Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The fetus depends entirely on the mother for its nutritional needs, of which glucose is the principal energy substrate that fuels fetal growth and metabolism. At birth, when the maternal supply is discontinued, the neonate must adjust to an independent existence. This transition to the extrauterine environment is often perturbed by alterations in the mother's metabolism or by intrinsic fetal and placental problems that result in changes in the neonate's glucose homeostasis. An understanding of the normal physiologic adaptation of the maternal–fetal nutritional relationship during pregnancy and of fetal glucose homeostasis and glucose metabolism during the transition to extrauterine life serves as a framework for evaluating disordered glucose metabolism in the neonate.
The fetus is entirely dependent on the mother for nutrients supplied by the placenta. Yet the fetus is hormonally independent of the mother ( Fig. 86.1 ), since no maternal peptide hormones are transported to the fetus in any significant amounts. The fetal endocrine and paracrine responses are mediated by the transport of nutrients such as glucose and amino acids.
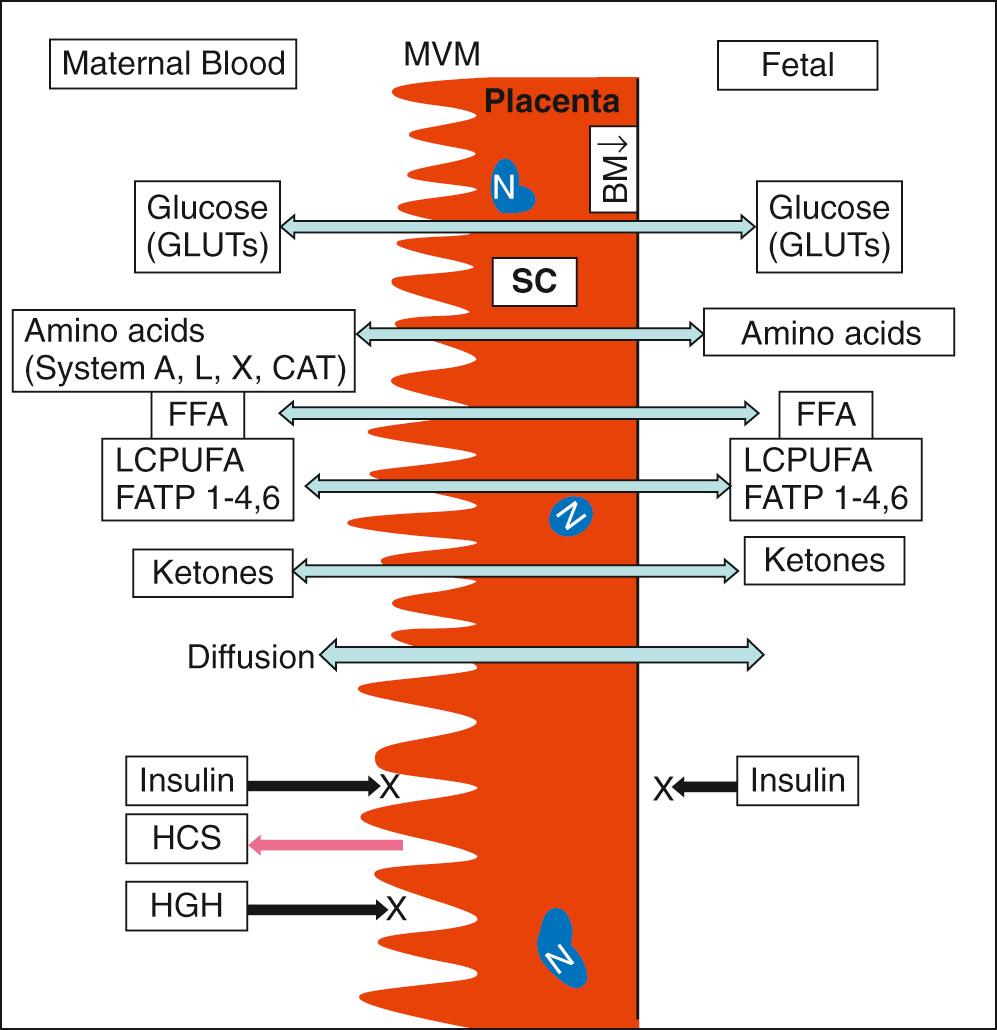
Glucose is the primary substrate for fetal energy metabolism for which the fetus depends entirely on maternal glucose because of minimal fetal gluconeogenesis. Glucose is transported to the fetus along a downward concentration gradient via facilitated transport mediated by glucose transporters (GLUTs) encoded by the SCL2A genes. A higher concentration of GLUT 1 is present on the maternal side of the syncytiotrophoblastic (SC) membrane compared to the fetal side of the SC basal membrane, suggesting a rate limiting step in glucose transport. In normal pregnancies, the plasma glucose concentration of the fetus is about 70%-80% of that of the mother. Because the range of glucose concentrations observed in human pregnancy does not saturate maternal–fetal glucose transfer, even when complicated by diabetes, fetal glucose uptake becomes excessive as the maternal glucose concentration increases. When fetal glucose uptake exceeds the requirements of energy production and growth, the excess glucose is stored as glycogen and triglycerides. Glucose transfer by placenta is also affected by the placental surface area, glucose metabolism, and blood flow.
A significant amount of fatty acids are transported to the fetus, as they are a critical source of energy and are an essential component of cell membranes, tissue development (white adipose tissue), and organogenesis (brain). The transfer of nonesterified fatty acids (NEFA) from the mother to the fetus occurs by simple diffusion. A progressive shortening of chain length from C16-C8 is associated with an increased transfer rate. Protein binding appears to slow the rate of transfer. A number of fatty acid transport proteins (FATP 1-4, and 6) that are present in SC membranes in placenta are involved in the cellular uptake of long-chain fatty acids. In addition, a significant amount of nonessential fatty acids appears to be synthesized de novo by the fetus in utero. Maternal adipose tissue and dietary intake are the primary sources of transplacental long chain polyunsaturated fatty acids (LCPUFA) to the fetus.
Amino acids are transported to the fetus against a concentration gradient by a carrier-mediated transport process. Amino acids may be transported through the placenta either unchanged or after placental metabolism and processing; for example, leucine can be transferred intact or as its keto analogue α-ketoisocaproic acid. The syncytiotrophoblast is usually considered the main transport epithelium of the term placenta (see Fig. 86.1 ). Amino acids are transported by means of energy-dependent processes through selective amino acid transport systems.
β-Hydroxybutyrate and possibly acetoacetate, the major ketone bodies, are transported along a concentration gradient. The concentration of β-hydroxybutyrate in fetal blood is significantly less than that in maternal plasma (≈50%); however, a linear correlation between the maternal and fetal concentrations has been reported.
There is a linear correlation between maternal and fetal glycerol levels, with the fetal level in most instances being somewhat lower than the simultaneously determined maternal plasma level.
Data on the maternal–fetal lactate relationship in human studies are conflicting. Experimental data in sheep demonstrated placental production and fetal use of lactate, but data in human studies showed higher levels of lactate in fetal than in maternal blood. In the few studies in which umbilical artery and vein samples were obtained simultaneously, the data could be interpreted as net fetal production and placental clearance of lactate.
