Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
![]() Please access Videos to view the corresponding videos for this chapter.
Please access Videos to view the corresponding videos for this chapter.
A patulous or abnormally patent (“stuck open”) eustachian tube (ET) causes an amplified perception of one’s own voice and breathing (termed autophony of voice and breathing) that can range from minimal to extremely disturbing. Although the condition is not known to cause pathology in the ear, the symptoms can motivate a patient to seek medical therapy and submit to surgical interventions. Identifying the diagnosis can be challenging because the reported symptoms may overlap with those of other conditions; consequently, it is probably far more common than was previously reported in the literature. The ET has a pivotal role in maintaining the proper volume and pressure homeostasis of the middle ear cleft, in expelling middle ear secretions, and in protecting it from the reflux of sound and material from the pharynx. Dysfunction of the tube may be divided into two groups: (1) obstructive ET dysfunction (OETD) with failure to open the ET valve adequately and (2) patulous ET dysfunction (PETD) with failure to close it adequately. This chapter is concerned with the diagnosis and treatment of the excessively open ET.
The ET is an organ composed of a skeleton made of cartilage and bone and the associated muscles, fat, connective and lymphoid tissues, nerves, and blood supply. The normal tube is maintained in a closed, resting position, and it opens by voluntary and nonvoluntary maneuvers, such as swallowing; yawning; and deliberate manipulations of the palate, pharynx, muscles of mastication, and mandible. The tube may also open passively by changes in the ambient air pressure or by forcing the passage of air, such as with a Politzer maneuver.
The active opening of the tube most commonly occurs with a swallow and usually lasts approximately 400 μs. It is the result of a coordinated effort of four muscles, the most important of which is the tensor veli palatini (TVP), which dilates the anterolateral membranous wall laterally. , ,
Although the opening mechanism of the ET has been studied extensively, the closing of the ET has received less attention. The part of the ET that is normally closed at rest and open upon dilation is referred to as a “functional valve.” The valve constitutes the approximately 15-mm-long segment of opposing mucosal surfaces within the middle of the cartilaginous portion of the ET. Closure of the ET is thought to be the result of the following factors: the recoiling memory properties of the cartilaginous ET and Ostmann’s fat pad (adipose tissue within the anterolateral wall of the cartilaginous ET), the relaxing bulk of the TVP muscle, and the pressure of the neighboring paraluminal tissue. The closed lumen is likely maintained by all of the above and aided by the surface tension of the apposed wet mucosal surfaces.
The cartilaginous canal is anchored to the basisphenoid bone along its superior wall. The medial cartilaginous lamina is mobile, principally by action of the levator veli palatini (LVP) muscle. Along the superior wall, the medial and much smaller lateral cartilaginous laminae come together to form a junction, rich in elastin fibers, that contributes to the spring-like quality of the cartilage (“J” portion).
The cartilaginous skeleton of the ET becomes increasingly deficient in the anterolateral wall as it courses distally toward the nasopharynx. Therefore, closure of the lumen is also partially dependent on the pressure of the abutting soft tissues. The relaxed paratubal muscles (mainly the TVP) and Ostmann’s fat pad contribute to lumen closure. The pterygoid muscles may also play a role in the dilation and closure process. The glandular tissue and Ostmann’s fat pad were found to be smaller in patients with patulous ET (pET) compared with healthy controls, as measured by computed tomography (CT) scans. Ostmann’s fat pad also decreases in size with age. The mucosal and submucosal tissue layers increase in thickness as they are relieved of tension with muscle relaxation and are in themselves a factor in the closure of the valve. A report of fluctuating patulous symptoms in a hemodialysis patient who described symptomatic relief during fluid retention and exacerbation following excretion demonstrated the close connection between a periluminal mass and the symptoms of pET.
