Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
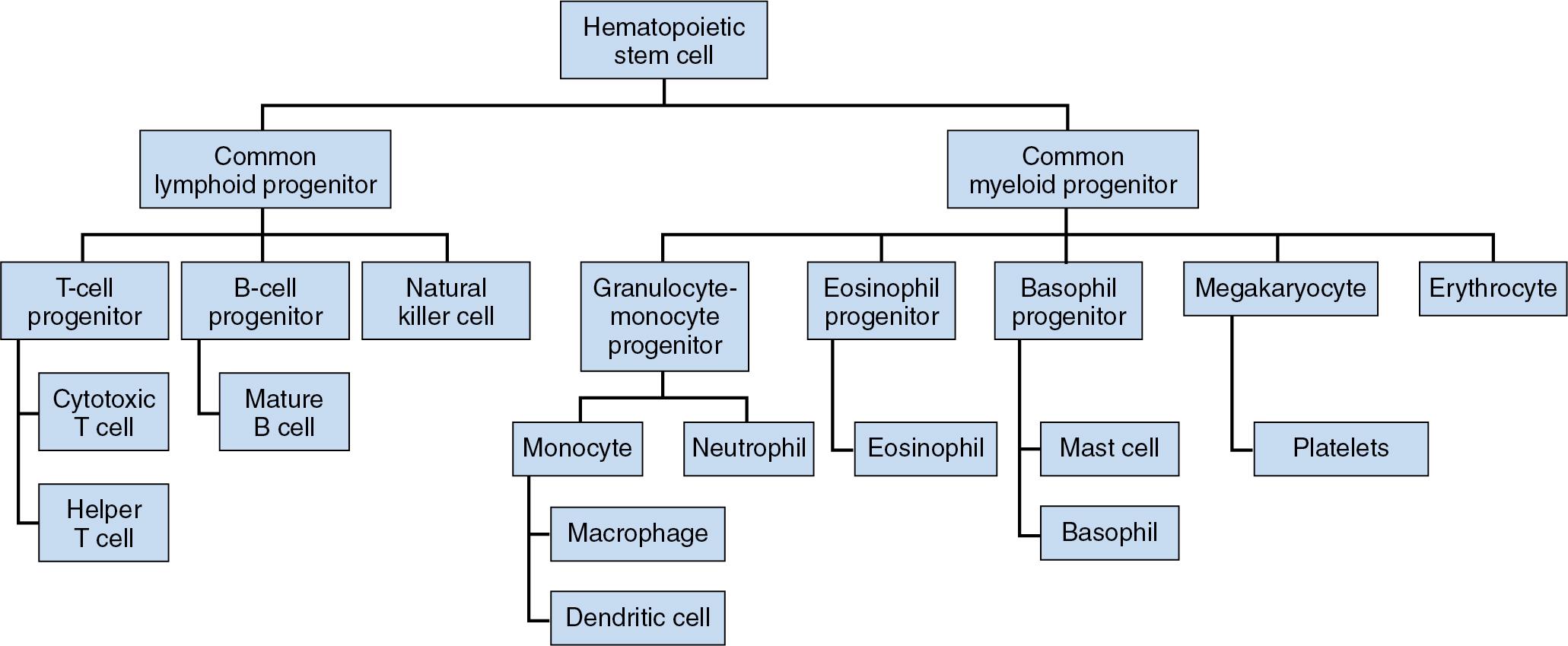
The human immune system is traditionally divided into two pathways: innate immunity and adaptive (also known as acquired) immunity. Each is comprised of a series of unique components, all of which function to protect the host against invading microorganisms. The innate immune response is rapid and nonspecific (i.e., recognizes targets that are common to many pathogens and requires no prior exposure to a target antigen). Its noncellular elements include physical barriers (tight junctions in the skin, epithelial and mucous membrane surfaces), complement factors, acute-phase proteins, and proteins of the contact activation pathway. Cellular elements include neutrophils, macrophages, monocytes, eosinophils, basophils, mast cells, and a subset of lymphocytes called natural killer (NK) cells ( Fig. 26.1 ). The adaptive immune response is an evolutionarily more mature system present only in vertebrates. Adaptive immunity has a more delayed onset of activation but is capable of developing memory and more specific antigenic responses. It consists of a humoral component mediated by B lymphocytes that produce antibodies and a cellular component composed of T lymphocytes. T cells are divided into two main subsets: cytotoxic (Tc) cells and helper/modulatory (Th) cells, distinguished by their different combinations of surface antigens. Tc cells express a predominance of CD8 antigen, while Th cells express a predominance of CD4 antigen. Precursor helper T lymphocytes differentiate into several distinct subsets, including Th1, Th2, Th9, Th17, regular T cells (Tregs), and follicular T cells (Tfh). Th1 cells produce interferon-γ (IFN-γ) and interleukin-2 (IL2) and promote cell-mediated immune responses. Th2 cells produce specific interleukins, including IL4, IL5, and IL13, which favor a humoral immune response and suppress cell-mediated immunity. Th9 cells produce IL9 and IL10 and have a role in tumor immunity, allergy, and autoimmune disease. They also help to protect against parasitic infections. Th17 cells are proinflammatory and influence the development of chronic inflammatory conditions, including some cell-mediated autoimmune diseases. In contrast, Treg cells promote tolerance and minimize autoimmune and allergic or inflammatory responses. Finally, Tfh cells play a role in the maturation of B-cell germinal centers, which ultimately lead to the production of antibodies. As a general rule, cytotoxic and helper T-cell responses are important in the effective response to trauma, infection, and tumorigenesis ( Table 26.1 ).
| Subset | Main Functions | Cytokines Produced |
|---|---|---|
| Helper T cells | ||
| Th1 | Macrophage activation Cellular cytotoxicity Protection against intracellular microorganisms Delayed hypersensitivity reactions |
