from the fluid surrounding the fetus, not from a mechanical vibrator. Although it is clear that the route of sound transmission to the fetal inner ear will have substantial effects on what the fetus experiences, the nature of these effects is not entirely predictable from the adult bone conduction. One clear implication of the differences between air- and bone-conducted sound is that infants who are born prematurely will be exposed to a qualitatively different set of sounds than they would have in utero.
The implications of having an immature conductive apparatus after birth lead to some predictions as to how sounds can be transformed by the developing structures. The resonance characteristics of a small ear canal and pinna suggest that for sounds of equal amplitude approaching the listener, high frequencies will reach the middle ear at relatively higher amplitude than low frequencies. If the ear canal is not fully open, of course, significantly less sound energy will reach the middle ear. The amount of attenuation due to a closed ear canal may be comparable to the attenuation due to another type of physical obstruction, such as earplugs; this has been described in detail by Lupo and colleagues. In addition, the immature tympanic membrane and ossicles may decrease the efficiency of the middle ear in transmitting sound to the inner ear, resulting in a biasing of the sound toward lower frequencies. Studies of the acoustic response of the conductive apparatus in nonhuman species confirm these predictions.
The acoustics of the external and middle ear have not been described in premature human infants, although data exist for 1-month postterm infants. The external ear data are in the form of sound-field–to–ear-canal transfer functions. These transfer functions show the level of sound in the ear canal as a function of frequency, when the intensity level of sound in the surrounding field is equal at all frequencies. In adults, this function shows a broad peak of approximately 10 dB between approximately 2000 and 5000 Hz as a result of the resonance of the ear canal and pinna ( Fig. 135.5A ). The peak resonance frequency of the adult external ear is typically given as 2700 Hz. Keefe and colleagues characterized the acoustic response of the infant external ear, with the youngest infants being 1 month of age. The average resonance frequency in this age group was 4000 to 5000 Hz (see Fig. 135.5B ), consistent with physical measurements of ear canal length. Thus, relative to adults, the level of sound reaching the neonatal middle ear at frequencies in the 1000 to 3000 Hz range is lower. They also found that the sound-field to ear-canal transfer function is not mature by 24 months postnatal age, and the age at which the transfer function becomes adultlike is not yet known.
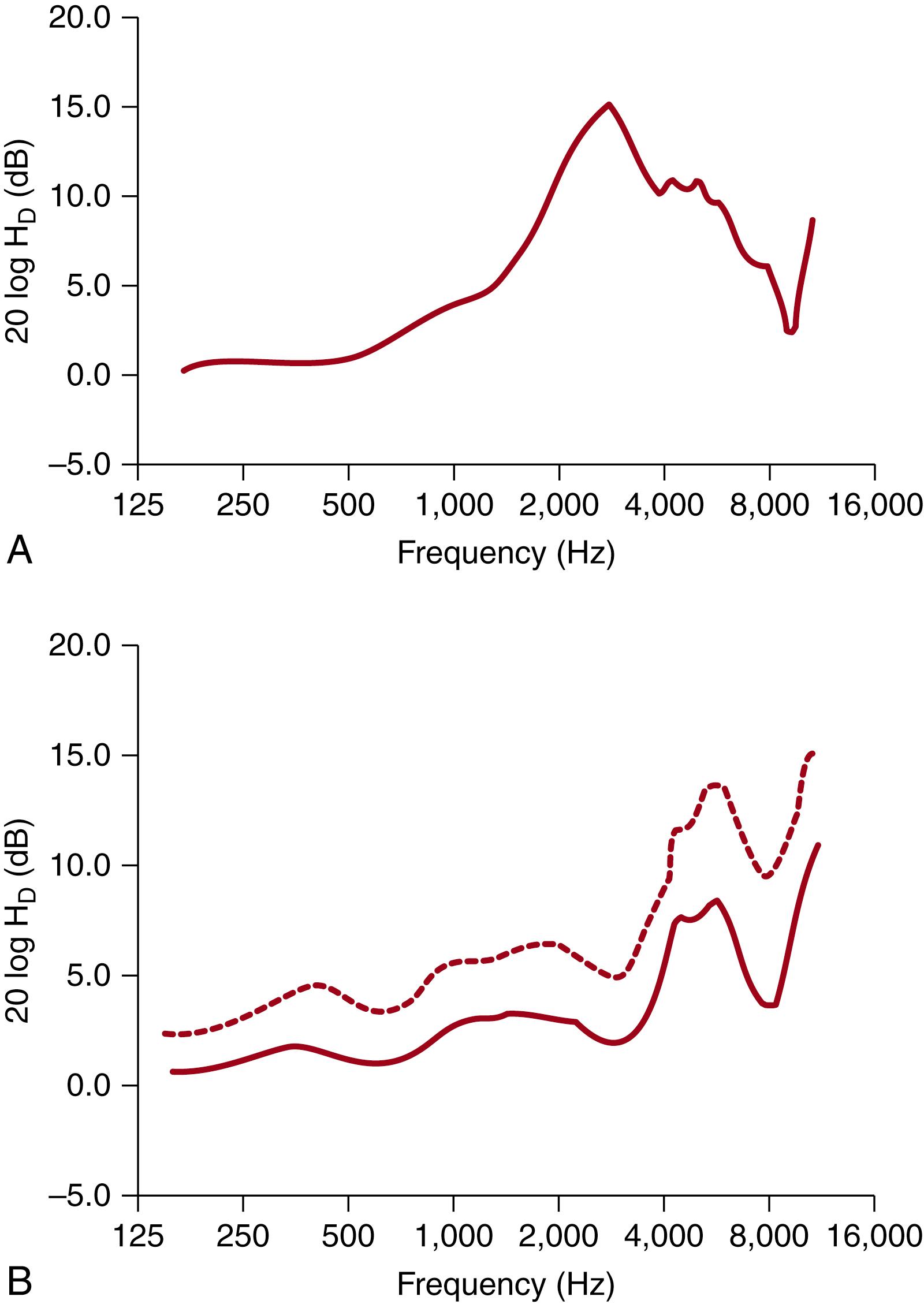
