Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
![]() Additional content is available online at Elsevier eBooks for Practicing Clinicians
Additional content is available online at Elsevier eBooks for Practicing Clinicians
While implantable cardioverter defibrillators (ICDs) were first approved by the U.S. Food and Drug Administration (FDA) for the secondary prevention of sudden cardiac death (SCD) in 1985, the advent of device-based therapy for the management of heart failure (HF) did not begin until 2001 when the FDA approved cardiac resynchronization therapy (CRT) to reduce the symptoms of moderate to severe HF. In 2003, the Center for Medicaid Services (CMS) issued the first coverage decision for the use of ICDs for the primary prevention of SCD in patients with HF and reduced ejection fraction (HFrEF) post–myocardial infarction (post-MI) followed later by more expansive coverage to include nonischemic HFrEF patients. After a hiatus of nearly two decades, several new therapeutic devices that target central sleep apnea (CSA), secondary mitral regurgitation (SMR), abnormal myocardial contractility, and autonomic imbalance have been approved by the FDA for the treatment of patients with HF. Devices have also been developed to monitor and transmit physiologic data that provide important information about the events preceding HF exacerbations, allowing testing of treatment strategies based on remotely monitored data to avert HF exacerbations, reduce HF hospitalizations (HFHs), and reduce mortality. This chapter reviews the use of CRT, ICDs, and newer approved devices for management of HF and discusses the ability of monitoring devices to provide data to improve symptoms and reduce HF exacerbations. Medical management of HF is discussed in Chapter 48, Chapter 49, Chapter 50, Chapter 51 .
Intraventricular conduction abnormalities are common in patients with chronic HF and are associated with increased morbidity and mortality. Conduction delay resulting in a QRS duration greater than 120 msec on the surface electrocardiogram has been termed electrical dyssynchrony. The difference in the timing of mechanical contraction or relaxation between different segments of the left ventricle that results from electrical dyssynchrony has been termed mechanical dyssynchrony and can result in suboptimal ventricular filling, a reduction in left ventricular (LV) contractility, greater degree and prolonged duration of mitral regurgitation (MR), and paradoxical septal wall motion. Using this definition of a QRS duration of greater than 120 milliseconds, about one-third of patients with HFrEF have ventricular dyssynchrony. CRT improves ventricular dyssynchrony with implantation of pacing leads to pace both the right and left ventricles. Optimal placement of two leads, one on the right ventricular septum and a second in the coronary sinus at the site of latest LV activation allows for simultaneous or near simultaneous activation of both ventricles and improves inter- and intra-ventricular synchrony. Early studies demonstrated a benefit of CRT in patients with HFrEF and ventricular dyssynchrony on hemodynamics, functional outcomes and quality of life (QoL) leading to the initial indications for this therapy. These results led to large-scale randomized controlled trials (RCTs) confirming the beneficial effects of CRT on functional status and demonstrating an important morbidity and mortality benefit.
The following RCTs are considered among the most important studies of CRT in the severe HFrEF patient population: the Multisite Stimulation in Cardiomyopathy (MUSTIC) study, the Multicenter InSync Randomized Clinical Evaluation (MIRACLE) trial, the MIRACLE ICD trial, the CONTAK CD trial, the Comparison of Medical Therapy, Pacing and Defibrillation in Heart Failure (COMPANION), and the Cardiac Resynchronization in Heart Failure (CARE HF) trial. To understand and compare the size and design, clinical benefits, baseline medical therapy, and limitations of CRT with or without an ICD, these studies are described in Table 58.1 with specific comments in the text that follows.