IFN-γ IL2 |
| Th2 | IgE production Eosinophil proliferation Protection against parasitic infection |
IL4 IL5 IL13 |
| Th9 | Tumor immunity Resistance to parasites |
IL9 IL10 |
| Th17 | Chronic inflammation, allergy, autoimmune diseases Protection against extracellular bacteria and fungi |
IL17 IL22 IL26 |
| Th22 | Chronic inflammation, allergy, autoimmune diseases | IL22 |
| Regulatory T cells (Tregs) | Prevention of allergic and autoimmune disease Downregulation of immune response/development of tolerance |
TGFβ IL10 |
| Follicular T cells (Tfh) | Germinal center B-cell development and maturation | |
| Cytotoxic T cells | Induction of cellular toxicity in infected or tumor cells Inhibition of microbial replication |
|
Immune dysfunction can be divided into three categories: (1) injury caused by an inadequate immune response, (2) injury caused by an excessive immune response, and (3) injury caused by a misdirection of the immune response.
Neutropenia is defined as a neutrophilic granulocyte count of less than 1500/μL. Normal neutrophil counts vary somewhat by age and ethnicity. For example, newborns tend to have higher granulocyte counts in the first few days of life, and Blacks tend to have lower average granulocyte counts compared to whites. It is not until the granulocyte count decreases to less than 500/μL that a patient is at significantly increased risk of pyogenic infections. Common infecting organisms include Staphylococcus aureus, Pseudomonas aeruginosa, Escherichia coli, and Klebsiella species, producing infections of the skin, mouth, pharynx, and lung. Broad-spectrum parenteral antibiotics are indicated in the management of these patients.
Several neutropenic syndromes can occur in newborns and children. Neonatal sepsis is the most common cause of severe neutropenia within the first few days of life. A transient neutropenia may occur in children born to mothers with autoimmune diseases or as a result of maternal hypertension or drug ingestion. Persistent neutropenia can occur due to defects in neutrophil production, maturation, or survival.
The autosomal dominant disorder cyclic neutropenia is a particularly well-studied cause of childhood neutropenia. It is characterized by recurrent episodes of neutropenia (not always associated with infection) that occur in regular cycles every 3 to 4 weeks. Each episode is characterized by a brief phase of reduced granulocyte production, followed by a period of reactive mastocytosis and then spontaneous recovery of normal granulocyte production. A characteristic feature is the presence of chronic mouth ulcers and gingival disease. In most cases, the condition is caused by a defect in the elastase gene, which halts myelocyte maturation in the bone marrow. Patients respond well to treatment with granulocyte colony-stimulating factor (G-CSF).
