Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Both type 1 and type 2 diabetes mellitus commonly target the nervous system. In the peripheral nervous system, complications include polyneuropathies and focal neuropathies. In the central nervous system (CNS), diabetes may be associated with cognitive decline, leukoencephalopathy, and heightened risk of both stroke and dementia. Acute changes in blood glucose levels are also associated with neurologic signs and symptoms. This chapter summarizes the acute and chronic neurologic complications of diabetes mellitus. For a detailed discussion of diabetes and the nervous system, with relevant reference citations, readers are also referred to a recent monograph on the topic.
Diabetic ketoacidosis in patients with type 1 diabetes is a medical emergency that may present with neurologic signs and symptoms. Anorexia, lethargy, thirst, polyuria, vague abdominal pain, and Kussmaul respiration are followed by confusion and a decreased level of consciousness. Rarely, diabetic ketoacidosis accompanies a primary CNS infection, such as bacterial meningitis. Cerebral edema complicates diabetic ketoacidosis and may present with headache, papilledema, and bilateral abducens neuropathies. It may develop on presentation or during correction of the metabolic disorder. Secondary complications include cerebral infarction, cerebral venous sinus thrombosis, and compression neuropathies.
Neurologic symptoms and signs may also reflect a nonketotic hyperosmolar syndrome, defined as a blood glucose level exceeding 33 mmol/L (600 mg/dL) and a plasma osmolarity greater than 320 mOsm/L, without accompanying acidosis or ketonemia. Polyuria, polydipsia, thirst, fatigue, and overall weakness are general symptoms; neurologic signs include decreased level of consciousness, hemiplegia, aphasia, brainstem abnormalities, dystonia, chorea, and seizures. The seizures are often focal and can include tonic, movement-induced, or continuous forms (e.g., epilepsia partialis continua). Unusual neurologic features are visual symptoms, hallucinations, hemichorea, tonic eye deviation, nystagmus, abnormal pupils, and meningeal signs. The correction of hyperglycemia may be more effective than using antiepileptic agents to treat the seizures.
Hypoglycemia most often presents with altered neurologic function. In diabetic subjects, hypoglycemia is common in the setting of insulin use. A high index of suspicion is required as patients may present with focal neurologic signs or seizures. Hypoglycemia is defined as a plasma glucose concentration less than 2.7 mmol/L (50 mg/dL), a level associated with a decline in cognitive function. Premonitory systemic symptoms include anxiety, tachycardia, perspiration, nausea, and tremor, but these signs may be absent in patients taking β-adrenergic-blocking medications or in patients with autonomic neuropathy. Early neurologic symptoms are decreased attention and concentration, drowsiness, poor memory, disorientation, behavioral changes, clumsiness, and tremor. Patients may progress to experience seizures and loss of consciousness. Seizures may be focal or generalized and may lead to status epilepticus. Rapid detection through expectant testing and early treatment are essential. Severe untreated hypoglycemia is associated with diffuse cortical, basal ganglia and dentate gyrus damage, leading to permanent disability.
Diabetes mellitus targets the peripheral nervous system in several ways. Polyneuropathy is a chronic, symmetric disorder that targets the distal terminals of axons first. Focal or localized neuropathies of a single plexus or nerve, also known as mononeuropathies, are also common and develop from mechanical compression, ischemia, or other, less well-defined causes. Autonomic neuropathy is the other major category of peripheral nervous system dysfunction in these patients.
Diabetic polyneuropathy is the most common form of peripheral neuropathy. With detailed evaluation, approximately 50 percent of both type 1 and type 2 diabetic subjects have evidence of polyneuropathy. Symptomatic polyneuropathy may occur in a smaller proportion, estimated as approximately 15 percent of these patients. Lower prevalence numbers have been derived from studies of hospitalized patients or utilizing exclusively clinical signs of polyneuropathy. Polyneuropathy is most often sensory, or sensorimotor but with lesser motor involvement. Positive sensory symptoms are common and include prickling, tingling, “pins and needles,” burning, crawling, itching, electric, sharp, jabbing, and tight sensations in the legs, feet, hands, and fingers. Warm stimuli may be inappropriately perceived as cold, and cold stimuli as warm or hot. Nocturnal burning of the feet accompanies allodynia (the generation of pain or discomfort from normally innocuous stimuli). Many of these symptoms are associated with pain, sometimes severe or intractable, as discussed later.