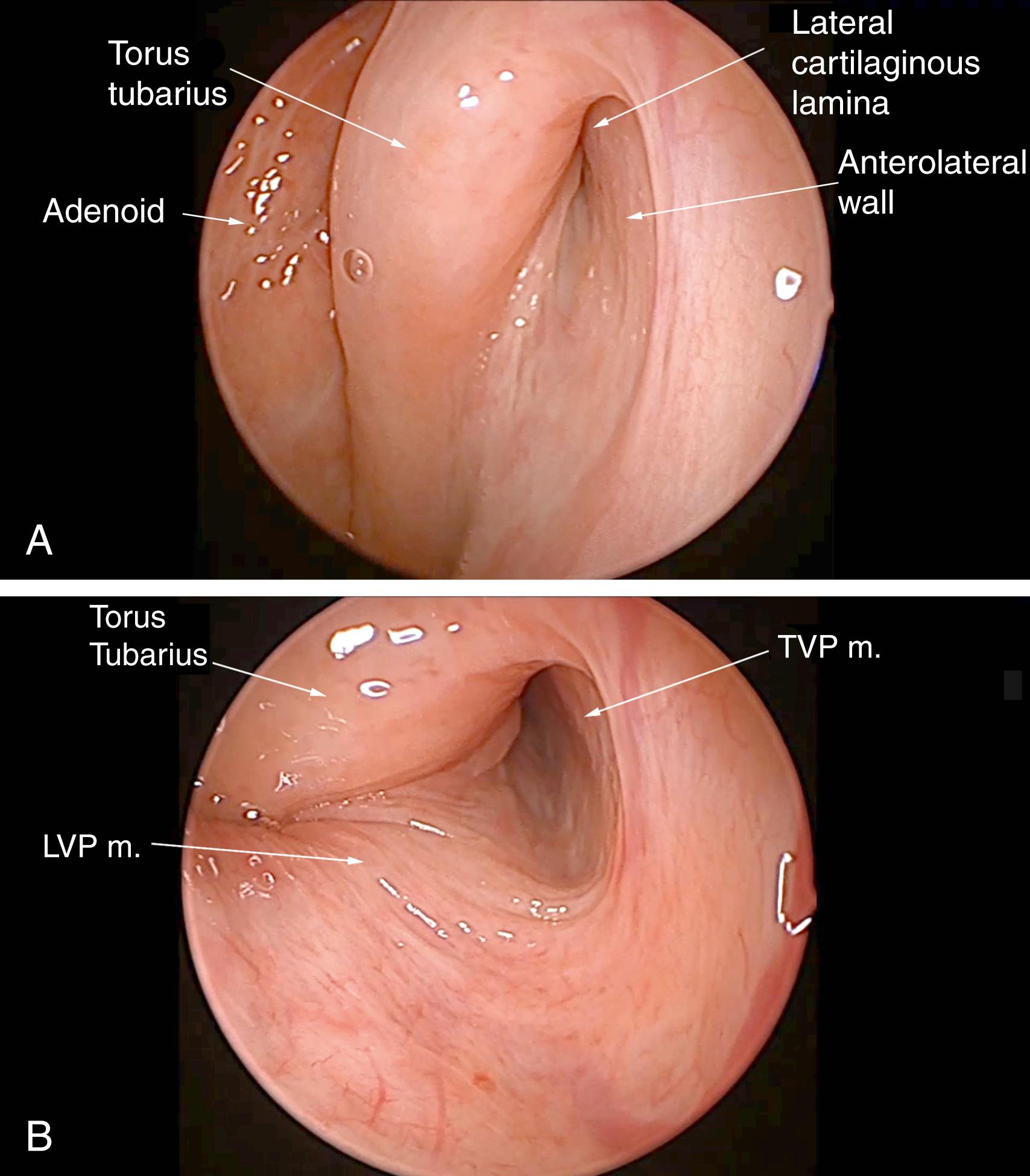
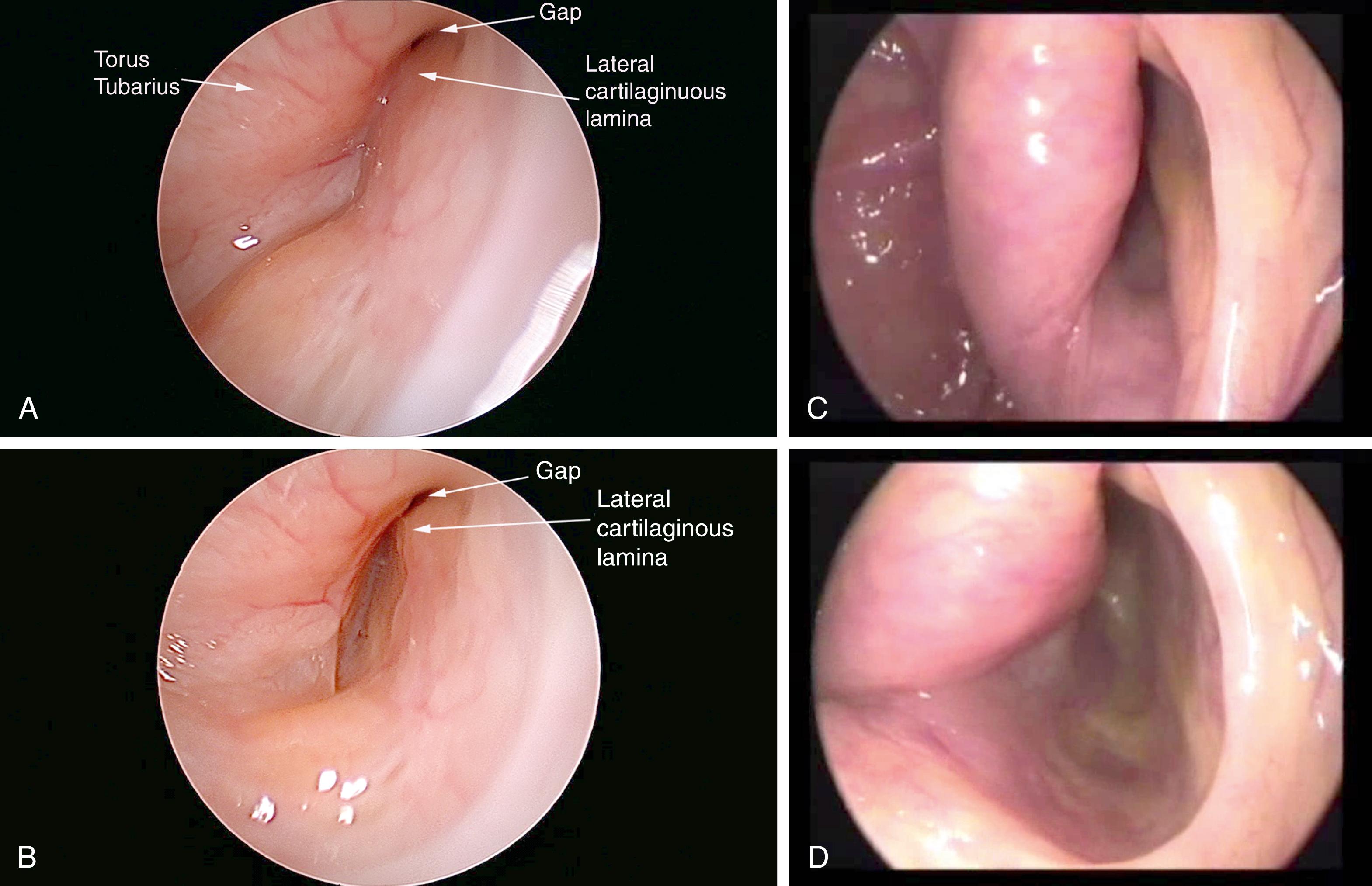
Transnasal endoscopy and surgery of pETs have revealed that the underlying pathology is a longitudinal concave defect through the length of the valve, which usually occurs in the anterolateral wall ( Figs. 6.1 and 6.2 ). This wall normally has a convex bulge into the lumen of the ET in the relaxed position. The defect represents a lack of tissue volume that can be due to a deficiency of the lateral cartilaginous lamina, Ostmann’s fat pad, TVP muscle bulk, submucosa, or mucosa. There is some early evidence that it may also be due to chronic tension in the TVP or pterygoid muscles, which could account for some of the anecdotal associations of pET in people under stress. Examples of these deficiencies, either in isolation or in combination, are observed in clinical cases. Temporary or permanent obliteration of the defect, even with some mucus, immediately relieves patients’ symptoms if the closure is of sufficient length within the lumen of the valve. A minimal closure such as occurs with a small amount of mucus might relieve autophony of breathing and the sensation of air passage, but not eliminate autophony of the voice (partially patulous ET).
An overly patent ET allows for the free passage of air and the sound from the nasopharynx to the middle ear, thus creating a pathological acoustic and pressure environment. The most prominent symptoms of a pET are aural fullness and autophony of a patient’s own breathing noises and voice. Symptoms are mostly related to free passage of air and sound but not reflux of material. Symptoms compatible with pET have been described since the second half of the nineteenth century. The constellation of symptoms compatible with those seen in patients with pET was reported by Jago in 1858, who later described his personal experience with the condition. Schwartze recognized the connection between the pET and excursions of the tympanic membrane (TM) with respiration (as reported by Ogawa et al. ). Zollner and Shaumbaugh emphasized the important symptom of autophony in a series of patients with patulous tubes (as reported by Bluestone and Magit ).
