Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
![]() Access video content for this chapter online at Elsevier eBooks+
Access video content for this chapter online at Elsevier eBooks+
Dupuytren’s disease (DD) is a primary fibroproliferative disorder of the palmar and digital fasciae of the hand. A similar disease process known as Ledderhose and Peyronie’s disease affects the feet and penis, respectively. Recent studies indicate that the condition occurs as a result of a complex interplay between genetic, epigenetic, immunologic, and environmental factors ( Fig. 17.1 ).
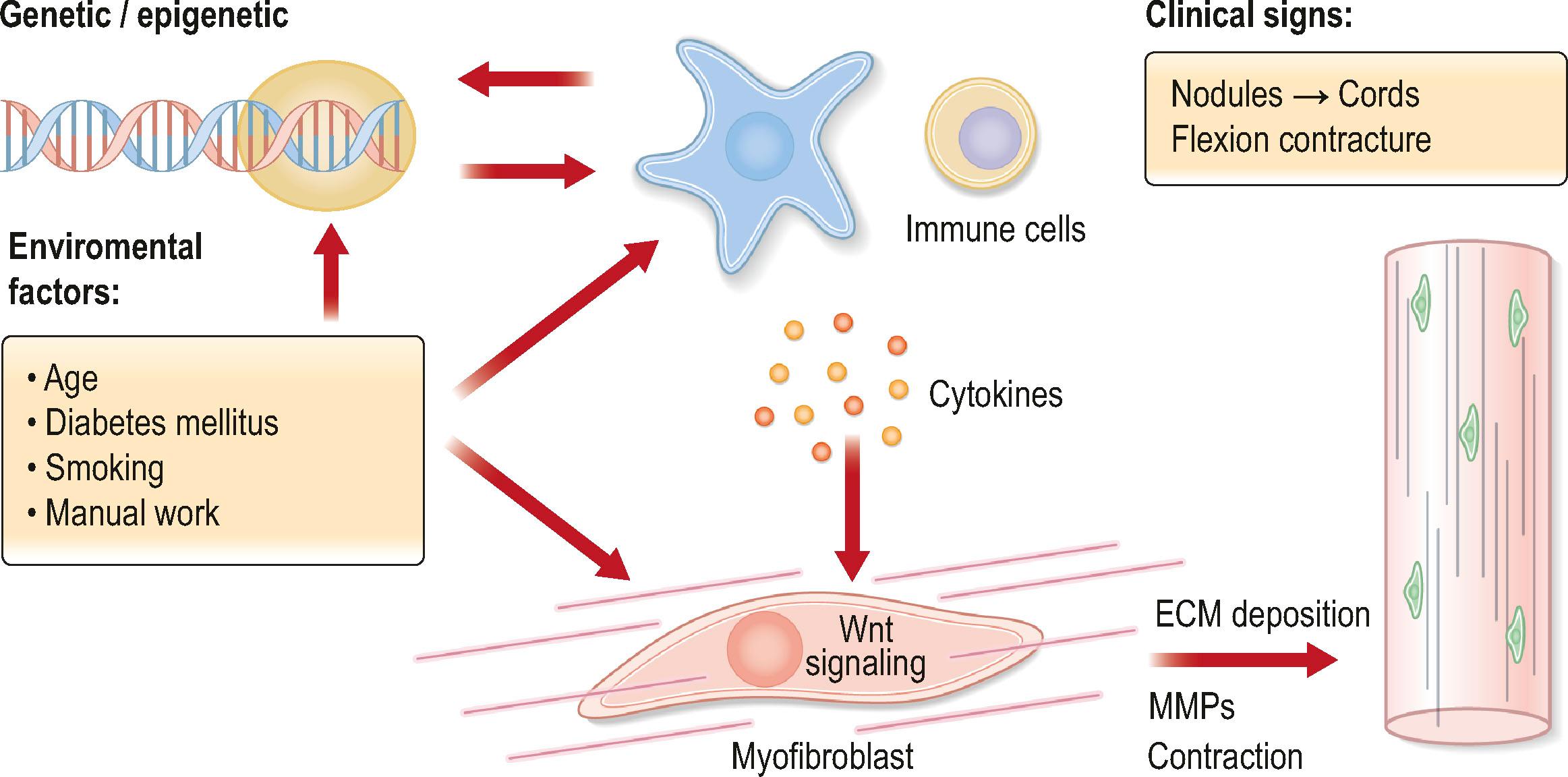
Epidemiological studies have shown that both hereditary and environmental factors contribute to the development of DD.
The prevalence of DD varies according to the population studied from 0.6 to 31.6%. In the UK, about 8% of the population is considered to be affected. The prevalence seems to be lower among populations of non-European descent, although the majority of studies to date have been of insufficient quality to draw robust conclusions.
A number of large population studies, largely undertaken in European countries, have demonstrated a strong heritable component. For example, a twin study from Denmark estimated the heritability, i.e. the degree to which the condition can be attributed to genetic variation, at 80%.
A strong genetic component may also have implications for the onset and severity of the disease. DD is more common amongst males than females. People with a positive family history of Dupuytren’s contracture require surgical intervention at a younger age, suggesting that a genetic component leads to earlier manifestation of the condition. Furthermore, patients with features of diathesis are more likely to carry alleles for single nucleotide polymorphisms associated with DD than patients without diathesis. The different prevalence of DD across various ethnic populations can be largely accounted for by 26 genetic risk variants. However, the generalizability of these data across all ethnic groups remains to be determined.
Other epidemiological studies have reported strong associations between DD and non-genetic, environmental factors, including advanced age, diabetes mellitus, cigarette smoking, and manual work exposure. In this respect, the association between field hockey playing and DD is also of interest. However, there are currently limited data to demonstrate causality. Interestingly, there is also some evidence that DD is associated with excess mortality secondary to a wide range of causes 12 years following diagnosis. Unexpectedly, an increased risk of cancer mortality independent of confounders was observed with a hazard ratio of 1.66 (i.e., patients with DD are 1.66 times more likely to die of cancer than non-DD patients 12 years post diagnosis), and it would be interesting if these findings were also replicated in other populations outside the UK.
DD is a fibroproliferative disorder affecting the palmar and digital fasciae of the hand. The early stages of the disease are characterized by the presence of palmar nodules that in 30–50% of individuals progress to cords which lead to flexion contractures of the digits. Dupuytren’s nodules are highly cellular structures comprising densely packed myofibroblasts in an irregular pallisadal pattern together with fibroblasts, immune cells and blood vessels. The immune cells comprise a small but significant population composed of M1 and M2 macrophages, mast cells and T cells. By contrast, cords, which tend to develop later than nodules, are composed of mature fibrillar collagen and far fewer, mostly stromal, cells.
The nodular cells are thought to secrete and shorten the matrix component which, as it is remodeled in the shortened state, progressively contracts the digit though a “slip and ratchet” mechanism. As the cord matures, myofibroblast numbers decrease, perhaps through apoptosis.
The pathogenesis of DD has been investigated at multiple levels to identify the genetic, epigenetic, chemokine and cytokine-dependent processes. A model of disease progression describes secretion of chemokines by a group of immune regulatory fibroblasts. These soluble mediators attract immune cells, which produce low levels of cytokines, that drive myofibroblast differentiation. However, it remains unclear how the disease is initiated and the myofibroblast precursors have yet to be clearly defined.
Genome-wide association studies (GWAS) have confirmed the epidemiological data that demonstrate the hereditary component of DD. A number of susceptibility genetic loci have been identified. Many of these involve genes that encode proteins in the Wnt signaling pathway as well as those associated with extracellular matrix (ECM) remodeling. Wnt signaling is a major regulatory signal transduction pathway of cell proliferation, migration, and differentiation. Aberrant Wnt signaling is associated with many other fibrotic diseases and activation of Wnt signaling has been reported in DD, with upregulated expression of Wnt signaling protein in Dupuytren’s nodules. Wnt signaling has also been shown to lead to myofibroblast activation. Interestingly, tumor necrosis factor (TNF) activates the canonical Wnt pathway to upregulate α-smooth muscle actin (α-SMA) and collagen I expression by myofibroblasts.
The restriction of DD to the palm led to the investigation of the role of epigenetic regulation in the pathogenesis of the disease and also provides a potential link with environmental factors. For example, histone acetylation has been found to be a key regulator of ECM production and contractility.
DD has been shown to be a localized low-grade inflammatory disease, with low levels of cytokines secreted by the nodular cells. Unlike in conditions such as rheumatoid arthritis, these cytokines were not identified in the systemic circulation. The authors proposed that low levels (~50 pg/mL) of TNF secreted by the local immune cells (M2 macrophages and mast cells) act via the TNFR2 receptor to promote differentiation of myofibroblasts, which secrete low levels of interleukin (IL)-33 that in turn acts on the immune cells to lead to further TNF production. This has led to clinical trials of local administration of anti-TNF (adalimumab) into the nodules. The phase IIa trial found that 40 mg of adalimumab in 0.4 mL resulted in downregulation of α-SMA and procollagen 1 proteins. A subsequent phase IIb clinical trial found that a course of four injections of this formulation of adalimumab at 3-month intervals resulted in reduction in nodule hardness (primary endpoint) and nodule size on ultrasound scan (secondary outcome) at 12 months. Both parameters continued to decrease for a further 9 months. The authors went on to show that repeated courses of four injections of adalimumab at 3-year intervals are likely to be cost-effective.
The myofibroblast is considered the principal cell responsible for the physical manifestation, i.e., flexion contracture, of the disease process. It expresses an intracellular protein, α-SMA, and excessive ECM proteins, including collagens, glycoproteins, and proteoglycans, which drive tissue contraction. The myofibroblasts coordinate their activities via gap, adherens, and mechanosensitive junctions, effectively functioning as a syncytium. The myofibroblasts span a spectrum that ranges from highly contractile cells that express CD82 to more fibroblast-like cells, and also contain a distinct small cycling population. Nodules also contain several distinct groups of fibroblasts, including an immune regulatory population.
The ECM is an extensive molecular network consisting of proteins, glycosaminoglycans (long polysaccharides consisting of repeating disaccharide units) and glycoconjugates (carbohydrates covalently linked to other compounds such as proteins or lipids). These components provide an environment within which cells reside and interact via cell adhesion receptors and molecules.
Under physiological, non-disease conditions, the ECM is maintained at homeostasis whereby deposition is balanced by degradation by matrix metalloproteinases (MMPs), a family of enzymes that degrade both matrix and non-matrix proteins and are major contributors to ECM turnover. Fibrosis is characterized by disruption of ECM homeostasis in favor of deposition in the presence of high matrix turnover. A common single nucleotide polymorphism identified by a GWAS study involves MMP14, with reduced activity of this enzyme found to be a risk variant for DD. Interestingly, MMP gene expression, including MMP14, has been found to correlate with clinical outcomes, including recurrence following treatment.
The ECM is not a passive product of the stromal cells but has important regulatory functions. For example, the stiffness of the matrix is a key determinant of myofibroblast phenotype. Studies investigating the cellular mechanotransduction pathways have found the transcriptional coactivators Yes-associated protein 1 (YAP1) and transcriptional coactivator with PDZ binding motif (TAZ) as nuclear relays of mechanical signals exerted by ECM rigidity and cell shape. These provide a mechanism for sensing and mediating mechanical cues in the cellular microenvironment. As fibrosis progresses, matrix protein is deposited, which increases tissue stiffness. This in turn promotes further matrix secretion and contractility of myofibroblasts. The matrix also indirectly modulates the embedded stromal cells. An important profibrotic cytokine TGF-β1 is secreted in a nascent form that is activated on contraction of the matrix.
DD follows anatomical pathways, and one of the most enigmatic aspects of DD is how the disease converts the normal anatomy of the fascial bands into diseased cords. In this section, accepted and new insights in the microanatomy of the fascia pertaining to DD are reviewed and related to surgical findings.
DD commonly starts in the palm where it involves the triangular-shaped fascia or palmar aponeurosis (PA) which is connected to the overlying skin by vertical fibers described by Grapow.
