Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
This chapter discusses the use of proteins, whose serum levels are often elevated in patients with malignancies, in the diagnosis and management of cancer.
Many of these are so-called oncofetal antigens —that is, proteins that are expressed in fetal tissue during development but are not normally found in the tissues of adults. These include α-fetoprotein and carcinoembryonic antigen, whose serum levels are frequently elevated in hepatocellular and colon cancers, respectively.
Other proteins, such as CA 19-9, CA 125, and CA 15-3, are expressed in epithelial cells and are also often present in the sera of patients with pancreatic, ovarian, and breast cancers, respectively.
Because these “tumor marker” proteins are not tissue specific and can also be expressed in diseases other than cancer, their sensitivities and specificities are not sufficiently high that they can be used as cancer-screening proteins. Their use is predominantly in following patients who are being treated for known malignancies.
The exception to this is prostate-specific antigen (PSA), a chymotrypsin-like enzyme that is expressed almost exclusively in prostate tissue and is elevated in the sera of most patients with prostate cancer. Therefore, elevated serum PSA levels have a high sensitivity for diagnosing prostate cancer. Because PSA is also elevated in other prostate diseases, such as benign prostatic hyperplasia, it has a lower specificity but nonetheless can still be used very effectively to screen patients for this disease.
New developments in the field of early tumor detection using serum markers are also introduced in this chapter. These include use of mitogenic proteins, such as HER2/Neu, known to function as signal transduction proteins whose levels are elevated in many types of cancers; detection of genes encoding these proteins in body fluids; and proteomic approaches involving patterns of expression of multiple proteins that typify specific cancers. These specific approaches are also discussed in depth in Chapter 77 .
The identification of a circulating tumor cell (CTC) as a biomarker is a fast-growing area of research. This chapter updates the recent advances on CTCs that are or will be applied in clinical application for diagnostic and prognostic values.
Another area of growing research and translation to clinical application in cancer biomarkers is circulating nucleic acid, including the levels as well as mutation and methylation status.
Although cancer mortality has decreased over the past seventy years by about 21%, it is still a leading cause of death despite the advancement of multidisciplinary treatment modalities. In contrast, dramatic decreases in mortality due to cardiovascular and infectious disease have been achieved. Studies have shown that early detection of cancer can lead to superior long-term survival. Thus, there is an urgent need to search for cancer biomarkers with high sensitivity and specificity to allow for early cancer detection, effective treatment, and decreased mortality. Much effort has been devoted to the discovery, characterization, and clinical application of tumor markers to detect the presence of cancer at an early stage. As a result, increasing numbers of biomolecules, mainly specific proteins and some specific ribonucleic acids (RNAs) and deoxyribonucleic acids (DNAs), have been identified and employed as markers potentially for screening purposes and for prognostic purposes in the daily clinical management of cancer patients. These markers can further be used not only to classify cancers but also to monitor response to neoadjuvant therapy. For example, estrogen receptor (ER), progesterone receptor (PR), and HER2/ neu oncogene protein are used in the diagnosis and management of breast cancer patients using surgical specimens. ER has been shown to be a prognostic marker for breast cancer and a predictive marker for hormonal treatment ( ). HER2/ neu amplification and overexpression were shown to be associated with poor prognosis and to be a predictor for therapeutic response in breast cancer ( ). PR has been shown to be associated with a good prognosis in ovarian cancer ( ; ).
Among these markers are a number of tumor markers present in circulating body fluids, including blood. Broadly, body fluid tumor biomarkers (principally, serum and urine) are divided into three categories: tumor-associated proteins, such as the oncofetal antigens, which seem to be expressed in many cancers but have also been found to be present in other nonneoplastic conditions; oncoproteins, which are involved in the regulation of the cell cycle and become overexpressed or mutated almost exclusively in neoplastic conditions; and recently discovered patterns of protein expression in serum that appear to be unique to specific types of cancer (i.e., proteomics). This chapter emphasizes the use of the first two classes of tumor marker. Chapter 77 discusses oncoproteins and the use of multiple protein profiles on body fluids, or proteomics, as biomarkers for early tumor detection.
Ideally, a tumor marker should become elevated in the serum only of patients with a malignant tumor and should not be elevated in the serum of disease-free individuals or of individuals with nonmalignant diseases such as inflammatory or infectious diseases. Also, a tumor marker protein should become elevated in the serum of cancer patients at an early stage, thereby enabling early tumor detection and the initiation of appropriate therapy. Although no one tumor marker has been found to meet all of these characteristics, progress toward the discovery of such markers is continuously being made.
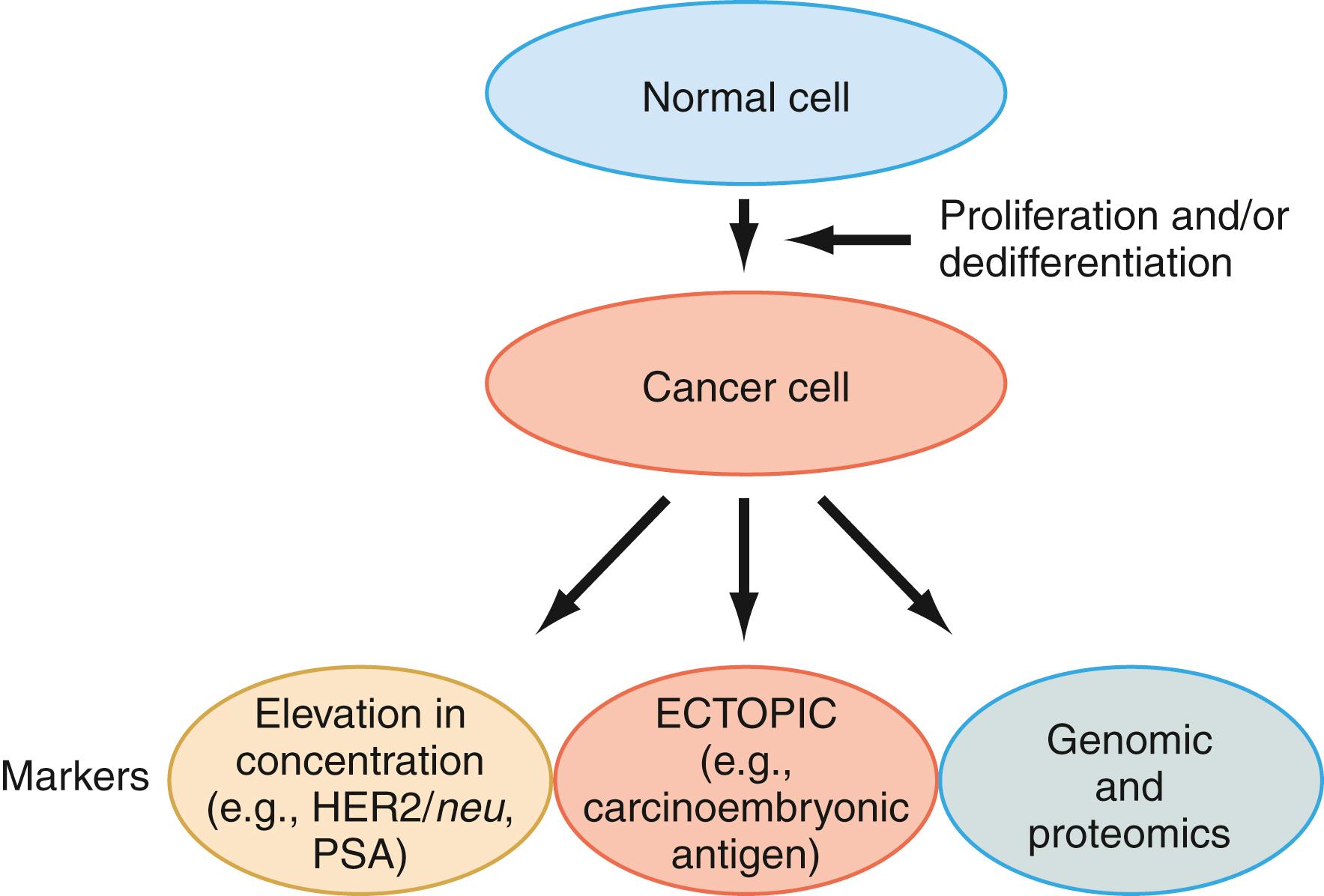
