Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
In the intensive care unit, it is not uncommon for today’s surgeon to take care of patients with multiple comorbidities unrelated to their surgical disease. A common knowledge of most major medical issues, and their potential consequences, is thus critical for any surgeon caring for inpatients after complex surgery. Given that the patients undergoing major surgery will often be placed in settings allowing for continuous monitoring techniques, the rapid identification and treatment of dysrhythmias has become common practice in the surgical intensive care unit (SICU). Cardiac dysrhythmias in the postoperative setting have several causes, with some of the most common including hypoxia, cardiac ischemia, catecholamine excess, routine medications, and electrolyte abnormalities.
Cardiac dysrhythmias can be diagnosed with a focused physical examination and a standard 12-lead electrocardiogram (ECG) and from the response to specific maneuvers or drug therapy. While some patients may present with symptoms, some important arrythmias may be even be present in asymptomatic patients. The acute management of most dysrhythmias is dependent upon the stability of the patient, an accurate classification of the dysrhythmia, and an understanding of the mechanisms causing the dysrhythmia. Management may range from simple pharmacologic intervention to cardioversion in the acutely unstable patient and may occasionally require percutaneous or transvenous pacing, pathway ablation, or implantation of pacemakers or defibrillators.
The incidence of cardiac dysrhythmias in the postoperative setting ranges from 9% in noncardiac surgical patients without a significant history of cardiac disease to over 40% in cardiac surgery patients. In one study that took place in a standard intensive care unit (ICU) admitting cardiac, noncardiac, and medical critical care patients, an average of 20% of admitted patients experienced significant dysrhythmias during their stay. The most commonly encountered dysrhythmia, other than sinus tachycardia, is atrial fibrillation (AF), which, in cardiac surgery patients, may be seen in nearly half of that patient population. Up to 15% of patients with inferior wall myocardial infarction (MI) will present with atrioventricular (AV) nodal conduction disturbances or complete heart block. Patients with preexisting cardiac or pulmonary disease have an increased risk of dysrhythmia, which is exacerbated by noncardiac surgery, trauma, or critical illness. Vasopressor requirement is associated with an increased risk of dysrhythmia, caused by the proarrhythmic properties of catecholamines on cardiac tissue. Only 10% of the dysrhythmias encountered are bradyarrhythmias. Dysrhythmias are classified based on their atrial or ventricular origins, with AF and ventricular tachycardia (VT) being most common, respectively. Of note, dysrhythmias have been shown to have an association with longer ICU stays and lower overall mortality in several studies, indicating that a dysrhythmia may be a marker of underlying critical illness.
Risk factors for the development of cardiac dysrhythmias have been examined in a number of retrospective studies. In patients undergoing cardiac surgery, risk factors include advanced age, the type of surgery performed (e.g., valve replacements combined with coronary artery bypass grafting [CABG] have higher rates of dysrhythmia than CABG alone), the need for pacemaker placement, any inotropic support during surgery, worsening New York Heart Association classification ( Table 1 ), and complicated weaning from cardiopulmonary bypass. Other risk factors include obesity, positive fluid balance during surgery, and metabolic syndrome. Additionally, some arrythmias, such as AF, may be associated with genetic predispositions for their development.