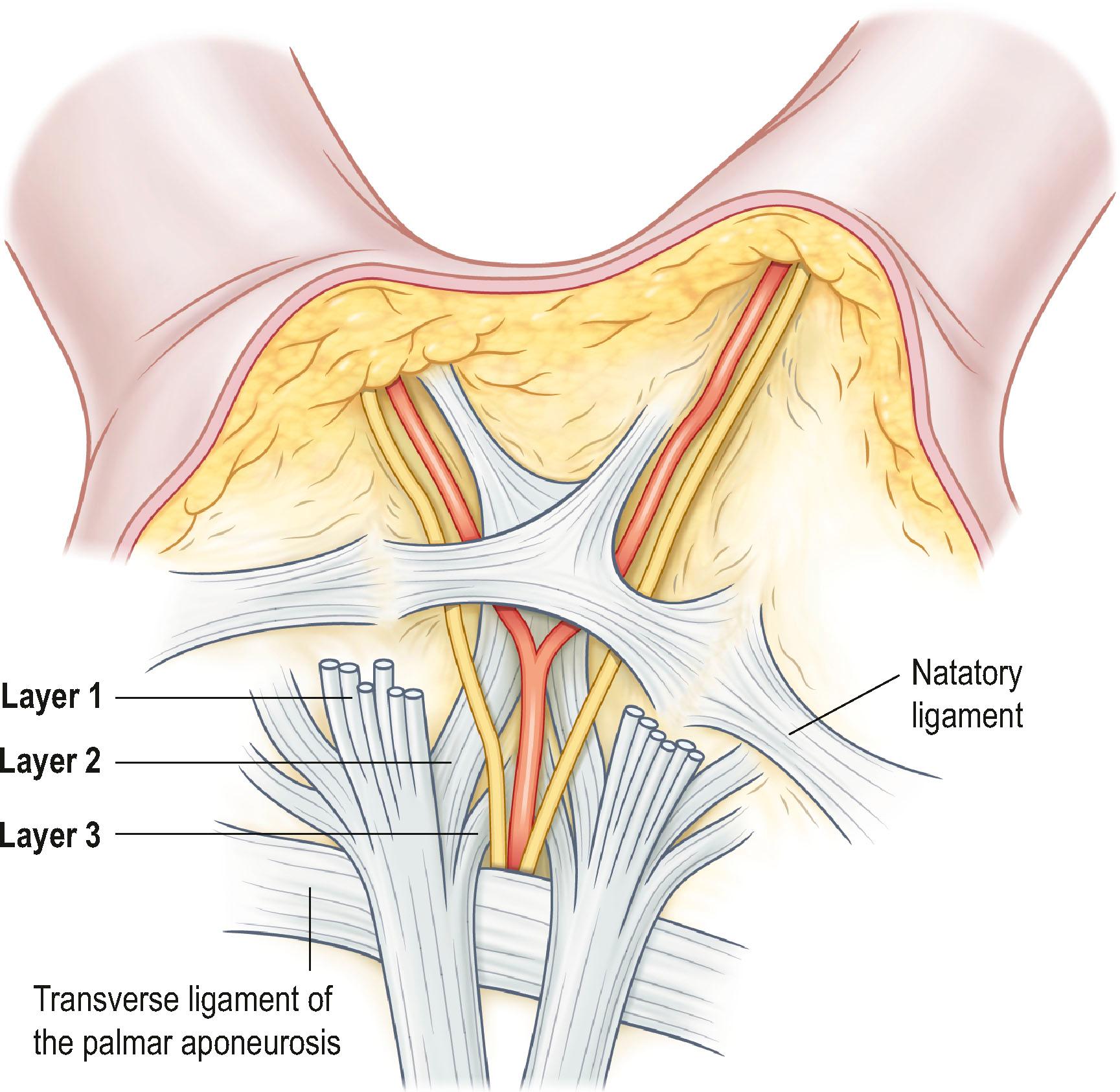
Involvement of the Grapow’s fibers results in the development of skin pits, one of the earliest signs of DD. Proximally, the apex of the PA is continuous with the tendon of the palmaris longus (PL) muscle, if present; otherwise, it attaches to the flexor retinaculum. It has been suggested that if the palmaris longus tendon is absent, the flexor carpi ulnaris (FCU) muscle acts as the longitudinal tensor of the palmar fascia. Dupuytren’s nodules have been described at the level of the wrist related to the PL or the FCU. The longitudinal fibers of PA are condensed into pretendinous (PT) bands that radiate distally towards the base of the fingers ( Fig. 17.2 ). A PT band for the thumb is also present, but less prominent than for the fingers. Between the PT bands the more delicate prelumbrical bands complete the palmar aponeurosis ( Fig. 17.3 ). The PA is bordered by the thenar and hypothenar eminences on either side and fixed to the interspatial septa that divide the palm in thenar, midpalmar, and hypothenar spaces. The base of the triangular sheet of the PA is located at the level of the transverse ligament of the palmar aponeurosis (TLPA), which is situated at a slightly deeper plane along a line that joins the proximal and distal palmar creases. TLPA is supported by the ligaments of Legueu and Juvara, which connect it to the deep transverse intermetacarpal ligament (DTIL, Fig. 17.3 ). The DTIL runs between the volar plates of the MCP joints and is not affected by DD.
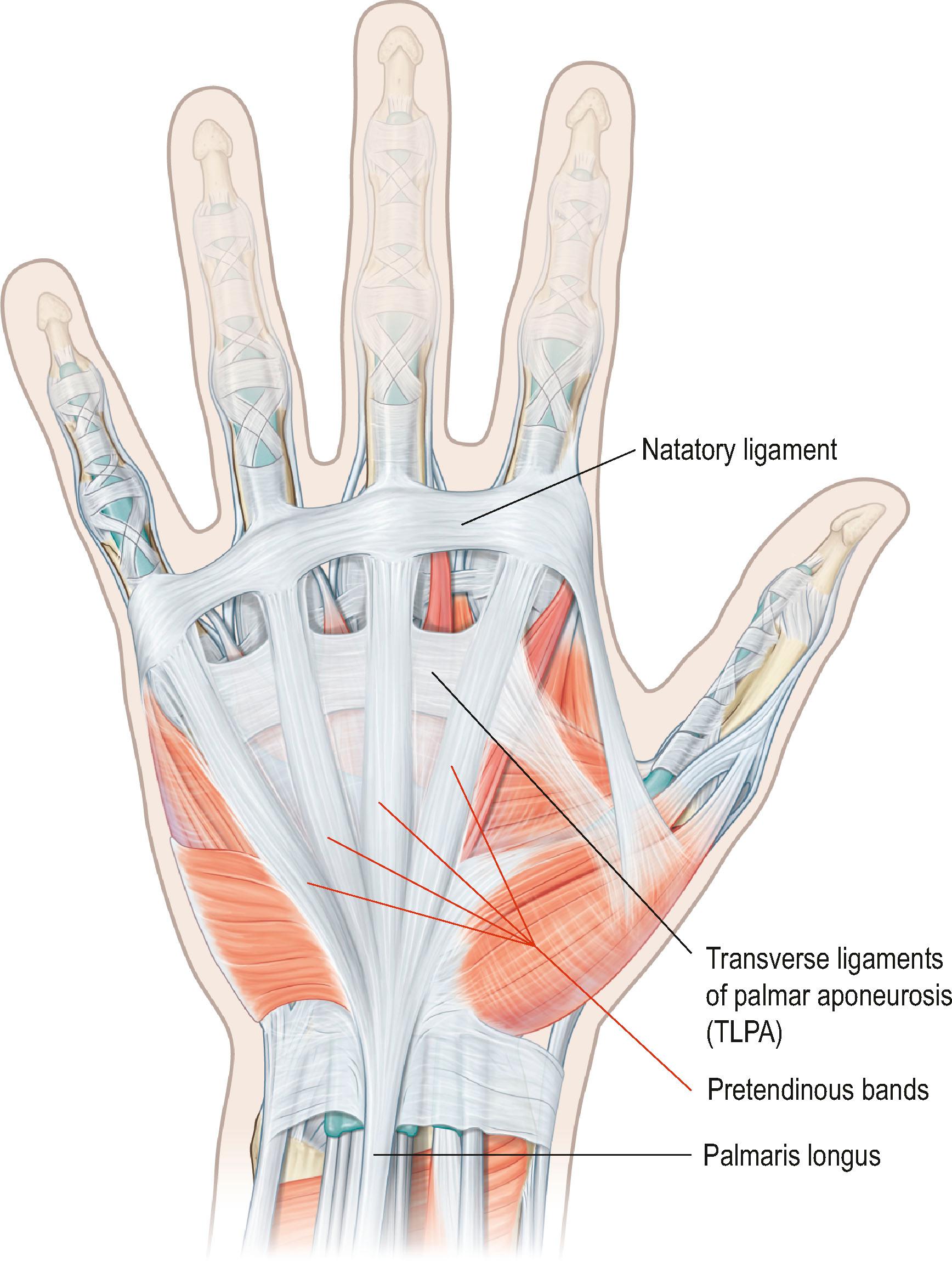
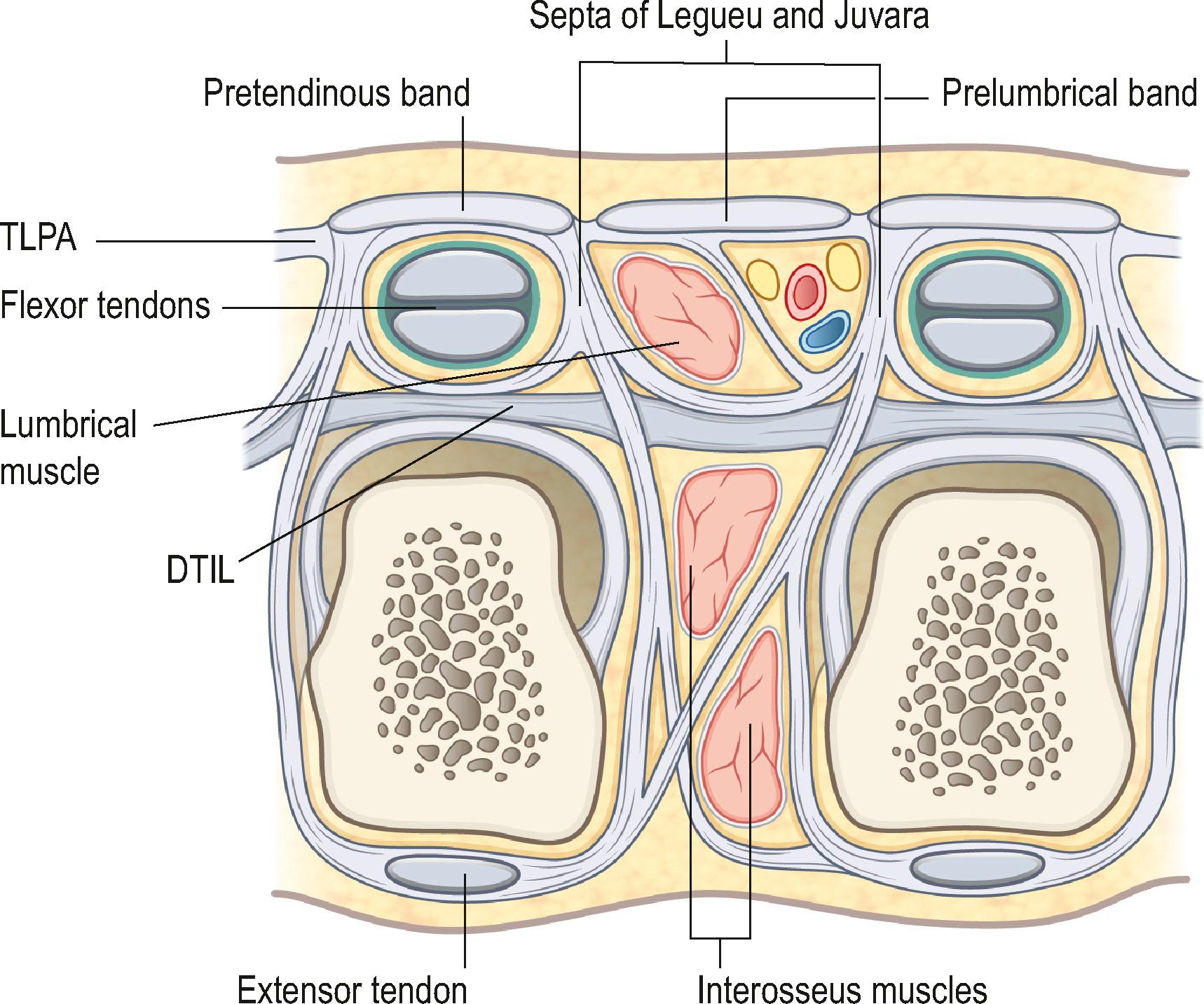
On cross-section, the TLPA and DTIL, together with the septa of Legueu and Juvara, form seven “boxes” (see Fig. 17.3 ). Each box encloses a lumbrical muscle and a neurovascular bundle, or the flexor tendons of each ray. Vertical septae separate the hypothenar from the midpalmar space, and the midpalmar from the thenar space, and need to be divided to remove the proximal part of the palmar aponeurosis.
The ulnar border of the TLPA merges with fibers of the hypothenar fascia.
At the radial border the TLPA is continuous with the proximal commissural band, which is a condensation of the fascia of the adductor pollicis muscle and runs through the first webspace to end in the thenar fascia at the level of the first metacarpophalangeal (MCP) joint. It is believed that TLPA is not affected by DD, whereas the proximal commissural band can give rise to an adduction contracture. The TLPA lies palmar to the neurovascular bundles but dorsal to the pretendinous and prelumbrical cords. As it is not involved in DD, most surgeons preserve it as it serves as a reference point for future surgery for recurrent disease.
More distally in the palm is located another superficial transverse ligament, the natatory ligament (NL). NL has attachments to the flexor tendon sheet in the midline ( Fig. 17.4 ). It lines the webs (see Fig. 17.2 ) and has extensions towards the fascial sheet on the lateral side of the digits, the lateral digital sheet of Gosset. DD commonly affects the NL leading to restriction of finger abduction. On the radial side of the palm the NL is continuous with the fascia of the first dorsal interosseus muscle, which may also be affected by DD, giving rise to the distal commissural cord ( Fig. 17.5 ).