In fact, studies show that one or more cancer biomarkers, including DNA, protein, or tumor cells, are almost always present in the serum of patients with cancer. These findings provide the rational basis for early detection of cancer using serum samples. Any circulating cell products, including DNA, RNA (including microRNA), proteins (enzymes, serum proteins, metabolites, receptors, carcinoembryonic proteins, oncoprotein, and proteins encoded by suppressor genes), and tumor cells, can be used as tumor markers if they are associated with events related to tumor formation and/or growth ( Fig. 76.1 ). Such events include malignant transformation, proliferation, dedifferentiation, and metastasis. The blood levels of serum tumor markers are determined by tumor proliferation, tumor volume, proteolytic activities in the tumor cell, and release from necrotic tumor cells. The recent improvement of instrumentation, especially in consolidation of specialized testing instruments as immunoassay analyzers to a more general chemistry analyzer, facilitated the analysis of a wide range of analytes with the same degree of accuracy, specificity, and precision. The improved sensitivities of the assays made serologic tests far superior to other clinical examinations based on physical methods. An additional advantage of serum testing over tissue-based methods is the noninvasive nature, more accurate quantification, and lack of interobserver difference as opposed to tissue-based methods. Because of these benefits, serum markers are frequently used to screen for, diagnose, and predict the behavior of many cancers.
The clinical value of any given tumor marker will depend on its specificity and sensitivity, as well as its intended clinical use. For example, prostate-specific antigen (PSA) can be used in the screening of prostate cancer, resulting in early detection and treatment. Serum HER2/ neu is used as a marker of prognosis and monitoring therapy for breast cancer. The difference in their usefulness is due to their difference in tissue specificity and cancer sensitivity: PSA is organ specific but not cancer specific, whereas HER2/ neu is cancer specific but not tissue specific and is found to be increased in breast cancers as well as lung and other epithelial cell tumors. The use of tumor markers as prognostic and risk factors has gained more popularity in recent years. Measurement of the level of risk factors has been found to be valuable in the assessment of the aggressiveness of a tumor and is helpful in selecting treatment strategies. The utility of tumor markers is adjunctive to medical and surgical management of malignancies, serving to help detect recurrences as well as predict prognosis.
To learn how to identify, select, and utilize tumor markers for the diagnosis of cancer and the management of cancer patients, it is essential to be familiar with the function of each individual or group of cancer serum markers. This chapter emphasizes the role of three specific classes of tumor markers that are commonly used in the diagnosis of human malignancies: oncofetal antigens, such as α-fetoprotein (AFP) and carcinoembryonic antigen (CEA), which are normally expressed during fetal development but do not occur normally in the tissues or sera of children and adults; proteins occurring in epithelial cells that become elevated in tissue and serum in adenosquamous and squamous cell carcinomas, such as the CA 19-9, CA 125, and CA 15-3 proteins; and polypeptide hormones, such as the β chain of human chorionic gonadotropin (β hCG), and specific enzymes, such as the placental isoform of alkaline phosphatase (ALP), that become elevated in the serum of patients with specific tumors. These latter two tumor markers are frequently elevated in the sera of patients with germ cell tumors. Additionally, hormone-like proteins are found in the sera of patients with different cancers, such as parathyroid hormone–like protein that induces hypercalcemia as part of the so-called paraneoplastic syndrome in such cancers as renal cell carcinomas.
Most of these proteins have been discovered on the basis of having been observed in the sera of cohorts of patients with specific types of cancer. However, many are also found in the sera of patients with nonmalignant (e.g., inflammatory) conditions. In addition, they may not occur in significant numbers of patients in whom these cancers have been diagnosed. The sensitivities and specificities of these tumor marker proteins are often low; thus, they are not useful for screening purposes. On the other hand, all of these proteins are quite useful for monitoring specific cancers. For example, CEA is often elevated in the sera of patients with colon cancer. Thus, if CEA serum levels are found to be elevated in patients who have had a colon cancer resected, this is evidence of tumor recurrence. As discussed later, the one exception is PSA, a chymotrypsin-like enzyme that occurs almost uniquely in the prostate gland and has proved exceptionally useful in screening for prostate cancer and in monitoring patients who are being treated for this disease.
Standard assays for all of these proteins are now well developed and have received approval from the U.S. Food and Drug Administration (FDA) for use in the monitoring of treatment of known cancers but not, except for PSA, for screening for human cancers. Investigations are currently focused on searching for proteins that are expressed only in cancer cells (see Chapter 77 ). This chapter summarizes some of the more recently discovered proteins that are almost always expressed in cancerous but less commonly in noncancerous diseases and are promising for screening for the occurrence of cancer and for monitoring therapy.