Kostmann syndrome is an autosomal recessive disorder of neutrophil maturation. Patients with Kostmann syndrome appear to have a normal population of early progenitor cells that somehow become suppressed, inhibiting normal maturation. If untreated, mortality in the first year of life approaches 70%. Treatment with G-CSF is effective in 90% of patients; in patients who do not respond to G-CSF, bone marrow transplantation may be considered.
Wiskott-Aldrich syndrome is another form of congenital neutropenia with an X-linked inheritance pattern seen almost exclusively in males. The pathophysiology relates to impaired T-cell signaling and an inability of NK cells to mount a cytotoxic response. In addition to susceptibility to infections, these patients also present with thrombocytopenia and eczema.
With the advent of G-CSF leading to increased survival in children with congenital neutropenic syndromes, it has become clear that these patients are at increased risk for the development of myelodysplastic syndrome (MDS) and leukemia in later life.
Acquired defects in the production of neutrophils in adults are very common. Typical causes include cancer chemotherapy and treatment of human immunodeficiency virus (HIV) with zidovudine. Neutropenia usually reflects the impact of a drug on stem cell and early myelocytic progenitor proliferation. In most cases, the marrow recovers once the drug is withdrawn. Many drugs have been associated with neutropenia. Among the most prominent of these are the injectable gold salts, chloramphenicol, antithyroid medications (carbimazole and propylthiouracil), analgesics (indomethacin, acetaminophen, and phenacetin), tricyclic antidepressants, and phenothiazines. However, virtually any drug can, on occasion, produce severe life-threatening neutropenia. Therefore, when neutropenia occurs in the course of medical treatment, the possibility that it is drug induced must be considered.
Autoimmune-related neutropenia can be observed as an isolated disorder or in the context of another known autoimmune condition. Antineutrophil antibodies are sometimes present. The two most common associations are with systemic lupus erythematosus (where the neutropenia can occur alone or be accompanied by thrombocytopenia) and rheumatoid arthritis. Conditions associated with splenomegaly often lead to granulocytopenia due to white cell sequestration. Felty syndrome is the triad of rheumatoid arthritis, splenomegaly, and neutropenia. Other causes of splenomegaly and neutropenia include lymphoma, myeloproliferative disease, and severe liver disease with portal hypertension. In these latter situations, it is often difficult to decide whether the granulocytopenia is caused simply by splenic sequestration or whether it also has an autoimmune component. In some patients, splenectomy may improve neutrophil production.
Acute, life-threatening neutropenia can occur as a result of certain infections. A decreasing white cell count in a patient with sepsis is a negative prognostic indicator. It reflects a rate of granulocyte use that exceeds the marrow’s ability to produce new cells. Alcoholic patients are especially susceptible to infection-induced neutropenia. Both folic acid deficiency and direct toxic effects of ethanol on marrow precursor cells compromise the host’s ability to produce new neutrophils in response to infection. HIV infection is a common cause of T-cell dysfunction. In these patients, loss of the Th subset and overexpression of the T-suppressor subset is associated with abnormalities of neutrophil production and function.
Benign ethnic neutropenia is an inherited condition most often seen in individuals of African descent, but also certain other ethnic groups (e.g., Sephardic Jews, Greeks, and Arabs). It is rare for the neutrophil count to drop below 1000/μL. The clinical course is benign for most patients.
Chronic granulomatous disease is a genetic disorder in which granulocytes lack the ability to generate reactive oxygen species. The granulocytes can migrate to a site of infection and ingest organisms but are unable to kill them. Staphylococcus aureus and certain gram-negative bacteria such as Serratia marcescens and Burkholderia cepacia that are normally killed by phagocytosis and lysosomal digestion are responsible for most infections in these patients. The condition is usually diagnosed during childhood or early adult life when patients present with recurrent microabscesses and chronic granulomatous inflammation. Persistent inflammation and granuloma formation can lead to multiorgan dysfunction, including intestinal obstruction, glomerulonephritis, and chorioretinitis. Aggressive treatment of infectious complications, prophylaxis with antibiotics and antifungal agents, and the use of recombinant IFN-γ has significantly improved survival in patients with this disease.
