Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Administration of omega 3 polyunsaturated fatty acids (n-3 PUFAs) is a biologically plausible, yet clinically untested, candidate therapeutic strategy to prevent or blunt neurologic disability after traumatic brain injury (TBI). The biologic plausibility of n-3 PUFAs rests on evidence that supports at least three putative mechanisms of neuroprotection after TBI. First, n-3 PUFA supplementation may address an acquired or existing n-3 PUFA deficiency which, if present, could impair recovery from TBI. Second, enzymatic and nonenzymatic metabolism of n-3 PUFAs may yield derivatives that modulate neuroinflammation after TBI. Third, n-3 PUFA supplementation may blunt oxidative injury after TBI via a free-radical scavenging mechanism. This chapter will review the current state of bench and clinical research in this area and will identify potential future avenues of investigation.
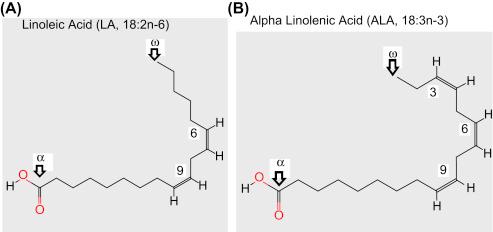
PUFAs are lipids with a hydrocarbon tail that is between 18 and 22 carbons long and includes at least one double bond. Omega 3 and omega 6 PUFAs are characterized by having a double bond located three and six carbons away, respectively, from the “omega” carbon or the methyl end of the hydrocarbon chain ( ).
Mammals must obtain the short-chain PUFAs alpha linolenic acid (ALA, 18:3n-3) and linoleic acid (LA, 18:2n-6) from the diet because they cannot perform de novo synthesis of these fatty acids. ALA and LA are depicted schematically in Fig. 18.1 . ALA and LA are commonly found in plant-based foods such as nuts, seeds, and their derived oils.
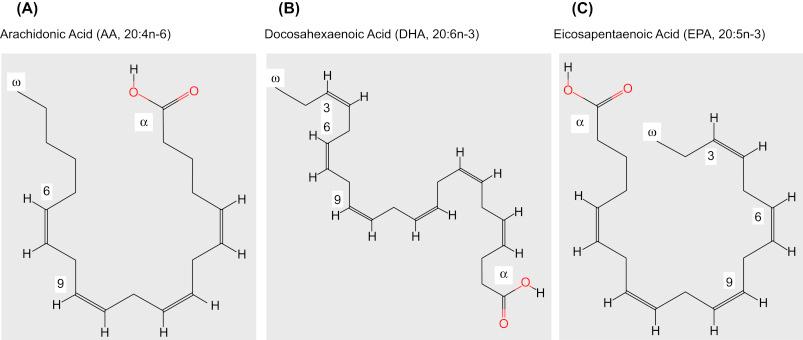
Conversion of ALA and LA to the respective n-3 and n-6 long chain PUFAs via elongation and desaturation occurs in a limited fashion, modulated by sex and the dietary ratio of ALA to LA, among other factors ( ). Long-chain n-3 and n-6 PUFAs are important components of cell membrane phospholipids, particularly in neural tissue where arachidonic acid (AA, 20:4n-6) and docosahexaenoic acid (DHA, 22:6n-3) predominate. Eicosapentaenoic acid (EPA, 20:5n-3) is another important neural PUFA, albeit present at concentrations about 250-fold lower than DHA ( ). AA, DHA, and EPA are depicted schematically in Fig. 18.2 .
Phospholipids are characterized by a glycerol backbone with a polar head to which two fatty acids are attached, usually via ester linkages. Typically, a saturated fatty acid is attached to the sn-1 position of the glycerol backbone, while omega 3 or omega 6 PUFAs are attached to the sn-2 position ( ).
Very little AA exists in the diet. Thus, the vast majority of AA derives from ingested LA. In contrast, since DHA and EPA are very inefficiently synthesized from ALA, they are generally derived from dietary sources such as salmon, tuna, other fatty fish, and algae. Only small amounts of EPA are found in neural cell membranes because dietary EPA is either metabolized or used as a DHA precursor. Thus, most of the DHA content of the mammalian central nervous system reflects dietary intake of DHA and EPA ( ). Immature as well as gestating mammals require higher dietary intake of DHA and DHA precursors than do adults, to account for the rapid DHA accretion of the developing brain. In utero, most DHA accretion occurs in the latter part of pregnancy while the rate of postnatal DHA accretion parallels brain development ( ).