| Trial (Year Published) | N | Inclusion Criteria | Study Design | Mean Follow-Up | Primary Endpoint | Secondary Endpoints | Medical Therapy ∗ |
|---|---|---|---|---|---|---|---|
| MUSTIC (2001) |
67 |
|
Single-blind, crossover RCT CRT on vs. CRT off |
24 wk | CRT on vs. CRT off 6MHWD (active vs. inactive pacing) 399 ± 100 m vs. 326 ± 134 m (p ≤ 0.001) |
CRT on vs. CRT off QoL 29.6 ± 21.3 vs. 43.2 ± 22.8 (p < 0.001) V o 2 16.2 ± 4.7 vs. 15 ± 4.9 mL/kg/min (p < 0.029) |
ACEI/ARB 96% BB 28% MRA 22% Diuretics † 94% Digoxin 48% |
| MIRACLE (2002) |
453 |
|
Double-blind prospective RCT CRT on vs. CRT off |
6 mo | CRT on vs. CRT off 6MWHD + 39 vs. +10 M (p = 0.001) QoL −18 vs. −9 points (p < 0.001) NYHA Improved (p < 0.001) |
CRT on vs. CRT off Measures of exercise performance (V o 2 ) and total exercise time, LVEF, area of MR jet, QRS duration—all improved (p < 0.001) |
ACEI/ARB 93% BB 62% MRA NR Diuretics † 94% Digoxin 78% |
| MIRACLE-ICD (2003) |
369 |
|
Double-blind, prospective RCT CRT-ICD implanted in all with CRT off in control group |
6 mo | CRT on vs. off 6MWHD +55 CI = 44-79] vs. +53 m [CI = 43-75] (p = 0.36) QoL −17.5 [CI = −21 to −14] vs. −11.0 [CI = −16 to −7] m (p = 0.02) NYHA Improved (p = 0.007) |
CRT on vs. off No significant differences in changes in left ventricular size or function, overall HF status, survival, and rates of hospitalization |
ACEI/ARB 93% BB 62% MRA NR Diuretics † 93% |
| CONTAK CD (2003) |
490 |
|
Single-blind, prospective RCT parallel-controlled CRT-ICD implanted with CRT off in control group |
6 mo | CRT vs. no CRT Progression of HF, defined as ACM, hospitalization for HF, and VT/VF requiring device intervention 15% reduction in HF progression with CRT vs. no CRT (p = 0.35) |
CRT vs. no CRT V o 2 0.8 mL/kg/min vs. 0.0 mL/kg/min (p = 0.03) 6MHWD 35 m vs. 15 m (p = 0.043) QoL (p = NS) |
ACEI/ARB 81% BB 45% MRA NR Diuretics † 92% Digoxin 72% |
| COMPANION (2004) |
1520 |
|
Prospective RCT Randomized 1:2:2 to OPT (optimal medical therapy) vs. CRT-D (CRT+ICD) vs. CRT-P (CRT alone) |
NR | Time to ACM or hospitalization for any cause CRT-P vs. OPT (HR= 0.81; p = 0.014) CRT-D vs. OPT (HR, 0.80; P = 0.01) |
ACM CRT-P vs. OPT (reduced by 24%, p = 0.059) CRT-D vs. OPT (reduced 36%, p = 0.003) 6MWD OPT vs. CRT-P vs. CRT-I 1 ± 93 vs. 40 ± 96 (p < 0.001) vs. 46 ± 98 (p < 0.001) QoL OPT vs. CRT-P vs. CRT-D −12 ± 23 vs. −25 ± 26 (p < 0.001) vs. −26 ±28 (p < 0.001) |
ACEI/ARB 89% BB 68% MRA 53% Loop diuretics 94% |
| CARE HF (2005) |
813 |
|
Unblinded, prospective RCT CRT vs. no CRT |
29.4 mo | CRT vs. no CRT Time to ACM or unplanned cardiovascular hospitalization HR = 0.63; 95% CI = 0.51-0.77; P < 0.001 |
CRT vs. no CRT ACM (HR= 0.64; 95% CI 0.48-0.85; P < 0.002) QoL difference (mean ± SD) at 90 −10 (−8 to −12) (p < 0.001) |
ACEI/ARB 95% BB 70% MRA 54% Loop diuretics 44% Digoxin 40% |
| REVERSE (2008) |
610 |
|
Double-blind, prospective RCT CRT on vs. off |
12 mo | CRT on vs. off Clinical composite response (improved, unchanged or worsened) CRT on vs. off 16% vs. 21% worsened (p = 0.19) |
CRT on vs. off LVESVI−18.4 ± 29.5 vs. −1.3 ± 23.4 mL/m 2 (p < 0.0001) |
ACE/ARB 96% BB 96% MRA NR Diuretics NR |
| MADIT-CRT (2009) |
1820 |
|
Unblinded, prospective double-blind CRT-ICD vs. ICD |
