Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
An understanding of the anatomy, physiology, and alternative treatment strategies for congenital heart defects is essential to provide the appropriate patient care. This includes an understanding of the guideline recommendations and the natural history of congenital heart defects. This knowledge is required to determine if there is an indication for treatment of these conditions. Pediatric and adult congenital heart patients offer distinct challenges, such as unusual cardiovascular anatomy and deranged or surgically corrected physiology, and for older patients there are demands such as pregnancy and exercise tolerability not found in traditional patients.
Diagnostic cardiac catheterization for children with heart disease and adults with congenital heart disease is the practice of percutaneous catheter-based techniques to assess cardiovascular hemodynamics and anatomy. Real-time fluoroscopy with contrast injection coupled with rapid digital angiography provides high-resolution images of the heart that are necessary for successful management decisions. In addition, the direct measurement of pressures within the cardiovascular system enhances the treatment of heart disease for this patient population by streamlining medical treatment options and defining surgical and interventional indications.
This chapter reviews current indications and best practice guidelines by proceeding through the diagnostic cardiac catheterization procedure from beginning to end in the order of anatomic structures evaluated.
In 1628 the court physician to James I and Charles I of England, Dr. William Harvey, presented the “movement of the heart and blood” in an experiment on a deer. He used his experiments and deductive logic to conclude that blood was pumped by the heart through systemic and pulmonary circulations. These experiments confirmed unidirectional blood flow in the blood vessels. In 1929 Werner Forssmann of Germany demonstrated on himself the ability to move a urethral catheter into his basilic vein in his left arm to his heart. He then walked to radiology and made an x-ray to prove the catheter course. After this report he was fired by his chief of surgery who said “for a real surgeon, there is only one thing, to operate, to operate, to operate.” Ultimately, he was awarded the Nobel Prize in 1956.
Over the next 6 decades, the field of structural and congenital cardiac catheterization evolved into primarily an interventional discipline. Accordingly, diagnostic cardiac catheterization evolved to mostly the preintervention evaluation and assessment. Meanwhile, the pure diagnostic cardiac catheterization regressed to a limited and select patient group. Currently the diagnostic cardiac catheterization and percutaneous interventional procedures are not mutually exclusive. It is currently recommended that percutaneous interventions are preceded by a diagnostic evaluation that most often determines the indications for that intervention (class I; level of evidence: A). Consequently, assessment of patient hemodynamics and, when necessary, assessment of anatomy by angiography should be accomplished before the interventional cardiac catheterization to confirm congenital or acquired heart disease in infants and children, as well as adults with congenital heart defects.
On the other hand, most pure diagnostic cardiac catheterizations will often include preprocedure discussions regarding potential interventions if indications for an interventional procedure are met during the diagnostic evaluation. Consequently, both the procedural preapproval process and the informed consent process for most diagnostic cardiac catheterizations include consent for any possible intervention. This is essential in the management of children but even more so for older children and adults where assent and consent from the patient is mandatory prior to sedatives. This preparation will allow congenital heart patients, who have often already undergone multiple procedures, to avoid more than one anesthetic to accomplish the clinical management deemed necessary during the diagnostic cardiac catheterization evaluation.
The Society of Cardiac Angiography and Interventions recognizes the importance of advanced training in pediatric and congenital interventional cardiac catheterization and recommends the development of guidelines and assessment tools for such programs. The society states that it is important for pediatric cardiologists who wish to perform cardiac catheterizations to become proficient in all aspects of cardiac catheterization through at least one additional year of advanced training. Individuals in these programs should perform procedures of gradually increasing complexity under the supervision of an attending interventional cardiologist until they become competent as the sole or primary operator. Mastery of the diagnostic component of this training will lead to successful interventions following appropriate guidelines and recommendations and lower the adverse event risk.
Diagnostic cardiac catheterization should not be considered routine for the diagnosis of congenital heart defects because the performance of complete right- and left-sided heart studies may subject patients to unnecessary risk and exposure to radiation. Complete cardiac echocardiographic imaging or alternative noninvasive imaging modalities such as cardiac magnetic resonance imaging (CMRI) or chest and cardiovascular computed tomography (CT) are indicated before invasive cardiac catheterization to facilitate planning of diagnostic data collection and performance of an intervention (class I; level of evidence: A).
Diagnostic cardiac catheterization has been impacted by advances in noninvasive imaging because it is no longer indicated in the routine preoperative evaluation of most congenital defects, such as atrial septal defect, complete or partial atrioventricular canal defect, ventricular septal defect (VSD), double-outlet right ventricle (RV), tetralogy of Fallot (TOF), TOF with pulmonary atresia, coarctation of the aorta, hypoplastic left heart syndrome, or any of the cyanotic neonatal heart defects. Details of anatomy, including situs, systemic and pulmonary connections, septal structure, severity of atrioventricular and semilunar valve function, coronary artery attachments and course, and aortic arch anatomy, are certainly established with noninvasive imaging to the degree of certainty required for surgical intervention.
In most instances, the 2011 indications for diagnostic cardiac catheterization were developed empirically, with no prospective trials, controlled or otherwise, that would validate superiority of cardiac catheterization over noninvasive diagnostic modalities. Therefore most indications are supported by patient series, retrospective case reviews, or the opinion of authorities (level of evidence: C). Most important, diagnostic cardiac catheterization should be considered only when the patient's congenital heart anatomy and physiology are inadequately defined by modern noninvasive methods such as two-dimensional (2D) or 3D echocardiography, cardiovascular CT, or CMRI.
It is recommended that hemodynamic and anatomic data be obtained (via angiography when necessary) at the time of a planned interventional cardiac catheterization procedure (level of evidence: A).