Symptoms are generally symmetric and initially confined to the toes, with later spread to more proximal parts of the feet and legs and to the fingers ( Fig. 19-1 ). Negative symptoms include loss of sensation to light touch, pinprick, and hot and cold. In more severe diabetic polyneuropathy, loss of protective sensation predisposes patients to the development of foot ulcers. Additional factors that promote foot ulceration include loss of sweating, abnormal foot architecture from muscle wasting, delayed healing, and both macrovascular (atherosclerosis) and microvascular disease.
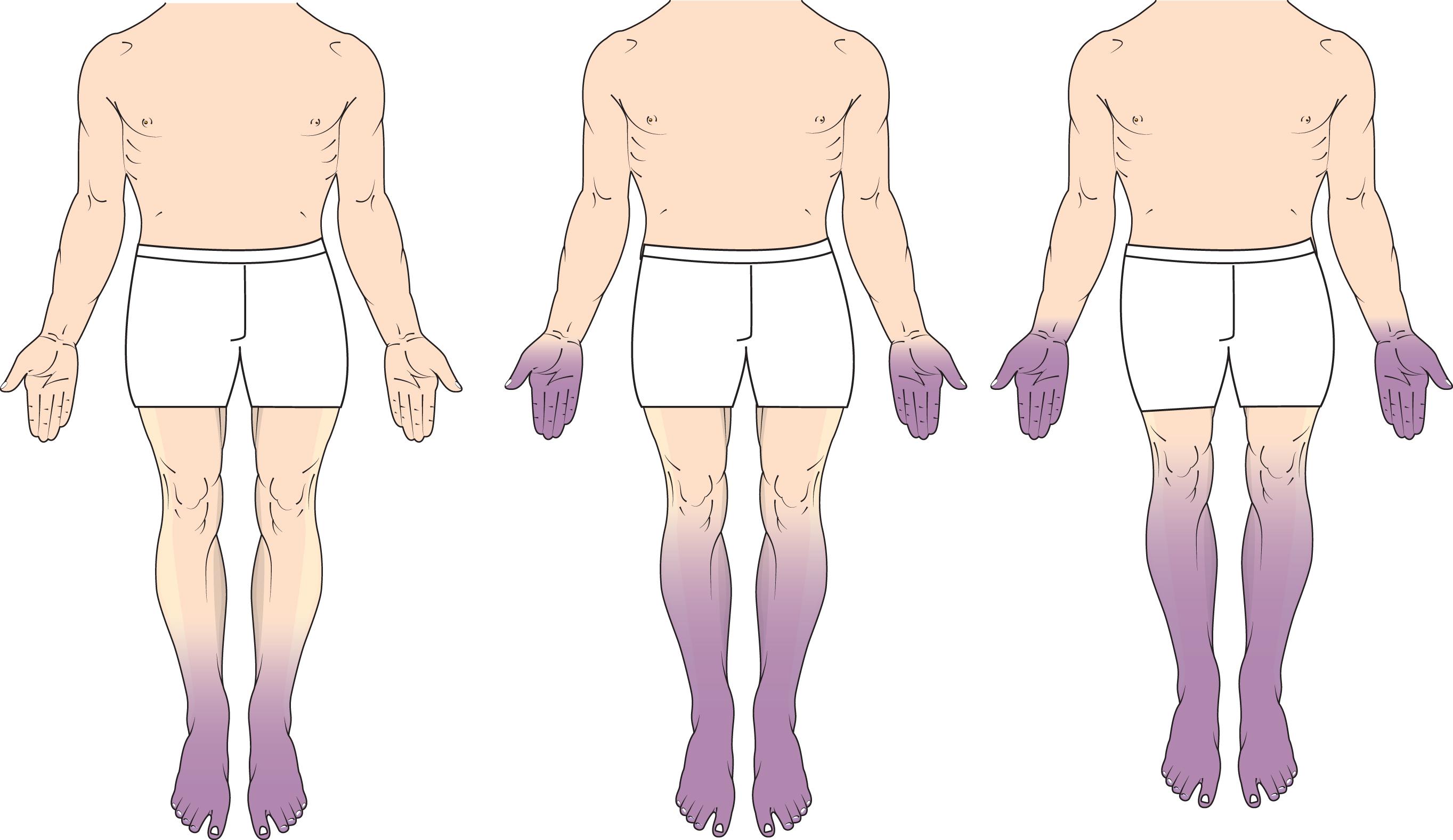
There is a stocking-and-glove pattern of sensory symptoms and loss. Motor involvement is less common in early diabetic polyneuropathy but may eventually lead to distal weakness of foot and toe dorsiflexion, predisposing patients to falls. Weakness accompanies wasting of intrinsic foot muscles. Symptoms from concurrent abnormalities of the autonomic nervous system are common. They include erectile dysfunction (ED) in men, distal loss of sweating, orthostatic dizziness, and bowel and bladder dysfunction.
A detailed neurologic examination provides a low-cost, patient-interactive means of direct evaluation. While some variation in findings, especially in patients with early disease, is expected, the examination remains the gold standard for diagnosis and is not replaced by quantitative methods or electrophysiologic evaluation, which are considered ancillary tests.
On sensory examination, there is distal loss of sensation to light touch, pinprick, cold, and vibration with a 128-Hz tuning fork. Some patients with more dense sensory loss are unable to distinguish sharp (pinprick) from dull (analgesia) or to feel light touch at all (anesthesia). The Semmes–Weinstein (10 g) monofilament test is a useful adjunct to the neurologic examination. The filament is pressed against the skin over the dorsum of the great toe or other selected areas of the foot until it bows into a C shape for 1 second, and the patient is asked whether the