| Characteristic | NYHA Class I (n = 10,592) | NYHA Class II (n = 3242) | NYHA Class III (n = 869) | p Value |
|---|---|---|---|---|
| Male sex | 8702 (82) | 2517 (78) | 651 (75) | <.001 |
| Age, mean ± SD, years | 60 ± 7 | 61 ± 7 | 60 ± 7 | <.001 |
| Current smoking | 1092 (10) | 353 (11) | 109 (13) | .04 |
| Hypertension | 3336 (32) | 1185 (37) | 318 (37) | <.001 |
| Diabetes mellitus | 1860 (18) | 718 (22) | 216 (25) | <.001 |
| Past myocardial infarction | 7749 (73) | 2222 (69) | 605 (70) | <.001 |
| Angina pectoris | 5285 (50) | 2913 (90) | 799 (92) | <.001 |
| Peripheral vascular disease | 326 (3) | 168 (5) | 65 (8) | <.001 |
| Body mass index, mean ± SD, kg/m 2 | 26.6 ± 3.4 | 26.9 ± 3.7 | 27.3 ± 3.9 | <.001 |
| Glucose, mean ± SD, mg/dL | 112 ± 44 | 118 ± 49 | 123 ± 56 | <.001 |
| Total cholesterol, mean ± SD, mg/dL | 224 ± 39 | 225 ± 41 | 225 ± 43 | .24 |
| HDL cholesterol, mean ± SD, mg/dL | 37.9 ± 10 | 37.7 ± 10.6 | 37.1 ± 10.2 | .07 |
| Triglycerides, mean ± SD, mg/dL | 155 ± 86 | 164 ± 100 | 167 ± 94 | <.001 |
| LDL cholesterol, mean ± SD, mg/dL | 156 ± 34 | 155 ± 35 | 156 ± 38 | .92 |
| Percent HDL out of total cholesterol, mean ± SD | 17.3 ± 5.0 | 17.1 ± 5.1 | 16.9 ± 5.0 | .03 |
| Antihypertensive medications | 8024 (76) | 2782 (86) | 768 (88) | <.001 |
| Antidiabetic medications | 1082 (10) | 451 (14) | 140 (16) | <.001 |
| Antiplatelets | 6497 (61) | 1706 (53) | 458 (53) | <.001 |
* Data are presented as number (percent in parentheses) unless otherwise indicated.
Although not as commonly encountered as tachyarrhythmias, a bradyarrhythmia can represent potentially life-threatening illness. In the ICU setting, bradycardia only accounts for 10% of arrythmias. These cardiac abnormalities can be classified according to the node from which they originate: the atrioventricular (AV) or sinoatrial (SA) node. Bradyarrhythmias may be caused by either extrinsic factors or intrinsic disease in the conduction system of the heart. Extrinsic causes include medications, myocardial ischemia, metabolic abnormalities, increased vagal tone, and acute respiratory failure. Further classification of bradyarrhythmias depends on the reversibility of the rhythm, whether the patient is symptomatic due to the arrythmia, and the likelihood of whether that particular rhythm will progress or recur. The treatment of such an arrythmia can vary, and it may be as simple as withholding a causative agent or as complicated as placing a permanent pacing device.
The normal heartbeat arises from the SA node, which serves as the pacemaker of the heart under normal conditions. Bradycardia arising from SA nodal dysfunction originates from either failure of impulse generation or failure of the conduction of that impulse. In the past, the term “sick sinus syndrome” was used to describe a range of conditions that reflect such dysfunctions. Currently, bradyarrhythmias arising from the SA node are described as one of the following: inappropriate sinus bradycardia, sinus pause or arrest, sinus exit block, and tachycardia-bradycardia (aka tachy-brady) syndrome.
Sinus bradycardia is defined as a heart rate below 60 beats per minute (bpm). This alone does not signify SA nodal dysfunction, as heart rates below even 40 bpm can be asymptomatic and considered normal in well-trained athletes or active individuals. Sinus bradycardia is considered pathologic, then, when patients are symptomatic (e.g., syncope, chest pain, shortness of breath) or when there is a failure to appropriately increase the heart rate during activity. Sinus pause or arrest occurs when the SA node transiently fails to exhibit automaticity and does not fire. Sinus exit block similarly results in a pause but the SA node still depolarizes normally. The impulse is either delayed or fails to propagate beyond the SA node, resulting in failure of atrial depolarization. Tachycardia-bradycardia syndrome refers to sinus node dysfunction with both tachycardia and bradycardia. Typically, bradycardic episodes follow the termination of tachycardia events and can be associated with the clinical symptoms of presyncope or syncope. Management can be challenging, as pharmacotherapy to treat fast rhythms often predisposes patients to slow ones, and vice versa. Commonly, insertion of a pacemaker in situations of tachycardia-bradycardia syndrome may be necessary to treat the symptomatic bradycardia, in conjunction with pharmacologic treatment for the tachycardia.