It is recommended that cardiac catheterization be used to assess pulmonary resistance and reversibility of pulmonary hypertension in patients with congenital heart disease or primary pulmonary hypertension when accurate assessment of pulmonary resistance is needed to make surgical and medical decisions (level of evidence: B).
Cardiac catheterization is indicated in patients with complex pulmonary atresia for the detailed characterization of lung segmental pulmonary vascular supply, especially when noninvasive imaging methods incompletely define pulmonary artery anatomy (level of evidence: B).
Cardiac catheterization is indicated in determination of coronary circulation in pulmonary atresia with intact septum (level of evidence: B).
Cardiac catheterization is indicated in patients being assessed for cardiac transplantation unless the patient's risk for catheterization outweighs the potential benefit (level of evidence: C).
Cardiac catheterization is recommended for surveillance of graft vasculopathy after cardiac transplantation (level of evidence: B).
It is reasonable to perform a cardiac catheterization to determine pulmonary pressure/resistance and transpulmonary gradient in palliated single ventricle patients before a staged Fontan operation (level of evidence: B).
Cardiac catheterization is reasonable in any congenital heart disease patient in whom complete diagnosis cannot be obtained by noninvasive testing or in whom such testing yields incomplete information (level of evidence: C).
Cardiac catheterization is reasonable for the assessment of cardiomyopathy or myocarditis with consideration for obtaining an endomyocardial biopsy (level of evidence: B).
Cardiac catheterization is reasonable for the assessment of coronary circulation in some cases of Kawasaki disease in which coronary involvement is suspected or requires further delineation or in the assessment of suspected congenital coronary artery anomalies (level of evidence: B).
Cardiac catheterization is reasonable to perform for the assessment of anatomy and hemodynamics in postoperative cardiac patients when the early postoperative course is unexpectedly complicated and noninvasive imaging techniques fail to yield a clear explanation (level of evidence: C).
Diagnostic cardiac catheterization is indicated:
Planned interventional cardiac catheterization
Pulmonary resistance and reversibility of pulmonary hypertension
Patients being assessed for cardiac transplantation
Congenital anatomy or coronary anomalies when noninvasive imaging is incomplete
Surveillance of graft vasculopathy after cardiac transplantation
Diagnostic cardiac catheterization is reasonable:
Palliated single ventricle patients before a staged Fontan operation
Assessment of cardiomyopathy or myocarditis
Kawasaki disease for initial anatomy and if suspicion of coronary stenosis
Assessment of anatomy and hemodynamics in postoperative cardiac patients when the early postoperative course is unexpectedly complicated and noninvasive imaging techniques fail to yield a clear explanation
The diagnostic cardiac catheterization provides accurate physiologic and anatomic information to the care of congenital heart patients but is not without risk. The following are potential adverse events associated with a diagnostic cardiac catheterization:
Risk of general anesthesia or sedation-related changes to the cardiovascular physiology.
Allergic reactions to medications, anesthetics, or contrast agent.
Latex allergy occurs uncommonly in the modern “latex-free” catheterization laboratories. If an allergic reaction occurs, there is a wide range of symptomatology, including urticarial to anaphylaxis; thus protocols are used for high-risk patients or patients with previous allergic history. There are no reports of this occurring with the use of catheters.
Hypothermia, especially in the neonates and infants.
Aggravation of hypoxia or hypoxemia secondary to medications and sedation.
Arrhythmias—atrial flutter, atrial fibrillation, ventricular arrhythmias, and heart block.
Peripheral vascular injury, cardiac perforation, and cardiac valve injury.
Blood loss that requires transfusion.
Renal insufficiency and injury secondary to contrast agent administration.
Diffuse central nervous system injury, embolic events, stroke, and mortality.
Exposure to ionized radiation, especially for patients requiring multiple procedures.
Most congenital catheterization labs in the United States have voluntary reporting of their procedural and safety data into the IMPACT (Improving Pediatric and Adult Congenital Treatment) database. This collects data about the patient, procedure type, outcome, and complications. The data are submitted quarterly to the National Cardiovascular Data Registry/American College of Cardiology and are compared with the other centers nationally. This is published in a way such that the center information is deidentified and that only each individual center can see how they rank among the other programs nationally.
The IMPACT database is the largest voluntary reporting database, but there are other systems such as the CCISC, C3PO, and MAGIC databases.
Aside from national reporting, each individual center offering pediatric and congenital catheterization should report any complications to the hospital monitoring board and division M&M (Mortality and Morbidity) conference. These should be vetted and assessed and severe/catastrophic complications fully evaluated, discussed, and necessary changes in policy/procedure made where appropriate.
For the elective diagnostic cardiac catheterization procedure, general recommendations include no solid food for 8 hours, milk or formula for up to 6 hours, breast milk up to 4 hours, or clear liquids up to 2 hours before initiation of sedation. Premedication is usually administered orally, although for older patients or those requiring preprocedural hydration, intravenous (IV) placement is performed prior to coming into the lab. For younger patients or those with higher level of anxiety, mask induction is often performed in the lab and the IV placed after sedation. Local anesthesia is used often at the percutaneous access site(s), including lidocaine infiltration (maximum 4.5 mg/kg; avoid intravascular injection, and lidocaine buffered with sodium bicarbonate diminishes the discomfort of lidocaine infiltration) and topical anesthetics such as lidocaine 2.5% or prilocaine 2.5%.
Effective sedation and analgesia are maintained using agents with consideration for the individual patient's cardiovascular condition. Often patients with ventricular dysfunction need to avoid cardiac depressant agents such as propofol, and at times agents that maintain or increase systemic vascular resistance, such as ketamine, are useful.
