Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The peripheral nervous system consists of nerve elements outside the brain and spinal cord. It contains both peripheral nerves and elements of the autonomic nervous system. Disorders of the autonomic nervous system can result in significant hemodynamic changes as well as abnormal responses to drugs that work via adrenergic receptors. Diseases affecting peripheral nerves often have perioperative implications, including choice of muscle relaxants and management of neuropathic pain.
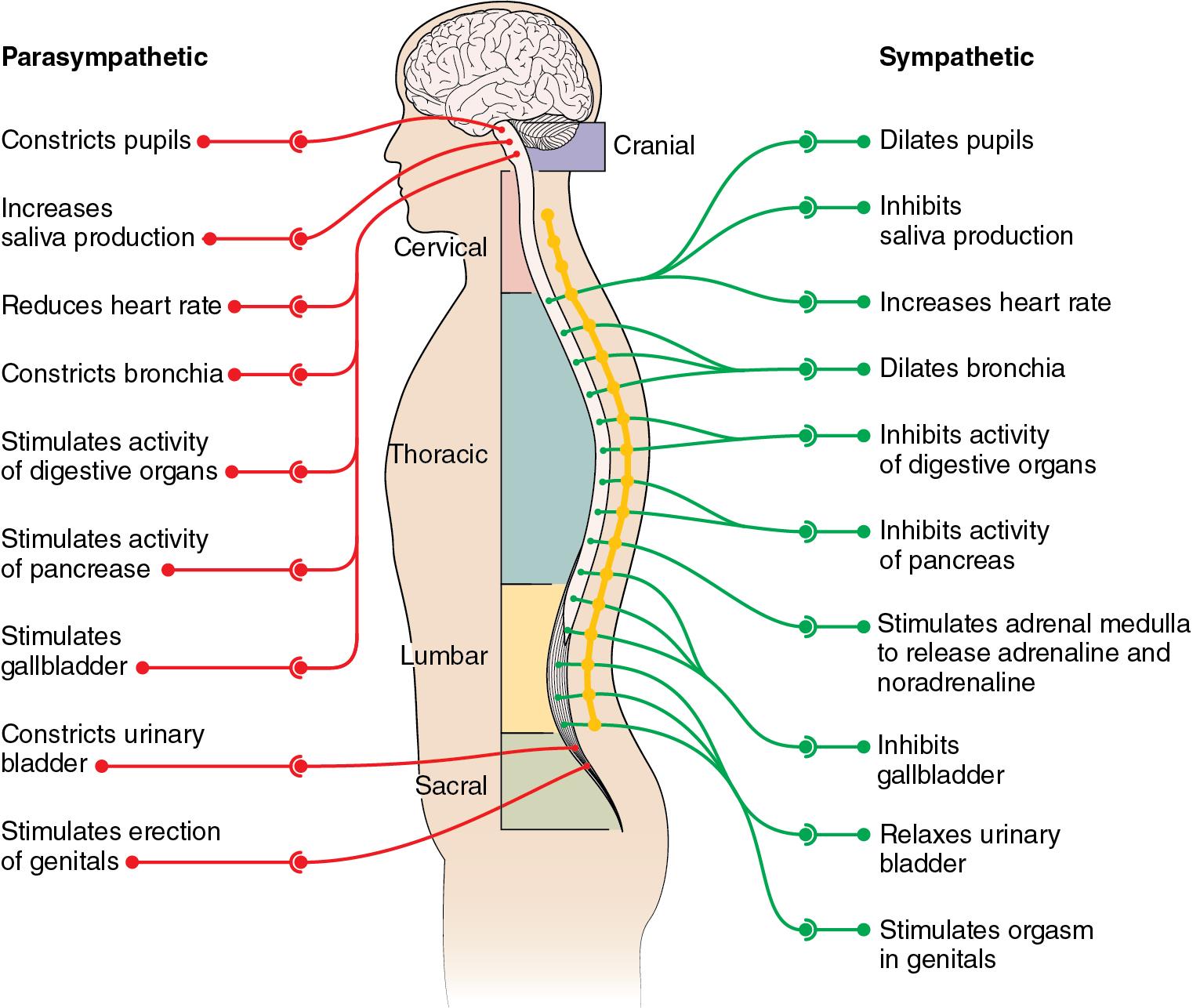
The autonomic nervous system is a part of the peripheral nervous system that acts involuntarily to regulate multiple bodily functions. The autonomic nervous system has two divisions: the sympathetic and parasympathetic nervous systems. A brief overview of the structure, end-organ innervation, and functions of the sympathetic and parasympathetic nervous systems are illustrated in Fig. 15.1 .