Treatment for SA node dysfunction depends on the clinical status of the patient and the presumed cause. If the bradycardia is transient and not associated with hemodynamic compromise, no therapy is necessary. Investigation into the cause of bradycardia should include correction of metabolic and electrolyte abnormalities, minimizing maneuvers that could increase vagal tone, and the cessation or reduction in dosage of potentially offending medications, such as beta blockers, calcium channel antagonists, and lithium. If the bradycardia is sustained, or severe enough to lead to hemodynamic instability, therapy with antimuscarinic agents such as atropine or beta-agonists should be considered. Percutaneous or transvenous pacing may be necessary in some patients in the acute setting and can act as a bridge for those patients until a permanent pacemaker is placed. Transcutaneous pacing can be used effectively in greater than 90% of patients, but should not be used long-term due to pain and loss of capture due to muscle impedance. Therefore, patients who require over 30 minutes of pacing would need conversion to transvenous pacing or permanent device. Patients who are hemodynamically stable but symptomatic from sinus node dysfunction almost always require permanent pacing.
Disturbances in conduction through the AV node or His-Purkinje system are classified as AV blocks. These blocks may be temporary or permanent, depending on the cause of delayed conduction. In adults, the most common causes are drug toxicity, coronary artery disease, and degenerative disease of the conduction system. Many other conditions, such as electrolyte disturbances, myocarditis, sarcoidosis, scleroderma, and hypervagal responses, can cause AV block. The PR interval is a measure of the conduction time through the AV node and bundle of His. When the PR interval is prolonged (more than 210 ms), a patient has first-degree AV block. Second-degree AV block occurs with intermittent failure of the conduction of the impulse to the ventricles. In Mobitz type I, second-degree AV block (Wenckebach block), there is progressive prolongation of the PR interval until failure of conduction to the ventricle occurs ( Fig. 1 ). The PR interval then shortens following the dropped beat. This failure in conduction originates from the AV node itself and the QRS complex