Vascular access is most commonly obtained in the femoral system using the Seldinger technique and ultrasound guidance. The right heart catheterization can be performed through the inferior vena cava (IVC) and the left heart catheterization through either antegrade also from the IVC through an existing congenital opening or retrograde from the femoral artery. The internal jugular vein is the most common access site when bilateral femoral veins are obstructed, there is Glenn physiology, or the IVC is interrupted. The internal jugular approach is either a high or standard entry or a low approach superior-inferior to the clavicular grove lateral from the supraclavicular notch. The low approach is mainly reserved for mid-internal jugular occlusion such as with patients with a history of vascular extracorporeal membrane oxygenation support via the neck. The subclavian approach is often used when there is internal jugular occlusion or as a primary access site if the postprocedure plan is to place a subclavian Broviac for long-term management. Other access options include umbilical artery and vein in neonates, axillary or carotid artery when femoral approach is nonconductive, and the transhepatic approach described in the mid-1990s.
For the transhepatic approach, a long 21- or 22-gauge needle with an obturator is most commonly used with a puncture along the mid to anterior axillary line. The needle can be guided by fluoroscopy and/or ultrasound toward the IVC and right atrial junction in a posteriorly, superiorly, and medially. The obturator is removed, and diluted (10% to 15%) contrast is injected during withdrawal until the hepatic vein is identified. Subsequently, the wire is advanced and the sheath placed is determined. Post procedure the track can be closed with a coil, plug, or device of preference, or the track can be rewired for placement of a transhepatic Broviac.
The cardiologist needs to have an understanding of and familiarity with the different catheters and wires available in the cardiac catheterization laboratory. Catheters are hollow, thus allowing blood sampling, transmission of pressure, and injection of contrast. These catheters can be classified as end-hole, side-hole, or both. Angiographic injections are primarily performed using catheters with a side-hole(s) to prevent vascular injury during the injection. Catheters may be straight, angled, or shaped either out of the package or by the physician using a wire or tip deflector apparatus.
This is typically performed using either an end-hole balloon-tipped catheter or end-hole deflectable catheter. This end-hole catheter is used for hemodynamic pressure measurement and blood gas sampling. If a balloon-tipped catheter is used, a small volume of CO 2 is placed in the balloon. The end-hole catheter is then moved through the vasculature and heart using a small amount of manual torque to advance the catheter with the flow of venous blood into right atrium (RA), across the tricuspid valve into the RV, and from the RV into the pulmonary arteries. The end-hole catheter may be directly advanced into a distal branch pulmonary artery to obtain a wedge pressure, or with the balloon-tipped end-hole catheter positioned in a distal branch pulmonary artery, gentle inflation of the balloon will allow for measurement of pulmonary artery wedge pressure. Angiography of the right heart is usually performed using a side-hole catheter or balloon-tipped angiographic catheter, which has side holes proximal to the balloon. Additional side-hole catheters used for angiography of the right heart can include but are not limited to pigtail catheters and straight flush–type catheters. Monorail catheters like the Multi-Trac can be used to obtain hemodynamics and perform angiography in situations where it is ideal to maintain wire position.
This is typically performed using smaller-caliber, thin-walled, but more rigid, catheter such as a pigtail catheter. A pigtail catheter is advanced to the descending aorta over a wire. With the wire removed, the catheter end curls, allowing it to be advanced and withdrawn in the aorta without engaging smaller branch arteries. To advance the pigtail catheter into the left ventricle (LV), a soft, typically J-tipped, wire is used to cross the aortic valve, which prevents leaflet damage. Pressure measurements, blood sampling, and angiography can all be performed using the pigtail catheter. In situations where there is a gradient across a left heart structure, an end-hole catheter is useful in helping to determine a more precise location of the gradient or area of obstruction, whereas the pigtail catheter (or side-hole catheter) measures the pressure across a longer segment, thus potentially giving a falsely high or low measurement.
There are multiple different options regarding wires that can be used to direct or stabilize catheters. Like catheters, wires come in many different diameters and lengths. Most wires have a soft distal end, which comes in various contours, including straight, J-tipped, and angled. Wires advanced through hollow catheters are used to probe and enter vessels that may be otherwise difficult to access with the catheter alone, such as stenotic branch pulmonary arteries or tortuous collateral vessels. Wires can also be used to cross a patent foramen ovale (PFO) for access to the left atrium (LA), pulmonary veins, and other left heart structures. Stiff wires are used to stabilize catheters for angioplasty and valvuloplasty. Stiff, extra-long wires are valuable for maintaining position while exchanging one catheter for another.
Many other catheters and wires of various sizes, lengths, and contours are available for use depending on the specific clinical scenario and need, but an extensive review and discussion is beyond the scope of this chapter.
A detailed discussion of catheter manipulation is also beyond the scope of this chapter, but several key points deserve mention. In the neonate and infant, cardiac tissue is thin, thus perforation can occur easily, especially in the atrial appendages, right ventricular outflow tract (RVOT), left ventricular apex, and aortic valve cusps. The risk of perforation can be decreased by gentle catheter manipulation, using small careful movements, the use of balloon-tipped or soft catheters and floppy-tipped wires, as well as a thorough understanding of the cardiac anatomy and the desired catheter route and destination. The importance of reviewing all previous imaging studies before the catheterization cannot be overemphasized. If a catheter is too straight to manipulate to the desired location (e.g., across the tricuspid valve), it may be safely curved outside the body, in a hepatic vein, or using a tip-deflecting or shaped wire, rather than within the heart. The small catheters used in infants and children can be damaged by vigorous manipulation. Thin-walled catheters, such as pigtails, should be advanced over a wire. Large catheter loops in the atrium or RVOT can cause hemodynamic instability owing to reflex bradycardia, heart block, or tricuspid valve insufficiency; therefore one needs to pay attention to all parts of the catheter, not just the tip. It is also important to note that it is possible to tie an overhand knot with a catheter that has a large loop, thus reinforcing the importance of careful monitoring of all parts of the catheter.