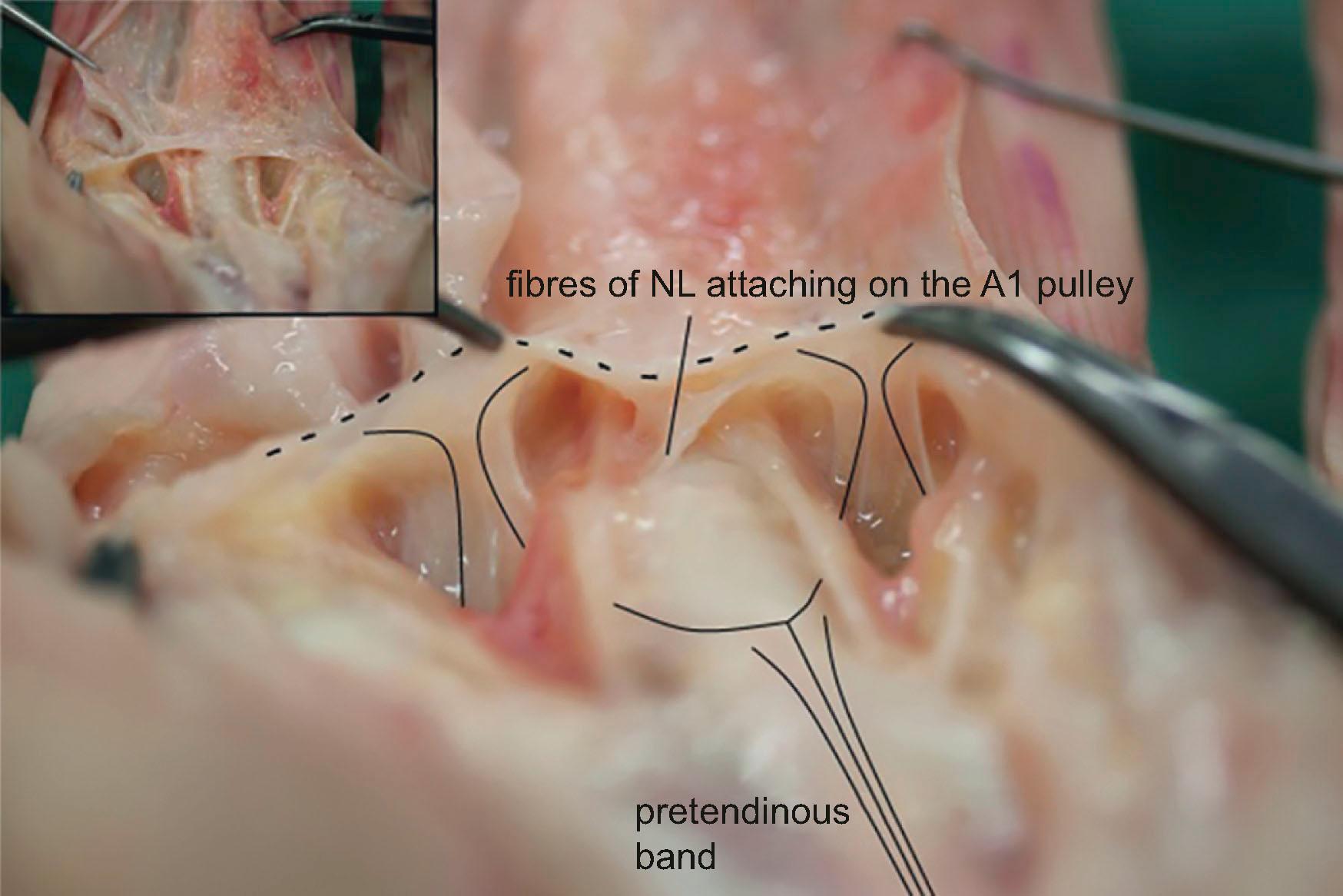
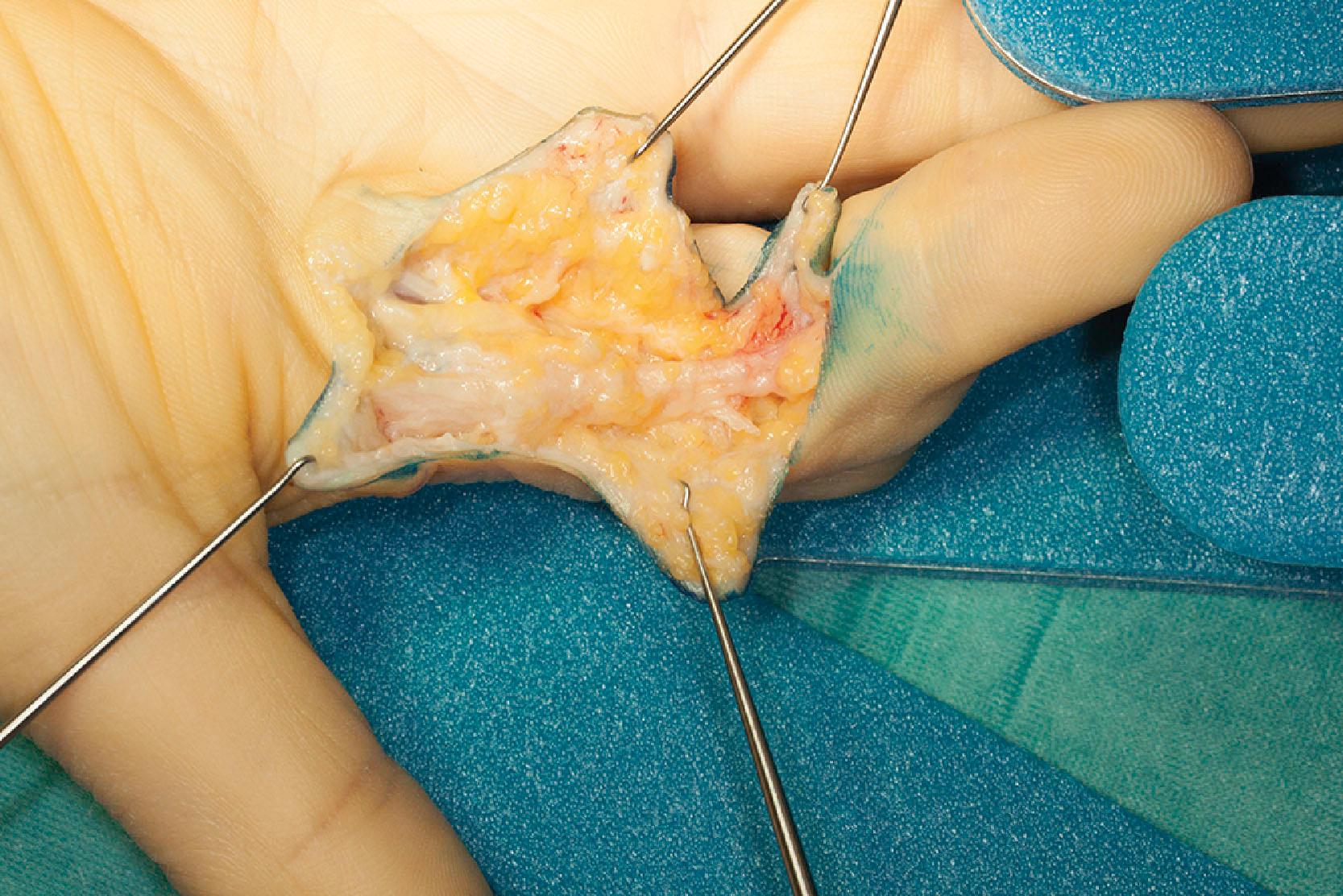
The normal distal commissural band lines the first webspace and blends with the fascia of the thenar insertions near the MCP joint and the PT band of the thumb. On the ulnar side the NL blends with the fascia of the abductor digiti minimi muscle. This is almost always affected if there is a PT cord of the little finger and, if not excised during limited fasciectomy, predisposes to early recurrence. It may also occur as an isolated cord ( Fig. 17.6 ).
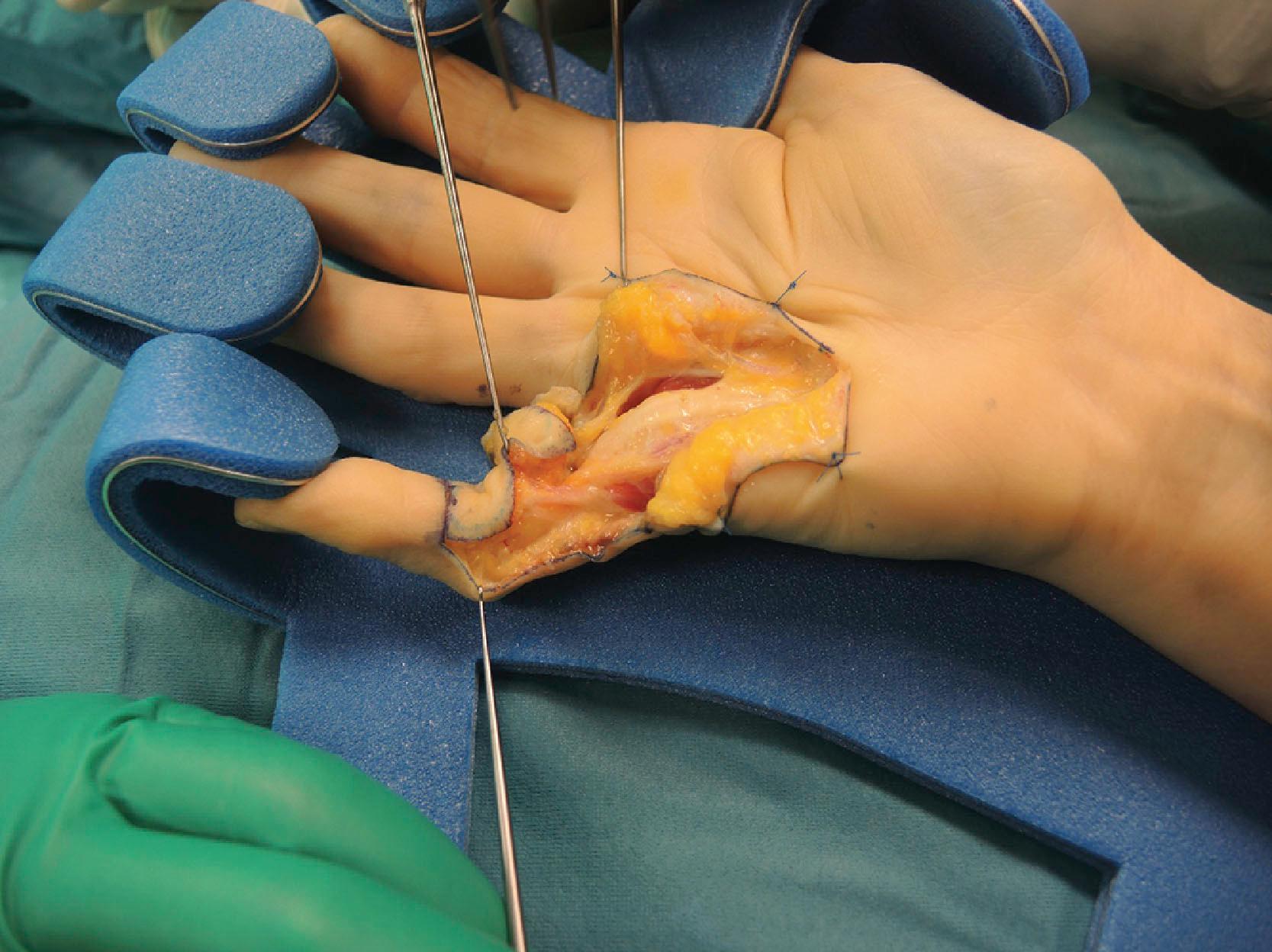
However, it is more common for the abductor cord to blend with fibers that are a continuation of the PT. The proximal edge of the confluence of the abductor and the PT cords is an important anatomical landmark to identify the ulnar digital neurovascular bundle, which can be found in the space immediately deep to where the two structures meet ( Fig. 17.7 ).
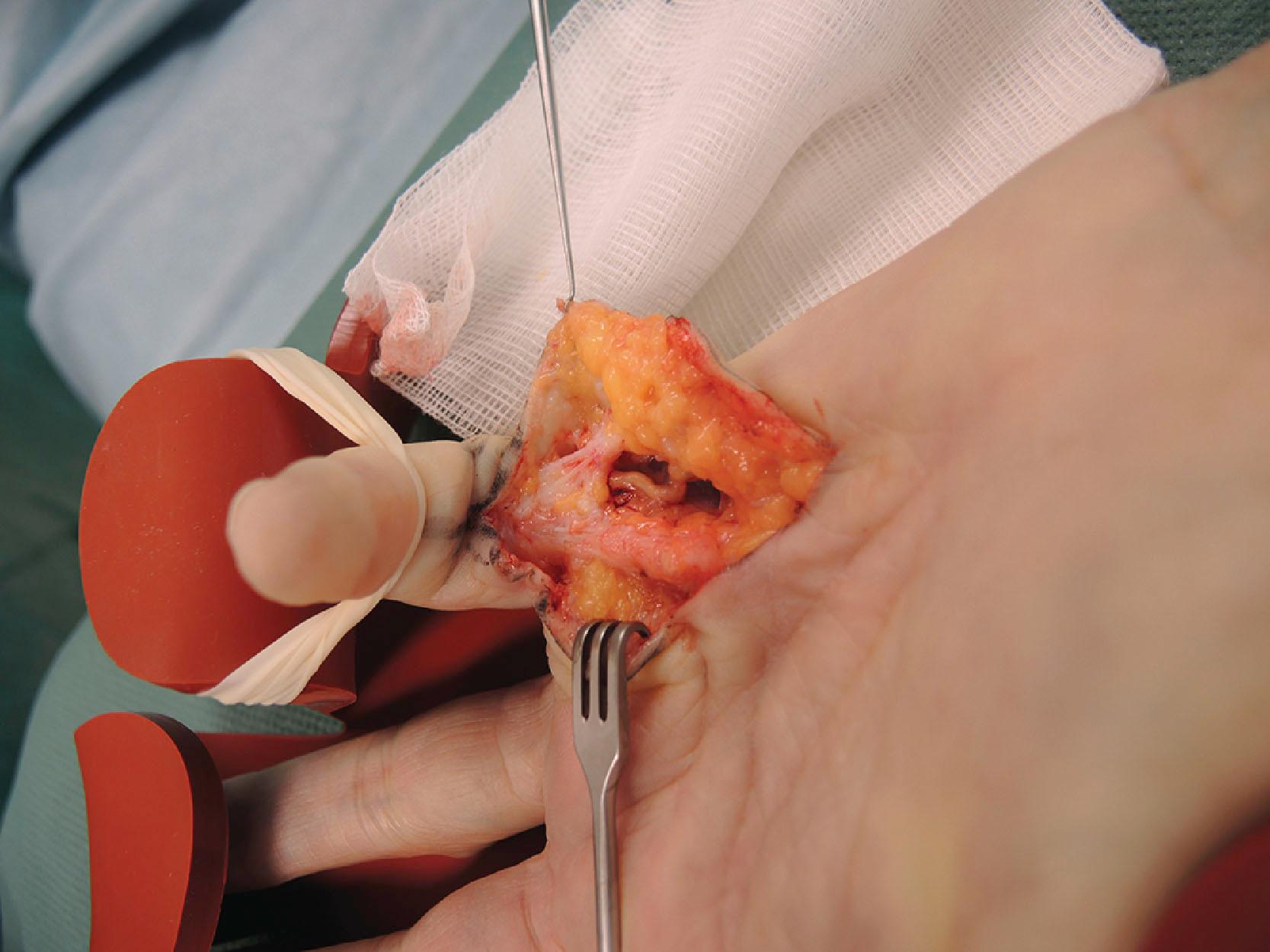
The region between the TLPA and NL defines the distal palm and contains a substantial amount of fat in between the flexor tendons of the fingers. Here, the common neurovascular bundles lie proximally and bifurcate more distally, and this is where the palmodigital spiraling system originates.