A recent study describing the clinical symptoms of patients with pET in a large cohort of 190 patients reported that over 93% presented with voice distortion, 92% with breath autophony, 57% with aural fullness, and 17% with related pulsatile tinnitus. In this cohort, symptom progression (deterioration) was seen in over 65% and the symptom duration was over 5 years. Additionally, pET was commonly bilateral, progressive, and slightly more prevalent in women. Autophony is described by patients as hearing their own voice and/or breathing noises. A patient’s own voice sounds to the person as if he or she were talking very loudly into a barrel, typically prominent on the pronunciation of “M” and “N.” Patients often have difficulty describing autophony, focusing on their perception of a “blocked or full” ear. For clarification, the symptoms can be simulated by talking and breathing into the diaphragm of one’s own stethoscope. Because the auditory feedback can be disturbingly loud, patients experience a wide range of cognitive and emotional responses, ranging from asymptomatic to suicidal ideation. A patient of the senior author took a fatal dose of tranquilizers and was rescued only because a family member unexpectedly changed plans and came home early. Autophony can be disturbing, leading to depression and the need for psychiatric medication or intervention. The perception of pressure changes in the middle ear with breathing can be a loud wind-like noise, constant ear blockage or pressure, and the sense of the medial and lateral excursions of the TM. Otalgia is occasionally reported if there are large pressure changes.