Glucose-6-phosphate dehydrogenase (G6PD) deficiency is an inherited disorder caused by a genetic defect in the enzyme G6PD. This enzyme is mostly present in red blood cells (RBCs), where it generates nicotinamide adenine dinucleotide phosphate (NADPH) and protects RBCs against oxidative injury. G6PD is also present in neutrophils. Patients with severe G6PD deficiency exhibit an impaired ability to generate the oxidase needed to kill ingested microorganisms. As with chronic granulomatous disease, neutrophil G6PD-deficient patients are at lifelong risk of infection with catalase-positive microorganisms.
Leukocyte adhesion deficiency is a relatively rare deficiency of a subunit of the integrin family of leukocyte adhesion molecules. This subunit is critical for cellular adhesion and chemotaxis. Although clinical severity varies, patients with leukocyte adhesion deficiency experience a higher risk of recurrent bacterial infections. Persistent granulocytosis is often present; however, the absence of pus is the most characteristic feature of the disease.
Chédiak-Higashi syndrome is an uncommon multisystem disease characterized by partial oculocutaneous albinism, frequent bacterial infections, mild bleeding diathesis, progressive neuropathy, and cranial nerve defects. The neutrophils of these patients contain characteristic giant granules. Patients exhibit multiple defects of immune function, including impairment in neutrophil chemotaxis, phagocytosis, NK-cell activity, and T-cell cytotoxicity. Many white blood cells (WBCs) are destroyed before leaving the bone marrow. In most patients, an accelerated lymphoproliferative syndrome leads to death; however, bone marrow transplantation can reverse immunologic dysfunction in some patients.
Neutrophil-specific granule deficiency syndrome is another rare congenital disorder characterized by neutrophils that exhibit impaired chemotaxis and bactericidal activity. Patients are prone to recurrent bacterial and fungal infections with abscess formation. Skin and pulmonary infections appear to predominate and most of these respond well to aggressive antibiotic therapy.
Patients with neutropenia or a qualitative disorder of granulocyte function often benefit significantly from treatment with G-CSF. Recombinant G-CSF reduces the duration of absolute neutropenia in patients receiving ablative chemotherapy and autologous bone marrow transplantation. It also shortens the length of antibiotic therapy and reduces the risk of life-threatening bacteremia and fungal infections. G-CSF therapy has been approved for the reversal of the neutropenia associated with HIV infection and the prevention of worsening neutropenia in patients on HIV therapy. Neutropenic patients undergoing elective surgery may benefit from a course of G-CSF preoperatively to reduce the risk of perioperative infection.
Complement refers to a family of serum proteins that are critical to the host response to infection. Complement activation may occur by pathogen-dependent (classical or lectin) or pathogen-independent (alternative) pathways ( Fig. 26.2 ). Complement proteins assist in the clearing of microorganisms by coating infectious agents with proteins that facilitate phagocytosis. Complement proteins also promote the inflammatory response. Certain complement components are unique to a particular pathway, but all pathways lead to the formation of C3 and the membrane attack complex. Deficiencies in virtually all the soluble complement components have been described. Defects in early components of the classical pathway of complement activation (C1q, C1r, C1s, C2, and C4) predispose to autoimmune inflammatory disorders resembling systemic lupus erythematosus. Deficiencies in the common pathway component C3 are often fatal in infancy. Deficiencies in the terminal complement components C5 through C9 are associated with recurrent neisserial infections, but these infections are usually mild, and the mortality rate is low. The liver is the primary organ of complement protein synthesis; therefore patients with advanced liver disease are often at increased risk of infection, especially pneumonia and sepsis caused by Streptococcus pneumoniae , Staphylococcus aureus , and Escherichia coli . Prompt recognition and treatment of infection and careful maintenance of routine vaccinations are the hallmarks of treating these patients.