Insulin-like growth factors (IGF I and II) are highly expressed in all fetal tissues. IGF I in fetus is nutrient sensitive and corresponds with fetal growth independent of growth hormone. Immunoreactive insulin has been demonstrated in both plasma and pancreatic tissue as early as 8 weeks of gestation; the source appears to be the fetal pancreas, because the placenta is impermeable to insulin. At 13-18 weeks of gestation, the fetal insulin response to sustained maternal hyperglycemia is negligible. However, at term, the fetus is capable of a significant response to prolonged hyperglycemia, although to a lesser degree than adults. When the fetus receives appropriate glucose from the mother, the requirement for an insulin response is minimal. With repeated episodes of hyperglycemia, as in maternal diabetes, a greater insulin response is seen, indicating that B-cell sensitivity is being induced or enhanced. That insulin may modify the growth rate in utero has been shown by the positive correlation between the fetal plasma insulin concentration and fetal weight. Human growth hormone (GH) has been measured as early as 9 weeks of gestation and increases rapidly between the 11th and 16th weeks. Because the transplacental transfer of GH is negligible, the fetal pituitary appears to be its source in the fetus. At term, fetal plasma GH levels are higher than those of maternal plasma. Unlike those of adults, fetal GH levels are not suppressed during hyperglycemia and actually show a paradoxic rise. Several studies have demonstrated the role of insulin as the growth-promoting hormone of the fetus and that the fetus can sustain normal growth in the absence of GH.
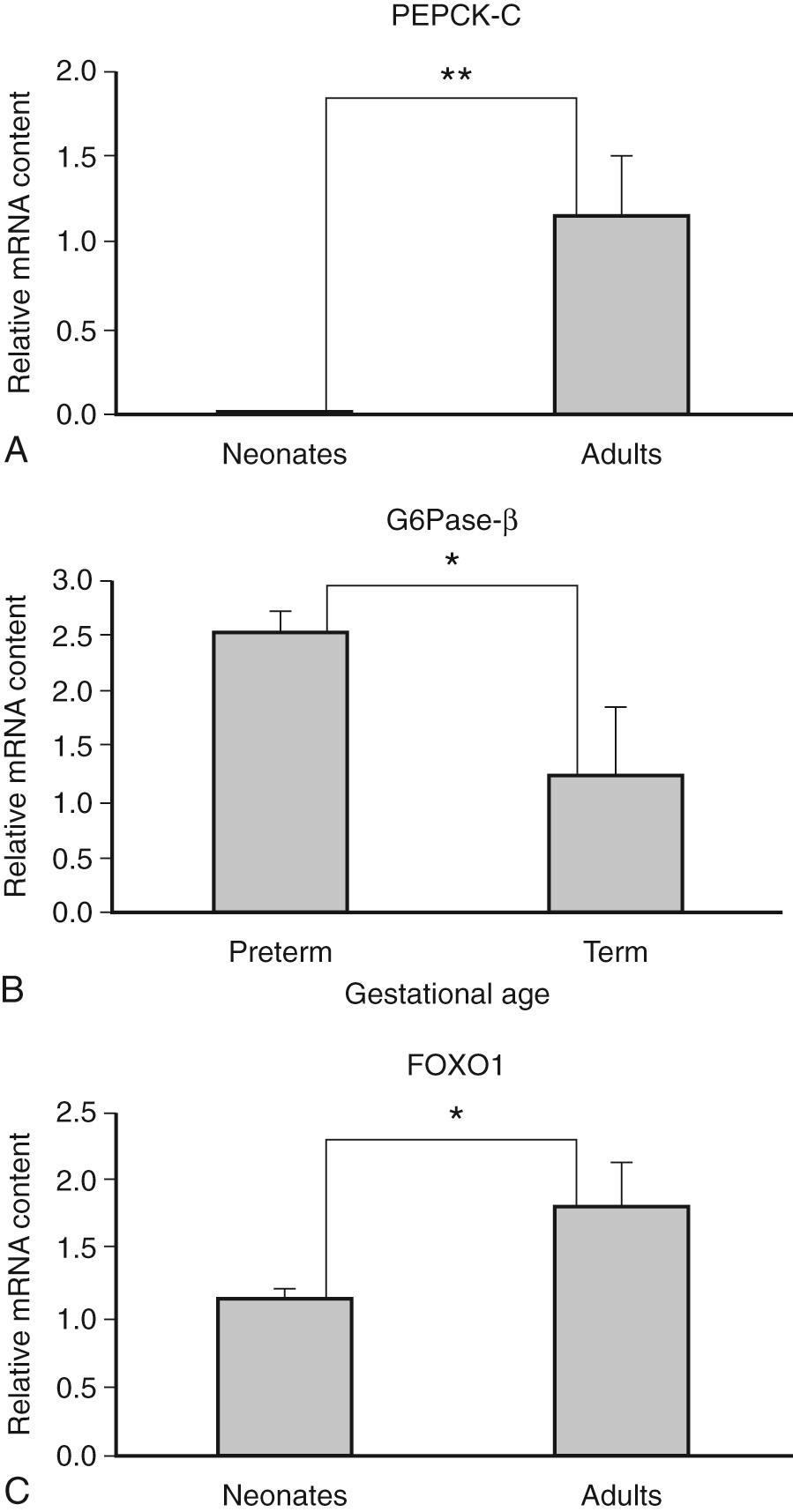
Under normal circumstances (i.e., in an uncomplicated, normal pregnancy), the fetus is entirely dependent on the mother for a continuous supply of glucose for both energy metabolism and the synthesis of other metabolic substrates. The fetal liver contains the full complement of enzymes required for the synthesis and breakdown of glycogen. Hepatic glycogen content is low early in gestation; a slow, continuous increase occurs between 15 and 20 weeks; and a rapid accumulation of glycogen in the liver is observed late. The fetus also has all the strategic hepatic enzymes involved in gluconeogenesis, although their levels are lower than those in adults. The only exception is cytosolic phosphoenolpyruvate carboxykinase (PEPCK), which, at least in the rat, is not expressed in utero and appears immediately after birth ( Fig. 86.2 ). Data in humans in vivo are not available; however, in nonhuman primates, mitochondrial PEPCK and PEPCK-C gene expression increase with advancing gestational age, while glucose-6-phosphatase-alpha (G6Pase-a) and its target gene FOXO1 expression are higher at preterm compared to term gestation. In the mammalian species studied, the gluconeogenic capacity is not expressed in utero under unperturbed circumstances, and the contribution of gluconeogenesis from lactate, pyruvate, or alanine to glucose is quantitatively negligible. Studies in humans and animals have consistently demonstrated that the fetus does not produce glucose and that maternal glucose is the only source of fetal glucose. Fluctuations in maternal blood glucose are rapidly reflected in parallel changes in fetal glucose concentration. These variations occur even in response to acute hypoglycemia induced by insulin infusion in the mother during sheep pregnancy. However, if maternal hypoglycemia is prolonged, the fetus begins glucose production. Whether such a response can occur in human pregnancy has not been examined and cannot be pursued without violating ethical standards. Glucose is the primary fuel for the fetus, accounting for about 80% of fetal energy consumption. The remaining 20% of fetal energy needs is provided by lactate, amino acids, and other means.
At birth, after separation from the umbilical circulation, the supply of glucose and other nutrients abruptly ceases, and the newborn infant has to mobilize its depots to meet energy requirements. This initial drop in glucose concentration may be an essential step in activating physiologic processes necessary for postnatal survival, such as promoting glucose production by gluconeogenesis, stimulation of appetite, adaptation to fast/feed cycles, and enhancement of oxidative fat metabolism. Acute surge in the levels of circulating epinephrine, norepinephrine, and glucagon and a fall in the levels of insulin coincides with clamping of the umbilical cord. These hormones concomitantly mobilize hepatic glycogen and stimulate gluconeogenesis, resulting in a steady rate of glucose production and maintenance of the plasma glucose concentration.