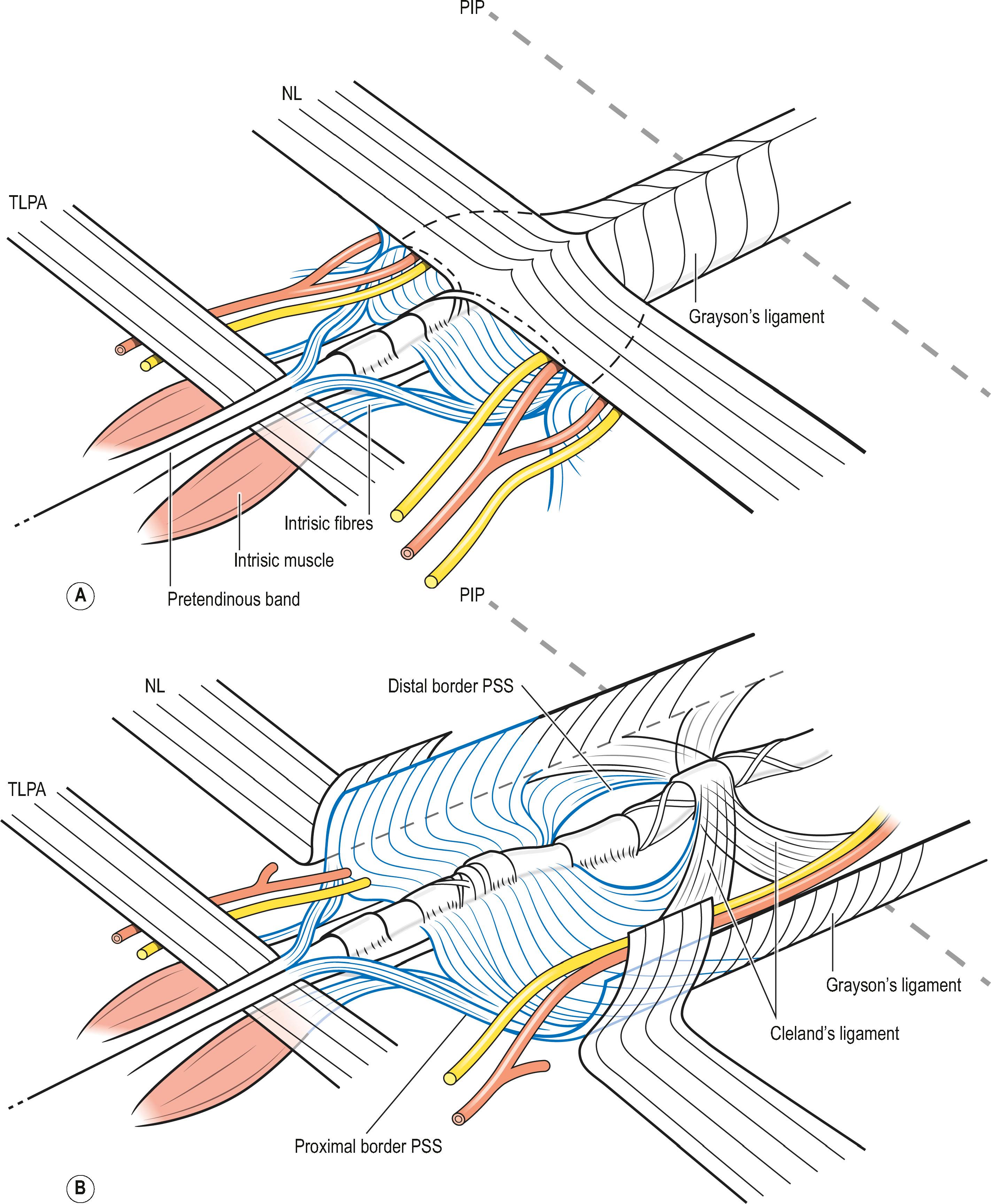
The pretendinous (PT) bands of the fingers pass volar to the TLPA and divide into three layers ( Fig. 17.8 ). The most superficial layer of the PT bands (layer 1) inserts into the palmar skin. Some fibers of this layer pass to the volar skin of the proximal phalanx, after crossing the NL superficially ( Fig. 17.9 ). Progressing distally, the number of cutaneous insertions reduces. When they turn into cords, these fibers may not be distinguishable from the fibers of the Grayson’s trabecular network of the proximal phalanx (see below). These layer 1 fibers can give rise to a central cord, leading to MCP joint contracture.
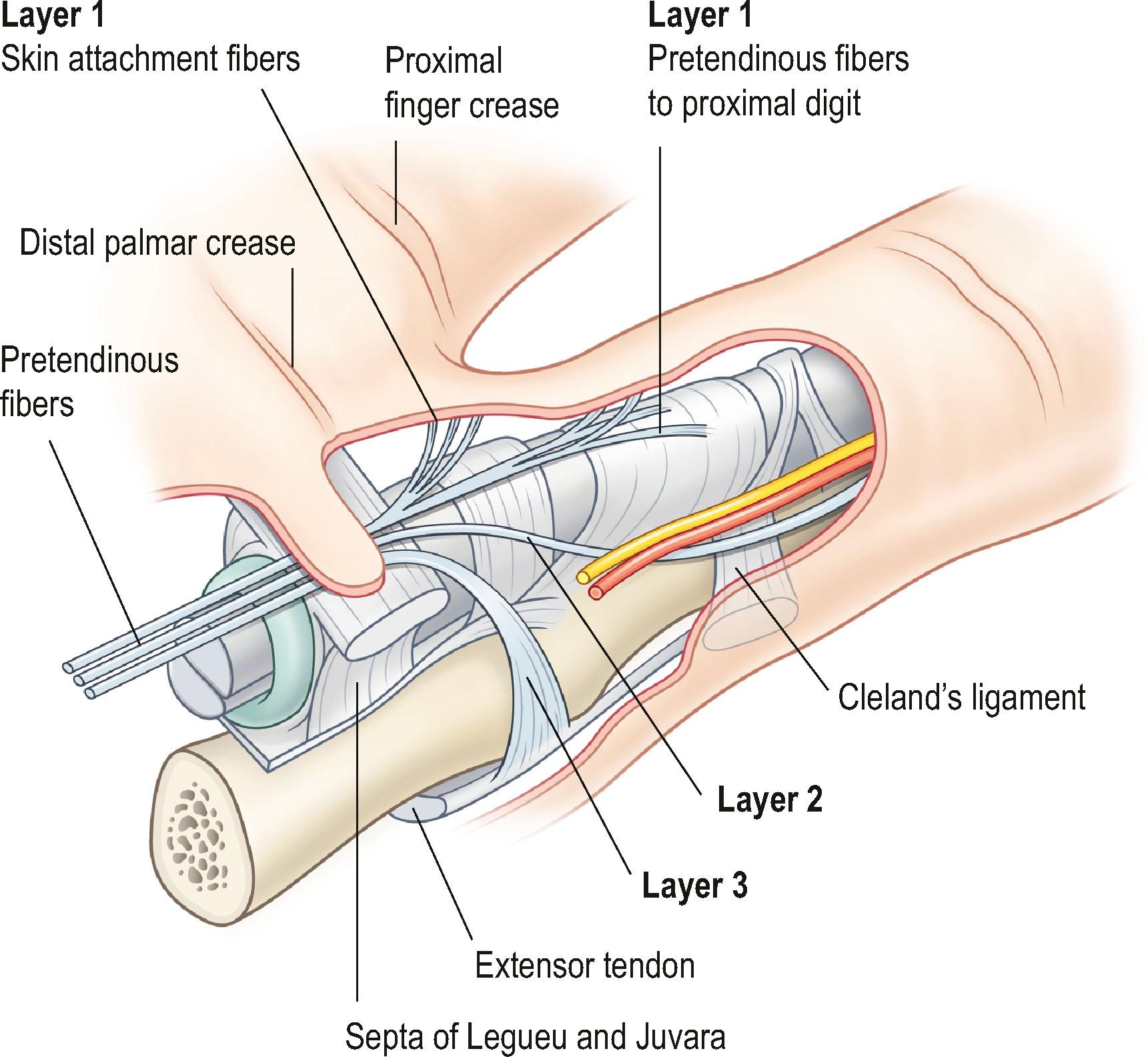
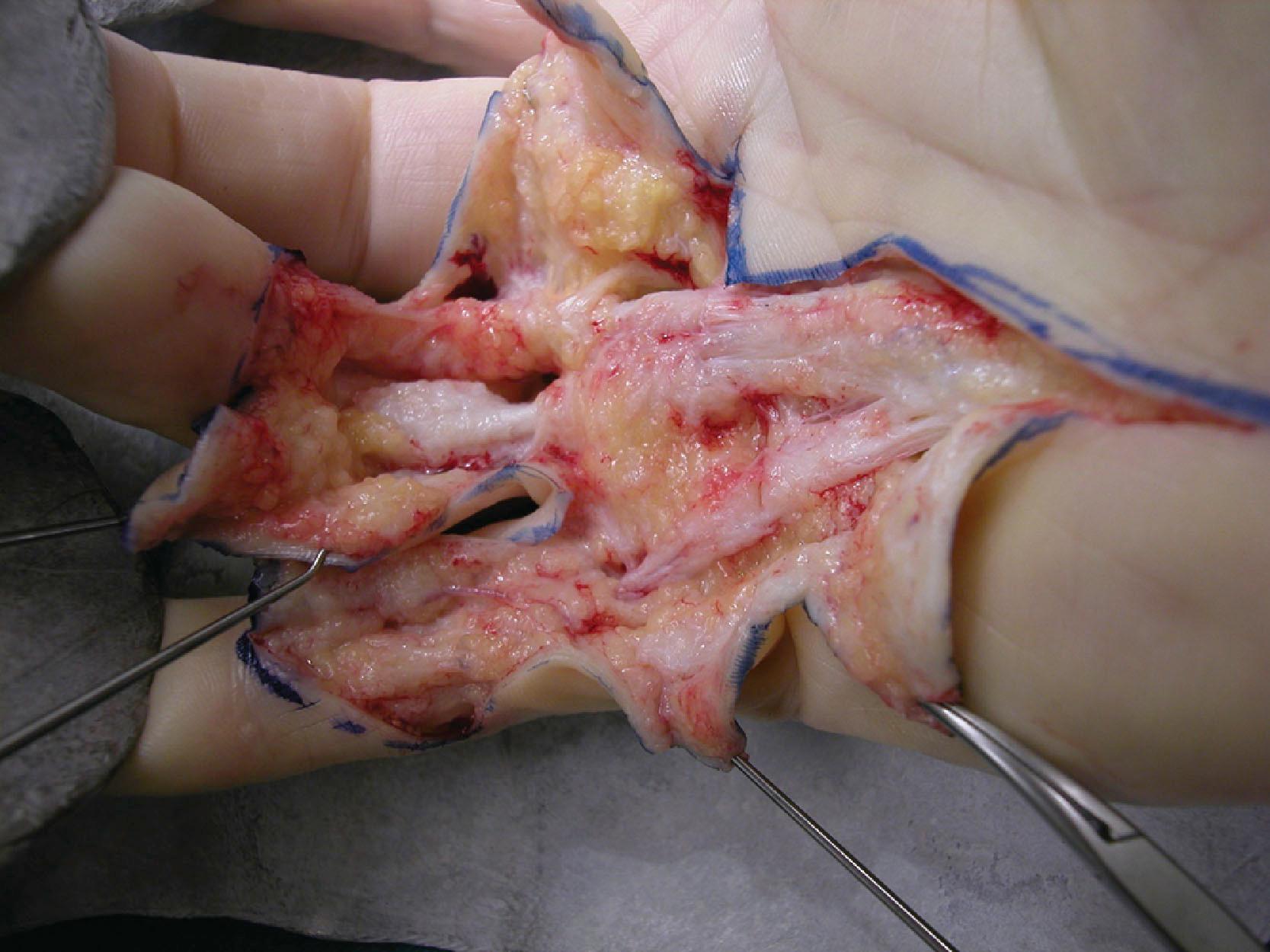
The second layer consists of two bands that diverge and run distally, passing deep to the neurovascular bundle. The classical description is that, distally, these fibers blend with the lateral digital sheet (LDS) of Gosset, a fascial condensation in the lateral plane of the finger ( Fig. 17.10 ). However, more recently it has been shown that these fibers have a different course and terminate on the dorsal aspect of NL, spiraling through a full 360° (see Fig. 17.4 ). These fibers are known in the literature as the spiral bands and form the most proximal contribution of the palmodigital spiraling system (PSS). When involved in DD these fibers thicken and shorten to form spiral cords that displace the neurovascular bundle medially and superficially, making the latter very vulnerable to injury during limited fasciectomy ( Fig. 17.11 ). Mobility of the skin overlying the pretendinous cord and the presence of fat in between the skin and the cord should alert the surgeon to the presence of a spiral cord and displacement of the neurovascular bundle (Short–Watson sign) ( Fig. 17.12 ).