Multiple system atrophy involves degeneration and dysfunction of diverse central nervous system structures such as the basal ganglia, cerebellar cortex, locus coeruleus, pyramidal tracts, inferior olivary nuclei, vagal motor nuclei, and spinocerebellar tracts. The extent of degeneration of these structures, individually or in aggregate, results in different clinical manifestations that were considered different disease states in the past. Examples include striatonigral degeneration associated with parkinsonian features, olivopontocerebellar atrophy associated with cerebellar dysfunction, and Shy-Drager syndrome associated with autonomic dysfunction. These disorders are now all categorized as multiple system atrophy.
Autonomic manifestations include orthostatic hypotension, syncope, urinary retention, sluggish pupillary reflexes, and sexual dysfunction. Baroreceptor function may also be impaired, resulting in the inability to appropriately produce tachycardia and vasoconstriction in response to systemic hypotension. Control of breathing may be compromised, especially later in the course of the disease. Nonautonomic manifestations of multisystem atrophy include ataxia and parkinsonism.
Treatment includes use of elastic stockings, consumption of a high-sodium diet to expand plasma volume, and administration of vasoconstricting α 1 -adrenergic agonists or α 2 -adrenergic antagonists. If symptoms of parkinsonism are present, they can be treated with drugs such as levodopa and anticholinergics. Patients with multisystem atrophy have an ominous prognosis, with death generally occurring within 8 years of diagnosis, usually due to cerebral ischemia from prolonged hypotension.
The keys to management of autonomic dysfunction include continuous monitoring of systemic blood pressure and prompt correction of hypotension. Fluids and vasopressors can be used to treat hypotension. If vasopressors are needed, a direct-acting vasopressor such as phenylephrine is preferred. Small doses of phenylephrine should be used initially until the response can be assessed, because upregulated expression of α-adrenergic receptors in the setting of chronic relative autonomic denervation can produce an exaggerated hypertensive response. Spinal or epidural anesthesia can be considered, although the risk of hypotension demands diligence and caution. Impaired baroreceptor function can impair physiologic responses to anesthetic-induced vasodilation, potentiating hypotension. Intravenous ketamine is associated with less hypotension. Administration of a muscle relaxant that has little or no effect on hemodynamics, such as vecuronium or cisatracurium, is preferred. Signs of light anesthesia may be less apparent in these patients because the sympathetic nervous system is less responsive to noxious stimulation. Any antiparkinson medications should be continued in the perioperative period.
Postural orthostatic tachycardia syndrome (POTS) is a chronic idiopathic disorder of the autonomic nervous system characterized by episodic and position-related hypotension. POTS is most often observed in young women. Symptoms include palpitations, tremulousness, lightheadedness, fatigue, and syncope. The pathophysiology is unclear, although possible explanations include enhanced sensitivity of β 1 -adrenergic receptors, hypovolemia, excessive venous pooling during standing, primary dysautonomia, and lower extremity sympathetic denervation. Treatment includes increasing intravascular fluid volume and use of α 1 -adrenergic agonists, such as midodrine.
Management of anesthesia for patients with POTS includes preoperative administration of fluids to expand intravascular fluid volume. Low-dose phenylephrine infusions may be cautiously administered, with recognition that lower extremity sympathetic nervous system denervation may cause upregulation of α 1 -adrenergic receptors and contribute to receptor hypersensitivity. β-adrenergic blocking drugs may be used to blunt tachycardia if needed, but care must be taken to avoid excessive hypotension.
Paragangliomas are neuroendocrine tumors that arise from neural crest cells. Distinct terminology based on tumor location, such as carotid body tumor and glomus jugulare, are currently falling out of favor. These tumors have a somewhat similar origin to pheochromocytoma except that paragangliomas exist in extraadrenal locations. They can develop within neuroendocrine tissues surrounding the aorta or within the lung, as well as in the head and neck. Paragangliomas rarely secrete vasoactive substances, but when they do, norepinephrine secretion is the most common one (mimicking a pheochromocytoma). Paragangliomas typically lack the enzyme that converts norepinephrine to epinephrine, thus epinephrine secretion is even less common than norepinephrine secretion. Other hormones that can be produced include cholecystokinin, thought to play a role in the high incidence of postoperative ileus following tumor resection; serotonin or kallikrein, which can cause carcinoid-like symptoms; and histamine or bradykinin, causing bronchoconstriction and hypotension. Small tumors are most often treated with radiation or embolization. Surgery may be required for large or invasive tumors.