To calculate pulmonary and systemic shunt volume and resistance, the initial measurement during a diagnostic cardiac catheterization is cardiac output ( Box 17.1 ). This cannot be measured directly; thus it is estimated by using an indicator dilution technique described by Fick. Oxygen, a common indicator used to calculate cardiac output, is carried in the blood stream attached to hemoglobin (Hgb) and dissolved in plasma. As the patient breathes room air, the vast majority of the oxygen in the blood is bound to Hgb. The amount of dissolved oxygen in the plasma (CdO 2 ) is directly proportional to the partial pressure of oxygen (PaO 2 ) know as Henry's law. The CdO 2 can be calculated using the solubility coefficient of oxygen at body temperature, and the PaO 2 . At 37°C (the normal body temperature), the amount of oxygen dissolved in blood is 0.03 mL/mm Hg per liter or 0.003 mL/mm Hg per deciliter. Thus in blood with a PaO 2 of 100 mm Hg, there is 3 mL of dissolved oxygen per liter of blood and 0.3 mL of dissolved oxygen per deciliter of blood. This amount of oxygen is trivial compared with the oxygen bound to Hgb; thus it is usually ignored in hemodynamic calculations. However, if the patient is on supplemental oxygen, and the PaO 2 is greater than 100 mm Hg, then the dissolved oxygen is considered for an accurate calculation. To calculate the oxygen capacity that is the amount of oxygen bound to Hgb in the blood, we multiply (Hgb in g/dL) × (1.36 mL O 2 /g of Hgb) × (10 dL/L) = the mL O 2 /L of blood.
Body surface area (BSA) of patient
Mosteller formula:
Oxygen consumption (VO 2 ) = BSA × VO 2 (from nomogram) = mL/min
Oxygen (O 2 ) capacity (mL/dL) = Hgb (g/dL) × 1.36
Dissolved oxygen (mL/dL) = Pa o 2 × 0.003 mL/torr/dL
Mixed venous O 2 saturation (MV):
Pulmonary artery saturation, pulmonary vein saturation, and systemic saturation
Oxygen content = (% saturation × oxygen capacity) + dissolved O 2
Systemic flow (Qs) = L/min (Fick equation):
Pulmonary flow (Qp):
Qeff = effective blood flow
Systemic vascular resistance indexed (units × m 2 )
Normal values: average = 15 units × m 2 and upper limits = 30 units × m 2
Pulmonary vascular resistance indexed (units × m 2 )
Normal values: average = 2.8 units × m 2 and upper limits = 5 units × m 2
Cannot be calculated if the systemic arterial saturation is >94%
Left to right shunt = Qp – Qs and right to left shunt = Qs – Qp
Q L−R = Qp – Qeff & Q R−L = Qs – Qeff
Oxygen consumption (VO 2 )—This is the oxygen taken up and used by the body (per/min). For hemodynamic calculations, such as cardiac output, oxygen consumption is often assumed, or calculated on a generic chart, which accounts for age, gender, and heart rate. Direct calculation obtained by exercise testing is rarely available prior to cardiac catheterization procedures. In addition, calculations for children younger than 3 years and adults older than 30 years of age are not available on the standard calculation charts. It is important to recognize that these assumptions in oxygen consumption are an important source of error in hemodynamic calculations.
Oxygen capacity —This is the maximal amount of oxygen that can be taken up by Hgb in blood (mL O 2 /100 mL or 1 dL of blood)
The total oxygen in blood is determined and dissolved oxygen is subtracted
Most often calculated: each gram Hgb takes up between 1.33 and 1.39 mL of oxygen; therefore the calculation is most often done with either 1.34 or 1.36.
Oxygen saturation —This is the percentage of oxygen that is actually oxygen bound Hgb.
If the saturation is 100%, then the amount is the same as the oxygen capacity.
Proportion of oxygen combined with Hgb to total amount of oxygen that can be taken up (percent oxygen saturation of oxygen capacity)
Measured by light reflectance
Transmission of light is different for bound and unbound Hgb (different at 630 to 660 µm given measurable ratio)
Can be measured on as little as 0.2 mL of blood
Oxygen content —This is the total oxygen in blood including bound to Hgb + dissolved oxygen
Calculated from Hgb concentration, oxygen saturation, and dissolved oxygen
(Hgb g/dL) × (1.36 mL/g) × (10 dL/L) × (0.99) + (PaO 2 mm Hg) × (0.003 mL/mm Hg/dL)
Cardiac output —This can be measured using and indicator dilution technique described by Fick. Essentially, the calculation determines the flow or cardiac output by dividing the rate of addition or removal by the change in concentration. The indicators commonly used are either oxygen or saline cooler than body temperature (thermodilution technique). One way to think about the Fick principle is to dump baseballs, at a constant steady rate, into the Mississippi River in Memphis, Tennessee. Then wait for the baseballs in New Orleans, Louisiana and compare what you see on a day when the river is flowing swiftly (high cardiac output) versus when the river is flowing slowly (low cardiac output). When the river is flowing swiftly, a greater amount of the baseballs will flow by New Orleans because less can be removed from the river along the way. In addition, the swift river will deliver the baseballs to New Orleans in a dilute fashion (a few here and there). On the contrary, when the river is running slowly a fewer amount of baseballs will flow by New Orleans because more will have been taken out along the way. The ones that do get to New Orleans will be in a dense concentration (filling the river).
