Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Parts of this chapter were adapted from sections of Chapter 134 by authors James A. Stockman III and Pedro A. DeAlarcon in the first edition of this book. We thank Erin Adair for assistance with illustrations.
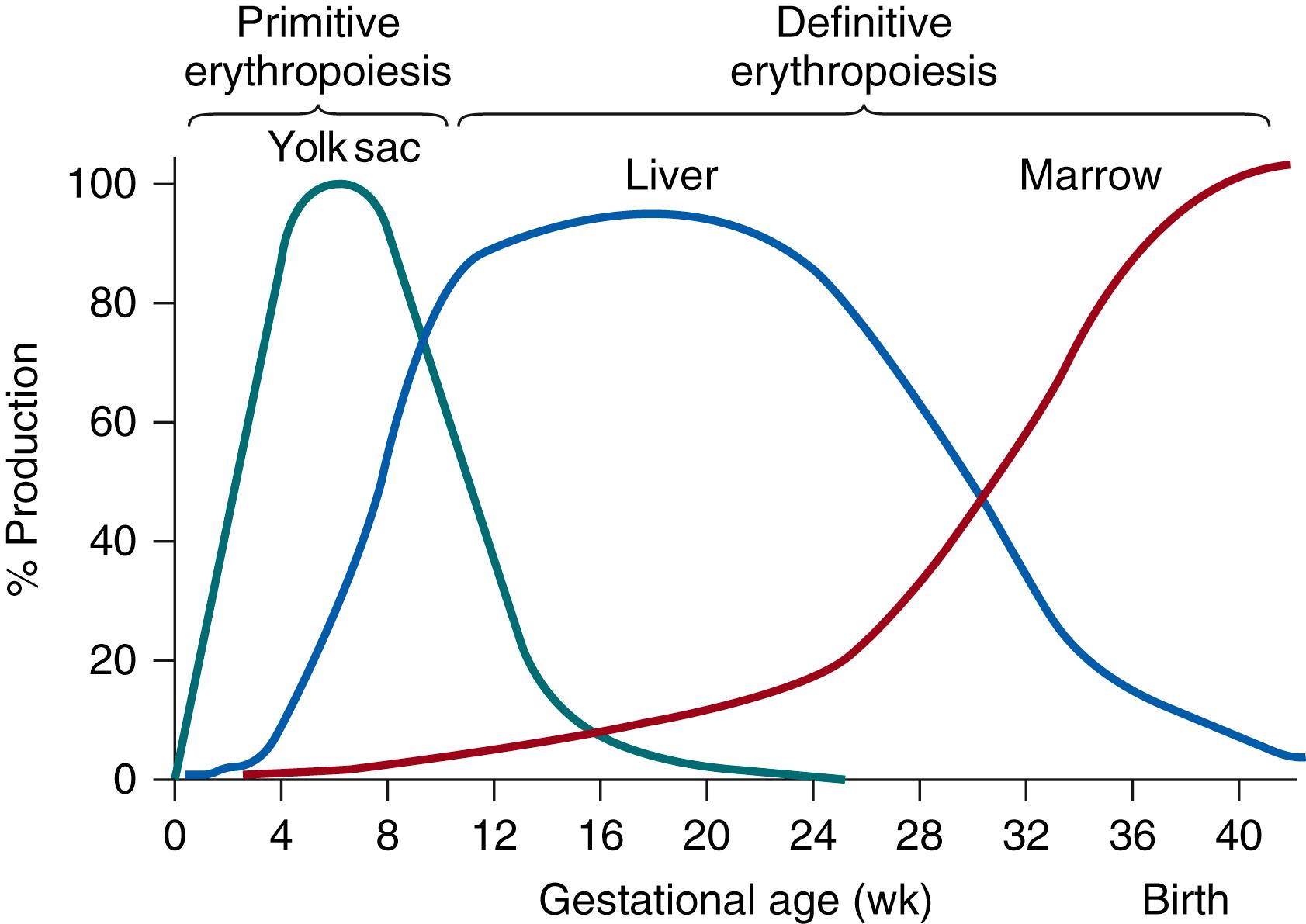
Extraembryonic erythropoiesis begins in the fetal yolk sac by 14 days’ gestation. Small nests of nucleated blood cells are present in the mesenchymal and endodermal layers of the yolk sac. These red blood cells, or hematocytoblasts, are the product of primitive megaloblastic erythropoiesis, and they differ from erythrocytes formed later in gestation when definitive normoblastic erythropoiesis occurs. Primitive erythroblasts are nucleated and macrocytic (20 to 25 μm in diameter). They have a mean corpuscular volume (MCV) of more than 180 fL and have a characteristic fine nuclear chromatin pattern and a polychromatophilic cytoplasm containing abundant hemoglobin. , Red blood cells enter the embryonic circulation at 3 to 4 weeks’ gestation, coincident with joining of the vitelline and umbilical circulations.