remains narrow. In Mobitz type II, second-degree AV block, there is intermittent failure of conduction reaching the ventricles that is not associated with progressive prolongation of the PR interval. There is not a shortened PR interval following the dropped beat. This failure in conduction is considered “infranodal” and originates from the His-Purkinje system. The QRS complex may be prolonged, and this type of AV block is more concerning. There is a significant likelihood of progression to complete heart block associated with inadequate ventricular response with this rhythm. Third-degree or complete heart block results from failure of all impulses through the AV node and His-Purkinje system, resulting in AV disassociation. The ventricles rely on their innate automaticity, which produces a typical wide QRS escape rhythm between 40 and 50 bpm. The atrial rate is commonly faster, producing multiple P waves with no relationship to the ventricular QRS complexes ( Fig. 2 ).
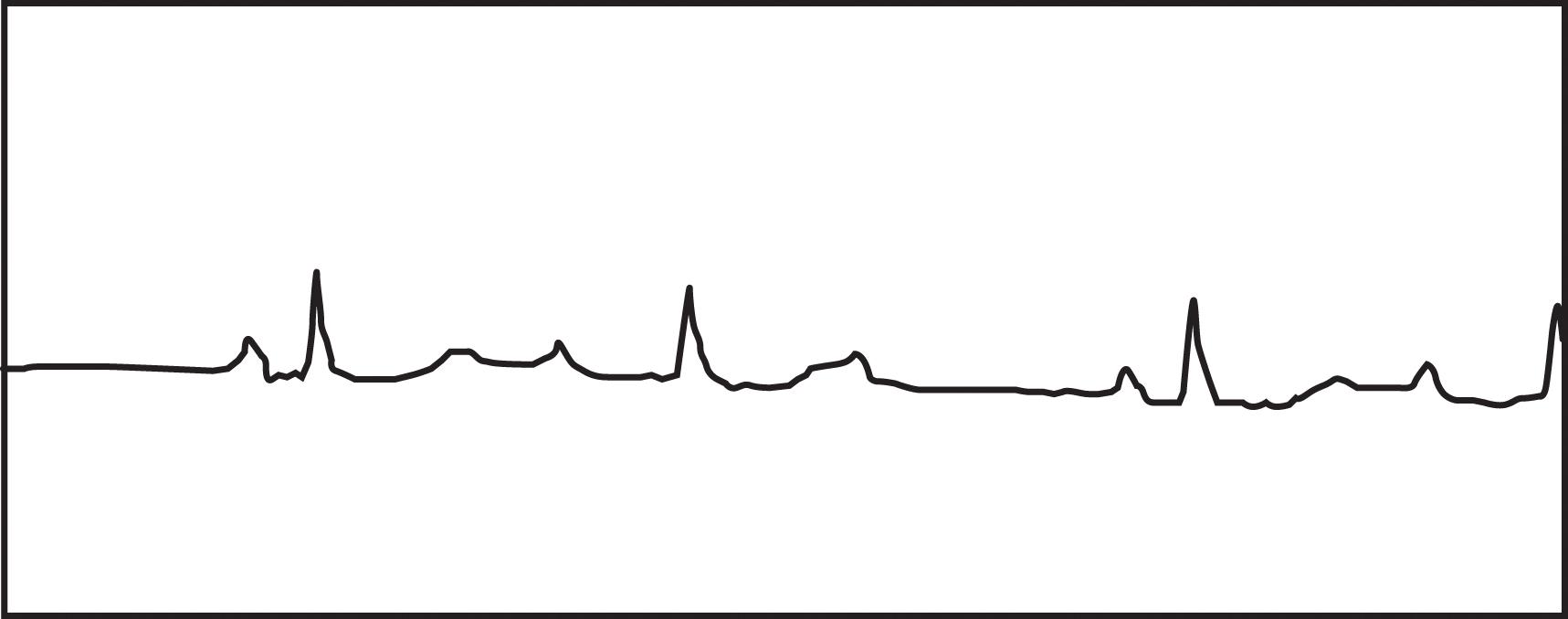
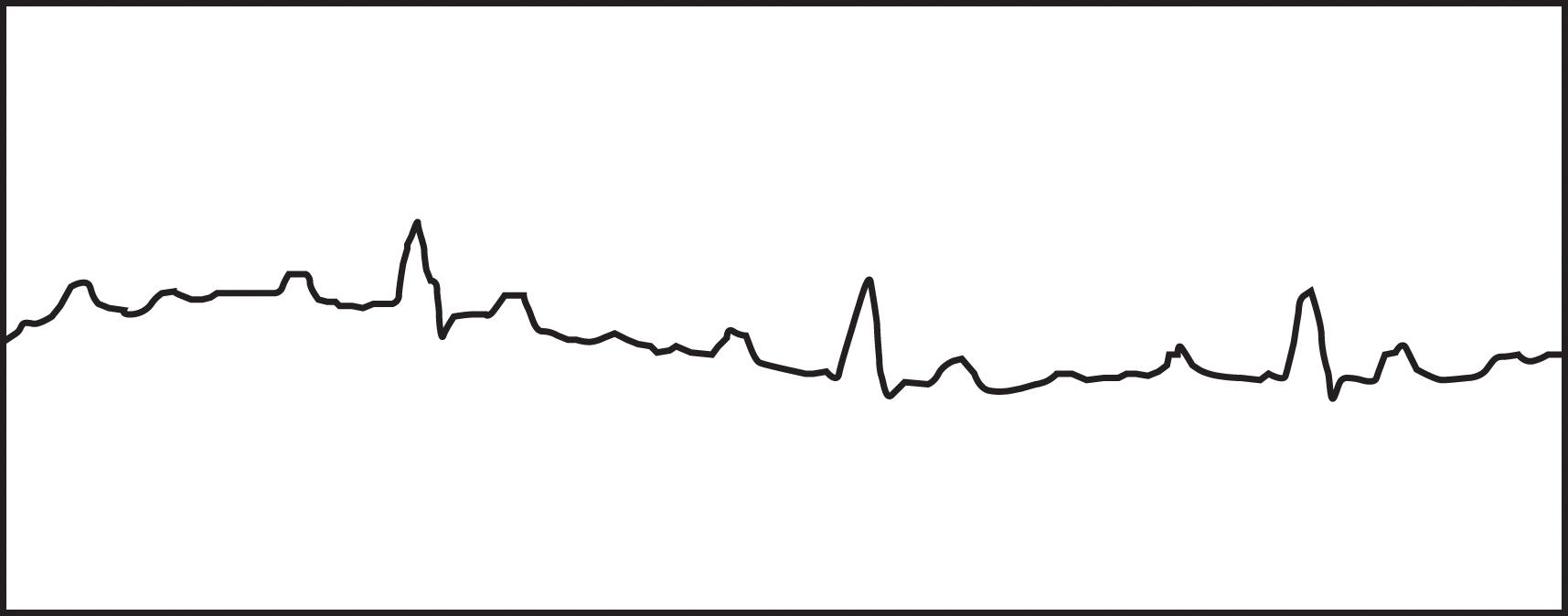
Management of AV block depends on the hemodynamic stability of the patient, the transient nature of the dysrhythmia, and where the focus originates from within the conduction system. Acute pharmacotherapy relies upon atropine and isoproterenol. Isoproterenol use should be avoided in patients with ischemic heart disease because of the associated increase in myocardial oxygen demand. There is no long-term pharmacotherapy for AV block, and removal of any of the common offending agents, such as digitalis or beta blockers, should first be attempted. Temporary pacing is used for those with ongoing instability, and permanent pacing is typically required for Mobitz type II second-degree AV block and third-degree AV block. It should be noted that the 2018 guidelines from the American Heart Association still recommend chronotropic pharmacotherapy, such as with dopamine or epinephrine, as an alternative bridge to a permanent pacing device or transvenous pacing, as it has been shown to be as effective as external pacing when atropine is ineffective.