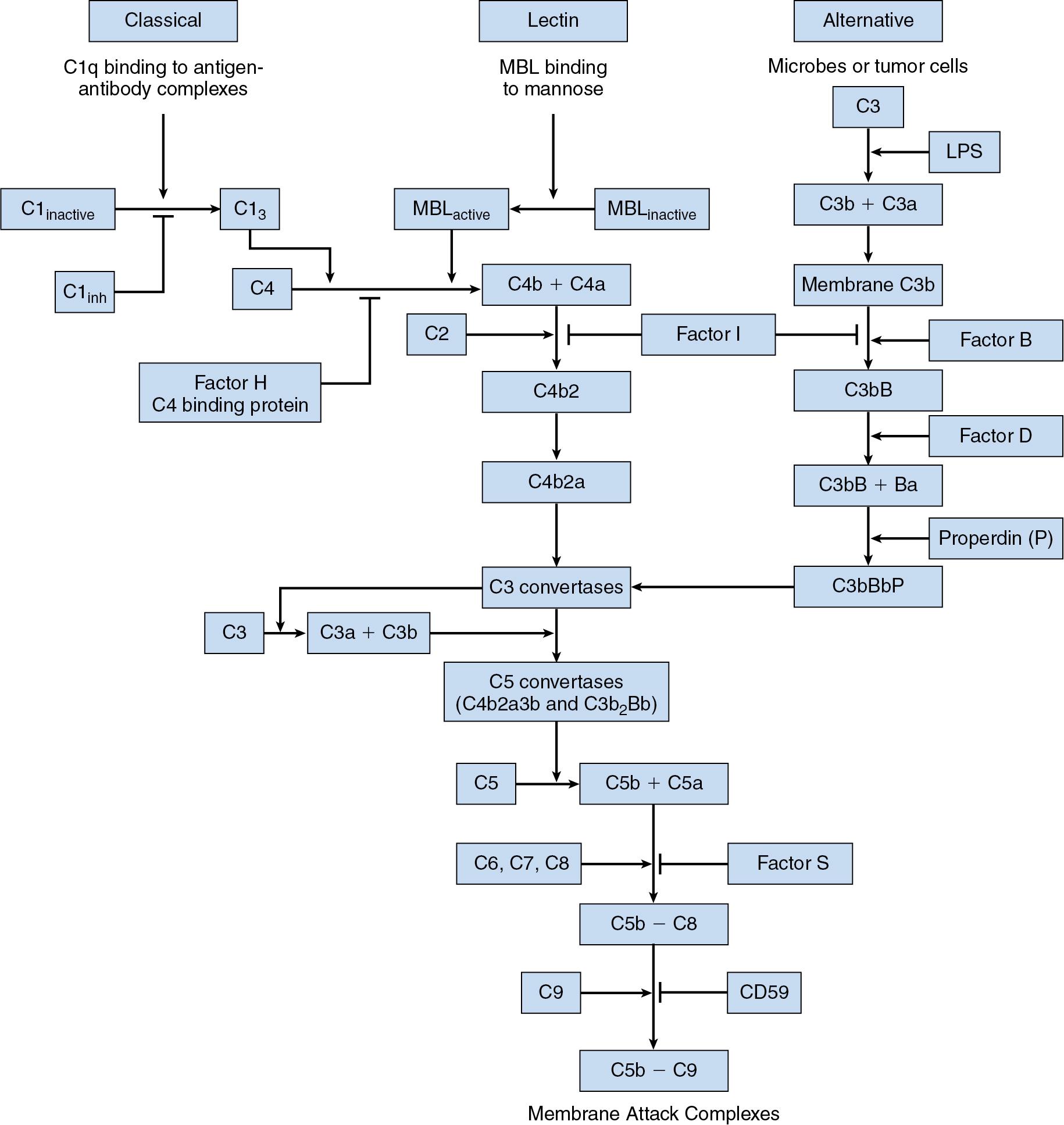
Tight regulation of complement activation prevents misdirected activation of the inflammatory and immune responses. The main inhibitor compound is C1 inhibitor. Deficiency of C1 inhibitor is responsible for hereditary angioedema, an autosomal dominant condition marked by episodes of subcutaneous and submucosal edema, caused primarily by excessive concentrations of bradykinin, which increases vascular permeability. Factor H is another regulatory protein of the complement cascade. Deficiency in factor H predisposes patients to hemolytic uremic syndrome and macular degeneration.