Definitive normoblastic erythropoiesis of the fetus begins in the liver during the early first trimester. By 6 to 8 weeks’ gestation, the liver replaces the yolk sac as the primary site of red blood cell production, and by 10 to 12 weeks’ gestation, extraembryonic erythropoiesis has essentially ceased. Red blood cell production occurs in the liver throughout the remainder of gestation, although production begins to diminish during the second trimester as bone marrow erythropoiesis increases ( Fig. 109.1 ). Erythroblasts are first noted in the marrow at 8 to 9 weeks’ gestation. By the end of the third trimester, almost all erythropoiesis is occurring in the bone marrow, although residual erythropoiesis may continue in the liver and may be found in other sites. In rodents, the spleen is also a site of erythropoiesis before the onset of marrow red blood cell production. As with the liver, this site ceases production shortly after birth. It is unclear whether the spleen contributes to erythropoiesis in the human fetus. Although erythroid precursors have been identified as early as 6 to 7 weeks’ gestation in human splenic tissue, it is not clear whether such cells are actually developing within splenic tissue or whether they are simply part of the circulation.
Red blood cell precursors in the yolk sac are extremely primitive cells, and they may either disappear or seed other areas where erythropoiesis later becomes prominent. The unicentric theory proposes that all hematopoietic stem cells originate from the yolk sac, then migrate from one hematopoietic site to another. Moore and Metcalf demonstrated circulating pluripotent stem cells in the peripheral blood just before the development of liver erythropoiesis. They suggested that the development of intraembryonic hematopoiesis requires an intact yolk sac, and migration of stem cells from the yolk sac to hematopoietic tissue is necessary for the development of intraembryonic hematopoiesis. The development of other organ systems via migration of cells (such as neural crest tissue) gives credence to this theory.
The multicentric theory suggests that a new clonal formation of hematopoietic stem cells occurs at different sites of hematopoiesis during fetal development. Yolk sac cells are capable of producing granulocytic, megakaryocytic, and erythroid colonies when transplanted into adult irradiated recipients and in conditioned newborn recipients, , a finding that supports the view that fetal stem cells possess the capacity to be pluripotent and their differentiation is controlled by microenvironmental factors.
Table 109.1 describes the differential counts in fetal liver, marrow, and circulating blood according to gestational age. The liver of the second-trimester human fetus is the primary organ for erythropoiesis, with a myeloid-to-erythroid ratio ranging from 0.07 at 14 to 17 weeks’ gestation to 0.2 at 21 to 24 weeks’ gestation. By 24 weeks the composition of the fetal bone marrow begins to resemble that of adult bone marrow, differing by the presence of a large number of stromal elements, the absence of plasma cells and lymph follicles, and an overall increased cellularity in the fetal bone marrow. Unlike adult bone marrow, large fat cells are not present in fetal bone marrow. Between 18 and 22 weeks’ gestation, the mitotic index of the fetal bone marrow becomes virtually identical to that of adult bone marrow. The fetal bone marrow myeloid-to-erythroid ratio starts to exceed the normal adult bone marrow ratio (1.5 ± 0.4) early in midgestation and remains elevated even at the time of birth.
| Differential Counts (Percentage ± SD) of Liver Cell Suspensions From Fetuses at a Gestational Age of 14–24 wk | |||
|---|---|---|---|
| Gestational Age (wk) | |||
| Cells | 14–17 ( n = 5) | 18–20 ( n = 7) | 21–24 ( n = 8) |
| Normoblast | |||
| Pronormoblast | 3.1 ± 1.4 | 3.4 ± 1.0 | 2.9 ± 1.8 |