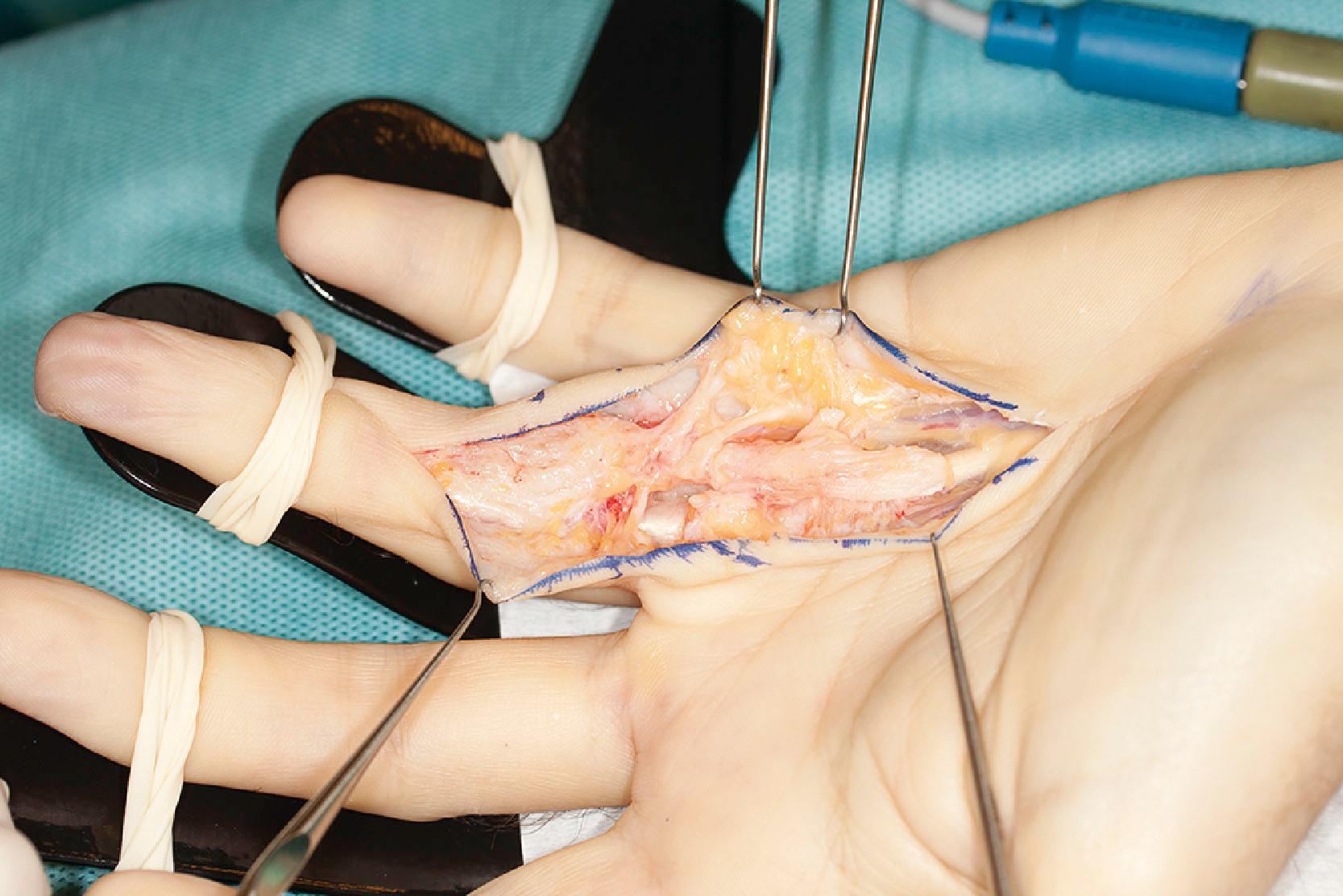
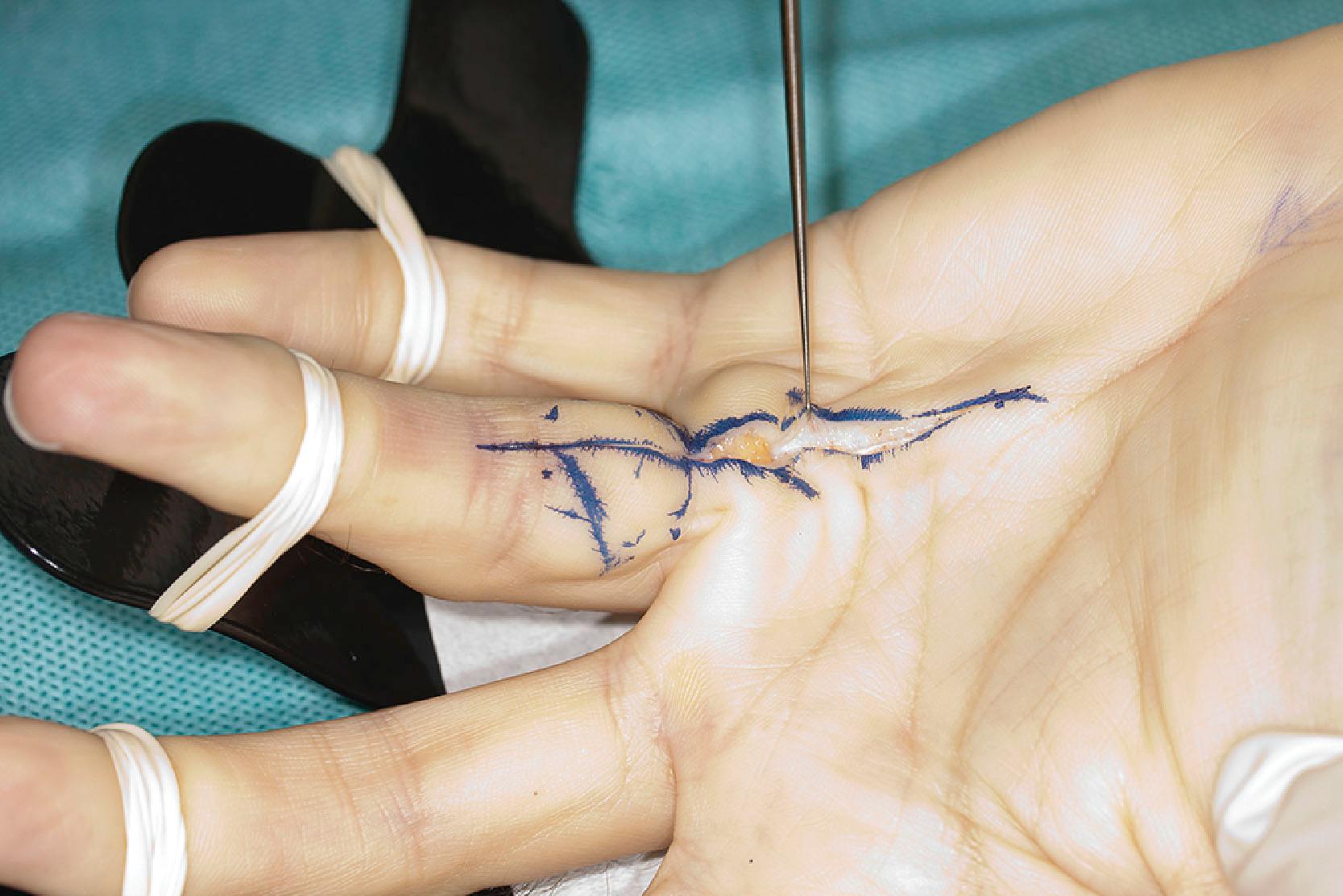
The blending of the spiral band with the undersurface of the NL is important because its proximal border defines the location at which a displaced (spiral) digital nerve in the palm crosses a spiral cord superficially. The intrinsic muscle fascia also contributes to the palmodigital spiraling system proximally, just before the spiral band inserts into NL ( Fig. 17.13 ).
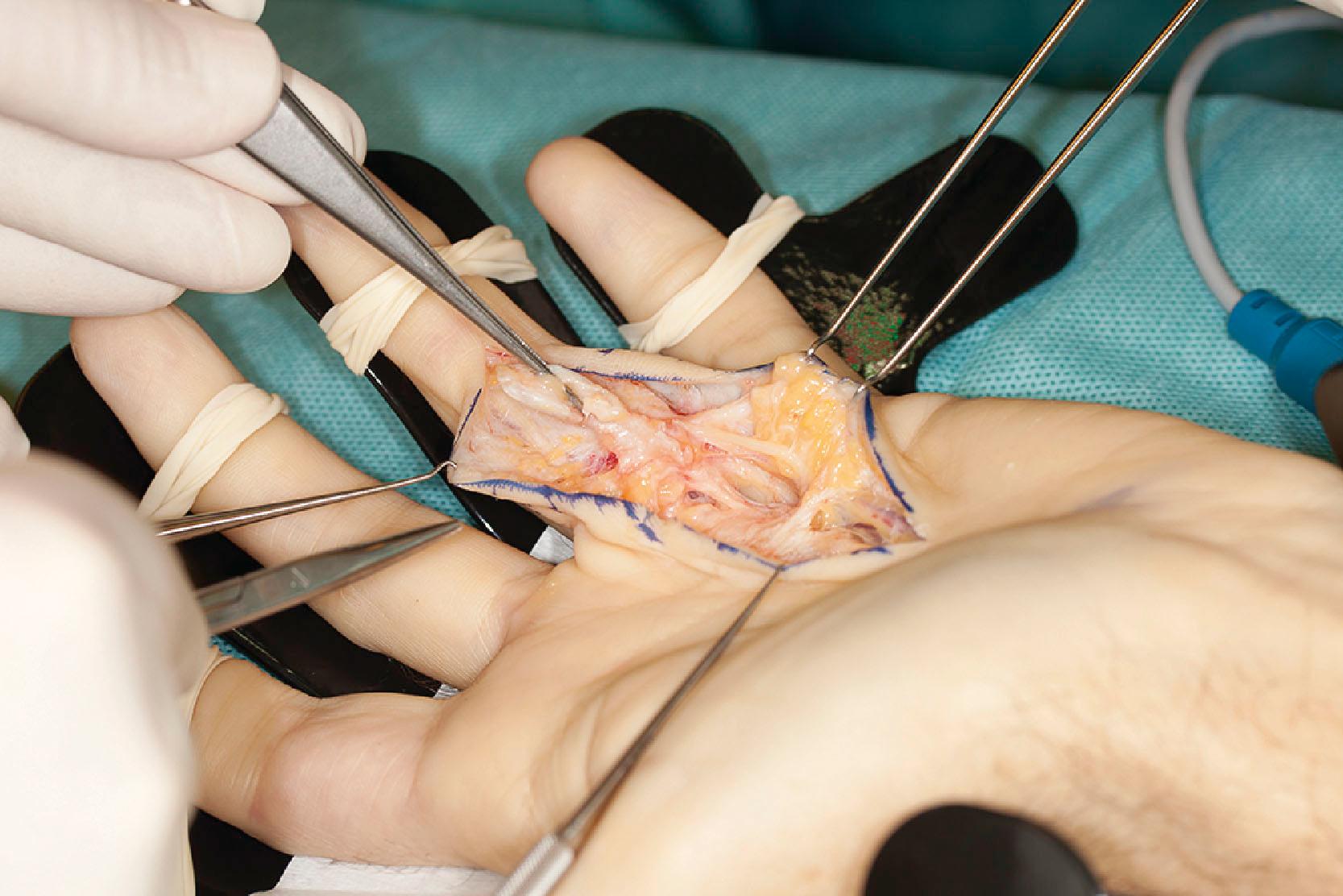
The spiral bands of adjoining fingers blend in the distal palm before inserting on the dorsal aspect of NL ( Fig. 17.4 ). This blending explains the formation of a Y-cord that seems to start in one ray and run into adjoining fingers. Therefore, a single strategically placed injection of collagenase can be performed to correct the MCP flexion deformities of two adjacent fingers.
Isolated layer 2 (spiral) as well as layer 1 (central) cords cause MCP joint contractures and can often be treated successfully and safely by percutaneous needle fasciotomy, providing the fasciotomy is not performed too close to the NL, especially in case of a Short–Watson sign. The clinical relevance of the various layers developing into central and spiral cords, and of contributions from the intrinsic fascia, is that not all MCP joint contractures can be completely released by percutaneous needle fasciotomy. The cords that are palpable immediately under the skin are a quick win. However, the spiral cord as well as the intrinsic contributions to PSS cords are located behind the neurovascular bundle and are too deep for safe access during a blind procedure.
