Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
A variety of disorders, including infectious or inflammatory conditions and malignant disease, will lead to activation of coagulation. In many cases, this activation of coagulation will not lead to clinical complications and will not even be detected by routine laboratory tests, but can only be measured with sensitive molecular markers for activation of coagulation factors and pathways. However, if activation of coagulation is sufficiently strong, the platelet count may decrease and global clotting times may become prolonged. In its most extreme form, systemic activation of coagulation is known as disseminated intravascular coagulation (DIC). DIC is characterized by the simultaneous occurrence of widespread (micro)vascular thrombosis, thereby compromising blood supply to various organs, which may contribute to organ failure. Because of ongoing activation of the coagulation system and other factors, such as impaired synthesis and increased degradation of coagulation proteins and protease inhibitors, consumption of clotting factors and platelets may occur, resulting in diffuse bleeding.
In view of the multiple, often contrasting mechanisms that occur in patients with DIC, a consensual definition of DIC had been a matter of debate. In 2001, the subcommittee on DIC of the International Society on Thrombosis and Haemostasis proposed a definition that reflects the central role of the microvascular milieu (i.e., endothelial cells, blood cells, and the plasma protease system, in the pathogenesis of DIC). This definition of DIC reads as follows: “DIC is an acquired syndrome characterized by the intravascular activation of coagulation without a specific localization and arising from different causes. It can originate from and cause damage to the microvasculature, which if sufficiently severe, can produce organ dysfunction.”
The diagnosis of DIC may be hampered by the non-specific nature of many indicators of coagulation activation, although newly developed scoring algorithms based on readily available routine laboratory parameters show promising diagnostic accuracy. Owing to the complexity of the clinical presentation, the variable, and unpredictable course, and the multitude of therapies given to patients with DIC, properly conducted clinical trials are difficult to perform and even to devise. Management relies on limited evidence from clinical trials in combination with small studies employing surrogate outcome endpoints and experience from case series, as well as from an understanding of the underlying pathophysiologic mechanisms.
Activation of coagulation in concert with inflammatory activation can result in microvascular thrombosis, which contributes to multiple organ failure in patients with severe sepsis. In support of this concept, postmortem findings in patients with coagulation abnormalities and DIC on the background of severe sepsis include diffuse bleeding, hemorrhagic necrosis of tissues, microthrombi in small blood vessels, and thrombi in mid-size and larger arteries and veins. Ischemia and necrosis were invariably the results of fibrin deposition in small and mid-size vessels. Importantly, intravascular thrombi appear to be the driver of the organ dysfunction. Fibrin deposition in various organs also is a characteristic finding in animal models of DIC. Thus, experimental bacteremia or endotoxemia causes intra- and extravascular fibrin deposition in the kidneys, lungs, liver, brain, and other organs. Amelioration of the hemostatic defect with various interventions in these models reduces fibrin deposition, improves organ function, and, in some cases, reduces mortality. Finally, the results of clinical studies also support the concept that the extent of activation of the coagulation system is an important determinant of clinical outcome. DIC has been shown to be an independent predictor of organ failure and mortality. In a consecutive series of patients with severe sepsis, 43% of patients with DIC were compared with 27% without DIC. In this study, the severity of the coagulopathy was directly related to mortality.
In addition to microvascular thrombosis and organ dysfunction, coagulation abnormalities may have other harmful consequences. Thrombocytopenia in patients with sepsis places them at increased risk of bleeding. For example, critically ill patients with a platelet count of less than 50 × 10 9 /L have a four to fivefold higher risk for bleeding than those with higher platelet counts. Although the overall risk of intracerebral bleeding in patients in the intensive care unit (ICU) is less than 0.5%, up to 88% of patients with this complication have platelet counts less than 100 × 10 9 /L. The use of anticoagulants in patients with thrombocytopenia further increases the risk of bleeding. Regardless of the cause, multivariate analyses indicate that thrombocytopenia is an independent predictor of ICU mortality and increases the risk of death by 1.9- to 4.2-fold. In particular, thrombocytopenia that persists for more than 4 days after ICU admission, or a 50% or greater decrease in the platelet count during the ICU stay is associated with a four- to sixfold increase in mortality. In fact, the platelet count appears to be a stronger predictor of ICU mortality than composite scoring systems, such as the Acute Physiology and Chronic Evaluation (APACHE) II or Multiple Organ Dysfunction Score (MODS). Decreased levels of coagulation factors, as reflected by prolonged global coagulation times, also increase the risk of bleeding. Prolongation of the prothrombin time (PT) or activated partial thromboplastin time (aPTT) to over 1.5 times the control is associated with an increased risk of bleeding and mortality in critically ill patients.