Tachyarrhythmias are classified according to their anatomic origin in relation to the AV node, and as such, the first step in the management of any tachyarrhythmia depends on determining whether the arrhythmia originates above the SA node (supraventricular tachyarrhythmias, or SVT), or below the SA node (VT). These can also be thought of as producing a narrow QRS complex (SVTs) versus a wide QRS complex (VTs) on ECG. Narrow complex tachyarrhythmias include sinus tachycardia, AV nodal reentrant tachycardia (AVNRT, sometimes called paroxysmal SVT), AV reentry from an accessory pathway (Wolff-Parkinson-White syndrome, also called AVRT, atrioventricular reentrant tachycardia), AF, and atrial flutter. In contrast, VTs originate from below the AV node and include ventricular tachyarrhythmia and ventricular fibrillation (VF). Important determinants of the malignant potential of tachyarrhythmias are the duration, the hemodynamic consequences, and the presence of significant structural heart disease. The acute management depends on a basic understanding of the mechanism, the choices for pharmacologic intervention ( Table 2 ), and the indications for urgent cardioversion for each situation. Interventional techniques, such as aberrant pathway ablation and implantation of pacemakers and defibrillators, have drastically improved long-term outcome once patients have left the ICU setting and have added significantly to our armamentarium in treating these dysrhythmias.
| Class | Examples | Mechanism of Action | Prolong QT Interval |
|---|---|---|---|
| Class IIA IB IC |
|
|
Yes |
| Class II | Metoprolol, esmolol |
|
No |
| Class III | Amiodarone, bretylium, sotalol, ibutilide |
|
Yes |
| Class IV | Verapamil, diltiazem |
|
No |
| Other |
|
|
No |
The mechanisms by which tachyarrhythmias arise are categorized into (1) abnormal automaticity, (2) triggered activity, or (3) reentry. Abnormal automaticity occurs when cells outside the normal conduction system generate spontaneous impulse formation. Triggered activity occurs during an “afterdepolarization,” which causes the membrane potential to reach threshold early and generate abnormal impulse formation. Reentry, the most common mechanism, occurs when an impulse can travel down two pathways separated by an area of unexcitable tissue. One of the pathways contains a unidirectional block, with slowed conduction, so that recovery and further excitation can subsequently occur. This defines an area of cardiac tissue that can self-propagate and thus becomes the focus for the generation of the tachyarrhythmia ( Fig. 3 ).