stimulus is felt. The Rydel–Seiffer tuning fork provides semiquantitative information about vibratory sensory perception and can contribute to grading the severity of the polyneuropathy. Vibratory loss may involve the distal toes, the foot below the ankle, or more extensive territories, depending on its severity. Testing for proprioceptive abnormalities in the toes is often normal except when the polyneuropathy is severe.
Distal motor wasting, such as in the extensor digitorum brevis muscle, and associated weakness especially involving foot and toe dorsiflexion, usually accompany more severe sensory loss. Patients may have foot ulcers or, less commonly, a destructive arthropathy from repetitive injury, known as a Charcot joint. Loss of the muscle stretch reflex at the ankle is common in early diabetic polyneuropathy; all the muscle stretch reflexes may be lost with more severe neuropathies. The feet may be dry from loss of sweating. Patients with concurrent atherosclerosis have loss of distal pulses and sometimes femoral bruits. Orthostatic vital signs should be assessed; in patients with involvement of the autonomic nervous system, a decline of 20 mmHg or more in the systolic blood pressure or 10 mmHg in diastolic pressure indicates postural hypotension.
Diabetic polyneuropathy has been divided into subcategories depending on whether large- or small-fiber involvement occurs. In large-fiber polyneuropathy, there is more prominent loss of sensation to light touch, vibration, and proprioception. Patients may have accompanying ataxia of gait. In small-fiber polyneuropathy, pinprick and thermal appreciation are impaired and autonomic dysfunction is common, as is neuropathic pain, especially at night. Neuropathic pain can accompany other forms of diabetic polyneuropathy as well.
Several scales have been developed to grade the severity of diabetic polyneuropathy for clinical trials, including the Modified Toronto Neuropathy Scale, the Utah Neuropathy Scale, the Michigan Neuropathy Scale, and the Mayo Clinic diabetic polyneuropathy classification.