Traditionally, DIC was thought to be the result of activation of both the extrinsic and intrinsic pathways of coagulation. The classical concept was that the extrinsic pathway was initiated by a tissue-derived component, which activated factor VII, leading to the direct conversion of prothrombin to thrombin. This process would proceed as long as there was tissue damage from systemic infection, trauma, placental abruption, or malignancy. In contrast, the intrinsic or contact pathway of coagulation was initiated by contact activation of factor XII which, together with its cofactors, kallikrein and kininogen, then activated factor XI with subsequent activation of factor IX. The initiators of contact activation were poorly understood until recently but were thought to include collagen and artificial surfaces. In recent years, the molecular mechanisms of coagulation pathways have been defined ( Fig. 137.1 ) (see Chapter 120, Chapter 124 ). This has provided new insight into the pathogenesis of DIC. In general, current thinking is that thrombin and fibrin generation in patients with DIC is largely driven via the extrinsic pathway; the role of the contact system is uncertain.
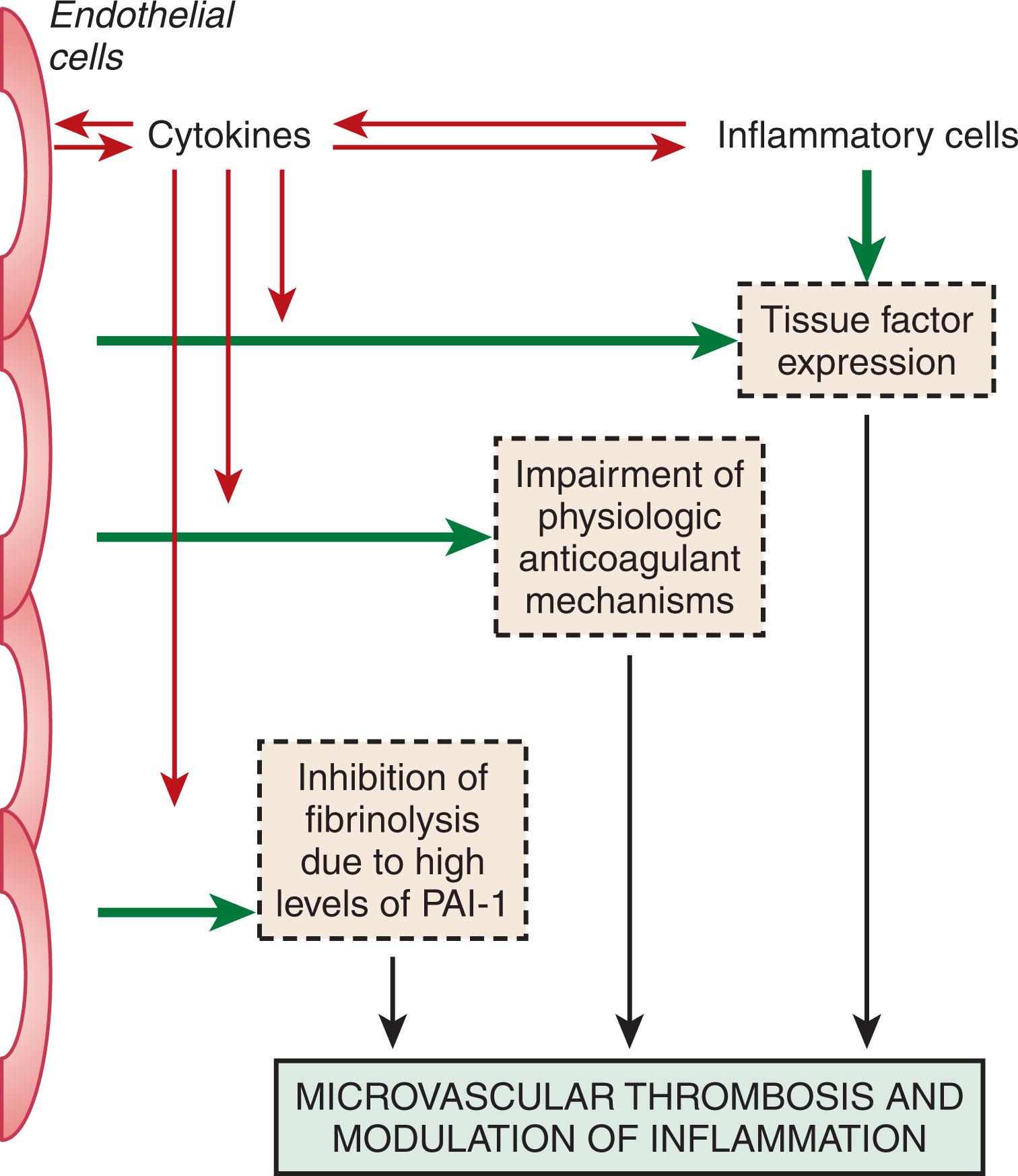
The extrinsic pathway is initiated by the tissue factor (TF)-factor VIIa complex. TF is a membrane-bound 4.5 kD protein that is constitutively expressed on cells that are mostly in tissues not in direct contact with blood, such as the adventitial layer of larger blood vessels. Subcutaneous tissue also contains substantial amounts of TF. When expressed on the cell surface, TF interacts with factor VII, either in its zymogen or activated form. The TF-factor VIIa complex catalyzes the activation of both factor IX and factor X. Factors IXa and Xa enhance the activation of factors X and prothrombin, respectively. In cells in contact with the blood, TF is induced by mediators such as cytokines, C reactive protein, and advanced glycosylation end products. Inducible TF is predominantly expressed by monocytes and macrophages. Monocyte TF expression is enhanced in the presence of platelets and granulocytes in a P-selectin-dependent manner. This may reflect nuclear factor-kappa B (NFκB) activation that occurs when activated platelets bind to neutrophils or mononuclear cells. These cell-cell interactions also stimulate the production of IL-1b, IL-8, monocyte chemotactic protein 1 (MCP-1), and TNFα. Under cell culture conditions, cytokines such as TNFα, and IL-1, can induce TF expression by vascular endothelial cells, but the in vivo relevance of this finding is uncertain. Studies in vivo suggest that IL-6 is the dominant inducer of TF expression by mononuclear cells.