2.4 yr | CRT-ICD vs. ICD ACM or HF event CRT-ICD 17.2% vs. ICD 25.3% (HR = 0.66, CI = 0.52-0.84, p = 0.001) |
CRT-ICD vs. ICD ACM 6.8% vs. 7.3% (p = 0.99) HF events CRT-ICD 13.9% vs. 22.8% (p < 0.001) |
ACEI 77% ARB 21% BB 93% MRA 32% Diuretics † 76% |
| RAFT (2010) |
1798 |
|
Double-blind, prospective RCT ICD-CRT vs. ICD alone |
40 mo | ICD-CRT vs. ICD ACM or HFH HR 0.75, CI 0.64-0.87 (p < 0.0001) |
ICD-CRT vs. ICD ACM HR 0.75, CI 0.62-0.91 (p < 0.0001) AE double in ICD-CRT compared with ICD at 30 days |
ACEI/ARB 96% BB 90% MRA 42% Diuretics † 85% Digoxin 34% |
| ECHO-CRT (2012) |
809 |
|
Prospective RCT CRT vs. control |
19.4 mo | CRT vs. control ACM or first HFH HR 1.20, CI = 0.92-1.57, p = 0.15 |
CRT vs. control ACM HR 1.81, CI, 1.11-2.93 (p = 0.02) |
ACEI/ARB 95% BB 96% MRA 61% Diuretics 86% |
| BLOCK-HF (2013) |
691 |
|
Prospective RCT CRT vs. RV pacing |
37 mo | CRT vs. RV pacing Time to ACM or urgent HF visit or a 15% increase in LVESVI HR, 0.74; CI, 0.60-0.90 |
CRT vs. RV pacing ACM + urgent HF care HR 0.73, CI 0.56-0.94 ACM + HFH HR 0.77, CI 0.58-1.00 ACM HR 0.83, CI 0.59-1.17 HFH HR 0.68 (049-0.94) |
NR |
The MUSTIC trial was a single-blind randomized controlled crossover study of CRT that enrolled 67 patients with enrollment criteria outlined in Table 58.1 . The CRT device was implanted in all patients and after a run-in period, patients were randomized to either VVI pacing at a fixed rate of 40 beats/min (“inactive pacing”) or atrio-biventricular pacing (“active pacing”) for 12 weeks followed by a crossover to the alternate treatment assignment. Forty-eight patients completed the study. The primary endpoint of peak exercise oxygen consumption ( Vo 2 ) improved with CRT vs. no CRT as did all the secondary endpoints. The blinded crossover design of this trial suggested substantial improvements in functional capacity and QoL with CRT that were dependent on ongoing pacing.
MIRACLE was the first prospective RCT designed to evaluate the benefits of CRT in patients with the inclusion criteria outlined in Table 58.1 . Patients assigned to CRT experienced an improvement in each of the primary endpoints at 6 months: 6MHWD, NYHA functional class, and QoL. The trial also provided evidence of substantial LV reverse remodeling with CRT as outlined in Table 58.1 . The results of this trial led to FDA approval of the InSync system in 2001, the first approved CRT system in the United States.
MIRACLE ICD was a prospective RCT designed to evaluate the benefits of an implantable cardiac defibrillator (ICD) + CRT vs. ICD alone. The inclusion criteria and primary endpoints were the same as in MIRACLE with the additional requirement that subjects have an indication for a secondary prevention ICD or have a history of inducible sustained ventricular tachycardia. With CRT, the improvements in QoL and (NYHA) classification were similar to those in MIRACLE but there was no difference in 6MHWD. However, V o 2 and total exercise time were improved. The ventricular remodeling benefits seen with CRT in MIRACLE were not reproduced in MIRACLE ICD. The combined CRT-ICD device used in this study was approved by the FDA in June 2002 for use in NYHA Class III and IV HFrEF patients with ventricular dyssynchrony and an ICD indication.