Preoperative determination of urinary catecholamine metabolites and serum 5-hydroxyindoleacetic acid (5-HIAA) may be useful to determine if the tumor is likely to secrete catecholamines or serotonin, respectively. Phenoxybenzamine or prazosin may be administered to patients with elevated urinary catecholamine metabolites, and octreotide should be given preoperatively to patients with elevated 5-HIAA concentration in serum.
Invasive arterial monitoring should be considered, especially in patients with vasoactive substance-secreting tumors. Given the risk of pheochromocytoma-like and carcinoid-like signs occurring intraoperatively, drugs used to treat both hypertension (e.g., rapidly titratable antihypertensive agents such as clevidipine, sodium nitroprusside, nicardipine) and carcinoid crisis (e.g., octreotide) should be immediately available.
With paragangliomas in the head and neck, cranial nerve deficits (vagus, glossopharyngeal, hypoglossal) may be present preoperatively or may occur as a result of tumor resection. Unilateral vocal cord paralysis is a risk after cranial nerve injury. In adults, this does not usually result in complete airway obstruction by itself but could produce airway obstruction in combination with airway edema or laryngeal distortion. Other complications can include impaired gastric emptying as a consequence of vagal nerve dysfunction and subsequent pulmonary aspiration.
Venous air embolism is a risk in head and neck surgery, especially if the internal jugular vein is opened to remove tumor. Appropriate monitoring to detect venous air is indicated when embolism is considered a risk. Sudden, unexplained cardiovascular collapse and death during resection of these tumors may reflect the presence of a venous air or tumor embolism.
If the surgeon finds it necessary to identify cranial nerves, skeletal muscle paralysis should be avoided to allow for monitoring of nerve integrity during surgery.
Carotid sinus hypersensitivity, exaggerated activity of baroreceptors in response to mechanical stimulation, is an uncommon entity. Stimulation of the carotid sinus by external massage, which in normal individuals produces modest decreases in heart rate and systemic blood pressure, can produce syncope in those with carotid sinus hyperactivity. Affected individuals have an increased incidence of peripheral vascular disease. Carotid sinus hypersensitivity is a recognized (although transient) complication following carotid endarterectomy.
Two distinct cardiovascular responses may be noted in the presence of carotid sinus hypersensitivity. In approximately 80% of affected individuals, a cardioinhibitory reflex, mediated by the vagus nerve, produces profound bradycardia. In approximately 10% of affected individuals, a vasodepressor reflex, mediated by inhibition of vasomotor tone, produces decreases in systemic vascular resistance and profound hypotension. The remaining 10% exhibit components of both reflexes.
Carotid sinus hypersensitivity may be treated with medications, implantation of a cardiac pacemaker, or ablation of the carotid sinus.
Anesthetic management in patients with carotid sinus hypersensitivity is often complicated by hypotension, bradycardia, and cardiac arrhythmias. Continuous invasive monitoring of arterial blood pressure can be valuable. Drugs to treat hypotension and bradycardia should be available. Further, external cardiac pacing can be useful to treat bradycardia that is unresponsive to pharmacologic therapy.
Hyperhidrosis is a rare disorder where individuals produce an excessive amount of sweat. The disorder can be either idiopathic or secondary to other conditions such as hyperthyroidism, pheochromocytoma, hypothalamic disorders (including that following central nervous system trauma), spinal cord injury, parkinsonism, or menopause. The disorder results from overactivity of sudomotor nerve fibers innervating eccrine sweat glands. Conservative treatments include topical astringents or antiperspirants. Although these sudomotor nerve fibers belong to the sympathetic nervous system, the primary neurotransmitter in sweat glands is acetylcholine. Thus patients may respond to anticholinergic drugs or to botulinum toxin injections. Severe cases may require surgical sympathectomy.
The sympathetic chain is most commonly accessed in the thoracic cavity via video-assisted thoracoscopy. One-lung ventilation will be required and is facilitated by placement of a double-lumen endotracheal tube. Successful sympathectomy will produce vasodilation in the ipsilateral upper extremity. This can be documented by an increase in ipsilateral hand skin temperature by more than 1°C or increase in cutaneous blood flow measured by laser Doppler flowmetry. In otherwise healthy patients, this surgery can be performed as an outpatient procedure. Patients often have minimal pain postoperatively, which responds well to opioids and nonsteroidal antiinflammatory drugs (NSAIDs). Common surgical complications include infection, Horner syndrome (resulting from injury to the stellate ganglion during the ablative procedure), and a compensatory hyperhidrosis elsewhere (e.g., trunk or lower extremity).