Brain DHA is found in cellular and organellar, including mitochondrial, membranes ( ). The vast majority of brain DHA is esterified in phospholipids, predominantly in a type of ether phospholipid, ethanolamine-containing plasmalogens ( ). Brain DHA is highly conserved, even in the face of dietary inadequacy that produces DHA deficiency in nonneural tissues. Experimental induction of brain DHA deficiency requires exposure to prolonged periods of dietary insufficiency because DHA consumption in the brain is limited to the small amounts lost to energy generation and to oxidative damage. Damaged DHA is deesterified from its sn-2 position by type VI calcium-independent phospholipases A2 (iPLA2 beta and gamma) or, possibly, by a plasmalogen-specific PLA2. Such free DHA may be metabolized enzymatically or nonenzymatically into biologically active compounds or it may be reintegrated into membranes. In health, only small amounts of free DHA are metabolized by lipoxygenases (LOX), cyclooxygenases (COX), and other enzymes; instead, released DHA is usually reincorporated into membrane phospholipids by remodeling processes that are not yet well understood, as outlined below ( ).
Free DHA may be reincorporated into membrane phospholipids by acetylation to coenzyme A (CoA) by an acyl-CoA synthetase (ACSL), an ATP-consuming process, followed by the action of an acyl-CoA:lysophospholipid acyltransferase (LPLAT). ACSL6 is a candidate ACSL given its enrichment in the brain and its specificity for DHA. While a type of LPLAT (LPEAT2) is highly expressed in the brain and has selective action on ethanolamine plasmalogens, the DHA specificity of LPEAT2 and other brain-enriched LPLATs is unknown ( ). In summary, though the details of brain DHA accretion are under active investigation, it is abundantly clear that brain DHA content is highly conserved in health.
DHA is the major n-3 PUFA esterified in retinal and brain cell membranes. Brain and retinal DHA content increases dramatically during early development, suggesting that DHA is important for normal neurologic function.
Animal studies have shown that decreases brain and retinal DHA content resulting from dietary DHA deficiency during development is associated with impairments in membrane fluidity, synaptic function, and neurotransmitter release and with decreased visual, cognitive, and behavioral performance ( ). DHA-deficient brains display a marked increase in the AA-derived DPA 5n-6, a change that would hypothetically decrease membrane flexibility and affect the functionality of integral membrane proteins ( ). DHA deficiency also decreases synaptic connectivity and the length and number of dendritic branches ( ).
In preterm infants fed a commercial, non-DHA supplemented formula, low red blood cell and plasma DHA levels correlated with decreased visual acuity as measured by visual-evoked responses at both 36 and 57 weeks postconceptional age, compared to preterm infants fed human milk or n-3 PUFA-fortified commercial formulas ( ). In adult humans, epidemiological and intervention studies have linked lower DHA intake and/or lower DHA levels in plasma and blood cell lipids to an increased risk of dementia and cognitive decline ( ).
In contrast to studies of DHA deficiency, clinical studies of n-3 PUFA supplementation have yielded mixed results. Possible explanations for such variability include inconsistent study design, small study sizes, and the inability to accurately assess baseline DHA sufficiency. Some studies of n-3 PUFA supplementation during pregnancy and/or infancy showed no effect, while others found that the n-3 PUFA-supplemented groups displayed improvements in specific neurodevelopmental outcomes such as visual-evoked potentials and neurodevelopmental scores ( ). Studies on the cognitive effects of DHA and/or EPA supplementation in school-aged children and adults are similarly inconclusive. Supplementation generally appears to improve cognitive performance, though effects are more often noted in those individuals with a lower baseline performance and/or poor DHA intake ( ). For example, a double-blind, randomized, placebo-controlled trial (RCT) in healthy children aged 7–9 showed that DHA supplementation improved reading performance limited to those children at the lowest reading percentile at baseline ( ) and a meta-analysis of interventional trials revealed that DHA improved episodic memory in those adults with mild memory complaints ( ).
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here