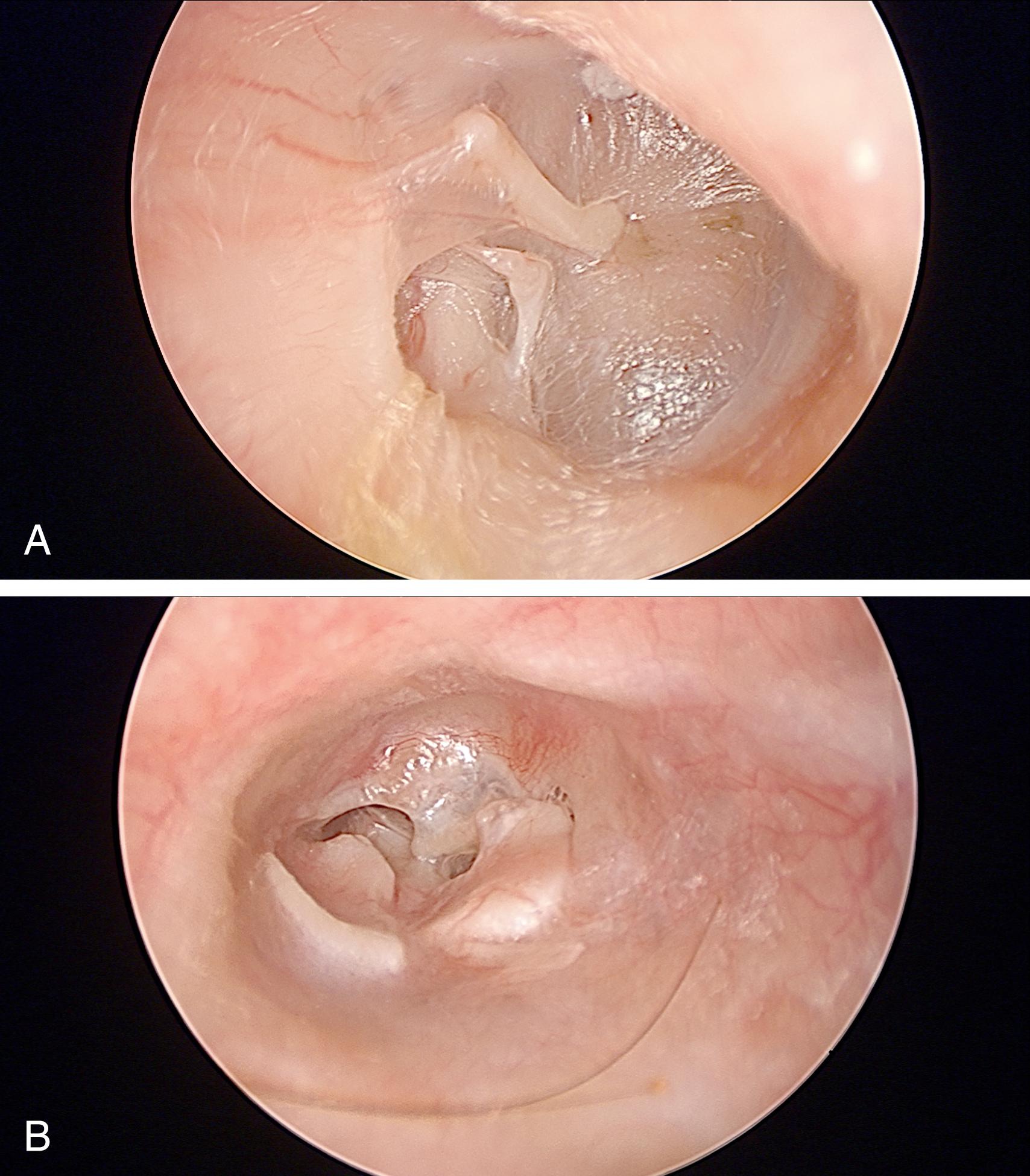
Aural fullness and the sensation of ear blockage can be misdiagnosed as OETD, especially because pET patients will usually describe their ears as “blocked.” In contrast with obstructive dysfunction, pET should not cause a conductive hearing loss or difficulties with barochallenges such as air travel and scuba diving. The diagnosis can be particularly difficult to recognize in patients who sniff frequently to try to close the valve of their ET. It can result in chronic negative pressure, even to the point of causing serous effusion, retraction pockets, and cholesteatoma ( Figs. 6.3 and 6.4 ). Previously termed “habitual sniffing,” this conscious or unconscious frequent behavior should trigger questions about autophony, which can reveal the underlying problem. The differential diagnosis for aural fullness can also include obstructive ETD, other ear pathology, temporomandibular disorders (TMD) including joint (TMJ) or related dysfunction in the muscles of mastication, Minor’s syndrome of semicircular canal or other otic capsule dehiscence, and endolymphatic hydrops. Because of the typical complaint of “ear blockage,” many patients are initially treated with medications directed against obstructive dysfunction, such as nasal decongestants, topical or oral steroids, and topical or systemic antihistamines, all of which will fail to help or may even aggravate the symptoms. The symptoms of pET can be unmasked by middle ear procedures such as tympanoplasty and stapes surgery.
Symptoms are mitigated by maneuvers and conditions that support tubal closure by increasing venous congestion in the tubal tissues, causing inflammation of the tissues, or restoring some hydration or mucus into the luminal surfaces. Conditions such as placing the head in a dependent position (supine or between the legs), applying pressure on the ipsilateral internal jugular vein, upper respiratory tract infections and allergies, and sniffing inward to “lock” the ET closed are all capable of generating temporary symptom relief in most patients. Clinicians should be aware that they may not always observe the classic sign of excursions of the TM on otoscopy that are synchronous with nasal breathing unless the patient is upright and actively symptomatic. The symptoms are typically relieved overnight and may start sometime after rising in the morning. They are often triggered by exercise, possibly because of dehydration or epinephrine-like hormones causing nasal decongestion. Caffeine and diuretics may have similar effects. Prolonged speaking and singing may provoke symptoms, possibly from desiccation of the mucosa from air movement. When susceptible, symptoms may initiate beginning with a swallow or yawn.