CONTAK CD was similar to MIRACLE ICD except NYHA Class II patients were enrolled. Both the study design (crossover) and primary endpoint (V o 2 ) were changed during the study to a parallel design and a composite HF progression endpoint. The 15% reduction in HF progression was not significant but Vo 2 and 6MWHD were improved. Significant reductions in ventricular dimensions and improvement in left ventricular ejection fraction (LVEF) similar to those seen in MIRACLE were demonstrated with CRT.
COMPANION randomized patients to optimal medical therapy (OPT), CRT-P (CRT alone), and CRT-D (CRT+ICD) and was the first reported large RCT to include mortality in the primary endpoint. COMPANION confirmed the results of earlier CRT trials in improving symptoms, exercise tolerance, and QoL for HF patients with electrical dyssynchrony. COMPANION was also the first large RCT to demonstrate the impact of CRT-D in reducing all-cause mortality (ACM) and suggested incremental benefit from combined ICD and CRT therapies.
Mean systolic blood pressure was significantly higher in both the CRT-P and CRT-D groups compared to the OPT group at 3, 6, and 12 months ( Fig. 58.1 ). This improvement in systolic blood pressure following CRT may allow uptitration of guideline-directed medical therapy (GDMT), further improving morbidity and mortality.
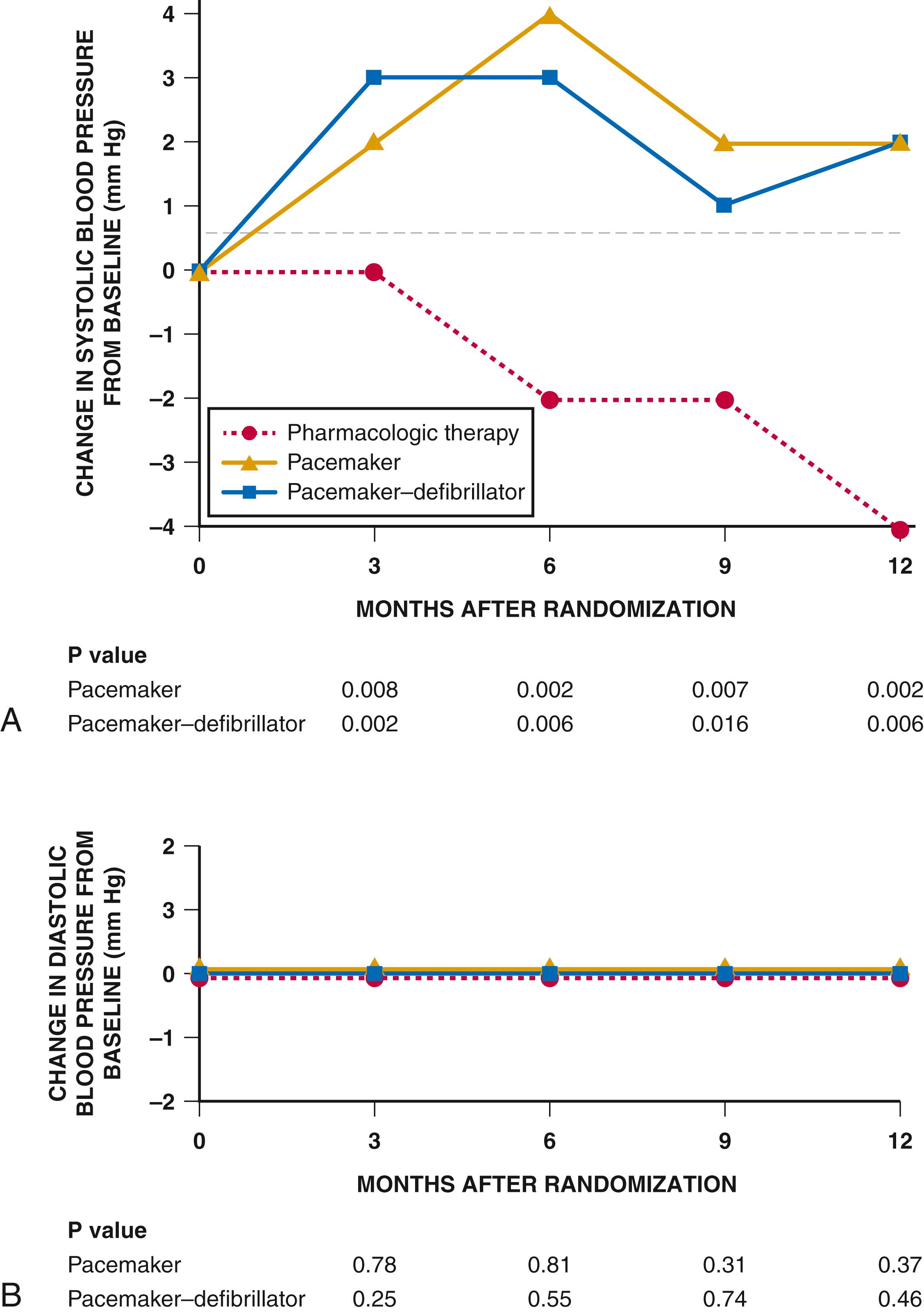
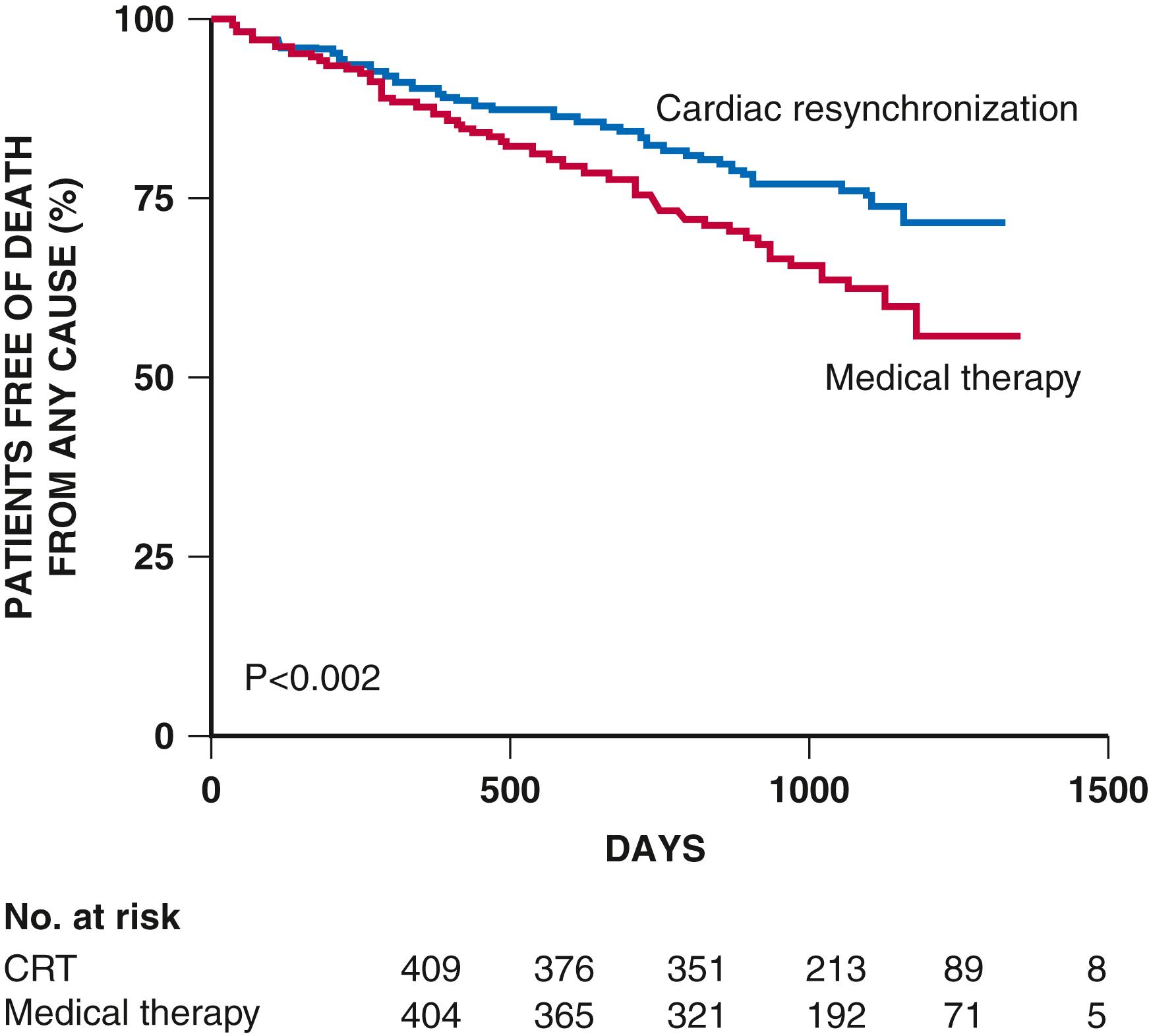