Increased monocyte TF expression and procoagulant activity has been demonstrated in DIC associated with sepsis, cancer, or coronary disease. Expression of TF appears to be localized to certain organs and vascular beds, but it is uncertain whether its expression is under genetic control in an organ-specific manner. With trauma, such as extensive surgery, brain injury, or burns, it is likely that constitutively expressed TF at the site of injury is the primary source of procoagulant material, but direct support for this concept is lacking.
The role of the intrinsic pathway in the pathogenesis of DIC is uncertain. Negatively-charged substances, such as phospholipids, polyphosphates, and glycosaminoglycans, are potential activators of the contact pathway (see Chapter 120, Chapter 124 ). Studies in patients with suspected DIC have identified elevated levels of markers of activation of the contact system. In patients with meningococcal septicemia, there was a negative correlation between plasma factor XII levels and the levels of factor XIIa-C1 inhibitor complexes. Although this finding implies consumption of factor XII and subsequent downstream activation of factor XI, an alternative explanation is that there is a negative acute-phase effect with reduced synthesis of factor XII in conjunction with thrombin-mediated activation of factor XI. However, blockade of the contact system with a factor XIIa-directed antibody failed to prevent DIC in a baboon model of Escherichia coli sepsis but diminished the development of lethal hypotension. These findings provide reasonable support for the current view that the contact pathway does not contribute to DIC but may play important roles in proinflammatory mechanisms related to vascular permeability, vascular proliferation (kininogen induces smooth muscle cell proliferation), and enhancement of fibrinolysis.
Activation of blood coagulation requires several cofactors (see Chapter 120, Chapter 124 ). For the development of DIC, the surfaces of cell remnants or intact cells, inflammatory mediators, and coagulation proteins are required. The stimulus for activation depends on the underlying disease and may range from bacterial cell compounds, such as endotoxin, TF on host or cancer cells, other cancer cell procoagulants, TF, or fat or amniotic fluid. Each of these triggers interacts with other mediators: TF assembles on anionic phospholipid surfaces, which can be provided by activated platelets, leukocytes, or by cancer cells. Cytokines interact with receptors and induce signaling pathways that induce TF expression and other proinflammatory components via the NFκB complex.