The spleen is a lymphopoietic organ involved with the removal of senescent RBCs, encapsulated bacteria, and other particulates from the circulation. The spleen is capable of enlarging significantly in response to infection. The spleen also normally contains about one-third of the total platelet mass. Splenectomy is the most common cause of splenic dysfunction, although various clinical conditions may lead to impaired splenic function (functional asplenia). Perhaps the most common of these is sickle cell anemia, which causes autoinfarction of the spleen due to vasoocclusive disease. Streptococcal pneumoniae is the most common cause of bacterial sepsis in postsplenectomy patients. Splenic dysfunction also increases risk of infection with Neisseria meningitides, Escherichia coli, Haemophilus influenzae , and malaria. As recommended for patients with complement deficiencies, management of hyposplenic patients relies heavily on prevention, mainly through vaccination against S. pneumoniae , H. influenza b, and N. meningitides . Daily penicillin prophylaxis is often administered postsplenectomy to children until the age of 5.
The earliest response to an infection is the migration of leukocytes out of the circulation and into the site of bacterial invasion. The rapidity and magnitude of the increase in the number of circulating leukocytes in response to infection is remarkable. Within hours of the onset of a severe infection, the leukocyte count increases two- to fourfold. This increase represents a change in the marginated and circulating pools of leukocytes as well as the delivery of new leukocytes from the bone marrow. The most common cause of leukocytosis is neutrophilia, since 60% to 70% of circulating leukocytes are neutrophils. Neutrophilia is defined as an absolute neutrophil count greater than 7700/μL, which is typically seen in patients with leukocyte counts greater than 11,000/μL. Major causes of neutrophilia are listed in Table 26.2 .
| Primary |
| Myeloproliferative disorders |
| Leukemias |
| Down syndrome |
| Secondary |
| Infection/inflammation |
| Cigarette smoking |
| Stress |
| Metabolic disorders (preeclampsia, diabetic ketoacidosis, thyroid storm) |
| Drugs (steroids, lithium, catecholamines) |
| Splenectomy |
Leukocytosis does not produce specific symptoms or signs unless the count exceeds 100,000/μL. Such marked leukocytosis can produce leukostasis resulting in infarction of the spleen and a reduction in the oxygen-diffusing capacity of the lungs. Leukocytes can also accumulate in the skin to produce nontender, purplish nodules called chloromas (also called myeloid sarcoma or leukemia cutis). Unlike immature blasts, mature granulocytes do not invade brain tissue, so neurologic complications do not result from reactive leukocytosis.
The clinical features associated with moderate leukocytosis vary depending on the primary disease underlying the condition. Deep-seated infections and peritonitis are associated with leukocyte counts of 10,000 to 30,000/μL or more. Parasitic infestations are typically associated with an elevated eosinophil count, whereas basophilia is seen in patients with chronic myelogenous leukemia. As a general rule, sustained leukocyte counts of 50,000/μL or higher are suggestive of a noninfectious malignant disease process such as a myeloproliferative disorder. The appearance of very immature myelocytic cells in the circulation and accompanying changes in other cell lines (increased or decreased platelets or RBCs) are also signs of a hematologic malignancy.
Neutrophilia is an expected side effect of glucocorticoid therapy because glucocorticoids interfere with the egress of granulocytes from the circulation into tissues. Patients receiving a prednisone dose of 60 mg/day or higher often have WBC counts of 15,000 to 20,000/μL. Other causes of neutrophilia include physiologic stress, drugs, and cigarette smoking (see Table 26.2 ).
Monocytosis occurs in conjunction with inflammatory disorders such as systemic lupus erythematosus, rheumatoid arthritis, and sarcoidosis and in the context of certain infections, including tuberculosis, syphilis, and subacute bacterial endocarditis. Monocytosis can also be seen in patients with primary neutropenic disorders or hematologic malignancies. Although important components of the immune system, the association between the circulating monocyte count and the propensity to infection is not as clear as in the case of neutrophils.