Keefe and colleagues , have also published comprehensive studies of the acoustic properties of the infant middle ear. They have estimated the acoustic conductance, or flow of sound energy, in the middle ear of newborns and 1- to 24-month-olds. The results for newborns, 1-month-olds, and adults are shown in Fig. 135.6 . The conductance of the infant’s middle ear is smaller than that of adults for frequencies greater than 500 Hz, with the greatest difference at higher frequencies. For frequencies greater than 1000 Hz, some improvement in conductance occurs in the first postnatal month, but at approximately 4000 Hz, infants would be expected to lose 10 to 15 dB relative to adults. The combined effects of external and middle ear immaturity could account for the loss of sound intensity at higher frequencies. Conversely, at frequencies lower than 500 Hz, both neonates and 1-month-olds appear to generate higher levels of sound intensity in the middle ear than in adults. In fact, conductance for low frequencies appears to decline between birth and 1 month and again between 1 month and adulthood. This result is consistent with the presence of a low-frequency resonance in the neonatal ear canal, perhaps resulting from the greater compliance of the ear canal walls. Although this could further explain why tympanometry is not the optimal diagnostic tool in young infants, , it can still be used as a screening method.

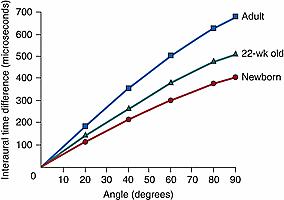
It is often not appreciated that the growth of the pinna, ear canal, and head also has important implications for sound localization. The primary cues used to locate a sound source in the horizontal plane are differences in the timing and intensity of the sound arriving at the two ears. Measurements in both human and nonhuman species indicate that smaller head dimensions produce smaller interaural differences. , Clifton and colleagues measured head diameter longitudinally in a group of infants from birth to 22 weeks of age. From these measurements, they estimated that the largest interaural time difference (ITD) that an infant’s head could produce increased from 420 microseconds at birth to 520 microseconds at 22 weeks of age. The average adult head, however, produces an ITD of approximately 700 microseconds.
Thus, for any sound source location, the infant experiences a smaller cue ( Fig. 135.7 ). Similar effects would be expected for interaural intensity differences. Interaural differences would also vary with sound frequency differently when the head is small: interaural intensity differences would be available at higher frequencies than in the adult, but interaural intensity differences in the midfrequency range would be relatively small. Finally, the transformation of the sound spectrum, due to the pinna and the ear canal, changes with the position of the sound source relative to the ear. These “spectral cues” are used to localize sounds in elevation and to distinguish sound source locations behind the head from those in front of the head. One would expect that smaller pinnae and ear canals would produce poor spectral cues for the localization of sound sources in the midfrequency range. However, the relative contributions of conductive, cochlear, and neural maturation to the development of sound localization remain to be established (although for a review, see Tollin ).
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here