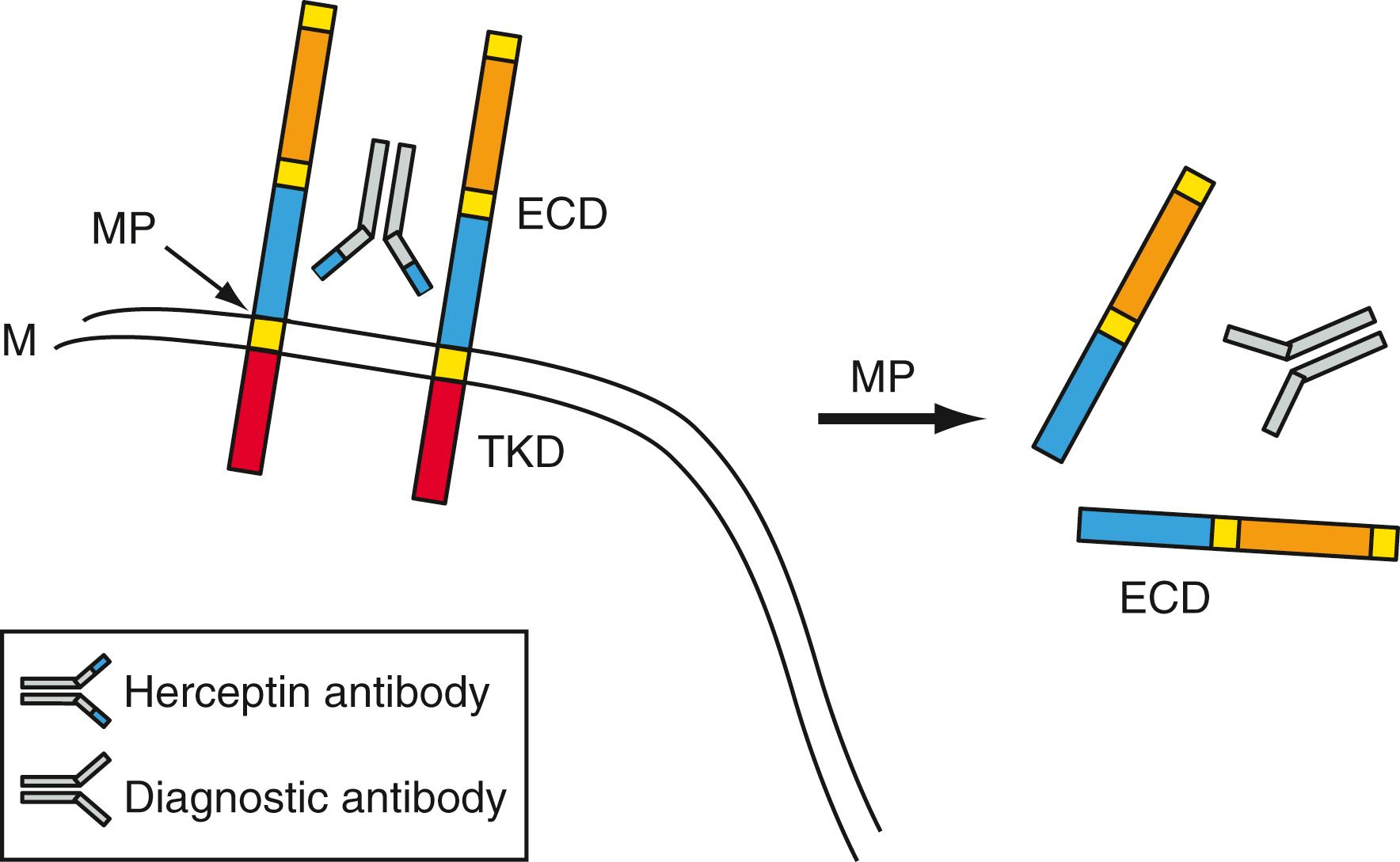
Oncoproteins are proteins that are directly or indirectly involved in the control of mitosis and are altered such that they continuously signal the cell to divide. These proteins either lie on signal transduction pathways carrying mitogenic signals from growth factors at the cell membrane to the nucleus or are involved in the regulation of transcription, activating the genes ultimately causing cell growth and mitosis (see Chapter 77 ). For example, as discussed in Chapter 77 , HER2/ neu is a transmembrane receptor with an extracellular domain (ECD), a transmembrane domain, and an intracytoplasmic domain containing a tyrosine kinase. Upon binding of extracellular growth factors, the intracellular kinase becomes activated, causing dimerization of the receptor and interaction with a cytosolic adapter protein, Grb-2, to relay the message next to a guanine nucleotide exchange factor, SOS. SOS, in turn, binds to the critically important protein Ras-p21 ( ). Oncogenic c-Myc is an example of a different type of oncogene transcriptional factor, which functions via the activation of its target genes, inducing synthesis of mitogenic proteins.
Often, these proteins can be detected in the serum of patients with abnormal cell growth (i.e., cancer or precancerous states). Extensive testing for the presence of oncoproteins has revealed that many oncoproteins are detected in the serum and/or other body fluids of cancer patients. A detailed discussion of each oncoprotein is found in Chapter 77 .
Malignancy is a disorder of the genome. Oncogenesis implicates intricate interactions between forces at the level of the DNA (including sequence and epigenetic framework), RNA processing, and the final protein product. Sanger DNA sequencing, also known as chain terminator sequencing , was developed in 1997 and has been the sequencing standard until the late 2000s ( ). More recently, next-generation sequencing (NGS) integrates the complex processes involved in oncogenesis by identifying recurrent patterns at the level of the genome and exploiting them for clinical gain ( ). NGS is a novel DNA sequencing technology that generates hundreds of millions of short DNA reads that can sequence a human genome quickly with lower expense, permitting determination of point mutations, genomic rearrangements, deletions, amplifications, inversions, translocations, and gene fusions ( ).
Clinical utility of NGS in cancer includes diagnosis, prognosis, prevention, and treatability. NGS-based gene panels have provided us with diagnostic tools enabling individualized care of cancer patients. It is now possible to determine the genomic changes related to disease in each individual patient. Although more than 20,000 genes have been identified in the human genome, only 500 genes are potentially related to cancer. An NGS-based gene panel test can identify gene alterations that are targetable by molecular target drugs ( ). An NGS-based gene panel test has been successful in identifying targetable driver mutations in lung adenocarcinoma such as EGFR, ROS1, and ALK ( ). Furthermore, patients with RET-rearranged non–small cell lung cancer can benefit from vandetanib, which targets RET ( ).