The etiology for the loss of tissue that creates a pET remains uncertain. Ward et al. reported that associated comorbidities including environmental allergy (50%), weight loss (35%), laryngopharyngeal reflux (33%), and stress and anxiety (31%) were risk factors for developing pET. The loss of tissue volume through weight loss or atrophy from chronic disease accounted for two-thirds of the patients and the leading associated condition was allergic disease. The loss of tissue volume from within the tubal lumen is cited as the most common pathogenesis and has been previously most commonly reported with weight loss. pET is observed in 21% of patients who lost significant weight after bariatric surgery, and the magnitude and rapidity of the weight loss were greater in the patients who developed pET. A recent study using 3-Tesla MRI demonstrated a smaller size of connective tissue surrounding the ET in patients with pET that includes Ostmann’s fat pad. More importantly, clinicians should be aware that patients with a significant history of OETD can convert to a patulous state secondary to a ‘burned-out’ focal mucosal atrophy. The authors have noted anecdotally that allergic rhinitis is most commonly associated with the phenomenon of converting from obstructive ETD to pET or even alternating between them.
Simonton grouped etiologies into positive and negative contributing factors. The positive factors were defined as those actively reducing tissue volume such as with scarring from previous procedures, chronic inflammation with mucosal atrophy, and radiation. , The negative factors were more passive because of a loss of tissue around the pharyngeal orifice, loss of tonic action of the TVP muscle, and conceivably reduced coiling properties of the ET. The hormonal factors include pregnancy, oral contraceptive use, and estrogen treatment for prostate cancer. Estrogen can reduce the viscosity of tubal secretions, reduce the elastic properties of the tubal cartilage, and elevate the level of surfactant via a change of prostaglandin levels. All of these factors would support the opening of the ET. The reflux of gastric contents and allergy are reported to be the cause in 3 of 11 patients in one series. Other contributors reported abuse of nasal decongestant and cocaine, poliomyositis, multiple sclerosis, other neuromuscular disorders or drugs for their treatment, cerebrovascular accidents, craniofacial abnormalities, and TMJ malfunction and malocclusion. Occasional association between pET and palatal myoclonus has been reported. The habit of sniffing or Valsalva maneuver can be acquired in patients attempting to aerate their middle ears at times of otitis media, but may ultimately result in a patulous tube. Sniffing has been observed to close the ET lumen as seen by fast sequences of CT scans.
The clinical history with the recognition of characteristic features on physical examination ensures the clinical diagnosis of pET. The diagnosis is certain when medial and lateral excursion of the TM with breathing is observed; however, this is only observed in 82% of patients with pET. Otoscopy should be performed with an otoscope or microscope, with the patient seated. If autophony is not exhibited at the time of the examination, it may be induced by physical activity. Excursions of the TM can be enhanced by the closure of the contralateral nostril during ipsilateral nasal breathing. This is the most important and useful tool for the diagnosis of pET.
High-quality endoscopy allows for a direct and detailed examination of the ET valve ( Figs. 6.1 and 6.2 ). Done with either a rigid or flexible endoscope, the view is angled to see into the longitudinal axis of the ET to view the opening and closing of the valve during swallows, yawns, and vocalization of “Ahhh.” A patulous ET will demonstrate a concave defect, most commonly in the anterolateral part of the valve, instead of the normal convexity. The defect prevents the normal closure of the orifice in the resting position, leaving an opening in the lumen that extends through the length of the visible portion of the lumen. Concave defects limited to the orifice are common, but if they are not observed to traverse the lumen, these can be normal variants.
Ancillary tests may be of some assistance in the diagnosis of the patulous tube. Impedance tympanometry while the patient experiences autophony may be the most sensitive test. It can be performed in the reflex decay mode to give a 10- to 15-second window in which ipsilateral nasal breathing (not forceful) may reveal breathing-induced fluctuations because of the variations in the middle ear pressure. Sonotubometry can directly the measure ET patency. , During the examination, a sound is emitted in the nasal cavity and is recorded by a microphone located in the external auditory canal of the examined ear. As the ET opens, the sound recorded in the external auditory canal intensifies, and an increase of 5 dB or more is considered to reflect opening of the ET reliably. The severity of subjective autophony was found to be correlated with the objective measurements of ET function by sonotubometry. This test is not available routinely in the clinical setting. Recently it has been suggested to use this principle when performing audiometry masked by a nasally presented noise. In subjects with patulous tubes, thresholds of low tones were significantly elevated more than in healthy controls because the sound presented in the ear was masked by the noise escaping the nasal cavity to the middle ear through the patent ET. The thresholds normalized in most patients after corrective measures were taken. , Newer data suggests that sound transmission assessed via sonotubometry could be more useful than is pressure transmission (impedance tympanometry) to predict the morphological severity of pET. However, newer data suggests that continuous impedance and tubo-tympanic-aerodynamic-graphy had the highest diagnostic accuracy.
Imaging is not routinely used for the diagnosis of the patulous tube. Until recently CT scanners required a recumbent examinee, a position that commonly results in the closure of the patulous tube. The availability of scanners allowing examinations in the seated position has given researchers the ability to record the patency of the ET both in resting during the Valsalva maneuver and sniffing. Imaging-based measurements of a group of patients with patulous tubes demonstrated some statistically significant differences from normal controls: an occlusive zone in the cartilaginous ET found in normal subjects was missing in patients with pET, and the distance of the open ET was longer in both the resting and Valsalva maneuver in patients with pET compared with the normal controls.