Endotoxin is a lipopolysaccharide compound of gram-negative bacteria that induces the sepsis syndrome and DIC. Gram-negative bacteria liberate endotoxin from their membrane, which interacts with cell surfaces via various pathways. In blood, endotoxin directly binds to CD14 on monocytes and binds to endothelial cells after complexing with lipopolysaccharide-binding protein (LBP) and the Toll-like receptor 4 (TLR 4) complex. Through these interactions, endotoxin induces signaling pathways that culminate in NFκB activation and initiates the expression of proinflammatory cytokines and TF. Likewise, exotoxin such as lipoteichoic acid (LTA) from gram-positive bacteria can induce proinflammatory cytokine expression in a similar manner.
The molecular mechanisms underlying endotoxin-induced activation of coagulation have been studied in primates and baboons. In endotoxin or E. coli models of sepsis, inhibition of the TF pathway abolishes the activation of coagulation, thereby highlighting the importance of TF. IL-6 is an important mediator of procoagulant effects, whereas TNFα is involved in the fibrinolytic response to endotoxin. Inhibition of TF with tissue factor pathway inhibitor (TFPI) reduces IL-6 levels in the baboon model, suggesting that there is extensive crosstalk between coagulation and inflammatory mediators (see the following section). Monocytes that express TF bind factor VII(a), shed TF, or bind to the damaged vessel wall. After interacting with platelets, circulating monocytes can trigger DIC. Microvesicles may accelerate this process, and the complex interaction among cells, membrane fragments, soluble mediators, and plasma proteins may trigger the DIC syndrome. The severity and duration of the consumptive process are mainly determined by the potency of the triggers and the capacity of inhibitory mechanisms.
In addition to activating coagulation protein zymogens, coagulation proteases also interact with specific cell receptors and trigger signaling pathways that elicit proinflammatory mediators. Factor Xa, thrombin, and the factor VIIa-TF complex have such effects. Factor Xa injection into rats induces localized inflammation, probably as a result of its interaction with effector-cell protease receptor-1 (EPR-1) and not because of thrombin generation. Exposure of cultured endothelial cells to factor Xa stimulates the production of MCP-1, IL-6, and IL-8 and upregulates the expression of adhesion proteins that tether neutrophils to the cell surface. Further evidence for the crosstalk between inflammation and coagulation comes from the observations that IL-6 and IL-8 elicit TF-dependent procoagulant activity in monocytes and the identification of IL-6 as the critical mediator of procoagulant activity either on its own or after endotoxin challenge in vivo. Therefore, cytokine production induced by factor Xa may be an important driver of coagulation in DIC.
In addition to its procoagulant functions, thrombin has a variety of non-coagulant effects. Thrombin induces the release of MCP-1 and IL-6 from fibroblasts, epithelial cells, and mononuclear cells in vitro. Thrombin also induces IL-6 and IL-8 production by endothelial cells, which has a procoagulant effect that is TF-dependent. Cell activation by thrombin is likely mediated by protease-activated receptors. The factor VIIa-TF complex also activates cells by binding protease-activated receptor-2 (PAR-2).
Direct evidence of the in vivo relevance of these phenomena comes from a study showing that recombinant factor VIIa infusion in volunteers induces an increase in plasma levels of IL-6 and IL-8. Although the concentrations of factor VIIa infused far exceed those found in patients with sepsis, it is possible that factor VIIa-induced cytokine production is of physiological importance. Thus, this information adds to the concept that several coagulation proteases induce proinflammatory mediators that augment procoagulant activity and amplify the consumptive process. Endogenous anticoagulant pathways are essential to regulate these proteases and prevent uncontrolled DIC.