Separate from oncogenes but equally important is a group of suppressor genes. Proteins encoded by suppressor genes are responsible for suppressing cell growth, either causing cell growth arrest in the cell cycle or apoptosis. Frequently, these suppressor genes undergo deletions or mutations, resulting in the production of inactive gene products. Among the tumor suppressor genes, the antioncogene protein p53 has been widely investigated and best characterized for its role in various cancers. It is involved in apoptosis, cell-cycle arrest, cell senescence, and DNA damage response. Deletions or mutations of the p53 gene greatly predispose cells to malignant transformation. p53 mutations have been identified in nearly 50% of human malignancies ( ). Molecular assays are available to detect mutations in the serum DNA of tumor suppressor genes. In addition, antibodies against the abnormal tumor suppressor proteins can be used as a biomarker for cancer.
Molecular methods, such as PCR-SSCP (polymerase chain reaction single-strand conformation polymorphism) and DHPLC (denaturing high-performance liquid chromatography), have been described to detect serum p53 mutations in cancer. Recently, detection and quantification of tumor-specific p53 mutations using droplet digital PCR at the level of circulating tumor DNA has shown promise in the posttreatment surveillance of head and neck cancer patients ( ). More significantly, antibodies against the abnormal tumor suppressor p53 proteins have been detected in the serum of cancer patients ( ). Interestingly, the presence of p53 antibodies is correlated with the p53 mutation ( ; ). In breast cancer, several studies indicated that the presence of p53 antibody in the serum correlated with increased proliferation antigen (Ki-67) and lack of ER expression, indicating that it may serve as a breast cancer prognostic marker ( ; ).
The discovery of two breast cancer susceptibility genes (or tumor-suppressor genes), BRCA1 and BRCA2 , has generated tremendous interest. Studies suggest that mutations in BRCA1 are responsible for approximately half of all cases of inherited breast cancer ( ; ; ). In addition, carriers of BRCA1 mutations are also at an increased risk for ovarian, colon, and prostate cancer ( ). BRCA2 , the second susceptibility gene for breast cancer, is thought to account for approximately 70% of cases of inherited breast cancer that are not due to BRCA1 mutations and is associated with an increased risk of breast cancer in men.
The suppressor genes and their products are potentially useful as tumor markers for the screening and identification of families or high-risk individuals. Development of immunoassays to measure both BRCA1- and BRCA2 -encoded proteins are under development and may be useful for the identification of high-risk individuals and their families.
Tumor metastases involve several major steps ( ). First, the tumor cells have to penetrate their adjacent surroundings, after which they invade vascular or lymphatic vessels. The tumor cells are then carried to distant sites until they are lodged in the venous or capillary beds of a distant organ. In this new environment, these tumor cells must again penetrate the vascular walls in order to grow at the distant site. Cell adhesion molecules, including integrins, selectins, cadherins, and cell adhesion molecules of immunoglobulin gene families, regulate many steps of the metastatic process. The changes in their levels of expression reflect the malignant behavior of cancer cells. For example, serum levels of E-selectin, intercellular adhesion molecule (ICAM), and vascular cell adhesion molecule (VCAM) are increased, in particular, in late-stage breast cancer patients ( ; ). Elevated serum VCAM level may be used to predict a shorter survival. Another study indicated that postchemotherapeutic levels of serum E-selectin and ICAM are associated with a response to treatment of patients with Hodgkin disease ( ). Furthermore, increased E-selectin, ICAM-1, and VCAM are suggested to be a prognostic factor for survival in patients with gastric cancer ( ). Therefore, the appearance of these cell adhesion molecules in blood circulation might indicate the risk or occurrence of metastases or a poor prognosis.
The development of hybridoma technology has greatly impacted the identification of tumor markers ( ). Rather than dealing with a whole molecule of known protein structure, it is now possible to focus on only a small surface area, an epitope or antigenic determinant, using monoclonal antibodies. It is no longer necessary to purify the antigen for the preparation of polyclonal antibodies in animals. The complete characterization and identification of the molecule carrying the epitope is also no longer needed. A hybridoma can be prepared by injecting a mouse with an enriched fraction of the tumor cell membrane or the whole tumor cell. Hybridomas producing the monoclonal antibodies of interest are selected through the subsequent screening procedure. Once a hybridoma is established, there will be an unlimited and consistent supply of monoclonal antibody (MAb) for various uses.
By combining the MAbs with the solid-phase sandwich test design, new assays have been developed that have eliminated many problems associated with polyclonal assays, involving poor reproducibility, lot-to-lot variations, poor specificity, and nonspecific cross-reactivity ( ). It also reduces the differences between different kits and widens the linear concentration range for the assay. Whenever an MAb is available, its use is recommended. To achieve higher test sensitivity, the use of a combination of multiple MAbs has been found to improve the affinity between solid-phase-absorbed-multiple MAbs and the soluble antigen.
Tests for the MAb-defined tumor markers have been demonstrated to have a higher sensitivity and specificity than those using polyclonal antibodies. For example, CA 19-9, CA 125, and CA 15-3 are much more sensitive and specific than CEA for pancreatic, ovarian, and breast carcinomas, respectively. These markers are recommended to replace the polyclonal CEA test for the diagnosis and management of patients with these carcinomas. Various tumor markers derived from different tumors also share many tumor-associated epitopes. For example, CA 19-9, CA 15-3, and CA 125 are expressed by almost all carcinomas in varying degrees. In addition to the sharing of any given epitope by more than one carcinoma, it is also possible for a single molecule to express more than one epitope ( ). For example, it is likely that CA 15-3 and CA 125 are expressed by the same mucin molecule.
Many hormones (e.g., hCG, epinephrine, dopamine, and serotonin), serum proteins, enzymes (lactate dehydrogenase, ALP), and their metabolites, such as the metabolites of the neuroendocrine hormones (e.g., vanillylmandelic acid, homovanillic acid, and 5-hydroxyindoleacetic acid), may become elevated in tumors because of the high proliferation rate of tumor cells. Their serum levels rise to even higher levels when a benign tumor becomes malignant and metastasizes. Benign and nonmalignant diseases may also involve elevated levels of markers. Thus, these markers are not suitable for screening or for cancer diagnosis because of the large numbers of false-positive results that would be generated. These markers are more appropriately used for monitoring patient response during treatment.