The CARE-HF trial convincingly demonstrated the benefits of CRT+ medical therapy vs. medical therapy alone on morbidity and mortality in patients with NYHA Class III or IV HF and ventricular dyssynchrony ( Fig. 58.2 ). CRT also led to a significant reduction in MR area by echocardiography and significant myocardial reverse remodeling and reduced N-terminal pro B-type natriuretic peptide (NT-proBNP) at 18 months. These benefits were achieved irrespective of the use of beta blockers, mineralocorticoid receptor antagonists (MRAs), or digoxin. In addition, the mean systolic blood pressure was 5.8 mm Hg (CI, 3.5 to 8.2, p = 0.001) higher in the CRT group than in the control group at 3 months; this difference was maintained at 18 months, confirming the blood pressure improvements in COMPANION.
CRT studies described earlier in this chapter focused specifically on patients with HFrEF and NYHA Classes III and IV. These studies also provided preliminary data that informed the design of studies that allowed expanded indications to patients with an LVEF between 30% and 35% and NYHA Class I and II HFrEF patients.
The echo data from MIRACLE-ICD suggested a significant improvement in LV remodeling with CRT even in the small cohort of NYHA Class II patients enrolled similar to that seen in the more symptomatic patients in CARE-HF. This finding led to three important trials with CRT in patients with mild HF including the Resynchronization Reverses Remodeling in Systolic Left Ventricular Dysfunction (REVERSE) trial, Multicenter Automatic Defibrillator Implantation Trial with Cardiac Resynchronization Therapy (MADIT-CRT), and Resynchronization/defibrillation for Ambulatory Heart Failure Trial (RAFT).