The plasma glucose concentration in umbilical vein blood is about 80% of the prevailing maternal blood glucose concentration. After birth, the plasma glucose concentration falls in all infants, reaching its lowest value between 30 and 90 minutes after birth. Thereafter, in full-term healthy neonates, the plasma glucose concentration rises and is maintained at a steady level of 40-80 mg/dL. Full-term newborn infants can tolerate fasting without a significant change in the blood glucose concentration. Fasting up to 9 hours after a meal did not cause a decrease in the plasma glucose concentration. After 4-6 hours post feeding state, steady state plasma glucose concentration is maintained with interactions between insulin and counter regulatory hormones (glucagon, cortisol, GH, epinephrine, and norepinephrine). Prolonged fasting state triggers increased utilization of FFA and ketone bodies with decrease in hepatic glycogenesis and increase in gluconeogenesis in response to increase in circulating glucagon and decrease in insulin concentrations.
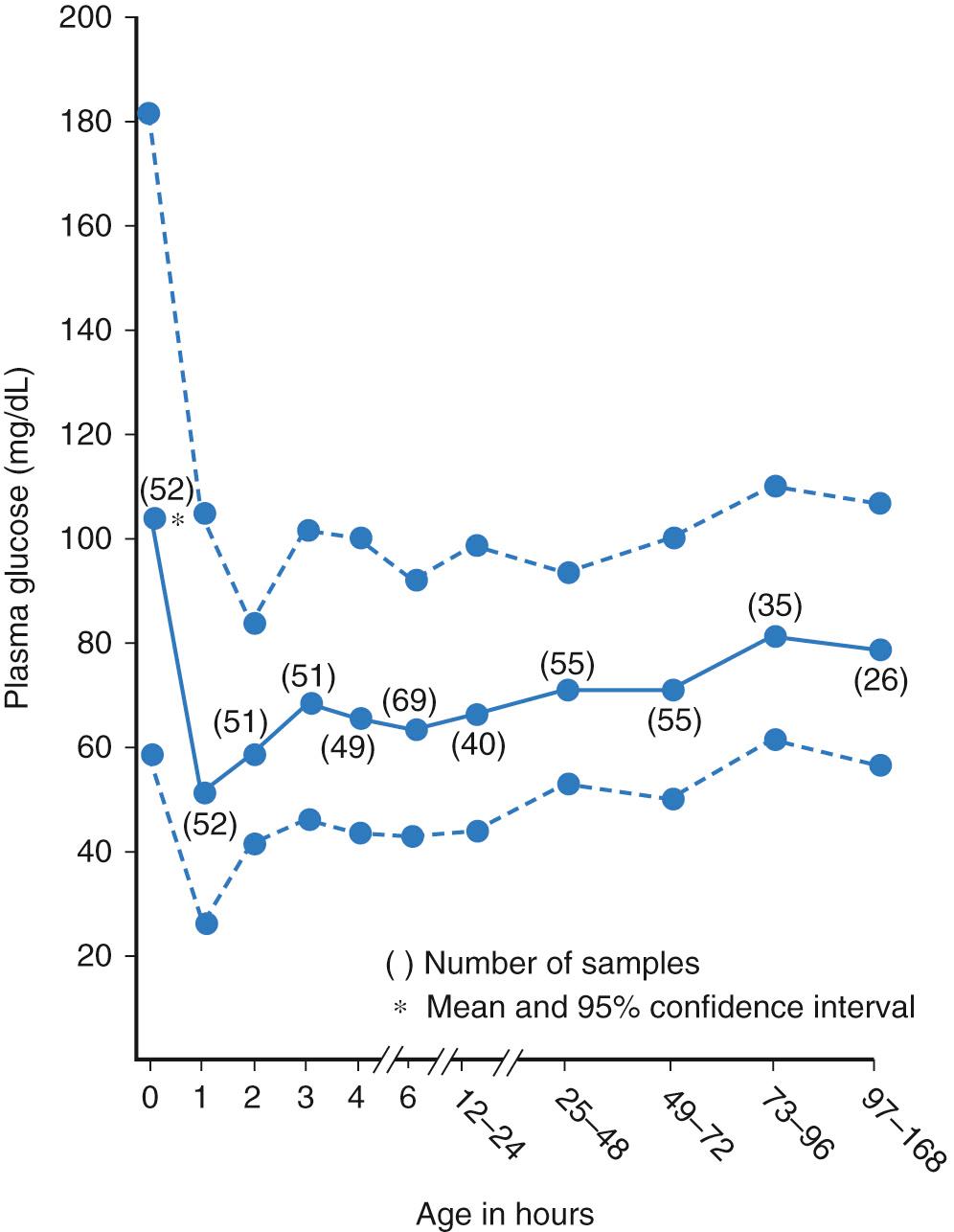
In full-term infants, the plasma glucose levels obtained at random intervals during the first week after birth have ranged from 40-100 mg/dL, with a mean of 80 mg/dL ( Fig. 86.3 ). These levels are higher than those previously reported in fasting infants and reflect changes in feeding practices and the random nature of measurements. The plasma glucose concentration in healthy, asymptomatic, breastfed babies has been reported to be lower with an average of 65 mg/dL (range 27-95 mg/dL) than that in formula-fed infants (mean 72 mg/dL, range 45-112 mg/dL) during the first 24 hours of life, but reveal higher blood ketone body concentration. The plasma glucose values in infants who are small for gestational age (SGA) and in premature infants are somewhat lower. Hypoglycemia in the neonatal period cannot be described by a single value. Most investigators consider a plasma glucose level lower than 45 mg/dL in the first 24 hours of life, and preprandial glucose less than 50 mg/dL up to 48 hours of age and less than 60 mg/dL after 48 hours of age as meeting the criteria of hypoglycemia. Additionally, there are differences between arterial and venous sample values, venous being 10% lower and when comparing whole blood versus plasma, whole blood being 15% lower.
During hypoglycemia, endogenous hepatic glucose production (EHGP) is derived from gluconeogenesis and glycogenolysis. The rates of endogenous glucose production in both full-term and preterm infants are significantly higher than in adults. In preterm infants, gluconeogenesis is attributed to account for 72% of EHGP. On average, the neonate produces glucose at rates between 4 and 6 mg/kg per minute. The higher rates of glucose production in the neonate reflect the higher ratio of brain to body weight, the brain being the major glucose-utilizing organ. In the first few days after birth, the rate of glucose production during fasting in full-term infants has been reported to decrease slightly. As the infant grows, the rate of glucose production expressed per unit of body weight decreases, so by adolescence, it approaches the rate seen in adults.
EHGP depends on (1) adequate glycogen stores, (2) sufficient supplies of endogenous gluconeogenic precursors, (3) normally functioning hepatic gluconeogenic and glycogenolytic systems, and (4) a normal endocrine system for modulating these processes. At birth, the neonate has glycogen stores that are greater than those in the adult. However, because of twofold greater basal glucose use, the stores begin to decline within 2-3 hours after birth. Muscle and cardiac carbohydrate levels fall more slowly. During asphyxia, the energy requirements are met by anaerobic glycolysis, an inefficient mechanism that results in a limited amount of energy and a decrease in glycogen stores. In premature infants, both total carbohydrate and fat content are reduced, and depletion of liver carbohydrates occurs. Endogenous gluconeogenic substrate availability is probably not a limiting factor, because the concentration of plasma amino acids is high at birth as a consequence of active placental transport. Similarly, other gluconeogenic precursors, such as lactate, pyruvate, and glycerol, are also increased. Nevertheless, active gluconeogenesis has been demonstrated in the human newborn soon after birth. Finally, EHGP is normally regulated; that is, it decreases in response to glucose as well as insulin infusion in both full-term and preterm healthy neonates. However, in extremely immature infants and in sick or stressed neonates, exogenous glucose infusion may not completely suppress endogenous glucose production.