One exception relates to the enzyme ALP discussed in Chapter 21 . There are multiple isozymes of this enzyme, one of which is the placental isozyme placental ALP (PLAP). This form becomes elevated in the serum of patients who have germ cell tumors. One particular use of PLAP is in the serum or, more effectively, in the cerebrospinal fluid (CSF) of patients with a mass in the pineal region with a differential diagnosis of germ cell tumor versus pinealoma. If PLAP is elevated in the CSF of a patient with a mass in the pineal area, a diagnosis of germ cell tumor can be made. As it happens, radiation therapy is curative, obviating the need for surgery.
The enzymatic activities of various tissue-specific glycosyltransferases are altered in tumor cells. Some of the elevated glycosyltransferases have been used as tumor markers. The sugar sequence and composition of the carbohydrate moiety of many serum glycoproteins, including blood group substances and mucins, such as CA 19-9, are tumor markers resulting from altered glycosyltransferase activity. The AFP isolated from patients with primary hepatocellular carcinoma has an additional fucose compared with the AFP from benign liver disease, an example of altered fucosyltransferase in hepatocellular carcinoma cells ( ).
Ectopic proteins are often expressed in cancer. Carcinoembryonic proteins, which are detectable in both fetal and tumor tissues but not in normal adult tissues, usually lack any known physiologic function and have blood concentrations at nanogram-per-milliliter levels. Therefore, measurements of carcinoembryonic proteins in the circulation must rely on immunoassays. The specificity and sensitivity associated with these proteins, although not 100%, are much higher than those of enzymes and metabolites that have been used as tumor markers in the past. The serum concentration of these carcinoembryonic proteins not only correlates well with tumor activity but can also be used to predict prognosis. However, carcinoembryonic proteins in general are not suitable for screening because the polyclonal antibodies directed against these proteins often cross-react with other, normal proteins, and these carcinoembryonic proteins do not appear sufficiently early in the blood from cancer patients to detect the tumors at initial stages. However, they have been used as adjunct tests for cancer diagnosis and are extremely useful for monitoring the success of treatment and for detecting recurrence.
The serum tumor markers are currently used for screening, diagnosing, and predicting prognosis and treatment response. Use of tests for screening of disease, even those with high sensitivity and specificity, should be confined as much as possible to populations at risk for the disease. This is because the positive predictive value depends on the prevalence of the disease. Because most of the tumor markers described in this chapter are expressed both in neoplastic and benign conditions, their use is confined to following possible tumor recurrence in patients being treated for specific types of tumor.
The recommendation of screening for prostate cancer by the measurement of serum PSA in combination with a digital rectal examination (DRE) in men older than age 50 years is due to the high tissue specificity of PSA ( ) and the high prevalence of prostate cancer. Combination of the PSA test and DRE provides the least costly approach to the early detection of prostate cancer ( ). PSA screening is especially recommended for African American men because the incidence of prostate cancer for African Americans is nearly twice that of the general population and the death rate is up to three times higher. Screening permits the treatment of organ-confined, potentially curable prostate cancer discovered in men with a life expectancy of longer than 10 years.
Although not approved for screening for hepatocellular carcinoma in the United States, AFP has been used to screen for primary hepatocellular carcinoma in China because of the high incidence of liver cancer in that country. The diagnosis of ovarian cancer has traditionally relied on imaging and discovery at surgery (e.g., exploratory laparotomy). However, in most cases, at the time of detection, the tumor has advanced to a stage at which the possibility of cure is low. The feasibility of screening for ovarian cancer in women by measuring serum CA 125 is being investigated.
Several approaches have been suggested recently to improve the diagnostic yield of many tumor markers. The use of multiple markers is one approach that has received wide acceptance. As is also noted in Chapter 77 , specific patterns of multiple tumor markers seem to be associated with individual malignant diseases. Another approach to improving both the specificity and sensitivity of tumor markers, as in the case of a serum PSA test, involves the measurement of the velocity (the rate of increase in PSA concentration over time) and the density (e.g., by dividing the serum PSA concentration by the volume of the prostate gland, determined by transrectal ultrasound) ( ). These efforts aim at a better discrimination between benign and malignant states. For example, a mildly elevated serum PSA level associated with a small prostate gland may indicate cancer, whereas the same PSA value in a patient with a large gland may indicate benign prostatic hypertrophy (BPH).
The assessment of tumor aggressiveness and the prognosis for the outcome of a cancer patient has received much attention in recent years. The knowledge of tumor aggressiveness helps in the development of a proper therapy for the patient. For example, the detection of tumor markers, highly associated with malignancy and metastases, will suggest a more rigorous and systemic treatment. Monitoring tumor markers for the detection of recurrence following the surgical removal of the tumor is the second most useful application of tumor markers. It is well known that the appearance of most of the circulating tumor markers has a lead time of several months (3–6 months) before the stage at which many of the physical procedures could be used for the detection of cancer. The specificity of tumor markers does not present a problem for this application. The ease of drawing blood and the sensitivity of tumor marker tests make this noninvasive monitoring process now widely accepted.
Most tumor markers become increasingly elevated when the tumor metastasizes. Very few tumor markers have a clear-cut boundary between benign and malignant stages. Proteins that reflect the risk factors associated with the process of tumor metastases, such as proteases and adhesion molecules, are usually better markers for predicting prognosis. However, most of these markers are still measured in tumor tissues and tissue lysates. The finding of the ECD of c- erb B-2 protein in the serum of cancer patients ( Fig. 76.2 ) and the correlation of the serum ECD with the levels of other serum tumor markers are encouraging. Another area of intensive study is to explore the use of serum marker surrogates in the prediction of cancer patient survival.
One of the most useful applications of tumor markers involves monitoring the course of the disease, especially during treatment. The serum level of tumor markers reflects the success of surgery or the efficacy of chemotherapy. Detecting elevated levels of a tumor marker after surgery would indicate incomplete removal of the tumor, recurrence, or the presence of metastases. The measurement of serum tumor markers during chemotherapy also gives an indication of the effectiveness of the antitumor drug used and a guide for the selection of the most effective drug for each individual case.
When ordering tumor markers as an adjunct diagnostic test for managing cancer patients, the following recommendations should be kept in mind in order to avoid misinterpretation of the test results:
Never rely on the result of a single test. Because of low specificity associated with most tumor markers, it is difficult to differentiate between malignant and benign diseases on the basis of a single test result. Most tumor marker elevations found in nonmalignant diseases may be transient, whereas with cancer, the level often either remains elevated or rises continuously. Ordering serial testing can help detect falsely elevated levels due to transient elevation. For example, elevated serum AFP can be detected in patients with either primary hepatocellular carcinoma or benign liver disease. On a subsequent testing 2 weeks later, the serum AFP will remain elevated in patients with cancer, whereas in patients with benign conditions, the serum AFP may return to normal levels.