The deepest PT fibers dive deep on both sides around the MCP joint to insert dorsally in the extensor sheet. These fibers perforate the DTIL on their way to the extensor sheet and are, therefore, also known as perforating fibers. If both sides of the flexor tendon are affected, they may cause triggering.
In addition to the proximal part of PSS described above, intermediate and distal parts can also be distinguished. The intermediate part of PSS is formed by fibers that originate from the A1 pulley (see Fig. 17.14 ). These fibers rotate up to 180° and join the best-developed part of Grayson's ligament, that covers the neurovascular bundle on the palmar side, and usually end at the A4 pulley, thus giving rise to a digital spiral cord in the finger.
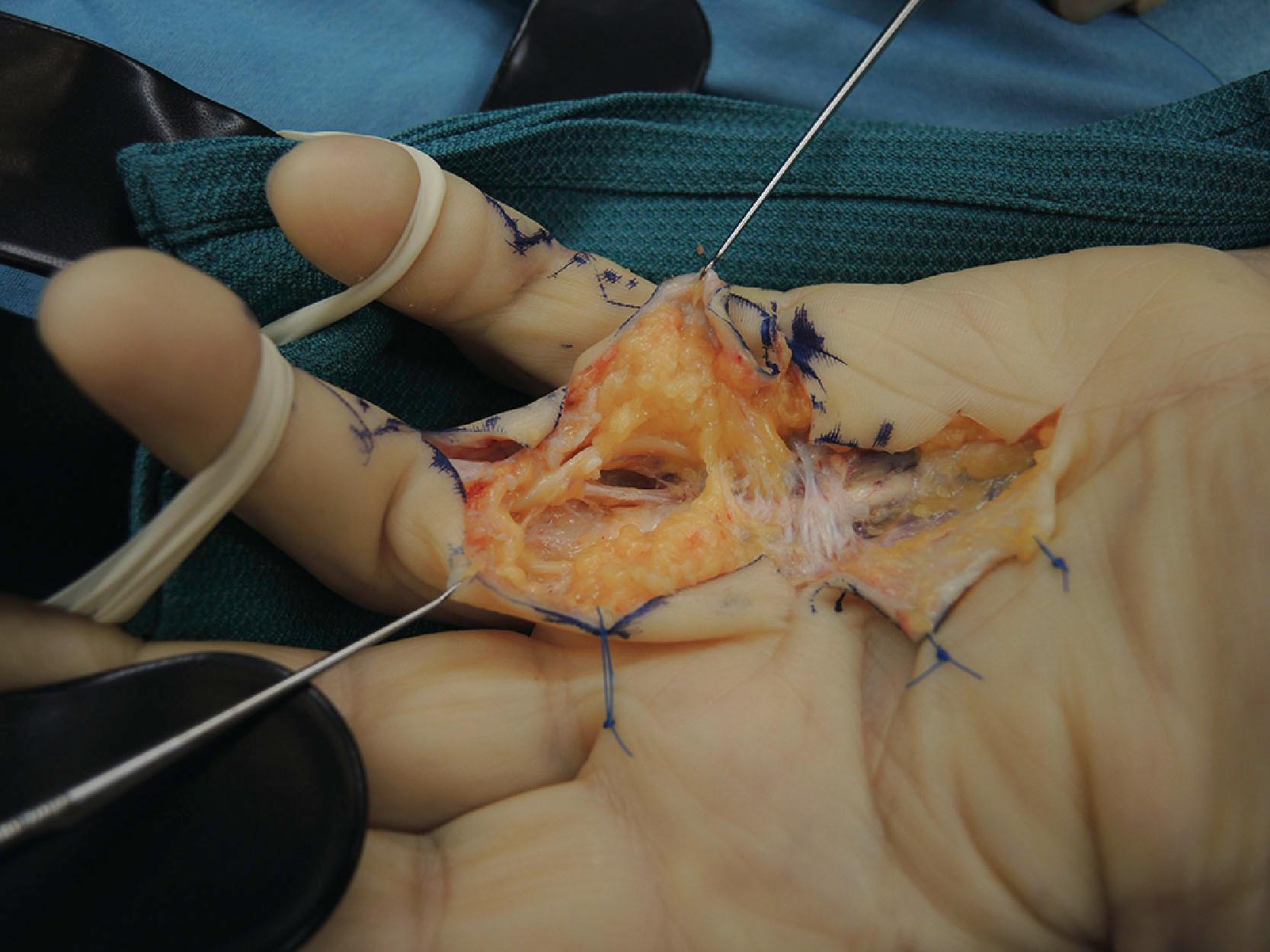
In addition, some of these fibers together with the more distal origin of PSS on the A2 pulley blend with the part of Cleland's ligaments that runs immediately deep to the neurovascular bundle ( Fig. 17.15 ). The fibers that run from PSS to the lateral digital sheet can give rise to a cord that may also contribute to MCP joint contracture and have to be sought behind the neurovascular bundle during limited fasciectomy. We routinely remove them in an effort to prevent or delay recurrence formation.
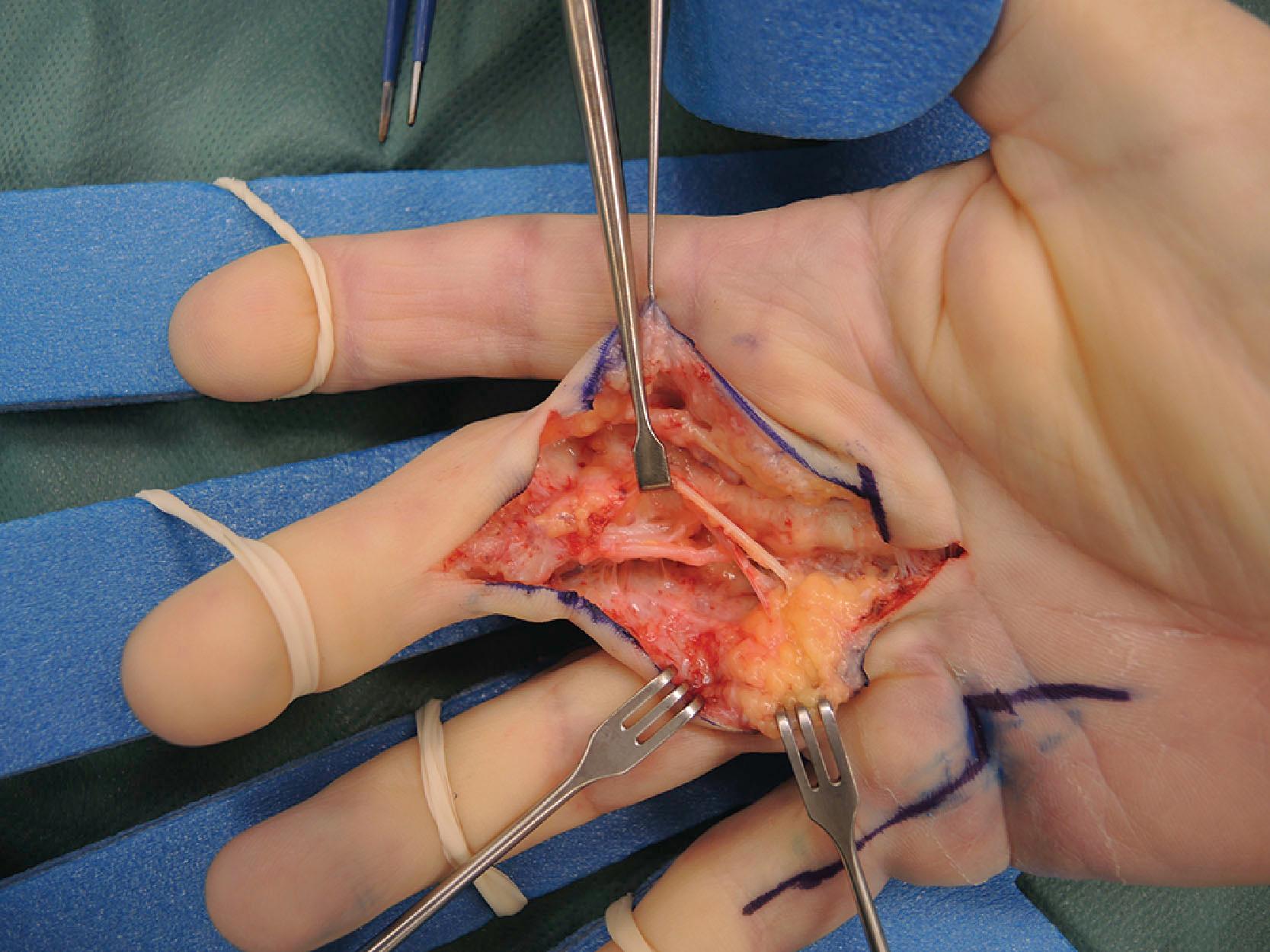
The anatomical description of the digital fascial structures relevant to DD that bear the names of Cleland and Grayson has changed significantly since their first description and there are conflicting illustrations depicting their course ( Fig. 17.16 ). More recently their microanatomy has been re-evaluated. Grayson’s ligament is the most condensed part of a trabecular network of fibers located volar to the neurovascular bundle. It consist of fibers that originate from the outer surface of the flexor tendon sheath and terminate proximally on the contralateral side of the finger in an inverted V-shaped orientation, which becomes less pointed during flexion ( Fig. 17.17 ). This trabecular network ( Fig. 17.18 ) encompasses fat lobules and facilitates the adaptation of the skin to grasp objects when the fingers are flexed.