Pressure measurements
Pressures are measured using fluid-filled catheters connected to a pressure-sensitive transducer. The system is calibrated to an arbitrarily assigned zero point, the center of the patient's heart.
The change in pressure (force per unit area) in the cardiac chamber or vessel measured is transmitted along a column of incompressible fluid (saline or blood) contained within a nonexpansible tube (catheter) to a transducer. The transducer contains a diaphragm that moves a small distance, in a linear fashion, in response to the change in the patient's pressure. This movement of the diaphragm is transmitted to an electronic strain gauge that converts the pressure into voltage changes. The voltage changes are converted to an electric signal, which is amplified and recorded.
Inaccurate pressure waveforms frequently are related to deterioration of frequency response or to overdampening or underdampening. Troubleshooting includes looking for loose connections, air in the system, inaccurate calibration or baseline drift, catheter motion, obstruction, end-hole artifact, or entrapment, and pulse wave amplification.
Valve areas
The pressure gradient across a valve is a component of both the flow across the valve and the effective orifice of the valve. At normal flow rates the valve presents minimal flow resistance. At increased flow rates (e.g., anemia, pregnancy, and fever) a small pressure gradient may develop across a normal valve (e.g., left-to-right shunting atrial septal defect and tricuspid or pulmonary flow resistance; left-to-right shunting VSD or patent ductus arteriosus mitral or aortic flow resistance).
The Gorlin formula states that the aortic valve area (cm 2 ) is equal to the flow through the aortic valve during ventricular systole divided by the square root of the mean systolic pressure gradient across the valve times a constant, 44.5.
A valve area of less than 0.8 cm 2 is considered to be severe aortic valve stenosis.
Resistance calculations
Often, assessment of pulmonary vascular resistance is necessary to determine operability or medical therapy options for pediatric heart patients and adults with congenital heart disease. It is crucial that these data are collected carefully with knowledge of the limitations and potential sources of error. Vascular resistance is the pressure drop across the vasculature divided by the cardiac output or flow across the same vasculature (see Box 17.1 ). These calculations are based on the laws of Poiseuille and Ohm, which is used for electrical resistance and does not perfectly apply to the cardiovascular system. Important differences include: (1) the movement of blood is pulsatile, and blood itself is not laminar or homogeneous, (2) the cardiovascular system is distensible rather than rigid, and (3) the vascular resistance is dynamic and changing rather than fixed. This latter source of error is best managed by maintaining the patient is a steady consistent state during the hemodynamic evaluation.
When calculating the pulmonary vascular resistance in patients with diminished pulmonary blood flow, a small 2 mm Hg difference in transpulmonary gradient can change the pulmonary vascular resistance calculation significantly. Consequently, accurate measurement of flow and pressures is critically important.
Cardiovascular angiography through an invasive procedure such as a diagnostic cardiac catheterization is now a secondary role due to highly developed noninvasive imaging techniques. The equipment for fluoroscopy and angiography should be of the highest quality and should be capable of producing high-resolution images. The fluoroscopy and angiography cameras must be regularly serviced, maintained, and regularly replaced or upgraded to maintain the high quality of imaging required in pediatric and adult congenital cardiology. Full use of reduced dose rates should be used whenever possible during diagnostic evaluations.
Rules relating to the measurement of radiation dosage, the reduction of radiation, and maximum radiation protection for the patients, as well as the staff in the catheterization lab, must be strictly enforced. These include protection of the pelvic areas of the patients and protection of the thyroid gland and the eyes of the operators. Fluoroscopy times and radiation doses for the catheterization laboratory personnel must be measured, recorded, and audited regularly.
The goal for angiography should be determined based on preprocedure noninvasive data and hemodynamic findings. Contrast agents affect osmolality, sodium content, and serum calcium and thus change the hemodynamics; these are why angiography is routinely performed after baseline hemodynamics are completed. Ionic media are not recommended in pediatric patients, as the nonionic media is routinely used. This is well tolerated with the typical maximum amount of 4 to 6 mL/kg and adequate hydration during and after the procedure. Allergic reactions are uncommon without prior exposure, and thus known contrast allergy patients are pretreated with corticosteroids and antihistamine therapy.
Cineangiography delivers considerably more radiation to the patient; thus fluoroscopy saved images are considered when deemed adequate for image quality and assessment of anatomy. For general catheter manipulation, the fluoroscopy frame rate can be set lower, thus minimizing the total radiation dose. In addition, avoiding use of biplane unless indicated, collimation of the x-ray beam using a virtual stored image, and wedge filters should be positioned to improve image quality and decrease radiation exposure to the patient and staff. Catheters used for angiography are thin walled and have side holes for rapid delivery of contrast at high pressures without catheter recoil. The cardiovascular anatomy including vessel diameter, and ventricular function can be quantitated to obtain anatomic and function data. Accurate calibration is routinely available through automated calibration references with the patient positioned at isocenter on the table. The catheter size (French size of the catheter divided by 3 [i.e., 6 Fr catheter divided by 3 = 2 mm for calibration use]) may be used as a reference for calibration; however, calibration errors can occur if the structure is larger than the catheter itself.