To date, the diagnosis of the pET is mainly clinical and is based on the history and physical examination. Patulous excursions of the TM during nasal breathing are pathognomonic for the condition, but will generally be seen only while symptoms are active. Patients may be asked to run or walk up and down stairs for several minutes to generate the symptoms when not initially present. Sometimes ten seconds of deep knee bends in the office can provoke symptoms. It is important to remember that patulous symptoms and signs (e.g., TM excursions) will only be present while the ET valve is in the open position. If the patient complains of active fullness and autophony symptoms, but there are no patulous TM excursions and no visible ET valve defect at that moment, another cause for the symptoms must be sought. Ancillary tests may be important to distinguish this pathology from other diagnoses with similar symptoms.
The other causes for aural fullness or ear blockage should be systematically considered ( Fig. 6.5 ). Chronic OETD will cause negative pressure in the middle ear or an effusion that should be recognizable on otologic examination and tympanometry. An endoscopic examination will reveal evidence of an obstructive problem with mucosal swelling or inflammation, or a dynamic disorder with failure of muscular dilation. If the symptoms of ear blockage only occur with barochallenges, the ET and ear may appear normal or nearly so, but the pattern of complaints limited to air travel or diving helps to establish the diagnosis.
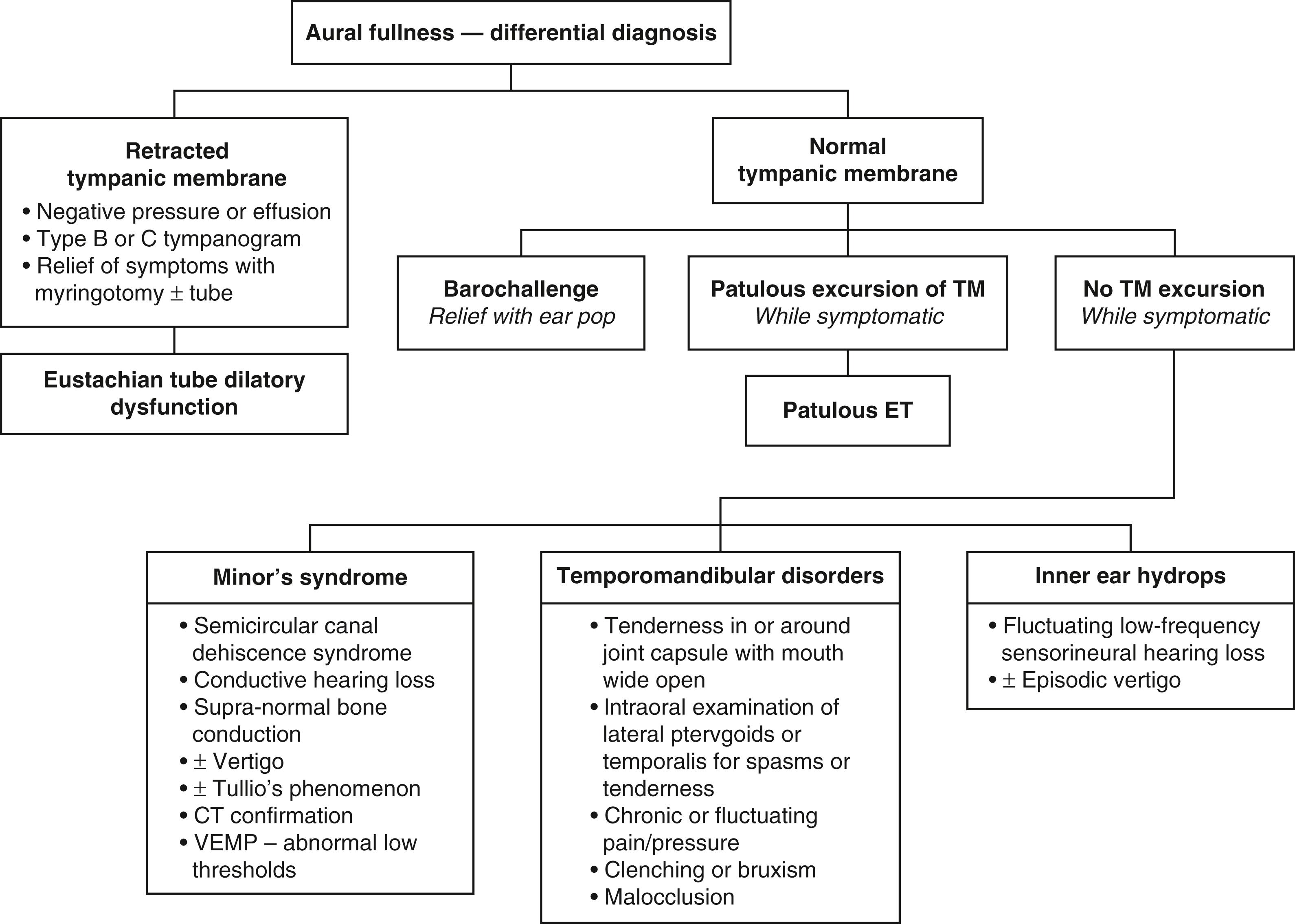
Minor’s syndrome of a dehiscence of the labyrinth or “third window,” commonly called superior semicircular canal dehiscence or SSCD, must also be entertained before diagnosing a patulous tube. These two very different pathologies can present with surprisingly similar symptoms of autophony of the voice, but autophony of breathing sounds is much more pronounced in the pET. Differential diagnosis is made more difficult because Minor’s syndrome may present itself without vestibular symptoms. Older series of patients assumed to have a pET most likely included patients who in fact had Minor’s syndrome. Close to 20 years before the first description of SSCD by Minor et al., O’Connor and Shea described some patients thought to have pETs who experienced vertigo accompanied by nystagmus induced by a sharp increase in nasopharyngeal and intratympanic pressure. They attributed the symptoms and signs to stapedial or round window membrane movement, although in retrospect, it is highly likely that the patients suffered from Minor’s syndrome. Out of the senior author’s (D.P.) series of patients with SSCD, 94% complained of autophony of their voice, and half of them found relief by placing their head in a dependent position. The relief of autophony while lying supine has long been thought to be pathognomonic for pET, but it appears that changes with intracranial or intralabyrinthine pressure with the head dependent probably also influence the impedances of the dehiscences that cause Minor’s syndrome. Patients with Minor’s syndrome tend to have unremitting autophony once present, as opposed to patulous symptoms that are generally intermittent. Vertigo and nystagmus induced by sound or pressure, supranormal bone conduction, and conductive hearing loss with intact stapedial reflexes should all raise the suspicion of SSCD. The diagnosis is confirmed by the demonstration of a bony dehiscence on a high-resolution temporal bone scan (best recognized in the reconstructed Poschel and Stenvers planes) and by lower-than-normal thresholds with cervical vestibular evoked myogenic potential testing (cVEMP) or higher-than-normal amplitudes with ocular VEMP (oVEMP). The recording of otoacoustic emission or stapedial reflexes in the presence of a conductive hearing loss can also point toward the inner rather than the middle ear as the source of an audiometric air-bone gap.