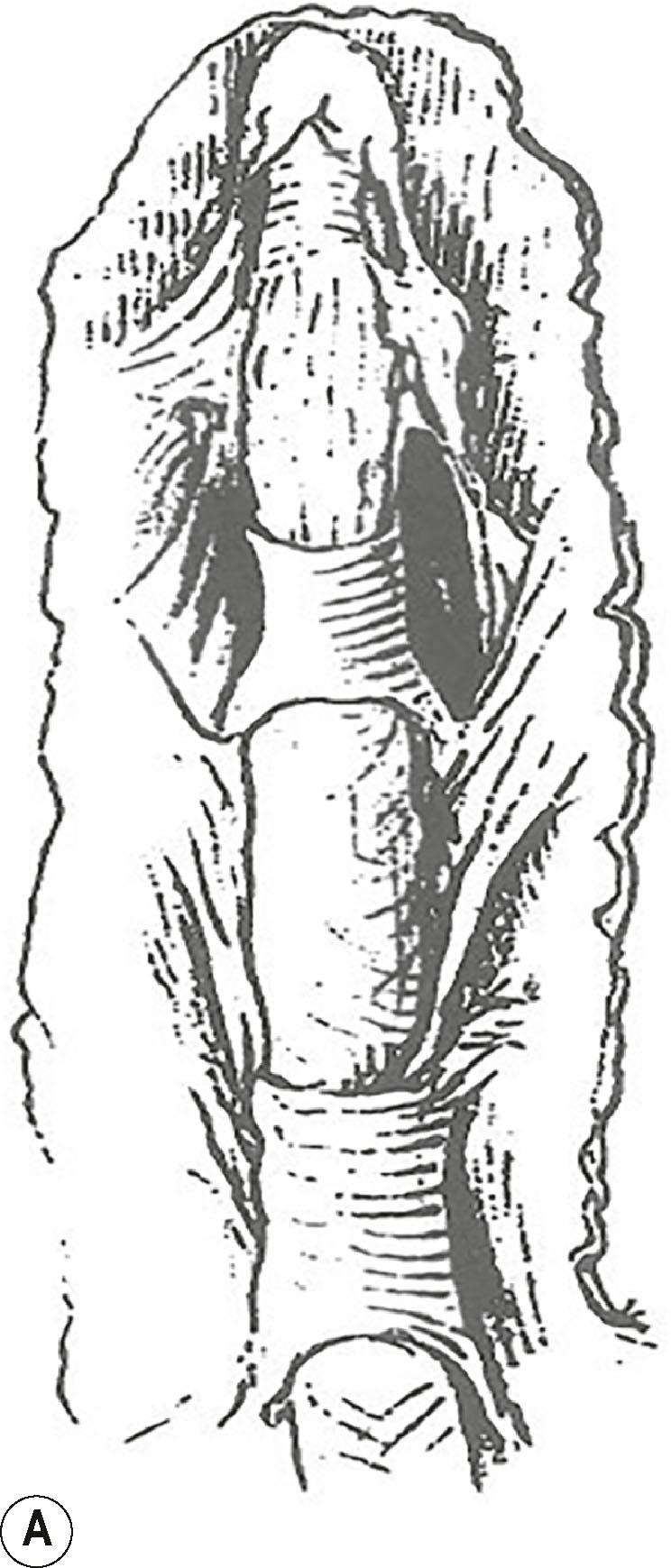
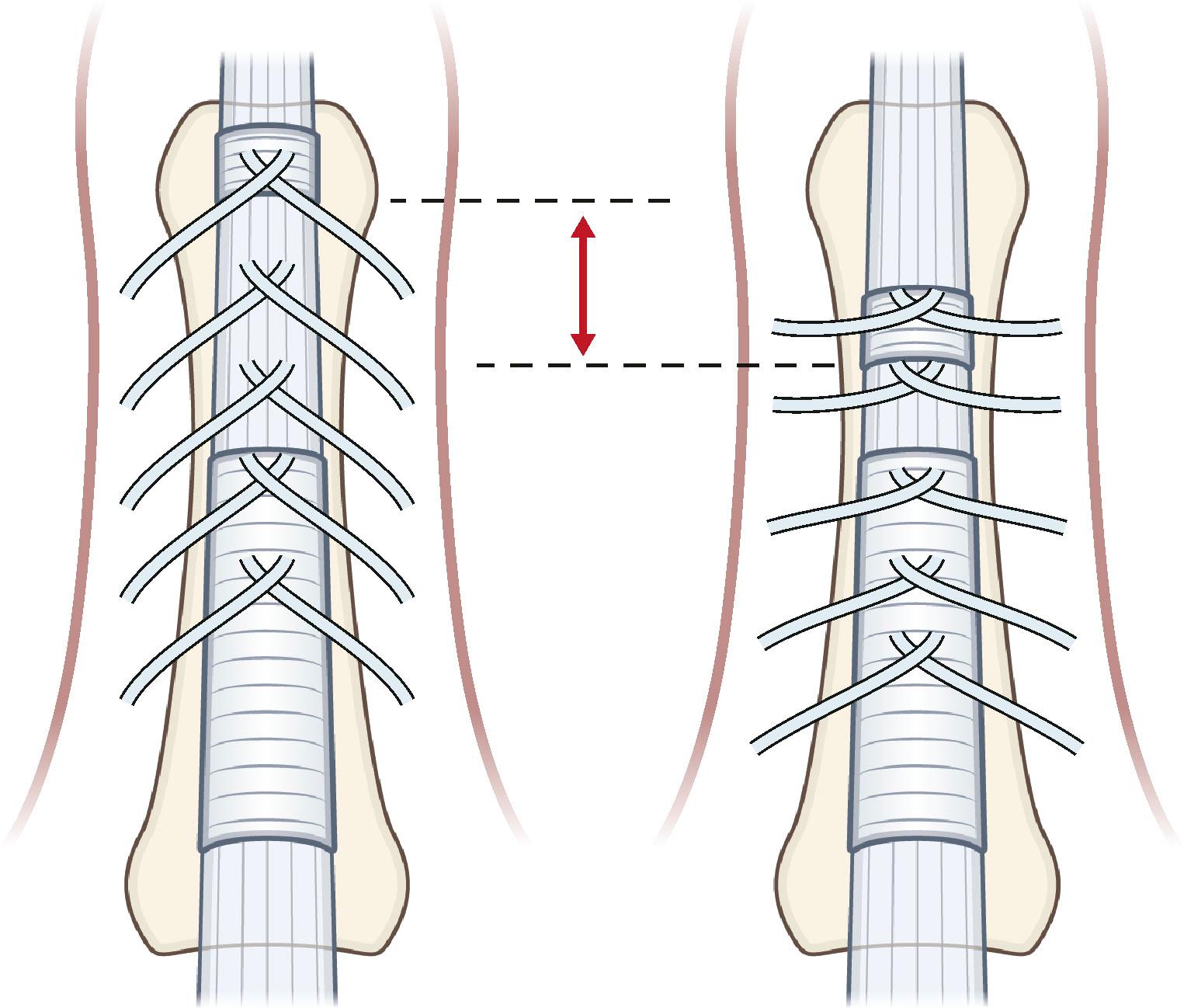
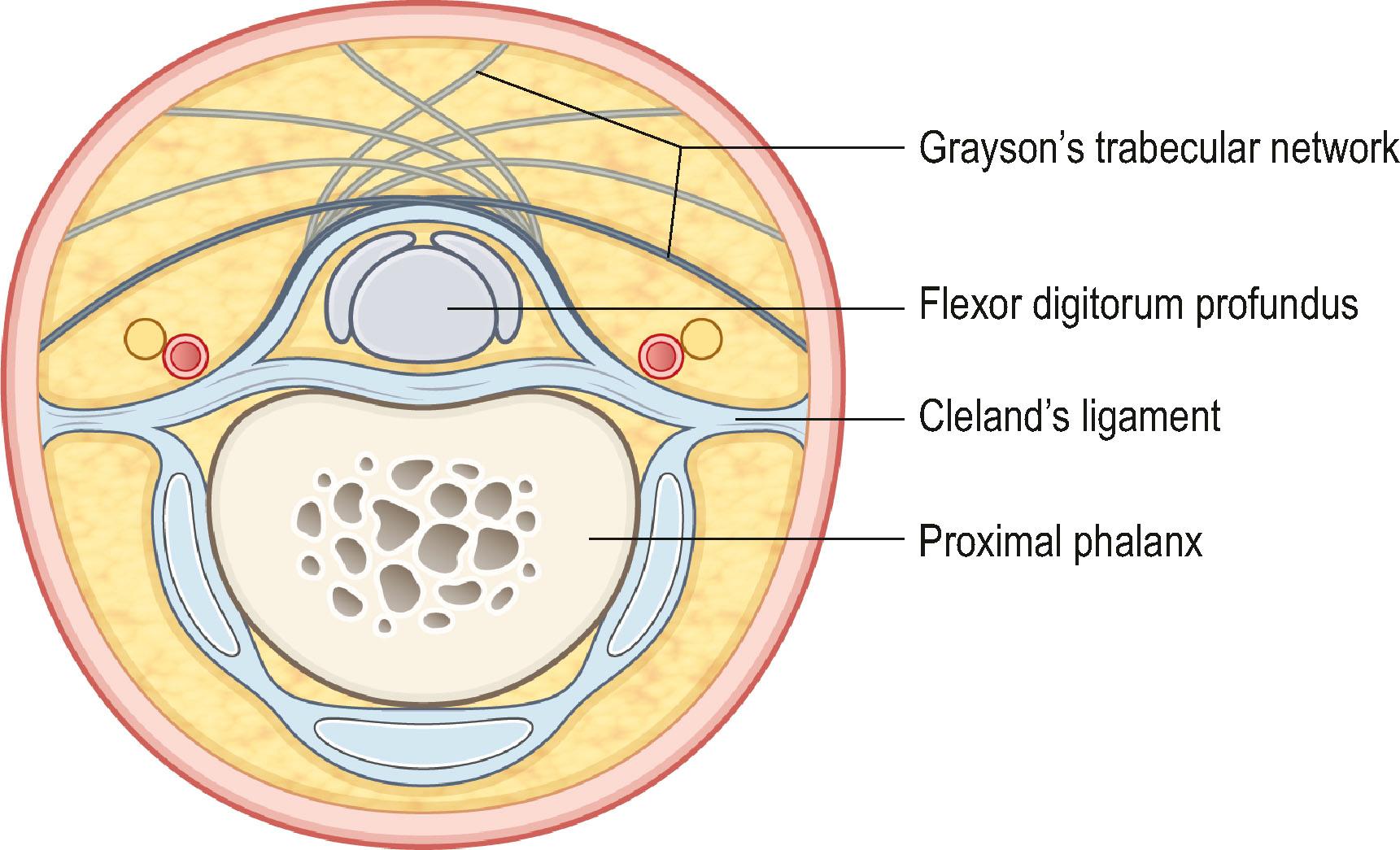
On the sides of the fingers, the fibers end in the deep dermis and also connect to the lateral digital sheets (LDS). The Grayson fibers that become affected form a cord that is located proximally in the finger lateral to the neurovascular bundle and distally blends with the A4 pulley. Therefore, it crosses the neurovascular bundle at the level of the proximal interphalangeal (PIP) joint and causes a PIP joint flexion deformity. In the case of a central cord with involvement of the midline part of the Grayson’s trabecular network, the contracture of the fibers can reverse the direction of the inverted V, resulting in the cord running from the midline at the level of the proximal phalanx to laterally beyond the PIP joint and further contributing to the PIP joint flexion contracture ( Fig. 17.19 ).
Cleland’s ligaments form paired structures located on each side of the finger, originating at the level of the PIP and distal interphalangeal (DIP) joints and course proximally and distally ( Fig. 17.20 ). Three layers have been distinguished at the level of the PIP joint: the most volar emerges from the fibers of the C1 and A3 pulleys of the flexor tendon sheet, the middle layer from the volar plate and the most dorsal layer, which is actually a double layer, encases the extensor apparatus. After joining just lateral to the volar plate, they fan out proximally and distally. They run dorsal to the neurovascular bundles (see Fig. 17.18 ). Although typically considered not to be involved with DD, a recent description has challenged this.
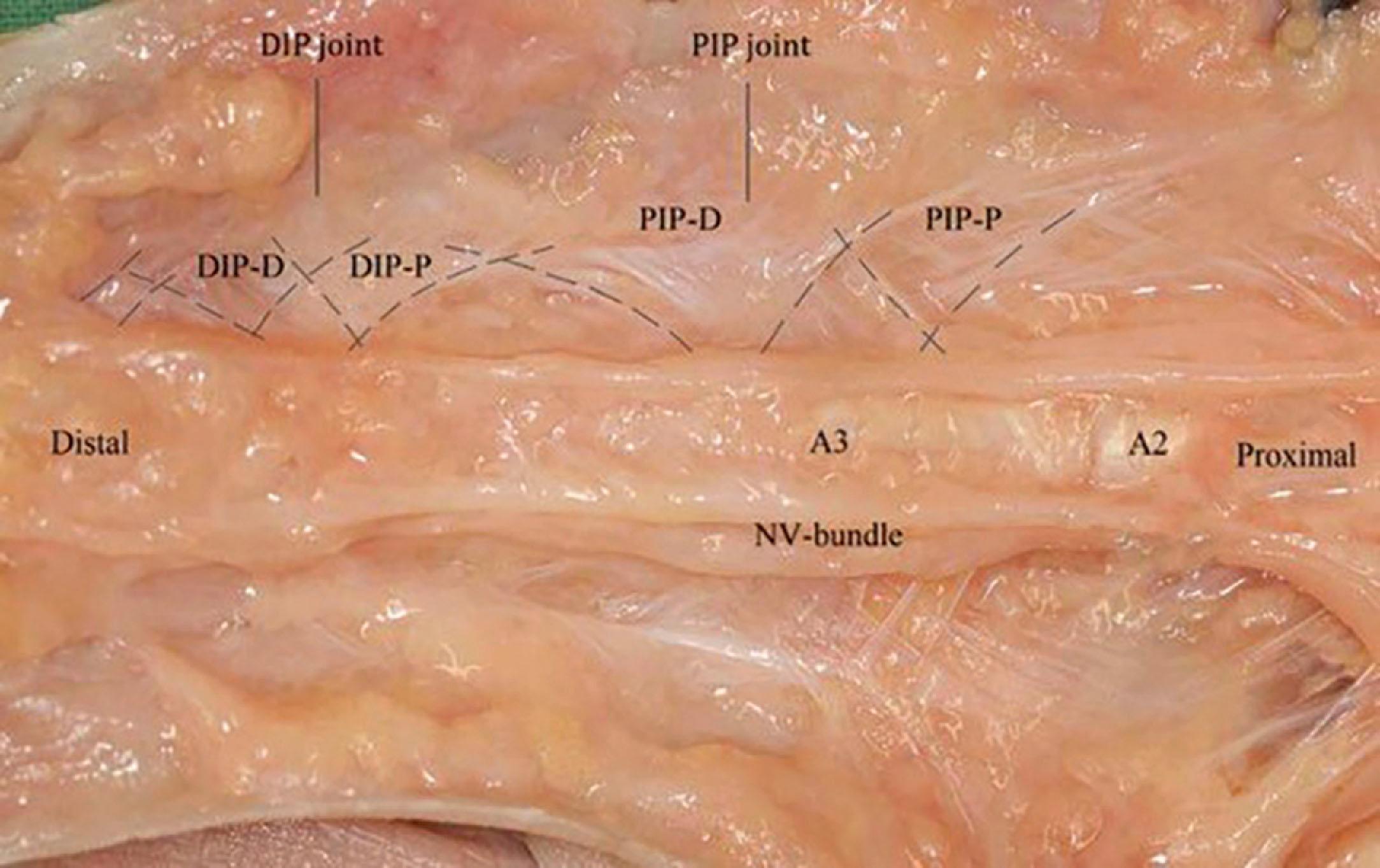
Recent microanatomical studies have revealed that the descriptions of the digital ligaments as forming a box-like structure may be too simplistic. Some of the digital fascia has been described as forming a continuous spiral around the neurovascular bundle, starting at the level of the proximal border of the NL and continuing distally at least beyond the PIP joint ( Fig. 17.21 ).
A detailed understanding of the spiraling nature of the normal fascia together with the observation that all parts may be involved in DD provides an explanation for the various patterns of cords encountered in clinical practice. However, whilst some patterns may be expected, there is always a chance of an unexpected finding during limited fasciectomy, leaving the neurovascular bundles vulnerable to inadvertent injury ( Figs. 17.22 & 17.23 ).