When one describes imaging angles, it is common to refer to the image intensifier or flat panel detector as the “anteroposterior camera.” Technically, the camera or x-ray tube is posterior to the patient on the table and the beam goes toward the image intensifier that is anterior to the patient; thus it is actually the “posteroanterior camera.” Standard angiography projections are outlined in Table 17.1 , contrast dosage recommendations by anatomic location are outlined in Table 17.2 , and angiography angles by anatomic location are outlined in Table 17.3 .
| FRONTAL CAMERA STANDARD ANGLES FOR CONGENITAL CARDIAC CATHETERIZATION | ||
| Direct PA | 0 Degrees | |
| “Sitting up” (cranial) | 0 Degrees frontal/+20–40 degrees cranial | |
| “Laid back” (caudal) | 0 Degrees frontal/30–40 degrees caudal | |
| RAO | 20–40 Degrees RAO | |
| LATERAL CAMERA STANDARD ANGLES FOR CONGENITAL CARDIAC CATHETERIZATION | ||
| Direct lateral projection | 90 Degrees | |
| LAO | 20–60 Degrees LAO | |
| Long axial oblique | 70 Degrees lateral/30 degrees cranial | |
| Hepatoclavicular view | 45 Degrees lateral/45 degrees cranial | |
| Aortic outflow tract view | 100 Degrees lateral/30 degrees caudal | |
| Injection Site | Dose (mL/kg) | Injection Rate (mL/kg/s) |
|---|---|---|
| Normal ventricles | 1.25–1.5 | 0.5–1.0 |
| Enlarged ventricles | 1.5–2.0 | 1.0–2.0 |
| Normal flow aorta | 0.8–1.0 | 1.0–1.5 |
| Increased flow aorta | 1.5–2.0 | 1.5–3.0 |
| Pulmonary arteries | 0.75–1.0 | 1.0 |
| Pulmonary wedge | 0.5–1.0 | 0.25 mL/s |
| Pulmonary vein wedge | 0.3–0.6 | 0.5 mL/s |
| Anatomic Location | Frontal (PA)(°) | Lateral(°) | Additional Considerations |
|---|---|---|---|
| Systemic veins | 0 | 90 | SVC/Glenn anastomosis may require PA—“sitting up” |
| Right atrium | 0 | 90 | |
| RV | RAO 20–30 | 90 | PA—RAO |
| RV outflow pulmonary valve | Cranial 30–40 | 90 | PA—“sitting up”—cranial |
| Pulmonary arteries | Cranial 30–40 | 90 | PA—“sitting up”—cranial |
| Sano shunt | Steep caudal | 90 | PA—“laid back” caudal |
| Pulmonary veins | 0 | 90 | |
| Left atrium | 0 | 90 | |
| Left ventricle | RAO 25–30 | 90 | PA—RAO |
| Aortic valve | RAO 25–30 | 90 | PA—RAO |
| Ascending aorta | RAO 25–30 | 90 | PA—RAO |
| Aortic arch | LAO 40–50 | 90 | PA—“laid back” with LAO |
| Caudal 20–40 | |||
| BT shunt | Steep caudal angulation | 90 | PA—“laid back” caudal |
| PDA | RAO 40 | 90 | Ductus in lateral over the trachea |
| Caudal 30 | |||
| AP collaterals | 0 | 90 | Best view to assess total flow to lungs related to AP collaterals, and anatomy of the renal or ileofemoral vessels. |
| Renal arteries | |||
| Femoral arteries |
The standard right heart catheterization is performed from the femoral veins. Access is obtained using the Seldinger technique and/or with vascular ultrasound guidance. Femoral venous occlusion should be documented only in the chart if there is angiographic or ultrasound evidence of occlusion. Revascularization of the occluded femoral veins can be accomplished when necessary but requires clinical experience with this technique.
The IVC is accessed from the femoral veins. In general, hemodynamic data are important from the IVC to determine the mixed venous saturation and assess venous pressures in the presence of Fontan pathway anastomosis, transposition of the great vessels inferior baffle, or history of venous thrombosis. The IVC can be interrupted in the presence of heterotaxy of the polysplenia type proceeding as an azygous continuation behind the heart to the superior vena cava (SVC). During the diagnostic catheterization this is obvious when the catheter is behind rather than within the heart in the lateral projection. If diagnostic evaluation is necessary across an IVC filter, distal IVC angiography can be performed and the filter crossed with a hemodynamic catheter to perform the right heart evaluation.
Information is usually not necessary except in patients with concern for portal hypertension such as Fontan patients. Then the hepatic vein mean pressure and mean hepatic vein wedge pressure are used to calculate the transhepatic gradient. The other time the hepatic veins are important is when the patient is undergoing a pre-Fontan evaluation and the location of the hepatic vein in relation to the IVC is important in preparation for the Fontan operation. Heterotaxy patients can, in 25% of the cases, have ipsilateral hepatic veins. The hepatic veins are also important in the presence of systemic venous occlusions to perform transhepatic access for the right heart evaluation.
This access is achieved using the Seldinger technique and ultrasound guidance. It is usually used for posttransplant patients due to the shorter recovery compared with the femoral access. In addition, it is used for the pre-Fontan catheterization or anytime a patient after a Glenn anastomosis operation is undergoing a diagnostic catheterization. The right heart catheterization is easily achieved from this route, but crossing the atrial septum or entering the left innominate vein can be more difficult to achieve.
When the internal jugular veins are occluded, the right or left innominate vein can be used as an access point by using a low internal jugular venous access approach. In this approach, the needle enters superior-posterior to the clavicle and posterior to the natural grove on the clavicle, thus entering the right or left innominate vein. During the right heart catheterization, usually a mixed venous saturation is obtained from one or both innominate veins ( Fig. 17.1 ). These vessels are the most common location for venovenous collaterals to the pulmonary or hepatic veins in the presence elevated central venous pressure. This commonly occurs in palliated single ventricle or Mustard/Senning baffle repair for transposition of the great vessels.