When ordering serial testing, be certain to order every test from the same laboratory using the same assay kit. Each different commercial kit may generate different results even though all are designed for the same tumor marker. Ordering from the same laboratory also ensures a more consistent performance. It is important to ensure that any change observed during the monitoring process is due to a change of tumor volume or other tumor activities and not to laboratory variability.
Be certain that the tumor marker selected for monitoring recurrence was elevated in the patient before surgery. Because none of the tumor markers are 100% sensitive to the detection of any particular cancer, it is important to be certain that the tumor marker ordered to detect recurrence was elevated before surgery. Otherwise, multiple markers should be measured before the surgery in order to select the tumor marker showing the highest elevation as the marker for monitoring the disease activity. Multiple markers may be used to monitor the therapeutic effects for increased sensitivity.
Consider the half-life of the tumor marker when interpreting the test result. Before surgery, estimate the time required for the level to decline to the normal or, in the case of PSA, to an undetectable level, based on the known half-life of the tumor marker. It is important that the success of surgical removal of a tumor as determined by tumor marker concentrations is not assessed earlier than 2 weeks postoperatively. If possible, it is preferable to wait 1 month to allow the preexisting tumor marker in the serum adequate time to decline to lower levels. For example, the half-life of serum PSA is approximately 3 to 4 days. Therefore, it takes 30 days for a serum PSA at 50 ng/mL to drop to an undetectable level following successful surgery.
Consider how the tumor marker is removed from or metabolized in the blood circulation. Elevated serum tumor markers are frequently detected in patients with renal or liver disease depending on whether the tumor marker is removed through the kidney or metabolized by the liver. For example, serum CEA is often elevated in patients with liver disease because the impaired liver fails to remove CEA efficiently from the blood circulation, whereas an elevated serum β 2 -microglobulin (β 2 M) has been frequently found in patients with renal failure in which even the small β 2 M molecule has difficulty passing through the glomerular membrane in a normal fashion.
Consider ordering multiple markers to improve both the sensitivity and the specificity for diagnosis. Tumors are made of heterogeneous types of cells. Some may still be normal, while others may be heterogeneous tumor cells as a result of different sequences of multiple mutations. Each type of cell may express a single marker, such as those shown in Table 76.1 , or a number of characteristic tumor markers. The same marker may also be produced by different types of cells. Some cells may never produce any unique marker. Certain types of cancer are heterogeneous in their cellular composition. Consequently, more than one tumor marker may be required to provide a high sensitivity of detection. The heterogeneity in cell composition and the percentage of cell distribution of each tumor explains why a number of tumor markers may be required to reach a high (>90%) detection sensitivity and is the reason that the sensitivity of an individual marker differs among cancer patients. Multiple tumor markers associated with individual malignant diseases are listed in Table 76.2 ; the appearance of individual tumor markers in various malignancies is listed in Table 76.3 . This explains why none of the tumor markers presently employed is 100% sensitive and specific and why the use of multiple markers will improve the sensitivity of detection. However, a unique pattern of multiple markers may be identified with tumors derived from the same tissues. Therefore, ordering multiple tumor markers may also improve test specificity. For example, a specific pattern seems to be associated with colon, breast, ovarian, and pancreatic carcinomas when all four MAb-defined tumor markers—CEA, CA 19-9, CA 15-3, and CA 125—are measured simultaneously. This information is clinically important, because more than 60% of diagnosed human cancers are epithelial-cell-derived carcinomas ( ). Multiple markers were used to develop a more specific screening strategy for ovarian cancer. The use of CA 15-3 and CA 72-4 in combination with CA 125 can increase the apparent specificity of the CA 125 assay for distinguishing malignant from benign ovarian disease ( ). Another example is the combination of CEA, CA 19-9, and CA 72-4. Use of this combination improves the diagnostic accuracy of gastrointestinal cancers ( ). During the selection of multiple tumor markers, only markers that are complementary to one another should be selected. Many tumor markers, which run parallel to one another when correlated with tumor activities, should not be selected for this purpose.
| Tumor Marker | Major Malignant Disease |
|---|---|
| CA 125 | Ovarian carcinoma |
| CA 19-9 | Pancreatic carcinoma |
| CA 15-3 | Breast carcinoma |
| CA 72-4 | Gastric carcinoma |
| HER2/ neu | Breast carcinoma |
| Malignant Disease | Major Marker | Other Markers |
|---|---|---|
| Neuronal Tumors | ||
| Brain tumor | Desmosterol | Polyamines |
| Neuroblastoma | VMA | HVA, NSE, cystathionine, ferritin, metanephrines |
| Head and Neck Tumors | ||
| Squamous cell carcinoma | CYFRA 21-1 | |
| Endocrine System | ||
| Pituitary tumors | Growth hormone | IGF-I |
| Adrenal pituitary tumors | Cortisol | Free catecholamines, DHEA, 17-ketosteroids, prolactin |
| Cushing syndrome | ACTH | Endorphin, lipotropin |
| Hypercalcemia of malignancy | PTH-related peptide | |
| Endocrine pancreatic tumors: | Pancreatic polypeptide: | Chromogranin AC-peptide, IGF-I binding protein I |
| Gastrinoma | Gastrin | |
| Glucagonoma | Glucagon | |
| Insulinoma | Insulin | |
| Medullary carcinoma of thyroid | Calcitonin | NSE |
| Microadenomas (pituitary) | Prolactin | |
| Multiple endocrine neoplasias | Chromogranin A | |
| Papillary and follicular thyroid cancer | Thyroglobulin | |
| Parathyroid tumors | PTH-intact | |
| Zollinger-Ellison syndrome | Gastrin | |
| Pheochromocytoma | Metanephrine | Chromogranin A, plasma catecholamines |
| Pituitary tumors | Free β hCG | FSH, LH, prolactin, TSH |
| Bone and Skeletal Muscle System | ||
| Osteosarcomas | Alkaline phosphatase | |
| Breast Cancer | ||