The fall in blood glucose that normally occurs after birth is accompanied by an increased level of GH, a relatively low concentration of immunoreactive insulin, and an increase in plasma glucagon. The neonate shows an attenuated but significant insulin response to a variety of stimuli. After the oral administration of glucose, the insulin response in the normal neonate is similar to that in adults with chemical diabetes, that is, a lag in insulin response and a delayed peak. The premature infant has a minimal and variable insulin response, but the values are markedly increased by the intravenous administration of glucose plus amino acids. The physiologic role of increased GH levels has not been defined; GH values are high during the first 48-72 hours and gradually decline but are still elevated at 8 weeks of age. In addition, the newborn shows a paradoxic rise in GH after glucose infusion. Plasma glucagon values at birth are similar to or slightly higher than maternal levels. Glucagon and GH are not suppressed during hyperglycemia. In healthy newborns, intravenous and oral alanine feedings raise plasma glucagon and glucose levels.
Adaptation to prolonged starvation in adult humans is facilitated by the ability of the brain to derive much of its energy from the oxidation of ketone bodies, which decreases the need for gluconeogenesis and spares muscle protein. The enzymes involved in ketone body utilization pathways are present in the brain tissue of human fetuses and newborns. That ketone bodies can be used by the brain of infants and children has been shown by measurements of arteriovenous differences across the brain.
The transition from an intrauterine environment to independent extrauterine life is also characterized by intense lipid mobilization, as shown by a rapid increase in plasma glycerol and FFAs. In addition, there is a shift in the sources of energy for oxidative metabolism, as evidenced by a decline in the respiratory quotient. An increased contribution of fat to oxidative metabolism is reflected in a drop in the respiratory quotient from near 1.0 at birth to a level between 0.8 and 0.85 within a few hours after birth. Evidence of significant lipolysis in the neonate has been obtained by using isotopic tracers to measure glycerol kinetics. These studies showed that lipolysis in the neonate occurs at rates almost threefold greater than those in adults and that the major metabolic fate of glycerol released as a result of lipolysis is conversion to glucose. Free fatty acids are used by the heart and muscle in the absence of readily available glucose, producing ketone bodies that readily cross the blood–brain barrier to be used as fuel sources for the brain. Therefore, the hypoglycemic neonate may remain asymptomatic because of the ability in utilizing alternate nutrients (ketones and lactate). However, neonatal hypoglycemia caused by hyperinsulinism leads to reduced concentrations of free fatty acids and ketone bodies, depriving the brain of an alternative energy source of ketones for fueling cerebral metabolism. This results in neuroglycopenic symptoms with severe neurologic injury and dysfunction.
Factors frequently overlooked in the interpretation of glucose concentration are the type of sample and the method of analysis. Whole blood includes red blood cells which, therefore, display a glucose concentration lower than that of plasma. Plasma glucose values are higher than those of whole blood by about 14%; the difference may be greater at very low glucose values (<30 mg/dL). Whole-blood glucose content also varies in accordance with the hematocrit. Neonatal red blood cells contain high concentrations of glycolytic intermediates; therefore, whole blood must be deproteinized before analysis. Capillary blood samples should be collected from a warm heel and kept on ice, because the rate of in vitro glycolysis is increased in red blood cells at room temperature; whole-blood glucose values may drop 15-20 mg/dL per hour if the sample is allowed to stand at room temperature. The most frequently used method for glucose determination in the laboratory is an automatic analysis technique with glucose oxidase or a commercial glucose oxidase immobilized electrode. Plasma or serum glucose concentrations are determined, and the results are very accurate.
In most newborn nurseries, the rapid point-of-care assessment of whole-blood glucose concentrations is accomplished by a glucose oxidase and peroxidase chromogen test strip method, either alone or with a reflectance colorimeter. However, all test strip methods show significant variations in glucose concentrations compared with laboratory methods, particularly in the low glucose range (<45 mg/dL). Devices and operator techniques vary, with confounding influences of incubation time and the hematocrit values. There should not be sole reliance on test strip devices, and tests resulting in borderline values (<40-45 mg/dL) should be repeated by using standard laboratory methods.
Continuous glucose monitoring using a subcutaneously placed microdialysis sensor has been validated in adults and children with diabetes. The feasibility, safety, and usefulness of these techniques have been examined in babies with low birth weight. However, similar to point-of-care devices, these methods cannot measure glucose levels less than 45 mg/dL with confidence. Thus far, continuous glucose monitoring has been used mostly for research studies, and its use in clinical setting is still limited.
Perturbations in glucose metabolism after birth caused by failure to adapt to the extrauterine environment, as a result of either alterations in maternal metabolism or intrinsic metabolic problems in the neonate, often result in hypoglycemia. Despite many years of research, uncertainty remains regarding the concentration of glucose responsible for long-term untoward effects of transient or recurrent hypoglycemia. The controversy arises partly because of the spontaneous decrease followed by recovery of the blood glucose concentration after birth and partly because many neonates have very low blood glucose concentrations without any clinical signs or symptoms (i.e., asymptomatic hypoglycemia).
Neuroglycopenia is known to cause seizures and permanent brain damage. The critical glucose concentration at which there is an inadequate supply of glucose for the brain has been suggested to be less than 47 mg/dL because of altered somatosensory-evoked potentials and lower scores noted for mental and motor development. However, a subsequent study of 2-year-old and 15-year-old children, who were born at less than 32 weeks’ gestation age with a blood glucose less than 47 mg/dL over at least 3 days when less than 10 days of postnatal age, found them to have no developmental or physical disability when compared to matched controls without hypoglycemia.
Another recent study from University of Arkansas for Medical Sciences on 1400 10-year-old fourth graders with transient hypoglycemia (glucose <45 mg/dL) reported an association with decreased literacy and mathematics proficiency at fourth-grade achievement testing when compared to age and size-matched controls.
McKinlay et al. reported no association between adverse neurodevelopmental outcome at 2 years and neonatal hypoglycemia (blood glucose <47 mg/dL) in 404 infants with birth weight less than 2500 g or greater than 4500 g and less than 37 weeks of gestational age. The glucose was monitored continuously in this cohort, and abnormal neurodevelopmental outcomes showed a U-shaped relationship with adverse cognitive outcomes associated with time spent outside the range of 54-72 mg/dL in the first 48 hours of life. In summary, inadequate blood supply to the brain or neuroglycopenia probably occurs at a range of glucose values. The harmful effect of blood glucose may be at a low, as well as unstable, high blood glucose values.
Clinical signs of hypoglycemia in the neonate are not specific to alterations in glucose concentration but rather reflect an immaturity of counter-regulatory hormonal stress response responsible for classical symptoms of hypoglycemia in older children and adults. Therefore, the presence or absence of such clinical signs cannot be used reliably to define neonatal hypoglycemia. The statistical definition of hypoglycemia is based on surveys of large numbers of infants. Abnormality is defined as a blood glucose concentration that falls outside a prescribed limit, for example, outside two standard deviations from the norm. AAP guidelines also recommend screening in the first 24 hours of life of all asymptomatic at-risk infants for neonatal hypoglycemia (preterm infants 34-36 6/7 weeks and term infants who were born to mothers with diabetes, small for gestational age, or large for gestational age). The AAP algorithm indicates lowest acceptable transitional glucose values of 25 mg/dL. Intervention is recommended between 25 and 40 mg/dL during the first 4 hours of life or during transition after birth in healthy neonates. These levels approximate epidemiologic data at the 5th-10th percentile for glucose. From 4-24 hours after birth, the lowest level requiring action is 35 mg/dL (range is 35–45 mg/dL). Early feeding initiation is critical for establishing breastfeeding as well as in maintaining plasma glucose levels. This practice is different from the 1960s when newborns were generally fasted between 8 and 24 hours after birth.