Paroxysmal sympathetic hyperactivity (PSH) is a phenomenon that can occur in up to 33% of patients with traumatic brain injuries and can persist for weeks or months after injury. This syndrome has also been referred to as autonomic storm, sympathetic storm, and dysautonomia. Symptoms include tachycardia, hyperthermia, hypertension, tachypnea, diaphoresis, hypertonia, and posturing. The pathophysiology of this syndrome remains unclear but may be attributed to disconnection of cortical inhibitory networks that control efferent sympathetic pathways leading to sympathetic disinhibition ( Fig. 15.2 ).
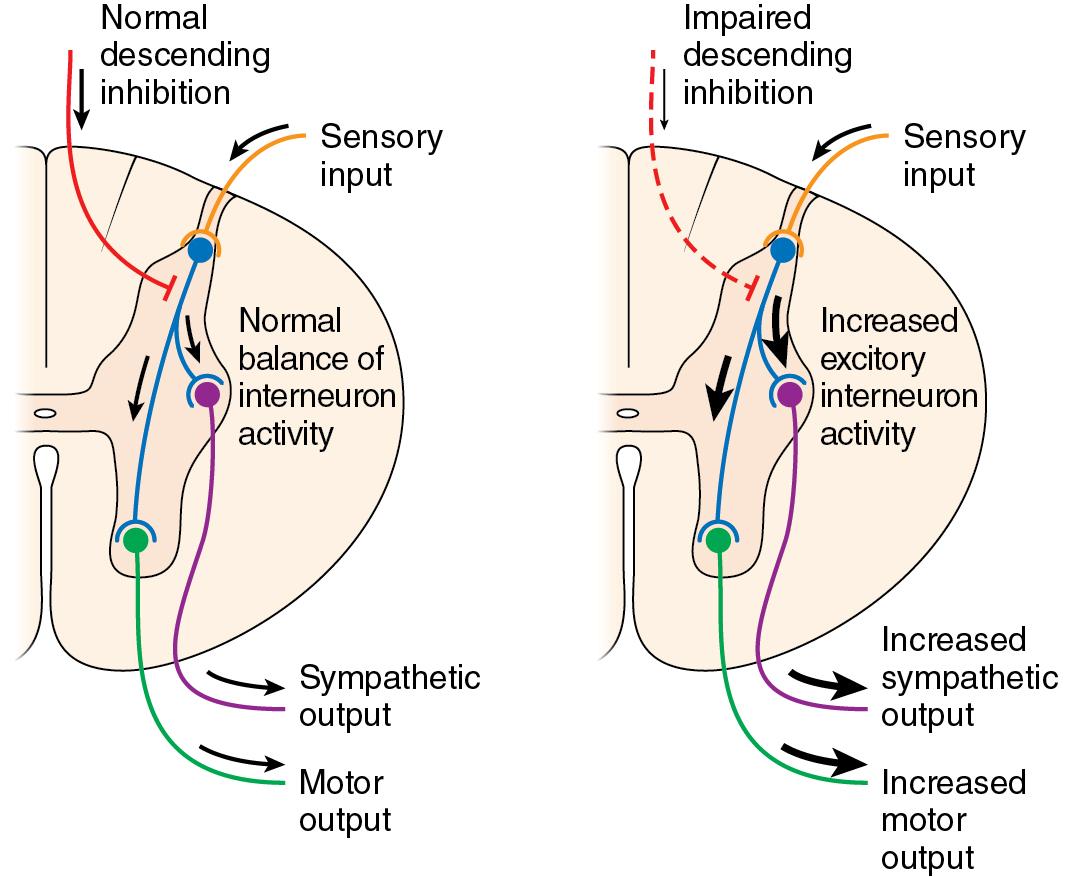
The main therapeutic goals of treating patients with PSH are to avoid triggers that provoke the syndrome, alleviate the sympathetic outflow, and provide supportive therapy. A major portion of patients experience paroxysms in response to stimuli such as pain, movement, or urinary retention. Thus it is important to avoid these triggers when they can be identified.
Opioids are most frequently used to mitigate pain responses. Other sedative agents such as benzodiazepines have anxiolytic effects and have also been used in treating PSH symptoms. β-adrenergic antagonists are commonly used to suppress central and peripheral adrenergic outflow. Propranolol is often chosen due to its lipophilicity and its ability to cross the blood-brain barrier and act centrally. α 2 -adrenergic drugs such as clonidine or dexmedetomidine can also be used in these patients to suppress adrenergic outflow. Dopaminergic agonists, such as bromocriptine, can decrease temperature and sweating in patients with PSH. Finally, dantrolene has been used to diminish fever caused by prolonged muscle contractions.
Another aspect of treatment of patients with PSH is supportive therapy to ensure that long-term sequela of the syndrome is avoided. Physiotherapy, nutritional management, and temperature control are all important things to consider.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here