The primary endpoint (an HF composite) in REVERSE was not significantly changed but the CRT-on vs. CRT-off group had a greater improvement in measures of LV remodeling. As noted in CARE-HF, the benefits of CRT on remodeling were present irrespective of the presence or dose of beta blockers. Despite the negative primary endpoint, this trial suggested a benefit of CRT on ventricular remodeling in mildly symptomatic HFrEF patients.
The MADIT-CRT trial was an unblinded RCT designed to determine if CRT + primary prevention ICD vs. primary prevention ICD alone reduced the risk of ACM and nonfatal HF events in HFrEF patients with NYHA Class I (ischemic etiology) and NYHA Class II (ischemic or nonischemic etiology) symptoms. The significant reduction in the primary endpoint was due to a reduction in HF events in both the ischemic and nonischemic groups. A subsequent analysis demonstrated that the benefit of CRT was seen only in those with a left bundle branch block (LBBB). A larger benefit of CRT was noted for women (HR = 0.37, CI 0.22 to 0.62) than men (HR = 0.76, CI 0.59 to 0.97, p = 0.01 for interaction) and in patients with a QRS of 150 milliseconds or longer. The MADIT-CRT trial led the FDA to expand the indication for CRT to NYHA Class II or ischemic Class I patients, with LVEF less than 30%, QRS duration longer than 130 milliseconds, and LBBB. MADIT-CRT also demonstrated substantial improvement in ventricular size and function in patients randomized to CRT, with the outcomes benefit directly related to the degree of reverse remodeling.