A recent recommendation has emerged from the Pediatric Endocrine Society. For high-risk neonates without a suspected congenital hypoglycemia disorder, they suggest the goal of treatment be to maintain a plasma glucose concentration greater than 50 mg/dL for those aged less than 48 hours and greater than 60 mg/dL for those aged greater than 48 hours. Higher treatment targets should be considered for those with a suspected genetic hypoglycemia disorder and for symptomatic neonates, because the risks of undertreatment outweigh those of overtreatment in these patients.
The definition of hypoglycemia for preterm infants should not be any different from that for full-term infants. Finally, hypoglycemia in the neonate should be described as transient or persistent, and in either or both of these cases, as symptomatic or asymptomatic. Such a description has implications for both clinical management and long-term consequences.
Transient hypoglycemia implies low glucose values that last only a short time if not corrected and that are confined to the newborn period. In contrast, persistent and recurrent hypoglycemia implies a form that requires prolonged management (glucose infusions for several days at high rates of infusion) and perhaps pharmacologic intervention. Several of these hypoglycemia syndromes may continue throughout infancy and childhood.
The clinical manifestations of hypoglycemia are nonspecific and similar to those of many disorders in newborn infants ( Box 86.1 ). The clinical signs and symptoms of hypoglycemia should improve with correction of the low glucose concentration. In addition, careful attention should be given to ensure that other associated disorders (e.g., sepsis, asphyxia) are not missed.
* Clinical signs should be alleviated with concomitant correction of plasma glucose levels.
Abnormal crying
Irritability
Apnea, cyanotic spells
Jitteriness, tremors
Feeding difficulty
Lethargy or stupor
Grunting, tachypnea
Seizures
Hypothermia
Sweating
Hypotonia, limpness
Tachycardia
Because of its implications for both long-term prognosis and clinical management, it is worthwhile to consider two types of neonatal hypoglycemia: those cases limited to the newborn period (transient hypoglycemia) and those cases continuing over an extended period of time or occurring more than once (persistent or recurrent hypoglycemia) ( Box 86.2 ). Transient hypoglycemia is often a consequence of changes in the metabolic environment in utero or ex utero, whereas persistent or recurrent hypoglycemia arises from intrinsic metabolic problems in the infant. Either type of hypoglycemia may manifest asymptomatically or symptomatically.
Associated with changes in maternal metabolism
Intrapartum administration of glucose
Drug treatment
Terbutaline, ritodrine, propranolol
Oral hypoglycemic agents
Diabetes in pregnancy: infant of diabetic mother
Associated with neonatal problems
Idiopathic condition or failure to adapt
Intrauterine growth restriction
Birth asphyxia
Infection
Hypothermia
Hyperviscosity
Erythroblastosis fetalis
Other
Iatrogenic causes
Congenital cardiac malformations
Hyperinsulinism
Congenital hyperinsulinism
Beckwith-Wiedemann syndrome
Endocrine disorders
Pituitary insufficiency
Cortisol deficiency
Congenital glucagon deficiency
Epinephrine deficiency
Inborn errors of metabolism
Carbohydrate metabolism
Galactosemia
Hepatic glycogen storage diseases
Fructose intolerance
Amino acid metabolism
Maple syrup urine disease
Propionic acidemia
Methylmalonic acidemia
Hereditary tyrosinemia
3-hydroxy, 3-methyl glutaric acidemia
Ethylmalonic-adipic aciduria
Glutaric acidemia type II
Fatty acid metabolism
Defects in carnitine metabolism
Acyl-coenzyme dehydrogenase defects
Neurohypoglycemia (hypoglycorrhachia) caused by defective glucose transport
As discussed, the fetus is entirely dependent on the mother for the supply of glucose, and fetal glucose concentration closely mimics that of the mother. An increase in maternal glucose concentration as a result of exogenous glucose infusion causes an increase in fetal glucose concentration, which in turn causes an increase in fetal insulin levels. Studies in animals have shown that fetal hyperinsulinism, caused by either direct infusion of insulin to the fetus or fetal hyperglycemia, results in an increased metabolic rate in the fetus, fetal hypoxemia, and metabolic acidosis. The acute administration of glucose during the intrapartum period—for example, to prevent hypotension during the conduction of anesthesia—has been shown to result in increased glucose, insulin, and lactate levels in the cord blood obtained at delivery. Fetal hyperinsulinism leads to hyperinsulinemia and hypoglycemia in the neonate. Similar observations have been reported in a diabetic pregnancy. Therefore, caution should be exercised in the administration of glucose to the mother during labor and delivery (see Chapter 27 ). Blood glucose concentrations in the mother should not be allowed to exceed those observed in the normal physiologic range.
Poor glycemic control during pregnancy has increased risk of diabetic embryopathy and congenital malformations. Insulin is the most common maternal therapy for gestational hyperglycemia; oral antihyperglycemic drugs are the other option. Among these agents, metformin alone or with insulin is used most commonly toward achieving timely glucose control and is associated with a decrease in macrosomia, respiratory distress, and need for NICU admissions. Glyburide is another oral antidiabetic drug with good efficacy, but it crosses the placenta and has a higher incidence of large-for-gestational-age (LGA) infants and neonatal hypoglycemia. Older antidiabetic drugs such as tolbutamide and chlorpropamide cross the placenta and produce pancreatic beta-cell hyperplasia and increased insulin release. Tolbutamide has a markedly prolonged half-life in the neonate and has been found in higher concentrations in the newborn after delivery than those found in maternal blood.
Benzothiadiazide diuretics may cause neonatal hypoglycemia by stimulation of fetal beta cells or secondary to an elevation in maternal glucose levels. Salicylates may cause hypoglycemia by uncoupling mitochondrial oxidative phosphorylation.
Oral beta-sympathomimetic tocolytic drugs such as terbutaline and ritodrine have caused sustained hypoglycemia and elevated cord blood insulin levels in infants delivered within 2 days after termination of tocolytic therapy (see Chapter 18 ). These agents may cause neonatal hypoglycemia through maternal hyperglycemia and fetal hyperglycemia and hyperinsulinemia.
Beta-adrenergic blocking agents such as propranolol cross the mammalian placenta, and their effects on the fetus are easily demonstrated. These drugs are used during pregnancy for the treatment of hypertension, hyperthyroidism, cardiac arrhythmia, and other conditions. They may interfere with the effects of the normal surge in catecholamine levels at birth. In animal studies, propranolol has been shown to impair fetal growth and may cause neonatal hypoglycemia and impair the thermogenic response to cold exposure.