In patients with established diabetes mellitus and typical symptoms of polyneuropathy, extensive additional testing may not be required. Exclusion of other causes of sensory polyneuropathy can be accomplished through judicious screening for hypothyroidism, vitamin B 12 deficiency, monoclonal gammopathy, and ethanol abuse. Table 19-1 is an extensive list of alternative diagnoses that may resemble diabetic polyneuropathy.
| Vitamin Deficiency |
|
| Infectious and Inflammatory |
|
| Endocrine |
|
| Drugs and Toxins |
|
| Metabolic |
|
| Congenital/Inherited |
|
| Vascular |
|
| Neoplastic |
|
Electrophysiologic testing is recommended for patients with unexpectedly severe or atypical forms of polyneuropathy including motor-predominant disease, rapidly progressive symptoms, asymmetric signs, or when another neuromuscular condition is suspected. It is important to choose a laboratory with appropriate certification, training, and experience in performing these techniques. Two major components are usually performed: nerve conduction studies and needle electromyography (EMG). In patients with only sensory symptoms and findings, nerve conduction studies alone may be sufficient. Initial changes in patients with diabetic polyneuropathy include reductions in the amplitude and conduction velocity of the sural sensory nerve action potential (SNAP) recorded from behind the ankle. Slowing of conduction velocity in fibular (peroneal) motor axons detected by recording over the extensor digitorum brevis muscle of the foot is an additional early abnormality. In severe neuropathy, there may be widespread loss of SNAPs and diffuse mild-to-moderate conduction velocity slowing in a number of motor and sensory nerve territories ( Fig. 19-2 ). In some patients with severe involvement, these findings may resemble the changes expected in a demyelinating polyneuropathy such as chronic inflammatory demyelinating polyneuropathy (CIDP), but it is usually possible to distinguish this condition. In CIDP, there are many more striking electrophysiologic features of primary demyelination such as motor conduction block or dispersion of compound muscle action potentials (CMAPs).
![Figure 19-2, Examples of nerve conduction abnormalities in a patient with moderately severe diabetic polyneuropathy (DPN) compared with waveforms in a normal subject. Note the decreased amplitude and prolonged latency of compound muscle action potentials and sensory nerve action potentials. The sural sensory nerve action potential is absent. Lines indicate nerve stimulation sites (recording site for the median motor nerve is the abductor pollicis brevis; for the median sensory nerve, the index finger; for the fibular [peroneal] motor nerve, the extensor digitorum brevis; and for the sural nerve, behind the lateral ankle). Figure 19-2, Examples of nerve conduction abnormalities in a patient with moderately severe diabetic polyneuropathy (DPN) compared with waveforms in a normal subject. Note the decreased amplitude and prolonged latency of compound muscle action potentials and sensory nerve action potentials. The sural sensory nerve action potential is absent. Lines indicate nerve stimulation sites (recording site for the median motor nerve is the abductor pollicis brevis; for the median sensory nerve, the index finger; for the fibular [peroneal] motor nerve, the extensor digitorum brevis; and for the sural nerve, behind the lateral ankle).](https://storage.googleapis.com/dl.dentistrykey.com/clinical/DiabetesandtheNervousSystem/1_3s20B9780128193068000198.jpg)
Loss of motor axons in more advanced diabetic polyneuropathy is detected by a decline or loss of CMAPs, initially in the lower and then the upper limbs. In these patients, needle EMG may detect abnormal spontaneous activity, including fibrillation potentials and positive sharp waves, in muscles that have undergone denervation. In the setting of partial loss of motor axons, remaining fibers sprout and innervate adjacent denervated muscle fibers. When activated, the motor unit action potentials recorded from these partially denervated muscles are enlarged but reduced in number, indicative of chronic denervation and reinnervation. Electrophysiologic testing is also valuable in identifying superimposed entrapment or compression neuropathies, which are discussed later.