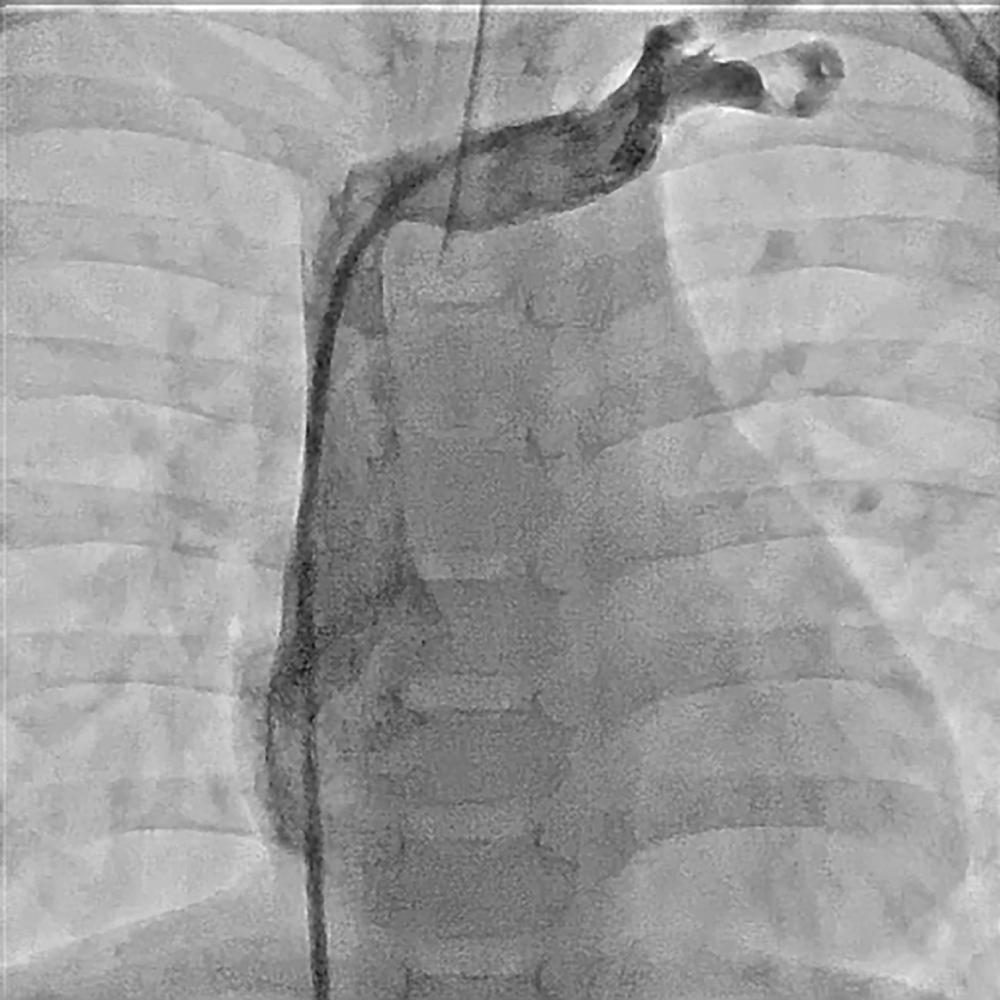
Access to the SVC from the femoral veins is achieved by moving the catheter into the mid RA, turning the catheter clockwise to move the tip toward the lateral wall, and moving the catheter gently forward against the wall, with a slight counterclockwise turn until the catheter falls into the SVC. The lateral camera is of greatest help during this maneuver. The SVC saturation is an important oxygen saturation for determination of the mixed venous saturation in the presence of intracardiac shunting. SVC stenosis can occur after surgical repair for anomalous pulmonary veins to the SVC, as well as with Mustard/Senning baffles ( Fig. 17.2 ) for transposition of the great arteries prior to the arterial switch operation.