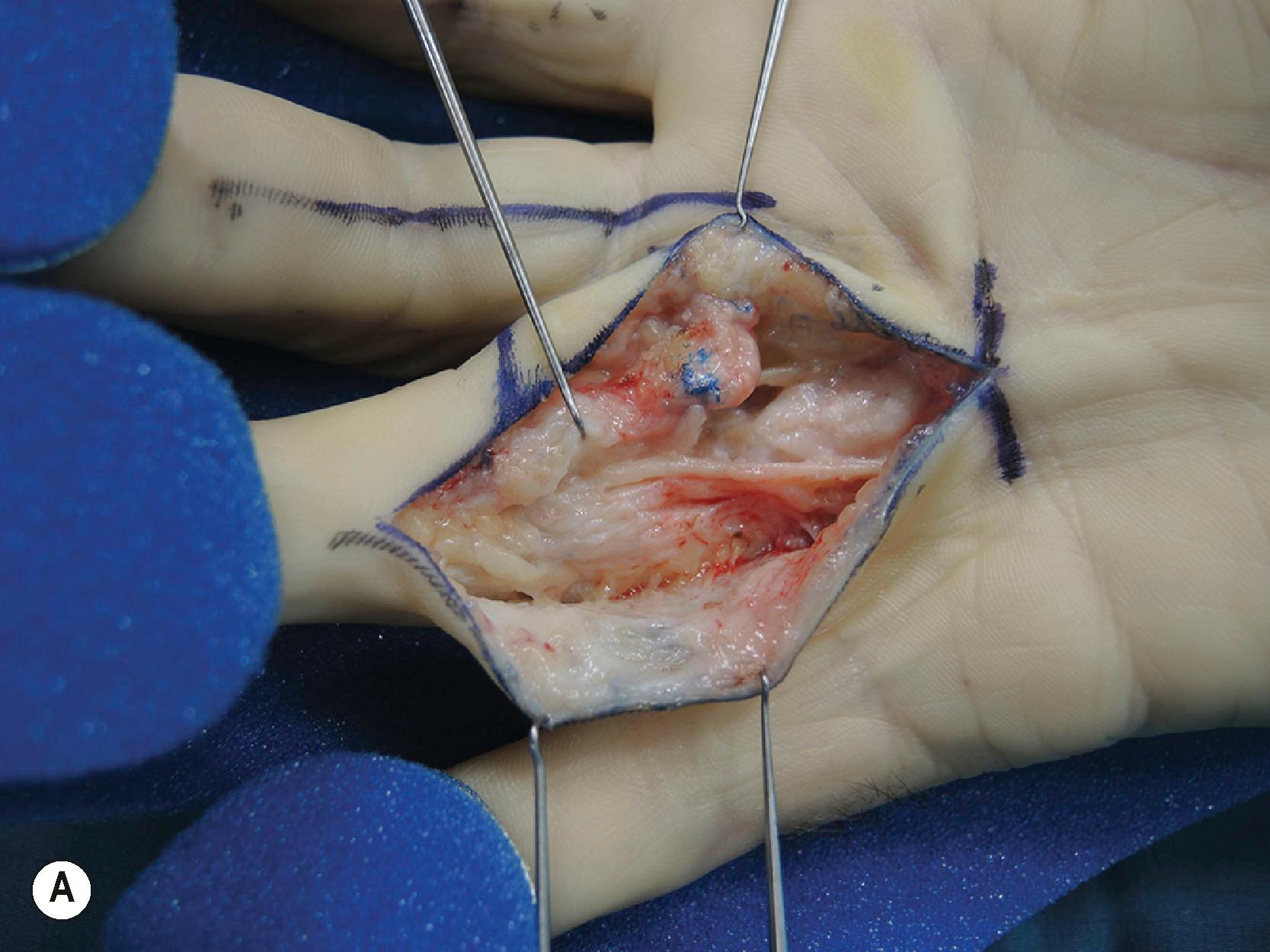
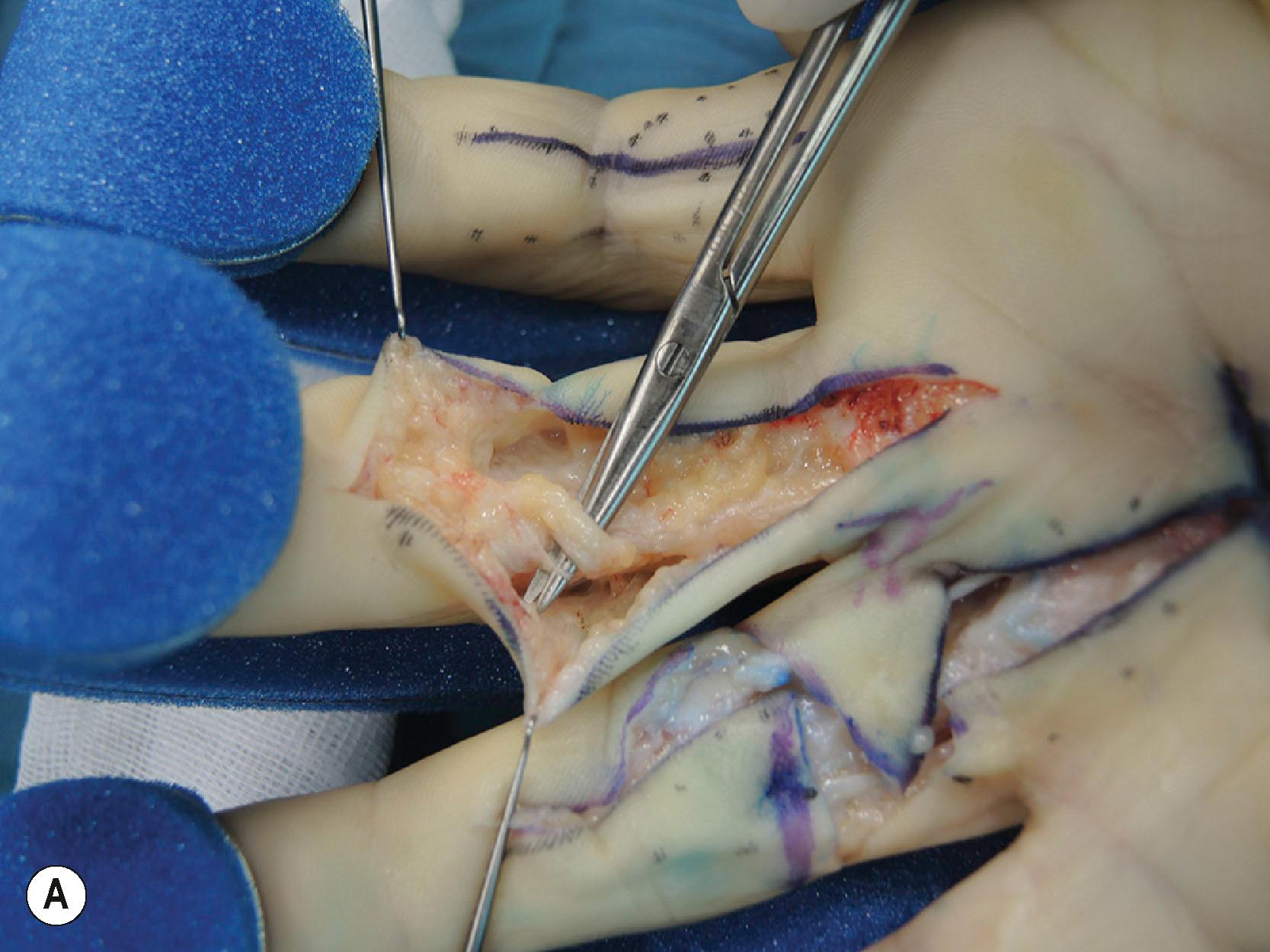
Approximately 20-40% of patients with early-stage DD progress to develop contractures and present with impaired hand function. Several subgroups can be distinguished based on their clinical presentation and likely outcomes ( Box 17.1 ).
Usually males of over 60 years of age; nodule(s) present for more than 10 year, no extra-palmar fibromatoses, no comorbidities such as diabetes mellitus, no excessive alcohol intake or smoking.
Isolated flexion contracture of the metacarpophalangeal joint of the ring or little finger of one hand.
Any modality will result in a good functional outcome and the risk of recurrence and need for reintervention is inversely related to the invasiveness of the treatment.
A small subgroup of patients responds poorly to treatment and often develops a stiff hand, and sometimes an early recurrence with worse flexion contractures than before treatment.
Dupuytren’s diathesis: strong family history of DD and disease onset typically at a young (<40 years) age. Affects both hands, also involves the radial side of the hand and commonly associated with fibromatoses such as knuckle pads, Peyronie’s, and Ledderhose disease.
Often earlier recurrences.
First treatment usually at a young age, often repeated interventions. A long-term management strategy is important.
Type I diabetics : Their convalescence period is longer and the functional outcome is often suboptimal, despite intensive postoperative hand therapy.
In the first group, which is also the most prevalent, the disease tends to follow a more benign course. The patient is usually a male of over 60 years of age who has had a nodule for more than 10 years, has no associated disorders such as Ledderhose or Peyronie’s or comorbidities such as diabetes mellitus, does not consume more than 14 units of alcohol per week, and is a non-smoker. He usually has an isolated flexion contracture of the MCP joint of the ring or small finger of one hand. Any treatment will result in a good functional outcome, and the risk of recurrence and need for reintervention is inversely related to the aggressiveness of the treatment, so that the less invasive procedures such as needle fasciotomy are associated with a higher rate of recurrence.
In a second group of patients, the disease follows a more aggressive course. This is the group Hueston described as possessing Dupuytren diathesis. These patients usually have a strong family history of DD and disease onset is typically at a young (<40 years) age. It affects both hands, also involves the radial side of the hand and is commonly associated with fibromatoses such as knuckle pads, Peyronie’s and Ledderhose disease. The earlier disease onset and greater propensity to develop contractures mean that treatment is often required at a younger age. These patients also tend to develop recurrence earlier, necessitating additional and repeated interventions. Therefore, it is important to agree to a long-term management strategy with the patient at the outset as each subsequent procedure is associated with its inherent risks.
A third subgroup of patients that imposes an even greater challenge to achieve a good and durable result are the insulin-dependent diabetics. Their convalescence period is longer, and the functional outcome is often suboptimal, despite intensive postoperative hand therapy.
Finally, there is a fourth subgroup of patients that very much resembles the first group, but for unknown reasons, pose challenges during the postoperative period. They do not have any clear risk factors to facilitate identification but often develop a stiff hand despite intensive hand therapy and judicious splinting, and sometimes an early recurrence with worse flexion contractures than before treatment.
A detailed history including specific questions about risk factors (family history of DD, alcohol consumption, smoking, exposure to vibration), associated fibromatoses (knuckle pads, Peyronie’s and Ledderhose disease, frozen shoulder), and comorbidities (diabetes type 1 or 2, liver disease, epilepsy), is essential for the initial risk assessment. Physical examination of both hands and, if indicated, the soles of the feet and penis, will further complete the picture. During examination of the hands, attention should be paid to the extent of the disease, the presence of bilateral disease and the location in terms of affected rays and joints, as well as the degree of flexion contractures, which should be accurately recorded using a goniometer (see below). Some authors have described the use of a tumorimeter for nodule size and a durometer for nodule hardness for research purposes, but the benefit of these additional measurements for clinical practice has yet to be defined.
The early signs of DD can be subtle irregularities adherent to the underside of the palmar skin, and the differential diagnosis should include inclusion cysts, occupational hyperkeratosis, callouses, ganglia, tenosynovitis, giant cell tumours, epitheloid sarcoma, or even metastatic disease. Sometimes a skin pit is the first sign of the disease. As the nodules become more clearly defined and cords begin to develop, the diagnosis is usually clear, especially if there is also a family history of DD. However, one may confuse DD with burn scars, congenital conditions such as camptodactyly, stuck trigger finger tendon bowstringing after pulley rupture as in rock climbers, tendon adhesions following infection or repair, tendon imbalances as in sagittal band rupture, intrinsic joint contractures, complex regional pain syndrome (CRPS) type 1, and cerebral or psychogenic spasticity.
The most practical grading systems are those of Iselin and Tubiana ( Table 17.1 ). Iselin’s staging system is more practical for quick scoring such as during prevalence studies. The original Tubiana’s system is easy to use but the 1986 modification is more sophisticated and makes the system relatively complex, hampering widespread application. Another disadvantage of the Tubiana system is that progression into a higher stage may involve 5–40° and is therefore not linear, but stepped. Hence, for follow-up studies it is better to register and report the active or passive extension deficit (AED or PED) by joint and its sum by ray (total active or total passive extension deficit – TAED or TPED), and active measurements have been found to be better reproducible. For the proximal interphalangeal joint, we would also advocate assessing the severity of fixed flexion deformity with the MCP joint flexed to determine what has been named dynamism.
| Described by | Stage | Meaning |
|---|---|---|
| Iselin | 1 | DD nodules and cords without contracture |
| 2 | MCPJ contracture | |
| 3 | MCPJ and PIPJ contracture | |
| 4 | Boutonnière deformity with MCPJ and PIPJ contracture and DIPJ hyperextension | |
| Tubiana | 0 | No signs of DD Disease |
| N | Nodules only. No contracture | |
| I | TPED smaller than 45 ° | |
| II | TPED in between 46–90 ° | |
| III | TPED in between 91–135 | |
| IV | TPED in between 136–180 |
There is no clear evidence for the benefit of treatment of any modality in the absence of flexion contractures. Surgical excision of nodules in the absence of flexion contractures may result in disease activation and progression in a similar fashion as distal radius fractures have been found to trigger development of Dupuytren nodules. A retrospective study showed that excision of early histological stage Dupuytren tissue was associated with recurrence. Furthermore, there are low-quality studies reporting the benefit of intralesional steroid injections or radiotherapy. However, these studies were largely retrospective, single-arm cohort studies with no control groups and the assessors and patients were not blinded. Trials are underway to assess the efficacy of radiotherapy and phase IIb data have recently been reported for intralesional injection of anti-TNF. Once flexion contractures develop, the general consensus is that impairment of hand function and progressive contracture of 20–30° of an MCP joint and any contracture of a PIP joint are good indications for treatment. As a simple guide, the patient with early-stage disease is asked to return when they cannot place their hand flat on the table (table top test).
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here