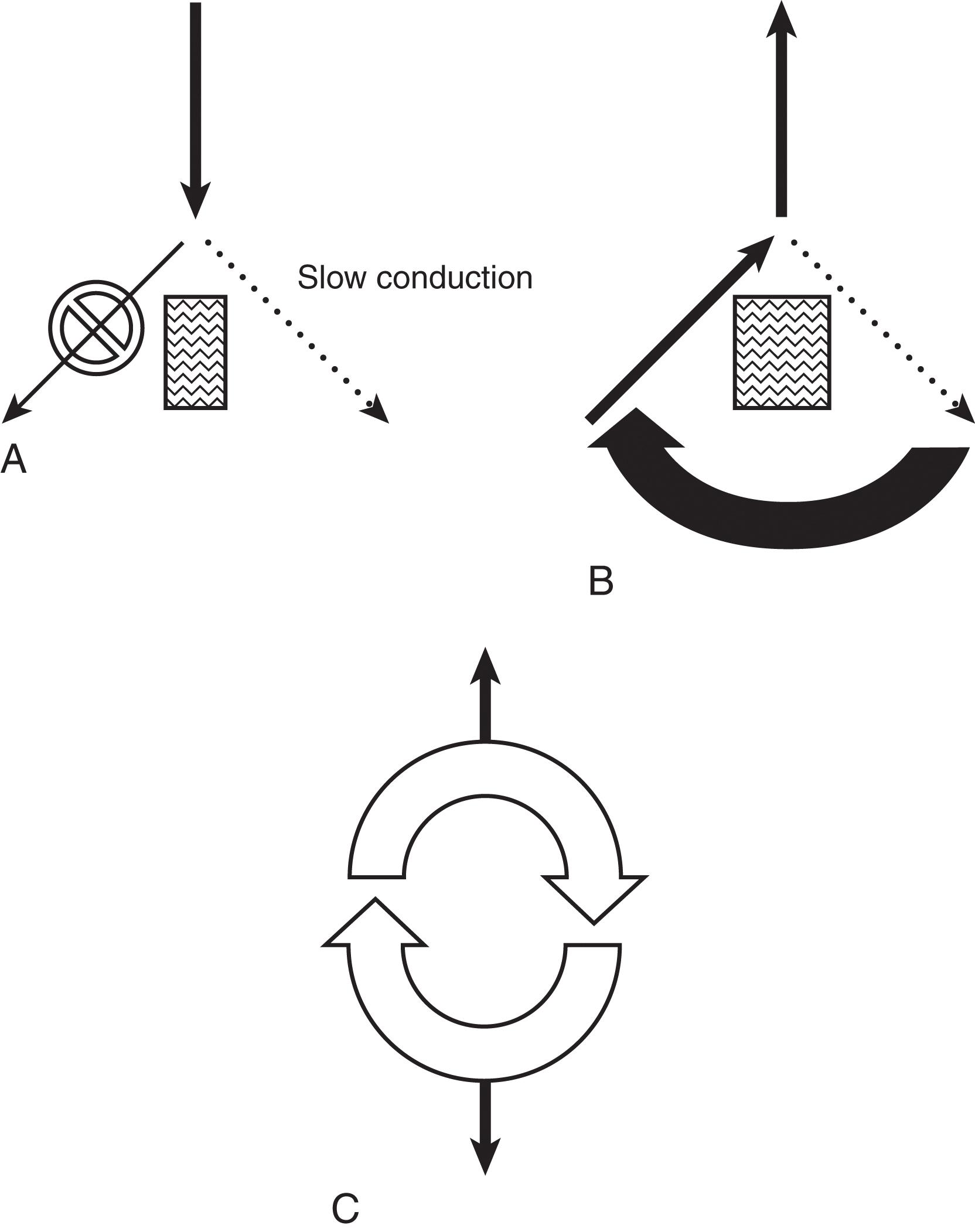
In attempting to classify a tachyarrhythmia seen on 12-lead ECG, maneuvers that increase vagal tone can assist in the diagnosis. Through carotid massage or administration of adenosine, AV nodal conduction time can be slowed and refractoriness increased. This can demonstrate to the clinician the presence of P waves on ECG or can interrupt reentry pathways. The response to such maneuvers can often suggest the type of tachyarrhythmia that the clinician is dealing with ( Table 3 ).
| Arrhythmia | Response to Increased Vagal Tone |
| Sinus tachycardia | Temporary slowing, with resumption of tachycardia |
| AVNRT | Abrupt termination or a transient slowing |
| Atrial fibrillation or atrial flutter | Increased AV block with slowed ventricular response rate |
| Multifocal atrial tachycardia | Increased AV block with slowed ventricular response rate |
| Ventricular tachycardia | No response |
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here