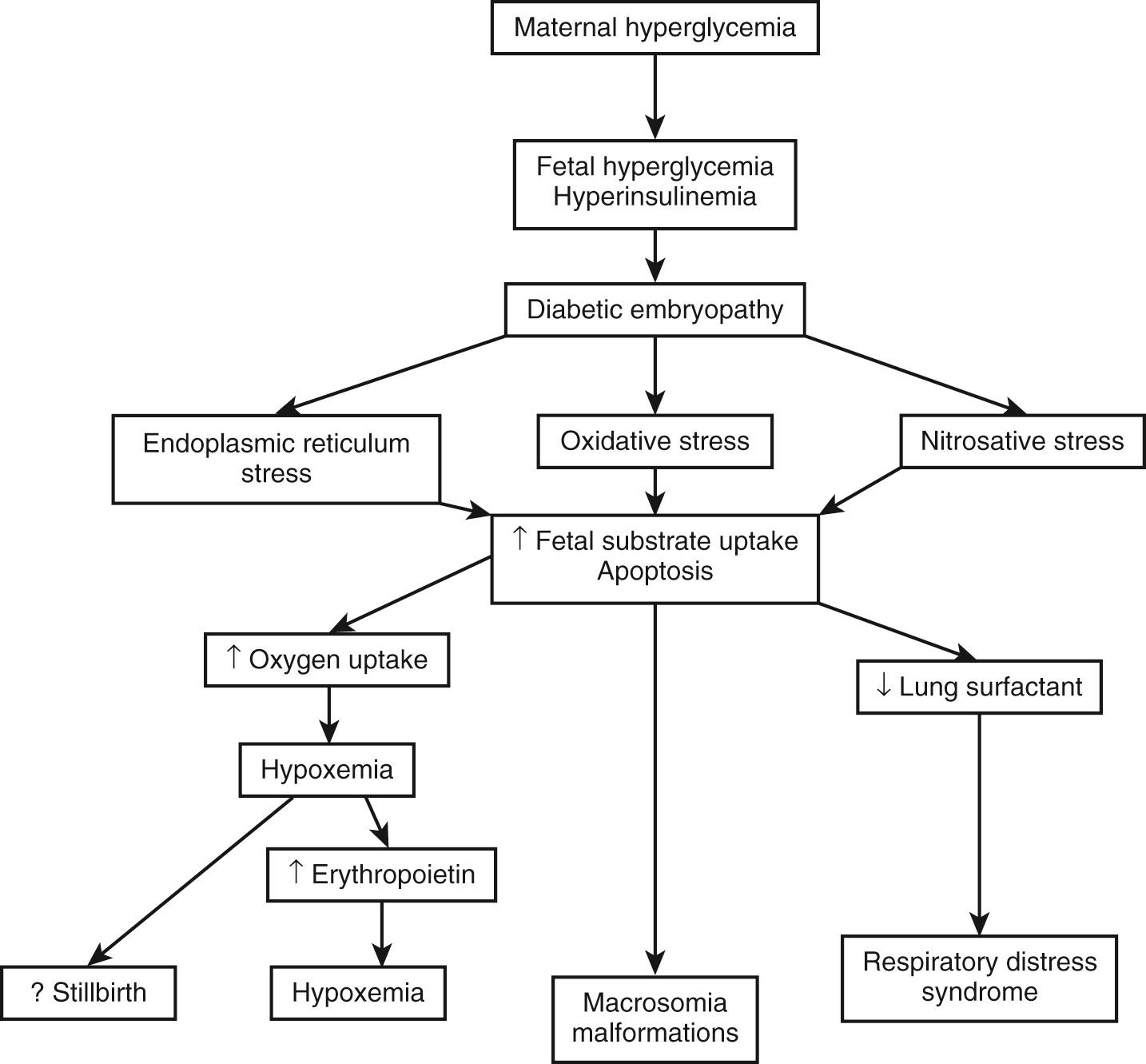
Despite advances in perinatal care, gestational and pregestational diabetes in the mother continues to result in neonatal morbidities involving major organ systems ( Box 86.3 ). Many maternal and fetal–placental factors are implicated in the development of insulin resistance and gestational diabetes (GDM). The recent worldwide increase in obesity and diabetes at younger ages raises the concern for fetal programming. Small changes in the fetal metabolic environment can result in epigenetic modifications and gene expression lasting a lifetime and modifying the phenotype. The pathogenesis of the spectrum of fetal and neonatal morbidities in the IDM has now been described by a number of careful studies in human and animal models. Intermittent hyperglycemia in the mother results in hyperglycemia that causes hyperinsulinemia in the fetus because of hypertrophy of fetal pancreatic islets and beta cells and increased secretion of insulin. Because of the lack of significant transfer of insulin from the mother to the fetus in humans, the circulating insulin in the fetal compartment is mostly of fetal origin. Increased intracellular glucose concentration, by upregulated glucose transporters, enhances mitochondrial oxidative phosphorylation. The resulting increase in reactive oxygen species production plays an important role in diabetic embryopathy. Research studies and animal experiments show that aberrations in intracellular conditions result in oxidative stress, nitrosative stress, endoplasmic reticulum stress, and hexosamine stress. These changes induce apoptosis and alterations in genetics and epigenetic systems, resulting in dysmorphogenesis. Chronically higher fetal metabolic rate and oxygen consumption lead to relative hypoxemia, which in turn results in upregulation of proangiogenic factors, such as leptin, vascular endothelial growth factor (VEGF), or fibroblast growth factor 2 (FGF2), and matrix metalloproteinases (MMPs) as MMP14 and MMP15. Fetal hypoxia caused by increased glycation of hemoglobin and reduced 2,3 DPG concentration, in addition to hyperglycemia and hyperinsulinemia, leads to increased erythropoietin and increased red blood cell mass resulting in polycythemia. In addition, hyperinsulinemia has been shown to suppress the production of surfactant in the lung and thus predisposes the infant to respiratory distress syndrome after birth (see Chapter 64 ). An excessive accumulation of glycogen in the liver, adipose tissue, and other tissues has also been observed in IDMs. The sequence of these metabolic events is outlined in Fig. 86.4 .
Congenital anomalies
Heart failure and septal hypertrophy of heart
Surfactant deficiency respiratory distress syndrome, transient tachypnea of the newborn, persistent pulmonary hypertension
Hyperbilirubinemia
Hypoglycemia, hypocalcemia, hypomagnesemia
Macrosomia, nerve injury related to birth trauma
Renal vein thrombosis
Small left colon
Unexplained intrauterine demise
Polycythemia
Visceromegaly
Predisposition to later-life obesity, insulin resistance, and diabetes
Although the excessive transport of glucose from the mother to the fetus has been thought to be primarily responsible for fetal metabolic and physiologic perturbations in the IDM, nonglucose metabolites (fatty acids, amino acids, and micronutrients) play an important role as well. The increased concentration of these nutrients in the fetal circulation stimulates fetal insulin secretion, which in turn stimulates excessive fetal growth.
After birth, the newborn IDM whose mother's disease has not received rigorous antepartum management appears to be unable to produce sufficient circulating glucose and alternative fuels. Even infants of mothers with rigorous management (i.e., those who have maintained normoglycemia throughout gestation) develop significantly lower blood glucose concentrations than those of normal infants. In addition, they do not mobilize fatty acids from adipose tissue; as a result, their circulating levels of FFAs remain low, although a normal increase in plasma glycerol has been observed. Such a metabolic picture suggests persistent insulin action and the lack of a counter-regulatory hormonal response. The latter is confirmed by the lack of an increase in circulating glucagon and catecholamine levels in IDMs during hypoglycemia. The combination of hyperinsulinism and insufficient counter-regulation results in decreased hepatic glucose production, increased peripheral glucose uptake, and impaired lipolysis. When measured by the isotope tracer dilution method, IDMs produce glucose at significantly lower rates than normal infants. In addition, their basal metabolic rates, or rates of oxygen consumption, have been reported to be lower than those of normal infants.
Several of these metabolic and morphologic abnormalities can be reversed with fastidious management of diabetes in the mother. Data from several studies showed that with rigorous management throughout pregnancy, IDMs do not become hypoglycemic and maintain normal rates of glucose production and basal metabolism.