| Breast cancer | HER2/ neu , CA 15-3 | CYFRA 21-1, CEA, calcitonin |
| Pulmonary System | ||
| Bronchogenic carcinoma | Prolactin | |
| Lung cancer (NSC) | CYFRA 21-1, NSE | ACTH, CK-BB, calcitonin, CA 72-4, CEA, AFP, ferritin, LASA-P, TPA |
| Carcinoid tumors | Histamine, ADH, bradykinin | |
| Oat cell cancer | ACTH, ADH, CEA, CK-BB, NSE, bombesin, calcitonin | |
| Gastrointestinal System | ||
| Colorectal cancer | CEA | CA 19-5, CA 19-9, CA 72-4, NSE |
| Gastric carcinoma | CA 72-4 | CA 19-9, CA 50, CEA, ferritin, CK-BB, hCG, LASA-P, pepsinogen II |
| Hepatocellular carcinoma | AFP | CEA, ferritin, ALP, TPA, γ-glutamyltranspeptidase (GGT) |
| Pancreatic carcinoma | CA 19-9 | CA 19-5, CA 50, CA 72-4, CEA, CK-BB, ADH, ALP |
| Vipoma (pancreas) | VIP | |
| Genitourinary System | ||
| Bladder cancer | T-antigen, urokinase inhibitor, TPA, cytokeratins | Glycosaminoglycans, uroplakins |
| Nonseminomatous testicular tumor | AFP | hCG |
| Prostate carcinoma | PSA | PAP, racemase |
| Renal cell carcinoma | Renin, erythropoietin, IL-4, PGA | |
| Testicular cancer | hCG | PLAP, Oct3/4 |
| Gynecological Tumors | ||
| Cervical cancer | SCC | CA 125, CEA, TPA |
| Ovarian carcinoma | CA 125 | UGF, inhibin, AFP, amylase isoenzyme, CEA, CK-BB, hCG |
| Choriocarcinoma | hCG | |
| Placental tumors | hCG | Free α hCG, CA 15-3, PTH, NSE, prolactin |
| Uterine cancer | SCC | |
| Teratoblastoma | AFP | hCG, ferritin |
| Hematologic Malignancies and Lymphomas | ||
| B-cell chronic lymphocytic leukemia | TdT | Serum β 2 M, LASA-P |
| B-cell malignancies | β 2 M | |
| Chronic myelogenous leukemia | TdT | |
| Hairy cell leukemia | IL-2 receptor | |
| Hodgkin disease | LASA-P, ferritin | |
| Leukemia | TdT | ALP, β 2 M, ferritin, LD, myelin basic protein, adenosine deaminase, PNP |
| Lymphoma | β 2 M | TdT, Ki-67, LASA-P |
| Multiple myeloma | Ig heavy and light chain | Bence-Jones protein, β 2 M, IgA, serum free light chain |
| Waldenström disease | IgM | β 2 M, serum free light chain |
| Melanoma | ||
| Melanoma antigen | Melanoma-associated LASA-P, l -dopa | C-reactive protein |
| Endoreticular System | ||
| Spleen tumors | Ferritin | |
| Other | ||
| Sarcoma | β 2 M | |
| Tumor Marker | ASSOCIATED MALIGNANT DISEASE | |
|---|---|---|
| Major Disease | Minor Disease | |
| α-Fetoprotein | Primary hepatocellular carcinoma | Teratoblastomas of the ovary and testes |
| β hCG | Pituitary tumors | Yolk sac tumor or mixed germ cell tumor |
| β 2 -Microglobulin | B-cell neoplasias | Multiple myeloma, B-cell lymphoma, B-cell chronic lymphocytic leukemia, and reticulum cell sarcoma; Waldenström macroglobulinemia |
| β hCG | Choriocarcinoma | Testicular cancers (nonseminomatous), trophoblastic tumors |
| Bence-Jones protein | Multiple myeloma | |
| Bombesin | Oat-cell cancer | |
| C-reactive protein | Melanoma | |
| CA 15-3 | Breast cancer | Various carcinomas |
| CA 19-9 | Pancreatic and gastric carcinoma | Various carcinomas |
| CA 72-4 | Gastric carcinoma | Various carcinomas |
| CA 125 | Ovarian carcinoma | Various carcinomas |
| CA 549 | Breast cancer | |
| CA M26 | Breast cancer | |
| Calcitonin | Medullary carcinoma | Cancer of the thyroid, liver cancer, renal cancer |
| Carcinoembryonic antigen | Colorectal carcinoma | Various carcinomas |
| c- erb B-2 oncoprotein | Breast carcinoma | Various carcinomas |
| Chromogranin A | Pheochromocytoma, neuroblastoma | Multiple endocrine neoplasias, small cell lung cancer, carcinoid tumors |
| CYFRA 21-1 | Squamous cell carcinoma of the lung | |
| DHEA | Adrenal/pituitary cancer | |
| Ferritin | Acute myelocytic leukemia | Hodgkin lymphoma, neuroblastoma and various carcinomas, teratoblastoma |
| Galactosyltransferase | Ovarian cancer | |
| Galactosyltransferase isoenzyme II | Pancreatic cancer | |
| Gastrin | Gastrinoma | Zollinger-Ellison syndrome |
| Her2/ neu | See c- erb B-2 oncoprotein | |
| Human chorionic gonadotropin | Choriocarcinoma | Gastric, ovarian, and breast carcinoma, trophoblastic or germ cell tumors, testicular cancer |
| Hyaluronic acid | Mesothelioma | |
| Immunoglobulin A | Multiple myeloma | |
| Insulin-like growth factor-I | Pituitary cancer | Insulinoma |
| Interleukin-2 receptor | Leukemia | |
| Immunoglobulins | Multiple myeloma | Mediterranean lymphoma, Waldenström macroglobulinemia, malignant lymphoma |
| Inhibin | Granulosa-cell tumors | |
| 17-ketosteroids | Adrenal/pituitary cancer | |
Be aware of the presence of ectopic tumor markers. The expression of tumor markers is under genetic regulation. For benign tumors, there are often proteins produced by the tumor that are cell specific and related to normal cell products at an elevated concentration (see Fig. 76.1 ). However, if a benign tumor becomes malignant, synthesis of these cell-specific proteins may no longer occur in the malignant cells. Conversely, proteins that are normally found at an early fetal stage and not in normal cells or in benign tumors of these cells may become constitutively expressed in the malignant cells. This is the reason that carcinoembryonic proteins and ectopic tumor markers are usually expressed in malignant cancers, often at advanced stages. Thus, the appearance of ectopic tumor markers is often associated with poor prognosis or metastasis. For example, elevated serum concentrations of AFP may be detected in patients with cancers of the gastrointestinal tract involving metastases even though the liver function tests are normal. Table 76.4 lists some of the known ectopic markers and their associated malignant diseases. One should be aware of the guidelines on gastrointestinal and breast cancer marker use published by the American Society of Clinical Oncology ( ). They recommend monthly breast self-examination, annual mammography of the preserved and contralat eral breast, and a careful history and physical examination every 3 to 6 months for 3 years, then every 6 to 12 months for 2 years, and annually thereafter. They do not recommend the use of tumor markers (e.g., CEA, CA 15-3, and CA 27.29) for screening, nor do they recommend routine bone scans, chest radiographs, hematologic blood counts, liver ultrasonograms, or computed tomography for screening. The American College of Physicians has also published clinical guidelines concerning the early detection of prostate cancer. They emphasize the importance of both screening PSA and performing DRE for the early detection of prostate cancer. Even though DRE is not as sensitive as PSA screening, it can detect cancer that would otherwise be missed by PSA measurement ( ; ).