Asthma is characterized by an exaggerated bronchoconstrictor response to certain stimuli (see also Chapter 2 ). Triggers for bronchospasm unrelated to the immune system produce intrinsic asthma. Placement of an endotracheal tube may trigger this type of asthma; other common triggers are cold, exercise, stress, or inhaled irritants. Mediators of intrinsic asthma are components of the innate immune system. By contrast, triggers that activate the immune system and release IgE produce extrinsic asthma and are part of adaptive immunity. Inhaled allergens such as pollen and pet dander are common causes of extrinsic asthma. Symptoms of extrinsic or allergic asthma are highly variable and can include cough, dyspnea, and wheezing. Treatment consists of administration of β agonists, corticosteroids, leukotriene inhibitors, and anticholinergics.
Angioedema may be hereditary or acquired. It is characterized by episodic, asymmetric subcutaneous, and submucosal edema formation, often involving the face, extremities, and gastrointestinal tract, that occurs rapidly and often resolves spontaneously. Two types of angioedema are recognized. One is caused by release of mast cell mediators and is associated with urticaria, bronchospasm, flushing, and even hypotension. The other results from bradykinin release and does not cause allergic symptoms. The most common hereditary form of angioedema results from an autosomal dominant deficiency or dysfunction of C1 esterase inhibitor. This serine protease inhibitor (serpin) regulates complement, contact activation, and fibrinolytic pathways. The absence of C1 esterase inhibitor leads to a release of vasoactive mediators that increase vascular permeability and produce edema via bradykinin. Patients deficient in this regulatory enzyme experience repeated bouts of facial and/or laryngeal edema lasting 24 to 72 hours. These attacks usually begin in the second decade of life and may be triggered by menses, trauma, infection, stress, or estrogen-containing oral contraceptives. Dental surgery can be an important trigger of laryngeal attacks. Abdominal attacks usually present with excruciating pain, nausea, vomiting, and/or diarrhea. The diagnosis of C1 esterase inhibitor deficiency is suggested by a low C4 level.
C1 esterase inhibitor deficiency can be acquired by patients with lymphoproliferative disorders. These patients have antibodies to C1 inhibitor, and this gives rise to a syndrome that closely mimics hereditary angioedema. Angiotensin-converting enzyme (ACE) inhibitors used for the treatment of hypertension and heart failure can also precipitate angioedema in about 0.2% of patients. This drug-induced angioedema is thought to result from increased availability of bradykinin made possible by the ACE inhibitor–mediated blockade of bradykinin catabolism. Interestingly, angioedema provoked by ACE inhibitors may develop unexpectedly after prolonged drug use. It is more commonly seen in Blacks, females, patients with a history of allergies, and smokers. Other drugs implicated in the development of angioedema include nonsteroidal antiinflammatory drugs (NSAIDs) and estrogens.
Treatment of angioedema depends on the suspected mechanism. Mast cell–mediated angioedema is treated with drugs typically used to treat allergy and anaphylaxis, including epinephrine, antihistamines, and glucocorticoids. For bradykinin-mediated angioedema, the preferred treatment for an acute attack is C1 inhibitor concentrate, a kallikrein inhibitor, or a bradykinin-receptor antagonist. Fresh frozen plasma has in the past been administered to replace the deficient enzyme, but this approach should only be employed if other alternatives are not available. For ACE inhibitor–induced angioedema, treatment is to stop the offending drug and administer supportive care. Glucocorticoids have been shown to increase the expression of ACE, which may accelerate bradykinin metabolism. C1 inhibitor concentrate, kallikrein, or a bradykinin-receptor antagonist have been used with variable results. Should upper airway obstruction develop during acute attacks, tracheal intubation may be lifesaving until the edema subsides. When performing laryngoscopy, it is important to have personnel and equipment available to perform tracheostomy if needed, but tracheostomy itself may be extremely difficult or impossible in the face of massive airway edema.
Patients experiencing recurrent angioedema, whether hereditary or acquired, require prophylaxis before a stimulating procedure such as dental surgery or any surgery requiring endotracheal intubation. It is prudent to ensure the ready availability of C1 inhibitor concentrate for intravenous infusion should an acute attack occur. In the past, androgens and transexamic acid have been used to prevent attacks of angioedema, but these drugs are used less frequently now, owing to the availability of more specific and effective therapies. Incidental trauma to the oropharynx, such as that produced by suctioning, should be minimized. Regional anesthetic techniques and intramuscular injections are well tolerated.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here