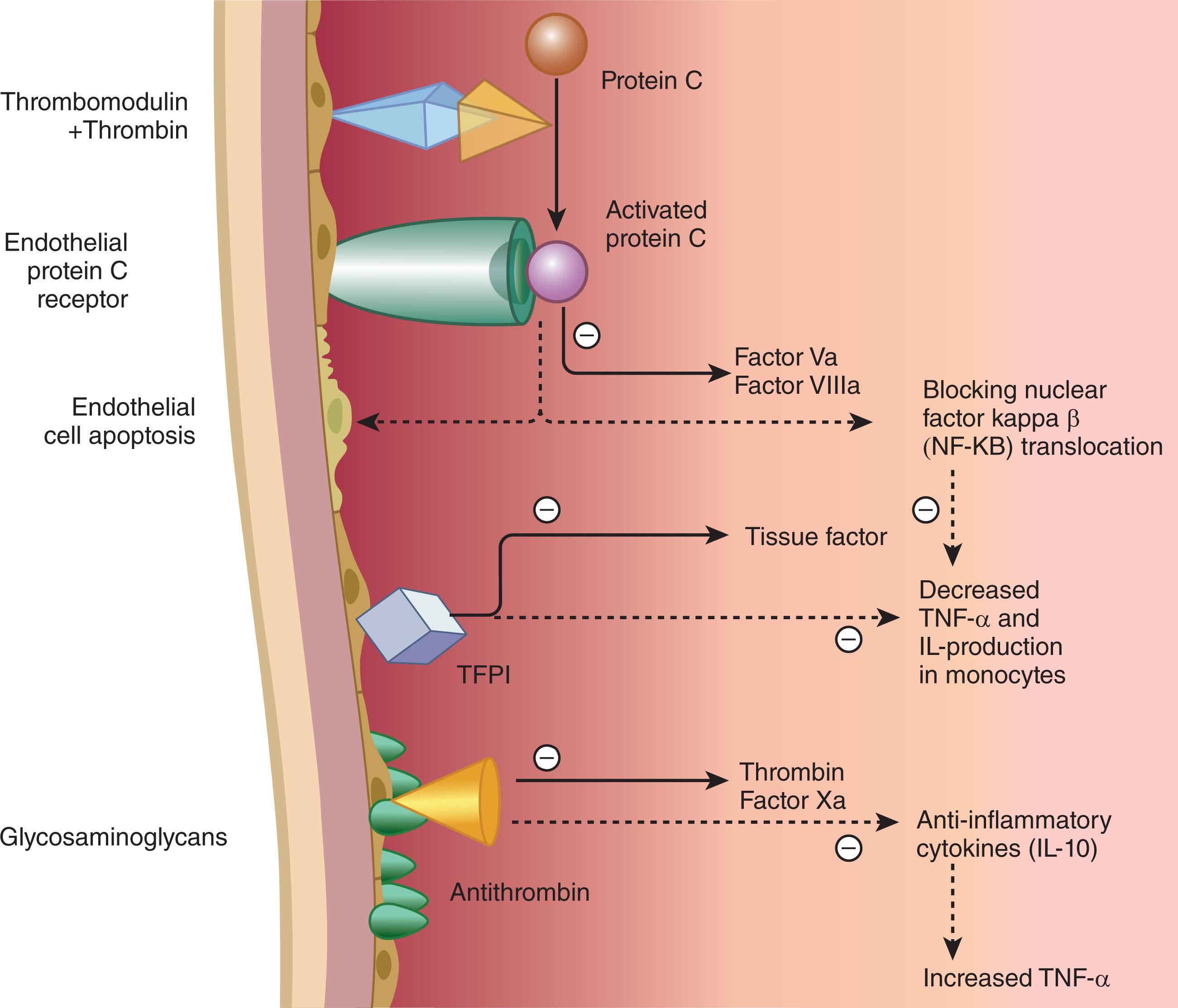
The development of DIC is counteracted by several mechanisms (see Chapter 120, Chapter 124 ). First, coagulation inhibitors regulate the coagulation mechanism. These inhibitors include antithrombin (AT), the protein C pathway, and TFPI ( Fig. 137.2 ). AT, which complexes and inhibits thrombin and factor Xa, is one of the most important inhibitors, and reduced AT levels are a characteristic of DIC. Reductions in AT levels reflect a combination of reduced protein synthesis increased clearance through the formation of protease-AT complexes and AT degradation by neutrophil elastase. In addition, cytokines may impair proteoglycan synthesis in the vessel wall, thereby reducing the availability of heparan sulfate for the potentiation of AT activity.
In animal models of experimental bacteremia, AT concentrate infusion increases survival, reduces the severity of DIC, and lowers the levels of IL-6 and IL-8. Therefore, in addition to its anticoagulant function, AT may also have an anti-inflammatory effect.