Patients with aural fullness complaints but normal TM findings should be asked about and evaluated for TMD, including TMJ and muscular disorders. Aural fullness with otalgia is especially suggestive of these problems. The joint capsule is examined with the patient’s mouth open as widely as is comfortable, and the space between the condyle and the glenoid fossa is palpated deeply to identify unusual subluxation, crepitus, or any tenderness. The open lower jaw is moved side to side during a portion of the examination, again palpating for areas of tenderness, especially superiorly and posteriorly to the condyle. Deep palpation is performed in the temporalis and masseter muscles surrounding the TMJ to examine for spasms and tenderness. Bimanual intraoral and extraoral examination of the masticator muscles is performed to identify spasms, tenderness, and hypertrophy. The examiner’s index finger is passed superiorly along the anterior edge of the mandibular ramus, palpating bimanually for the masseter muscle and then superiorly and medially into the pterygoid space for the insertions of the temporalis and lateral pterygoid muscle into the coronoid process of the mandible. If a patient does not have active periauricular pain during the office visit, it is possible that no findings will be made, so the patient is instructed in how to perform a self-examination during episodes of future symptoms, especially if there is otalgia. Inner ear conditions such as Ménière disease can cause aural fullness, but the symptoms would characteristically have a fluctuating pattern of sensorineural hearing loss and vertigo.
The treatment should be designed in a stepwise fashion. In some patients, the specific etiology can be determined and treatment initiated accordingly. Often, a correctable etiology cannot be identified. For most patients, reassurance will suffice once they are informed that the condition is physiologically harmless despite the disturbing symptoms. Others will find relief by swallowing, yawning, or placing their heads in a dependent position. Patients are advised to discontinue decongestants, avoid excessive consumption of caffeine and nasal steroids, observe good hydration, and use nasal saline drops, which are more effective than sprays. For the subset of patients experiencing unwarranted weight loss, appropriate evaluation is recommended. For patients with allergic rhinitis, non-decongesting treatments such as nasal saline irrigations, cromolyn sodium spray, and immunotherapy can be substituted.
When indicated, the treatment is directed toward augmenting the periluminal tissue, specifically filling the defect in the valve. This can be done either by adding bulk to the native tissue by inducing congestion or by the introducing mass from other sources (autografts, allografts, xenografts, or synthetic implants).
A number of agents affecting the mucosal lining of the valve can be used with good, albeit often temporary, results. Saline nasal drops can be instilled to moisten the mucosa of the lumen of the ET. If insufficient, hypertonic saline may be more effective due to its irritant effect. Four teaspoons of table salt in an 8-oz (∼200 mL) cup of water produces a concentration of hypertonic saline. Patients must be carefully instructed in how to administer the drops effectively. Beginning with lying supine with the head hanging at 15 degrees, three or four drops are instilled into the ipsilateral naris. The head is then turned 45 degrees toward the affected side. A twinge that radiates toward the ear should be experienced when the drops successfully contact the ET orifice. A more potent irritant nasal drop called PatulEND is available over the counter. It is a solution of ascorbic acid and supplements; three or four nasal drops are administered similarly, two to three times daily or for up to 2 months to attempt to achieve lasting relief. Premarin or other estrogen preparations have been employed more in the past, but, in the authors’ experience, they have been less effective and more expensive. They can be administered as a nasal solution resulting in mucosal edema. In an off-label use of the medication, a dose of 25 mg of intravenous Premarin is diluted in 30 mL of isotonic sodium chloride to be taken as three or four nasal drops three times a day for up to 6 weeks. The adverse effects include epistaxis and nasal irritation. Saturated solution of potassium iodide (SSKI), an expectorant designed to enhance the viscosity of the mucus, is taken orally three times a day as 8 to 10 drops diluted in water or juice. A powder of boric and salicylic acid (4:1 ratio) insufflated to the nasopharynx or instilled with a catheter causes local irritation and edema, offering temporary relief. The insufflation of mucosal irritants can also be used as a diagnostic test or temporary treatment for pET. Some of the other local irritants reported have included the application of silver nitrate, nitrate acid, and phenol. , These potent irritants have the potential to cause scarring of the mucosa and are no longer used.