It should be recognized that the clinical manifestations described here relate to diabetic mothers whose metabolism has not been well controlled. Data from a study in which maternal diabetes was rigorously managed by either an insulin infusion pump or split-dose insulin therapy are shown in Table 86.1 . Despite rigorous management and reduced maternal hyperglycemia, significant morbidity may persist.
| Parameter | Infants of Diabetic Mother ( N = 78) | Controls ( N = 78) |
P |
|---|---|---|---|
| Birth weight (g) | 3454 ± 817 | 3271 ± 621 | — |
| Gestational age (wk) | 37.9 ± 0.95 | 39.3 ± 1.7 | .0001 |
| K score * | 0.77 ± 0.95 | 0.18 ± 0.91 | .0001 |
| Macrosomia (>4 kg) | 19 (24%) | 8 (10%) | .03 |
| Large for gestational age | 32 (41%) | 12 (15%) | .0002 |
| Hypoglycemia | 11 (14%) | 1 (1%) | 0.0025 |
| Hyperbilirubinemia | 36 (46%) | 18 (23%) | .002 |
| Respiratory distress syndrome | 9 (12%) | 1 (1%) | .008 |
| Admission to neonatal intensive care unit | 21 (27%) | 9 (12%) | .01 |
* The K score is a method of adjusting the birth weight in grams for the infant's gestational age. A K score of 1 represents the 90th percentile for weight; scores of 0 and −1 represent the 50th and 10th percentiles, respectively. Data from Aucott SW, et al. Rigorous management of insulin-dependent diabetes mellitus during pregnancy. Acta Diabetol. 1994;31:126.
At birth, these infants are obese, plethoric, and large for gestational age (LGA) and show evidence of excessive fat as well as visceromegaly in the form of a large liver, spleen, and heart ( Fig. 86.5 ). Because the growth of the brain and possibly the kidney is not dependent on insulin, these two organs are normal in size. Careful management of maternal metabolism tends to reduce the incidence of macrosomia but does not prevent it entirely.
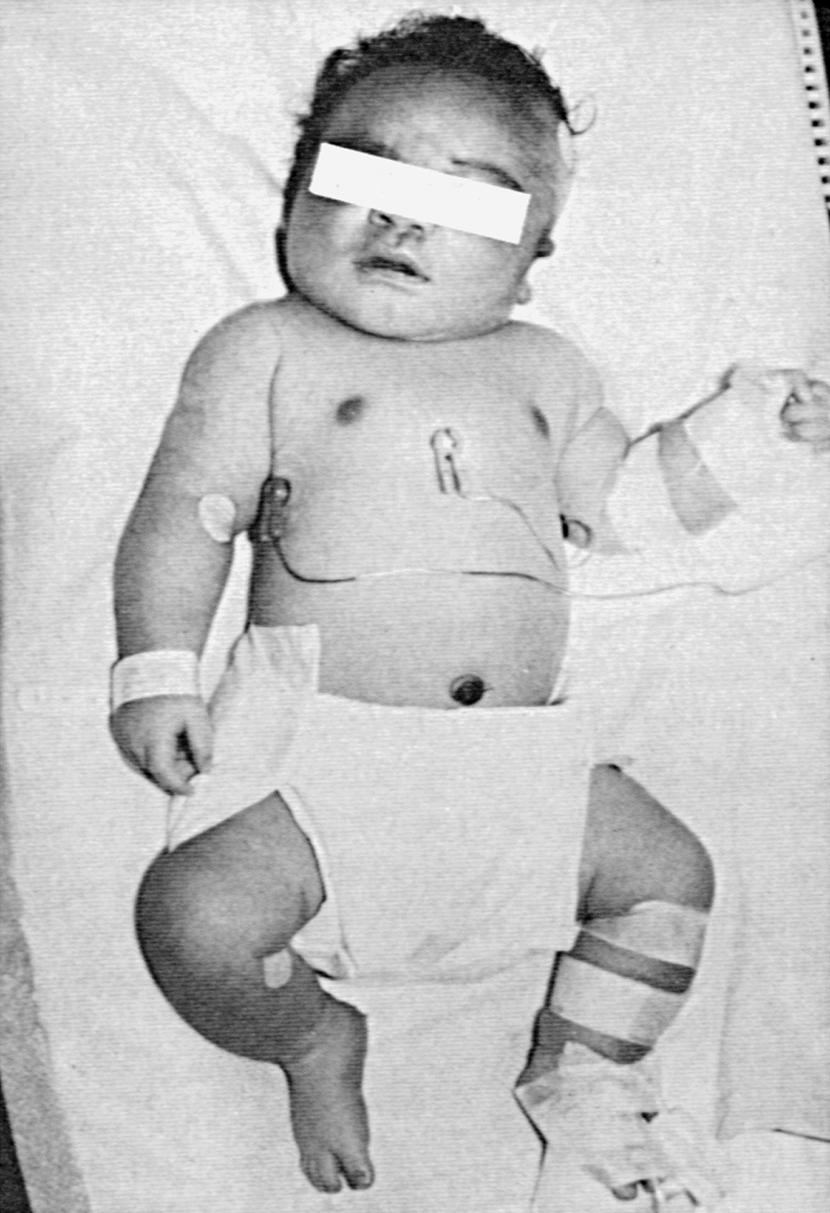
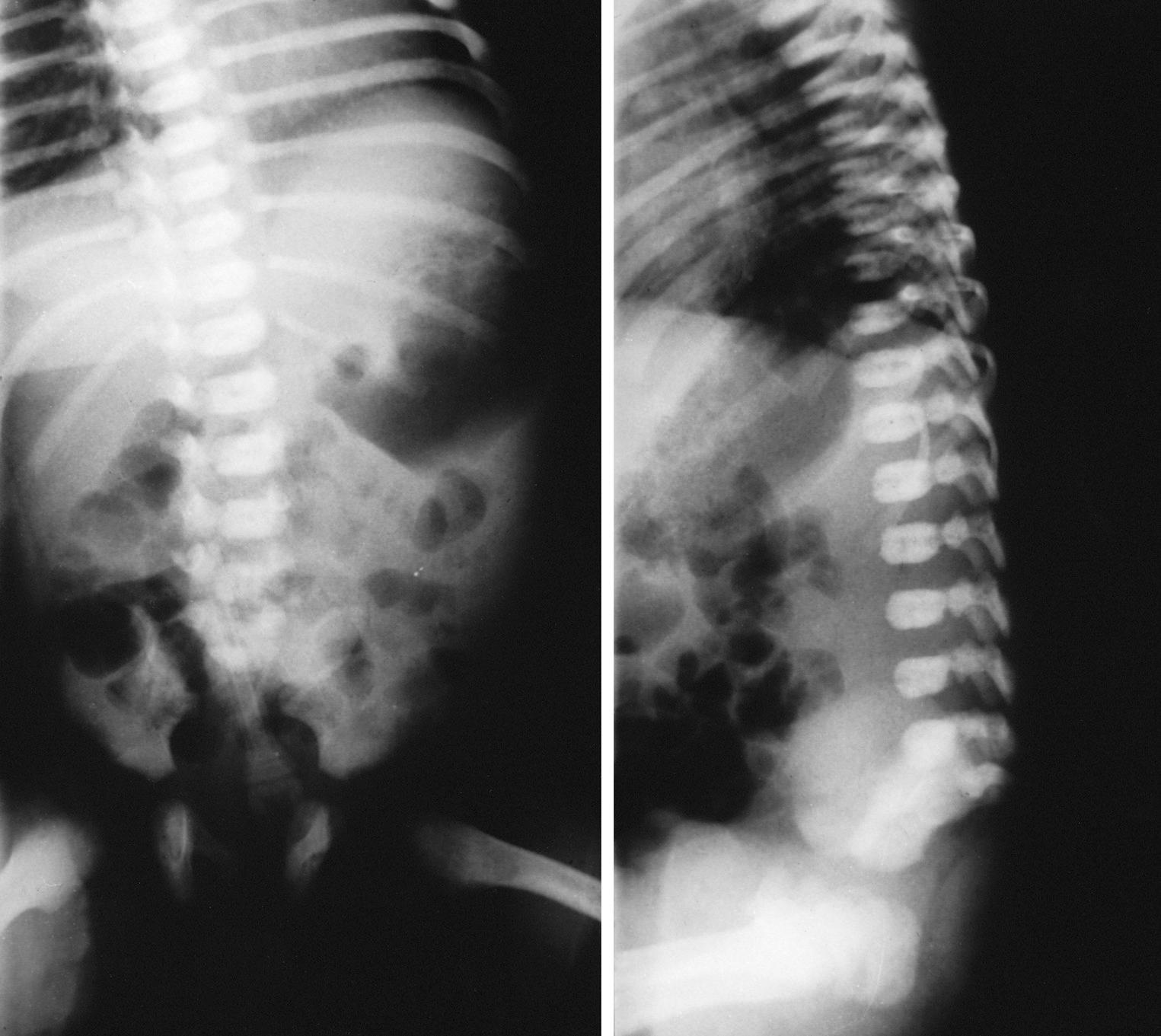
The incidence of congenital malformations is increased twofold to threefold in infants of insulin-dependent diabetic mothers compared with the normal population. The frequency of congenital anomalies is not increased in infants of gestationally diabetic mothers or in those of diabetic fathers. Chronic hyperglycemia in the diabetic embryo, causing accumulation of advanced glycosylation end-products (AGE) and glycated protein and oxidative stress, has been suggested to induce malformations. A number of congenital anomalies, including facial, skeletal, and neural tube defects, were induced in the diabetic environment created in a RAGE (receptor for AGE) knock-out mouse model. Dysmorphology of the cardiovascular system and genitourinary system has been reported. Caudal agenesis or dysplasia syndrome ( Fig. 86.6 ) is seen with markedly increased frequency, especially in IDMs. This syndrome consists of agenesis or hypoplasia of the femora in conjunction with agenesis of the lower vertebrae and sacrum. Data from animal models show a complex process of genetic and epigenetic processes enhancing apoptosis and resulting in diabetic embryopathy with multiple structural defects. The frequency of congenital anomalies is significantly increased in mothers with poor metabolic control, as evidenced by increased hemoglobin A 1c levels early in gestation, and a significant decrease in congenital malformations has been reported with rigorous metabolic regulation in the periconceptional period (see Chapter 18 ).