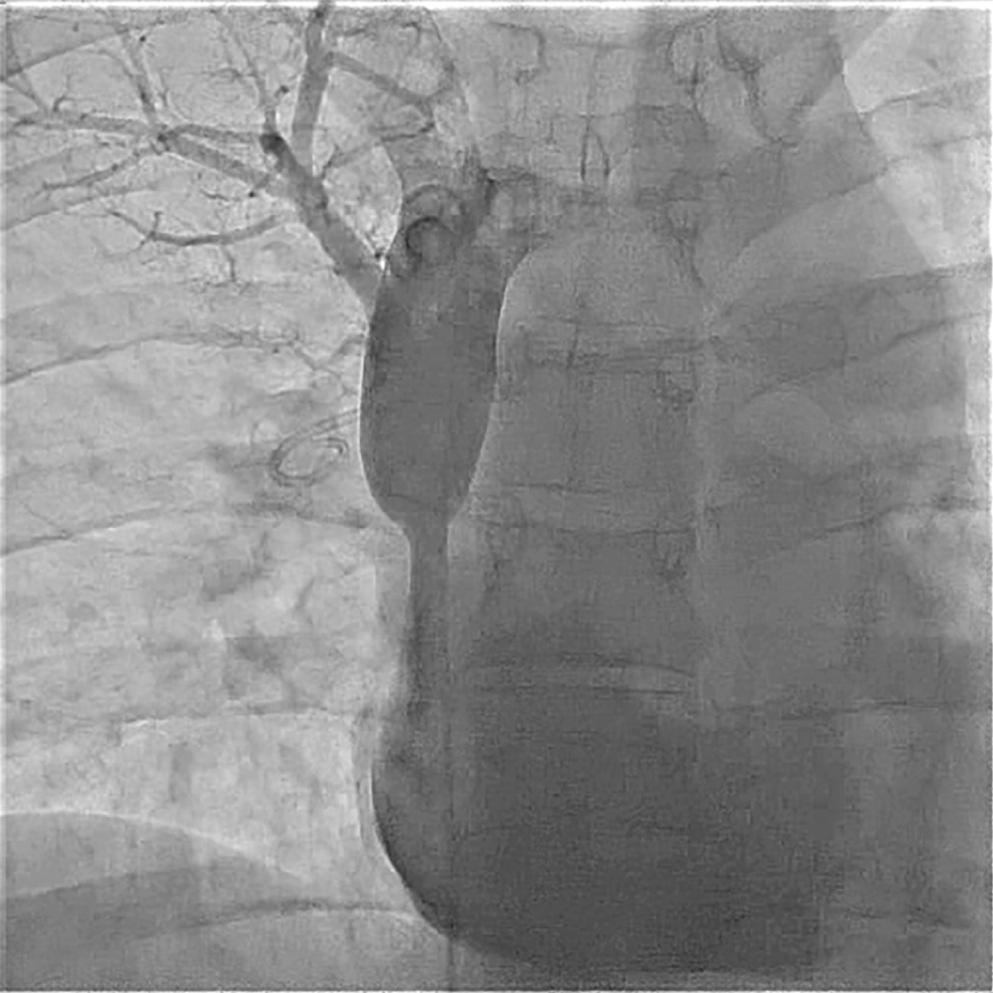
The azygous vein is a normal venous connection to the SVC that is ligated during single ventricle operations. This vessel can contain the entire lower body return other than the hepatic veins for heterotaxy polysplenia with interrupted IVC between the renal veins and hepatic veins. In addition, the azygous vein can act as a pop-off for the upper body venous return when the SVC is severely stenotic or occluded ( Fig. 17.3 ).
The most consistent value is obtained at the mid-lateral wall, and a significant increase in saturations (>7% to 9%) suggests a left-to-right shunt. Increases may occur in absence of shunt when the IVC and SVC are discrepant. In addition, a low coronary sinus saturation can cause a decrease in the saturation between the SVC/IVC and the pulmonary arteries.
A wave: atrial systole during the open tricuspid valve ( Fig. 17.4 ).
Normal: children 5 to 8 mm Hg and adults 7 to 12 mm Hg
Increased in right ventricular hypertrophy, reduced right ventricular compliance, tricuspid stenosis, atrioventricular dissociation
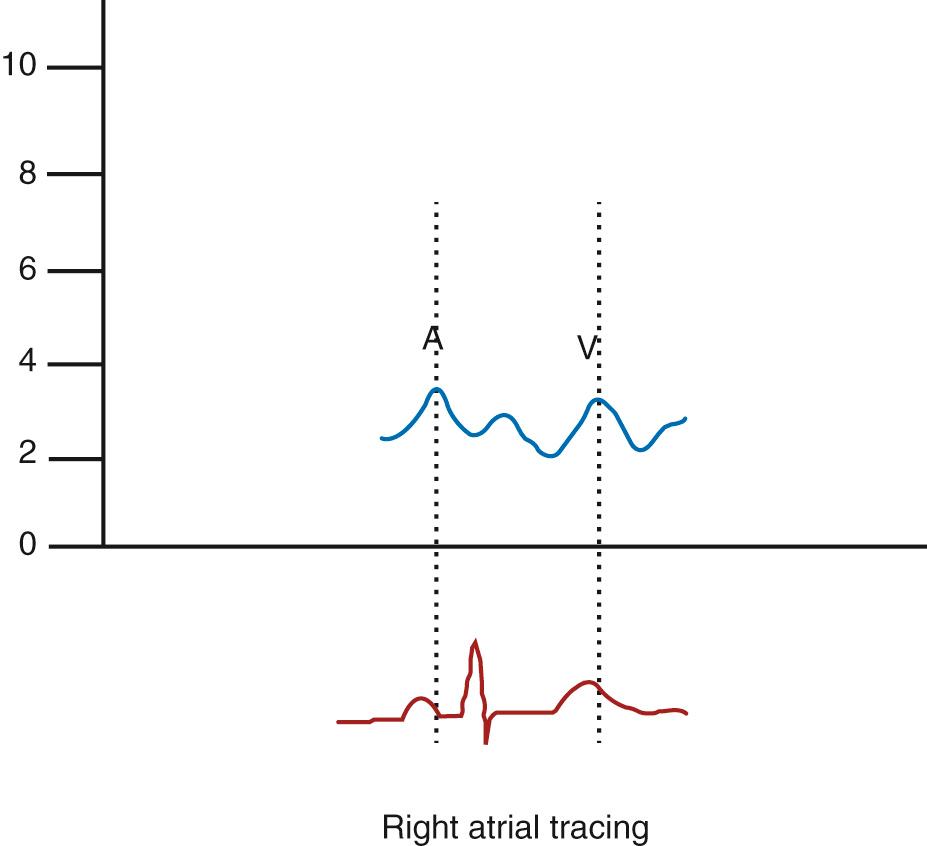
V wave: filling against closed tricuspid valve
Normal: children 3 to 6 mm Hg and adults 6 to 10 mm Hg
Increased in tricuspid regurgitation, left to right atrial septal defect
Normal mean RA pressure = 1 to 5 mm Hg
These structures can be prominent and consequently distort the catheter position within the RA.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here