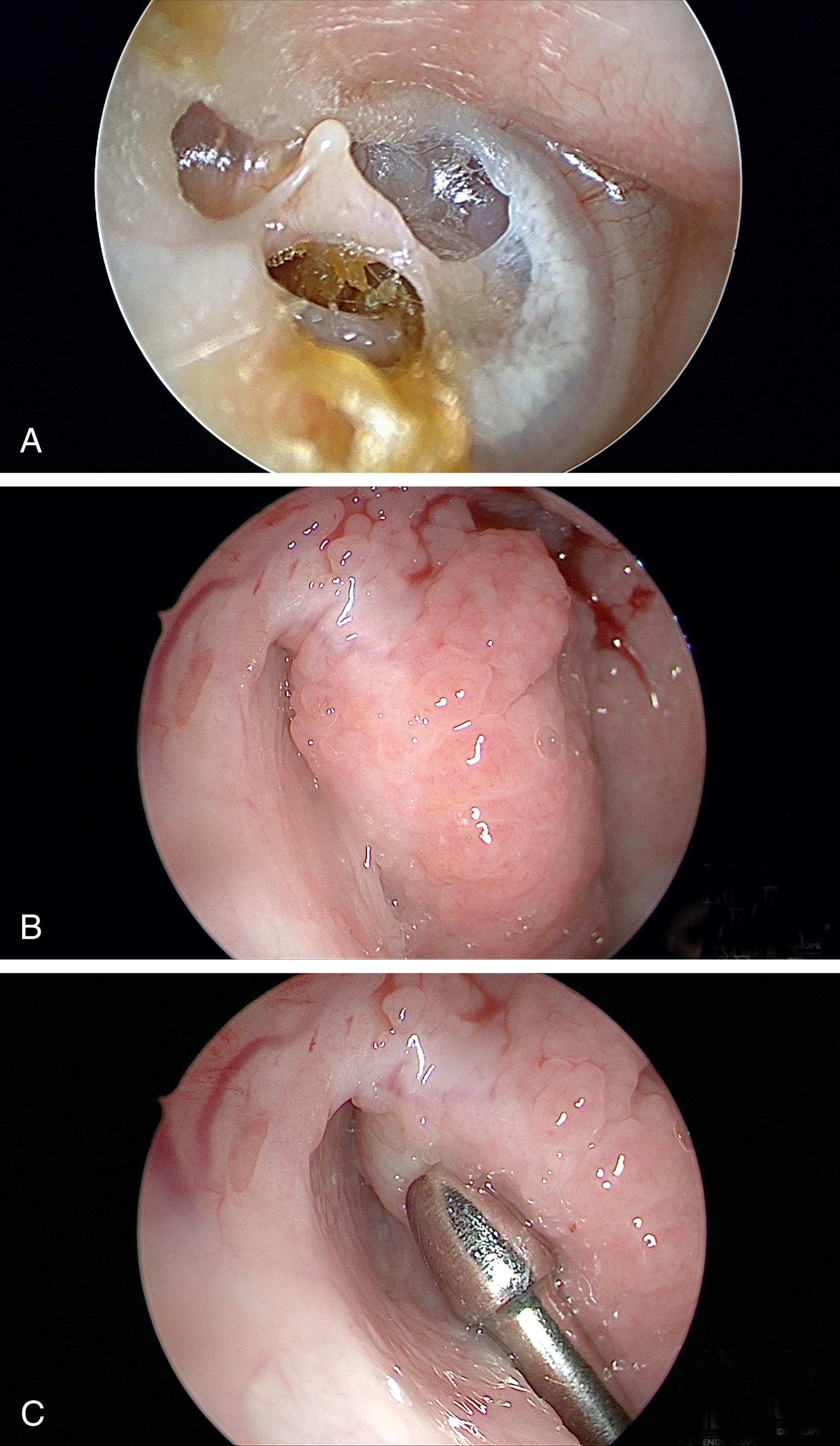
Surgical intervention is reserved for the minority of patients with symptoms that significantly affect their quality of life, when the less intrusive methods of treatment fail. Myringotomy with the placement of tympanostomy tubes is probably the most common surgical intervention, and it is reported to aid some 50% of patients. Tympanostomy tubes mostly alleviate the symptoms related to middle ear pressure changes and TM movements (aural fullness), but less so for reflux of sound. Mass loading of the TM by placing paper patches, topical ointment, or Blu-Tack on the posterior TM changes its resonance qualities and has been found to provide various degrees of relief of pET symptoms. If a significant degree of relief is obtained with these temporary measures, it may be predictive that mass loading with a tympanostomy tube or cartilage graft tympanoplasty may be beneficial. In patients with a floppy TM, cartilage reinforcement of the flaccid segment significantly reduced the symptoms of pET (autophony, breath sounds, and aural fullness).
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here