Activated protein C and its cofactor protein S form a second line of defense. Thrombin binds to thrombomodulin on the endothelial cell surface, and the thrombin-thrombomodulin complex converts protein C to its active form, activated protein C (APC). In addition, the thrombin-thrombomodulin complex converts thrombin activatable fibrinolytic inhibitor (TAFI) into its activated form, TAFIa. APC inactivates factors Va and VIIIa by proteolytic cleavage, thus down regulating the coagulation cascade. Endothelial cells, primarily those of large blood vessels, express endothelial protein C receptor (EPCR) on their surface. EPCR binds protein C and presents it to the thrombin-thrombomodulin complex for activation; a process that amplifies APC generation about 20-fold.
APC has anti-inflammatory effects on mononuclear cells and granulocytes, which may be distinct from its anticoagulant activity. Administration of APC prevents thrombin-induced thromboembolism in mice, mainly through its antithrombotic effect. In addition, several studies have demonstrated the anti-inflammatory effects of APC.
Defects in the protein C pathway enhance the vulnerability to inflammatory reactions and DIC. In patients with DIC, lower levels of protein C and protein S are associated with increased mortality. Mice with one allele targeted disruption of the protein C gene, causing heterozygous protein C deficiency, have more severe DIC and a greater inflammatory response than wild-type mice. Blockade of the activity of protein C by infusion of C4 binding protein converted a sublethal model of E. coli in baboons into a lethal model. Blockade of EPCR with a neutralizing monoclonal antibody also increased mortality in the E. coli baboon model. In contrast, infusion of PC in the same model protected the baboons from fatal DIC. Thus, the protein C pathway appears to be an important host defense against sepsis and DIC.
With systemic inflammation, TNFα and interleukin-1 may down regulate the expression of thrombomodulin by endothelial cells as they do in cell culture experiments. Although thrombin upregulates EPCR expression, by activating metalloproteases, thrombin may also induce EPCR shedding from the endothelium.
Thrombomodulin plays a central role in the pathogenesis of DIC. First, thrombomodulin is essential for the activation of protein C by thrombin; as such, thrombomodulin plays a key role in the anticoagulant and anti-inflammatory properties of the protein C system. Second, thrombomodulin binds thrombin, thereby preventing it from exerting prothrombotic and proinflammatory effects. Third, thrombomodulin-bound thrombin activates TAFI, which suppresses bradykinin activity and complement activation. In DIC, the expression of thrombomodulin is downregulated and activation of protein C is impaired. Thus, histological analysis of skin biopsies from patients with meningococcal sepsis reveals decreased endothelial expression of thrombomodulin in vessels with and without thrombosis.
TFPI is another inhibitor of coagulation. TFPI, which can be either endothelial cell–associated or lipoprotein-bound in plasma, inhibits the TF factor VIIa complex by forming a quaternary complex in which factor Xa is the fourth component.
The importance of TFPI in sepsis is uncertain because the majority of patients with sepsis have normal levels of TFPI. The relevance of TFPI in DIC is illustrated by three lines of experimentation. First, depletion of TFPI sensitized rabbits to DIC induced by TF infusion. Second, TFPI infusion protected against the harmful effects of E. coli in primates. TFPI not only inhibited DIC in baboons challenged with lethal amounts of E. coli but prevented mortality and improved organ function. Third, TFPI infusion blocked the procoagulant effects of endotoxin in human volunteers.
Although normal levels and functions of coagulation inhibitors are important for protection against DIC, there is no evidence that patients with congenital deficiencies of coagulation inhibitors are at higher risk for DIC. Additional work is needed to examine the impact of these deficiencies on the interaction between coagulation and inflammation.
In experimental models of DIC, there is an initial enhancement in fibrinolysis followed by marked impairment caused by the release of type 1 plasminogen activator inhibitor (PAI-1). The molecular mechanisms responsible for these findings include TNFα and IL-1 induced decrease in tPA release by endothelial cells and increase in PAI-1 production and TNFα-induced increase in urokinase-type plasminogen activator (uPA) production. Endotoxin and TNFα stimulate PAI-1 production in the liver, kidneys, lungs, and adrenal glands of mice. The net procoagulant state is illustrated by a late rise in fibrin breakdown fragments after E. coli challenge in baboons.