| Marker | Tumor |
|---|---|
| α-Fetoprotein | Gastrointestinal, renal, bladder, and ovarian carcinoma |
| Calcitonin | Endocrine tumors (islet cell, carcinoid, medullary thyroid, pheochromocytoma); lung, breast, and ovary carcinoma |
| Chromogranin A | Endocrine tumors (islet cell, carcinoid, medullary thyroid, pheochromocytoma), prostate cancer |
| Free α hCG | Colorectal carcinoma and pancreatic endocrine tumors |
| hCG | Gastric and pancreatic carcinoma, hepatoma, ovarian carcinoma, germ cell tumor of testis |
| PTH | Renal cell carcinoma, breast, squamous cell carcinoma, bladder and ovarian carcinomas |
| Thyroglobulin | Differentiated thyroid carcinoma |
Heterophilic antibody. The use of MAbs in immunoassays and the increasing clinical application of mouse MAbs for targeted imaging and immunotherapy create a new problem. Treated individuals apparently produce heterophilic antibodies against murine antibodies that interfere with many of the immunoassays for tumor markers ( ) although this is not a common encounter. The interference by the heterophilic antibodies in human sera can either increase or decrease the results of an immunoassay. These antibodies react in a way similar to antigens in terms of binding to both solid-phase-associated and signal-labeled antibodies. These heterophilic antibodies may bind to a site other than the analyte-binding site, cross-linking the signal antibody with the capture antibody and, thus, generating a false assay response. Conversely, they may block antibodies used in immunoassays from binding to the antigen (tumor marker), giving a falsely low result. Fortunately, interfering heterophilic antibodies in immunoassays are uncommon. The standard approach for reducing heterophilic antibody interference is to include excess mouse sera or nonspecific mouse immunoglobulins in the immunoassay or to avoid using mouse antibodies.
AFP is a major fetal serum protein and is also one of the major carcinoembryonic proteins. AFP resembles albumin in many physical and chemical properties. In the fetus, AFP is synthesized by the yolk sac and fetal hepatocytes and, to a lesser extent, by the fetal gastrointestinal tract and kidneys. Elevated AFP can be found in patients with primary hepatocellular carcinoma and yolk sac–derived germ cell tumors (mainly endodermal sinus tumor) and is the most useful serum marker for these cancers ( ). However, AFP is also transiently elevated during pregnancy and in many benign liver diseases. Because of the high prevalence of liver cancer in China and other countries in Southeast Asia, AFP testing has been used successfully in screening for hepatocellular carcinoma in that region of the world. Tests for both AFP and hCG are helpful in reducing clinical staging errors in patients with some testicular tumors and aid in the differential diagnosis of various germ cell tumors. Because an increase of fucosylation of AFP (hence, the lentil lectin reactivity of serum AFP) has been found in primary hepatocellular carcinoma, the determination of lentil lectin reactivity of serum AFP was found to be helpful not only to differentiate between primary hepatocellular carcinoma and benign liver diseases but also to provide an early signal indicating that hepatocellular carcinoma may begin to develop in patients with liver disease. Although the necessity for routine AFP screening needs further study, one study indicates that combined screening with AFP and ultrasonography results in increased sensitivity—from 75% to near 100%—in detecting hepatocellular carcinoma of patients with hepatitis B and hepatitis C ( ; ). Finally, AFP is currently offered for prenatal screening for neural tube defects and, in conjunction with free β hCG and unconjugated estriol, for Down syndrome (triple test) ( ; ). (See also Chapter 26 .)
Angiogenesis is the formation of blood vessels in situ, involving the orderly migration, proliferation, and differentiation of vascular cells. Blood vessel formation is also important in the pathogenesis of rapid growth and metastasis of solid tumors. Several angiogenic factors have been identified, including acidic and basic fibroblast growth factor (bFGF), described in Chapter 77 ; angiogenin; and vascular endothelial growth factor (VEGF) ( ). Both angiogenic and antiangiogenic factors have been found in the serum of patients with malignant disease ( ). Serum levels of bFGF and VEGF reflect their expression in individual tumors ( ; ). The significance of elevated serum VEGF in cancer patients has been evaluated in several studies, including breast, ovarian, hepatocellular, colorectal, and renal cell carcinomas and soft-tissue sarcoma. Elevated serum VEGF values in ovarian cancer patients were correlated with cancer differentiation, metastasis, and, more significantly, shorter average survival time ( ; ; ). Furthermore, elevated serum VEGF has been associated with shorter survival in renal cell carcinoma ( ) and colon carcinoma ( ).
β 2 M is the constant light chain of the human histocompatibility locus antigen expressed on the surface of most nucleated cells. The molecular weight of β 2 M is only 11.8 kDa. β 2 M is shed into the extracellular fluid and is elevated not only in solid tumors but also in lymphoproliferative diseases (including B-cell chronic lymphocytic leukemia, non-Hodgkin lymphoma, and, importantly, multiple myeloma) ( ). Serum concentration of β 2 M correlates with lymphocyte activity, making β 2 M a good marker for lymphoid malignancies of the B-cell line. It has been used as an indicator of the patient’s response to treatment ( ). CSF levels of β 2 M are useful for detecting metastases in the central nervous system. It is reported that serum β 2 M level is elevated in 75% of patients with multiple myeloma ( ) and is useful in following the efficacy of the treatment of this disease, although currently serum free light chain (FLC) assay has been found to be more effective in following this disease (see later discussion).
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here