The pathogenesis of hypoglycemia has been described already. Immediately after birth, there is a significant decrease in plasma glucose concentration, reaching a nadir between 30 and 90 minutes and followed by a spontaneous recovery in most infants. However, in some infants, the plasma glucose level remains persistently low (i.e., <45 mg/dL), necessitating intervention. Most of these infants are asymptomatic. Irrespective of symptoms, the current recommendation is to correct the hypoglycemia with an appropriate glucose infusion. It should be underscored that hypoglycemia in the newborn does not necessarily reflect the magnitude of antepartum metabolic control of the mother and may simply be the consequence of hyperglycemia during labor and delivery.
See Chapter 87 .
Alterations in calcium and magnesium homeostasis occur in about 50% of infants born to insulin-dependent diabetic mothers. Unlike hypoglycemia, hypocalcemia becomes apparent between 48 and 72 hours after birth. Plasma calcium concentrations of lower than 7 mg/dL are frequently observed. Hypocalcemia has been related to the severity and duration of maternal diabetes. In addition, it may be potentiated by prematurity and asphyxia. The mechanisms of hypocalcemia are probable failure of the IDM to mount an appropriate parathyroid hormone (PTH) response, persistently high levels of calcitonin, and possible alterations in vitamin D metabolism. Hypomagnesemia (≈1.5 mg/dL) has also been frequently observed. It is usually transient, and its pathophysiologic significance remains uncertain.
Both hypocalcemia and hypomagnesemia may be manifested with jitteriness and may require supplemental calcium therapy. However, in most infants, they are transient events that improve spontaneously.
The management strategies for other clinical problems, such as polycythemia (see Chapters 79 and 80 ), hyperbilirubinemia (see Chapter 91 ), and renal vein thrombosis (see Chapter 93 ), that occur with slightly higher frequency in IDMs are similar to those in otherwise normal infants.
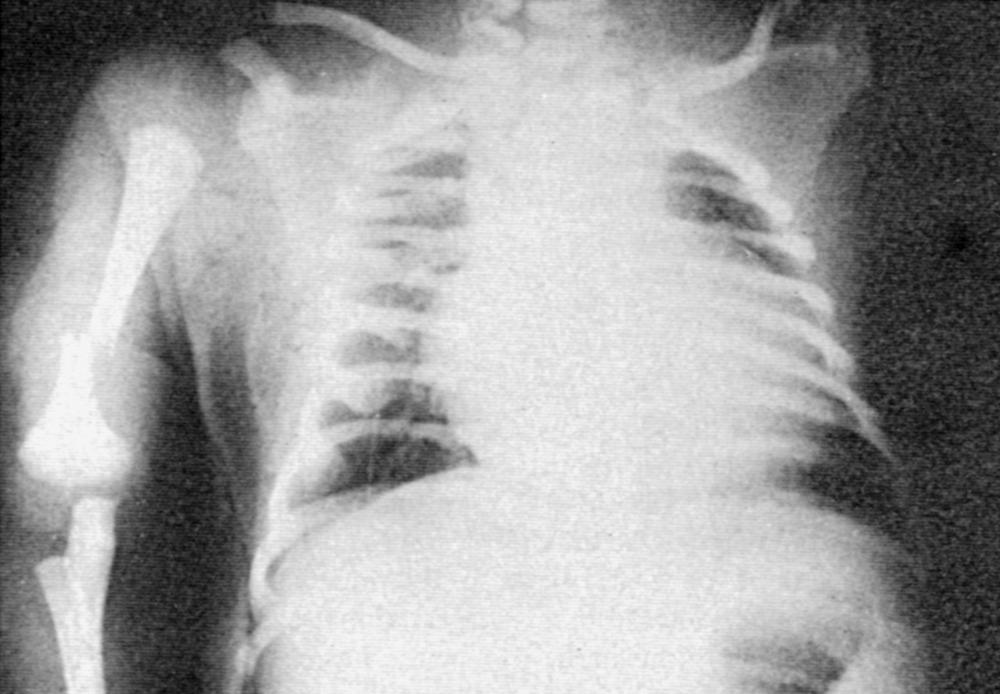
Septal hypertrophy and cardiomegaly are specific phenotypic consequences of diabetes in pregnancy and may be manifested with heart failure in the neonate ( Fig. 86.7 ). In addition, data in asymptomatic infants of gestationally diabetic mothers have shown alterations in diastolic function and decreased passive compliance of the ventricular myocardium. By serially evaluating cardiac growth in utero in the fetuses of diabetic mothers, it has been shown that despite good metabolic control, cardiac hypertrophy developed in late gestation (34-40 weeks). Although IDMs with septal hypertrophy may present with obstructive left heart failure, they may also be asymptomatic. Follow-up data show that cardiomyopathy in an IDM is a transient disease and usually disappears spontaneously within 6 months after birth. Otherwise minor functional changes, such as impaired diastolic filling, have been reported in infants of gestational diabetic mothers and are usually not clinically significant.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here