Initially the RAFT trial included patients in NYHA Class II and III but when the CARE HF trial showed a reduction in mortality for NYHA Class III patients, the protocol was revised to include only patients in Class II. The RAFT trial was the first to show a mortality benefit of combined CRT-ICD over an ICD alone, and a mortality reduction with the addition of CRT in patients in NYHA Class II HF. The results of REVERSE, MADIT-CRT, and RAFT resulted in the FDA expanding the indication CRT to include patients with mildly symptomatic HF.
Patients with HFrEF and a narrow QRS complex may demonstrate mechanical dyssynchrony using imaging techniques such as echocardiography. Small trials suggested a benefit in these patients but a larger trial, Echocardiography Guided Cardiac Resynchronization Therapy (EchoCRT), did not confirm these benefits. The primary endpoint was not significant, but the secondary endpoint of ACM was higher in the CRT group than in the control group ( Table 58.3 ), demonstrating the potential for harm in using CRT in narrow-QRS patients. Thus CRT is considered contraindicated in these patients.
Early studies of CRT specifically excluded patients with high degrees of atrioventricular block to avoid the confounding effects of right ventricular (RV) pacing with its potential to cause ventricular dyssynchrony. To determine whether CRT might reduce mortality, morbidity, and adverse LV remodeling in patients requiring RV pacing, the Biventricular vs. Right Ventricular Pacing in Heart Failure Patients with Atrioventricular Block (BLOCK HF) study was designed. Patients with standard guideline indications for CRT were excluded. Patients received a CRT and ICD (ICD if the patient had an indication for ICD therapy) and were assigned to standard RV pacing or CRT. Patients randomly assigned to CRT had a significantly lower incidence of the primary outcome than did those assigned to RV pacing ( Table 58.2 ) This led to a guideline recommendation to consider CRT in patients with and LVEF of ≤50% who require RV pacing.
| Trial (Year Published) | N | Inclusion Criteria | Study Design | % Ischemic | Mean Follow-Up | Primary Endpoint | Mortality/Year | Medical Therapy ∗ |
|---|---|---|---|---|---|---|---|---|
| MADIT II (2002) |
1232 |
|
RCT 3:2 ICD + medical therapy vs. medical therapy |
100% | 20 mo | ICD + MT vs. MT ACM HR = 0.69 (95% CI, 0.51-0.93; P = 0.016) |
ICD 8.5% Control 11.9% |
ACEI 68% ARB NR BB 70% MRA NR Dig 57% Diuretics † 72% |
| DEFINITE (2004) |
458 |
|
RCT ICD + MT vs. MT |
0% | 29 mo | ICD + MT vs. MT ACMHR = 0.65 (95% CI, 0.40-1.06; P = 0.08) Post hoc analysis of SCD HR = 0.20 (95% CI, 0.06-0.71; P = 0.006) (no. of events = 17) |
ICD 3.9% Control 7.0% |
ACEI 84% ARB 14% BB 86% MRA NR Dig 42% Diuretics † 87% |
| SCD-HeFT (2005) |
2521 |
|
RCT ICD + MT vs. amiodarone + MT vs. MT |
52% | 45.5 mo | ACM ICD vs. MT HR = 0.77 (97.5% CI, 0.62-0.96, p = 0.007) Amiodarone + MT vs. MT HR = 0.77 (97.5% CI, 0.86-1.3, P = 0.53) |
ICD 5.8% Control 7.6% |
ACEI/ ARB 94% BB 69% MRA 20% ‡ + Dig 67% Loop diuretics 82% |
| DANISH (2016) |
1116 |
|
RCT ICD + MT vs. MT |
0% | 67.6 mo | ICD + MT vs. MT ACM HR = 0.87 (95% CI, 0.68-1.12; P = 0.28) Other endpoints CV death HR = 0.77 (95% CI, 0.57-1.05; P = 0.10) SCD HR = 0.50 (95% CI, 0.31-0.82; P = 0.005) |
ICD 4.4% Control 5.0% |
ACEI/ ARB 96% BB 92% MRA 59% Dig NR Loop diuretics NR CRT 58% |
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here