To reproducibly detect or track sensory changes, quantitative sensory testing (QST) using a computer interface may be used. While its chief utility is currently for clinical trials, it may offer early detection should better therapy for diabetic polyneuropathy emerge. QST equipment is available from several manufacturers and most use calibrated electronic interfaces to measure thermal thresholds (warm, cold), pain, touch-pressure, and vibration. As expected, these thresholds in the feet are raised in diabetic polyneuropathy.
Specific testing of the autonomic nervous system is available for evaluating co-existing autonomic involvement (see Chapter 8 ). In some patients with diabetes mellitus, however, prominent and apparently selective autonomic damage occurs without polyneuropathy, as discussed later. Since postganglionic autonomic axons are unmyelinated, autonomic function testing may detect small-fiber forms of diabetic polyneuropathy.
Two additional forms of testing, not in routine clinical use for diabetic polyneuropathy, also evaluate small-fiber involvement. These include skin biopsy, using a 3-mm punch to count the number of epidermal axons, and corneal confocal microscopy, which provides a noninvasive measure of unmyelinated axons in the cornea and complements skin biopsy.
Biopsy of the sural nerve is not indicated for the routine evaluation of diabetic polyneuropathy and should be reserved for the diagnosis of unusual or progressive neuropathies that are atypical and suspected to be from another cause. In diabetes, sural nerve biopsies show loss of myelinated and unmyelinated axons that can accompany microvascular basement membrane thickening, endothelial cell reduplication, or vessel occlusion. Sural nerve biopsies leave the patient with a sensory deficit and therefore should be performed judiciously.
Cerebrospinal fluid (CSF) examination is not indicated routinely for typical diabetic polyneuropathy, although when performed it may show an elevated CSF protein concentration without pleocytosis.
Imaging studies are used to exclude spinal cord disease, spinal stenosis, or other central disorders with symptoms that may resemble diabetic polyneuropathy. There are no specific imaging characteristics of diabetic polyneuropathy although newer approaches—including ultrasound—are under evaluation.
A number of neurologic disorders may resemble diabetic polyneuropathy. Other polyneuropathies with prominent sensory involvement are listed in Table 19-1 . A detailed neurologic examination is essential to exclude other syndromes in diabetic patients; for example, spinal cord disease may present with limb tingling and numbness, but other signs of upper motor neuron dysfunction are usually present on examination. Lesions at the cervicomedullary junction may cause sensory symptoms that begin in one limb and then progressively involve all four limbs. “Pseudoneuropathy” is a term used to describe the combination of lower limb sensory symptoms from spinal stenosis with upper limb tingling caused by carpal tunnel syndrome. It is important to identify patients with CIDP because they may respond to immunomodulatory therapies.
A number of mechanisms have been considered in the pathogenesis of diabetic peripheral neuropathy, all with limited definitive evidence, and they are discussed elsewhere in recent reviews. Most have been evaluated in rat and mouse models of diabetes. Excessive flux of polyols (sugar alcohols), especially sorbitol, through the aldose reductase pathway is one such proposed mechanism. Aldose reductase inhibitors or protein kinase C inhibitors that interrupt this pathway have had limited clinical benefits, problematic side effects, or lack of penetration into the peripheral nervous system. Free radical oxidative/nitrergic stress and mitochondrial dysfunction along with impaired antioxidant defenses likely contribute to neuronal damage. Diabetic microangiopathy may lead to ischemic damage of neurons and axons. We have suggested that microangiopathy contributes to diabetic polyneuropathy later in the illness rather than serving as a primary trigger.
Trophic mechanisms that support neurons are impaired in diabetes. To date, separate clinical trials with nerve growth factor, neurotrophin-3, and brain-derived neurotrophic factor have been disappointing. In each case, the ability to protect all types of peripheral neurons targeted by diabetes has been limited. An intriguing alternative is insulin itself, an important growth factor which is neurotrophic; its receptors are widely expressed on most neurons of the peripheral nervous system. Novel forms of near-nerve or intranasal insulin have improved experimental diabetic neuropathy.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here