Experimental data also suggest that enhanced fibrinolysis clears fibrin from organs. Endotoxin-induced fibrin formation in the kidneys and adrenal glands occurs when PAI-1 levels increase. Furthermore, endotoxin does not induce fibrin formation in PAI-1 knockout mice, and fibrin deposition is increased in mice with functionally inactive thrombomodulin (TMProArg mutation). These experiments demonstrate that fibrinolytic action is required to reduce the extent of intravascular fibrin formation.
Fibrinolytic activity is regulated by PAI-1, the principal inhibitor of this system. Recent studies have shown that a functional mutation in the PAI-1 gene, the 4 G/5 G polymorphism, not only influences the plasma level of PAI-1 but was also linked to clinical outcomes in patients with meningococcal septicemia. Patients with the 4 G/4 G genotype had significantly higher plasma PAI-1 levels and an increased risk of death compared with those without this polymorphism. The PAI-1 polymorphism does not influence the risk of contracting meningitis, but likely increases the risk of fatal septic shock in patients with meningococcal infection. These studies provide the first evidence that genetically determined differences in fibrinolytic activity influence the risk of complications from gram-negative infection. In cohorts of patients with DIC, high levels of PAI-1 are one of the best predictors of mortality. These data suggest that DIC contributes to mortality in patients with sepsis. High levels of PAI-1 in such patients may contribute to mortality or may simply be a marker of severe inflammation because PAI-1 is an acute-phase protein.
The clinical manifestation of DIC may vary depending on the underlying disorder. At one end of the spectrum is the acute severe DIC that often occurs in the setting of sepsis, major trauma, obstetric calamities, and severe immunologic responses. Diffuse multiorgan bleeding, hemorrhagic necrosis, microvascular thrombosis, and thrombi in medium and large blood vessels are common findings at autopsy. Occasionally, patients with unequivocal clinical and laboratory evidence of DIC do not have confirmatory postmortem findings. Conversely, some patients whose clinical and laboratory findings were not consistent with DIC have typical autopsy findings. The lack of correlation among clinical, laboratory, and pathologic findings may reflect partly postmortem changes in the blood (e.g., hyperfibrinolysis) in some cases but is mostly unexplained. Diffuse microthrombi are most frequently found in the lungs and kidneys, followed by the brain, heart, liver, spleen, adrenal glands, pancreas, and gut. Immunohistology and ultrastructural analysis indicate that most thrombi consist of fibrin and platelets. In addition, activated mononuclear cells and evidence of inflammation are frequently present. In cases of persistent DIC, organization and endothelialization of the microthrombi are often observed.
Acute tubular necrosis is more frequent than renal cortical necrosis in patients with DIC. Clinically, thrombotic occlusive events occur first as a result of microthrombi that obstruct the microcirculation of organs. These thrombi result from clots that form either in the circulation or in situ in arterioles, capillaries, or venules. Circulatory obstruction produces organ hypoperfusion and can lead to ischemia, infarction, and necrosis. The process is disseminated throughout the microcirculation; therefore, all organs are potentially vulnerable.
In contrast to acutely ill patients with severe DIC, some patients have mild or subclinical disease manifested only by laboratory abnormalities. Subacute or chronic DIC generally occurs in patients with malignancy, particularly those with mucin-producing adenocarcinomas or acute promyelocytic leukemia (see Chapter 60, Chapter 63 ). Patients with acute promyelocytic leukemia frequently present with hemorrhage, whereas thrombotic manifestations predominate in those with adenocarcinoma. In addition, patients with solid tumors may develop nonbacterial endocarditis with subsequent systemic arterial embolism and infarction.
Another cause of subacute or chronic DIC is the retained dead fetus syndrome. These patients may be asymptomatic or may present with mild to moderate skin and mucous membrane bleeding.
It is important to stress that DIC is not a disease but is always secondary to an underlying disorder that causes the activation of coagulation. The underlying disorders most commonly associated with DIC are listed in Box 137.1 and are described in detail in the following section.
Sepsis/severe infection
Trauma/burn/heatstroke
Malignancy
Solid tumors
Acute leukemia
Obstetrical conditions
amniotic fluid embolism
Abruptio placentae
HELLP syndrome
Vascular abnormalities
Kasabach-Merrit syndrome
Other vascular malformations
Aortic aneurysms
Severe allergic/toxic reactions
Severe immunologic reactions (e.g., transfusion reaction)
HELLP, hemolysis, elevated liver enzymes, and low platelet count.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here