| Basophilic | 18.4 ± 7.6 | 13.7 ± 1.6 | 13.7 ± 4.7 |
| Polychromatophilic | 57.5 ± 12.6 | 55.3 ± 7.5 | 51.1 ± 5.5 |
| Orthochromic | 13.9 ± 5.2 | 15.9 ± 3.7 | 14.2 ± 5.0 |
| Total erythroid cells | 9.3 ± 6.3 | 87.8 ± 4.8 | 81.9 ± 5.0 |
| Neutrophil | |||
| Promyelocyte | 0 ± 0 | 0.2 ± 0.2 a | 1.2 ± 0.4 a , b |
| Myelocyte | 0 ± 0 | 0 ± 0 | 0.2 ± 0.2 a , b |
| Metamyelocyte | 0 ± 0 | 0 ± 0 | 0 ± 0 |
| Band | 0 ± 0 | 0 ± 0 | 0 ± 0 |
| Segmented | 0 ± 0 | 0 ± 0 | 0 ± 0 |
| Total neutrophils | 0 ± 0 | 0.2 ± 0.2 | 1.4 ± 0.5 a , b |
| Undifferentiated blast | 0.5 ± 0.6 | 3.1 ± 1.8 a | 2.2 ± 0.7 a |
| Macrophage | 0.5 ± 0.6 | 1.2 ± 4.0 a | 1.3 ± 5.0 |
| Lymphocyte | 5.4 ± 2.6 | 3.9 ± 3.6 | 11.3 ± 4.2 a , b |
| Eosinophil | 0 ± 0 | 0 ± 0 | 0 ± 0 |
| Other c | 0.8 ± 0.8 | 3.4 ± 2.4 | 1.9 ± 2.4 |
| Differential Counts (Percentage ± SD) of Marrow Cell Suspensions From Fetuses at a Gestational Age of 14–24 wk | |||
| Gestational Age (wk) | |||
| Cells | 14–17 ( n = 6) | 18–20 ( n = 6) | 21–24 ( n = 8) |
| Normoblast | |||
| Pronormoblast | 0.3 ± 0.2 d | 1.1 ± 0.4 d | 0.5 ± 0.2 d |
| Basophilic | 1.4 ± 0.6 d | 3.1 ± 0.8 d | 1.7 ± 1.0 d |
| Polychromatophilic | 9.2 ± 5.0 d | 12.9 ± 6.2 d | 12.3 ± 5.2 d |
| Orthochromic | 12.1 ± 11.1 | 20.7 ± 12.2 | 8.1 ± 4.4 |
| Total erythroid cells | 23.0 ± 13.3 d | 37.8 ± 16.8 d | 22.6 ± 9.0 d |
| Neutrophil | |||
| Promyelocyte | 5.9 ± 2.9 d | 5.8 ± 2.6 d | 5.0 ± 1.7 d |
| Myelocyte | 2.7 ± 1.4 d | 3.4 ± 1.6 d | 2.1 ± 1.0 d |
| Metamyelocyte | 2.1 ± 1.0 d | 2.5 ± 1.8 d | 1.7 ± 1.0 d |
| Band | 3.1 ± 1.8 d | 3.2 ± 2.7 d | 1.6 ± 1.2 d |
| Segmented | 1.2 ± 1.3 d | 1.1 ± 1.3 d | 0.4 ± 0.2 d |
| Total neutrophils | 13.8 ± 6.1 d | 16.1 ± 8.0 d | 10.7 ± 5.0 d |
| Undifferentiated blast | 8.9 ± 12.4 d | 11.4 ± 5.4 d | 11.6 ± 3.6 d |
| Macrophage | 5.4 ± 3.8 d | 4.2 ± 2.4 d | 3.1 ± 1.6 d |
| Lymphocyte | 35.3 ± 15.8 d | 28.8 ± 8.0 d | 50.3 ± 6.8 d |
| Eosinophil | 0.5 ± 0.6 | 1.1 ± 1.2 | 1.2 ± 0.8 d |
| Other e | 0.8 ± 0.4 | 0.5 ± 0.4 | 0.4 ± 0.2 |
| Differential Counts (Percentage ± SD) of Umbilical Cord Blood Samples From Fetuses at a Gestational Age of 18–29 wk | |||
| Gestational Age (wk) | |||
| Cells | 18–21 ( n = 186) | 22–25 ( n = 230) | 26–29 ( n = 144) |
| Normoblast (%WBCs) | 45.0 ± 86.0 | 21.0 ± 23.0 | 21.0 ± 67.0 |
| Basophilic | 0.5 ± 1.0 | 0.5 ± 1.0 | 0.5 ± 1.0 |
| Neutrophil | 6.0 ± 4.0 | 6.5 ± 3.5 | 8.5 ± 4.0 |
| Lymphocyte | 88.0 ± 7.0 | 87.0 ± 6.0 | 85.0 ± 6.0 |
| Eosinophil | 2.0 ± 3.0 | 3.0 ± 3.0 | 4.0 ± 3.0 |
| Monocyte | 3.5 ± 2.0 | 3.0 ± 2.5 | 3.0 ± 2.5 |
a P < .05 versus 14 to 17 weeks.
b P < .05 versus 18 to 20 weeks.
c Hepatocyte, megakaryocyte, or cell of undetermined origin.
Studies of bone marrow cells in tissue culture have identified specific committed red blood cell precursors, termed erythroid progenitors, on the basis of their characteristic growth in vitro. When bone marrow cells are placed in semisolid media culture systems for 5 to 7 days, an erythropoietin (EPO) -sensitive erythroid progenitor cell, termed colony-forming unit–erythroid (CFU-E), clonally matures into a single cluster containing 30 to 100 normoblasts ( Fig. 109.2 ). An erythroid-specific progenitor that is less differentiated than a CFU-E (and therefore a more primitive cell) is termed a burst-forming unit–erythroid (BFU-E). Twelve to 14 days after cultures of bone marrow cells are initiated, a BFU-E develops into a large, multicentered colony of normoblasts, in which each center contains 200 to 10,000 normoblasts. Finally, the most primitive erythroid progenitor cell identifiable through in vitro culture is termed a colony-forming unit–granulocyte, erythrocyte, macrophage, megakaryocyte (CFU-GEMM, or CFU-MIX). Twelve to 14 days after marrow cells are placed in culture, this multipotent progenitor develops into a mixed colony of both normoblast clusters and granulocyte-macrophage clusters.
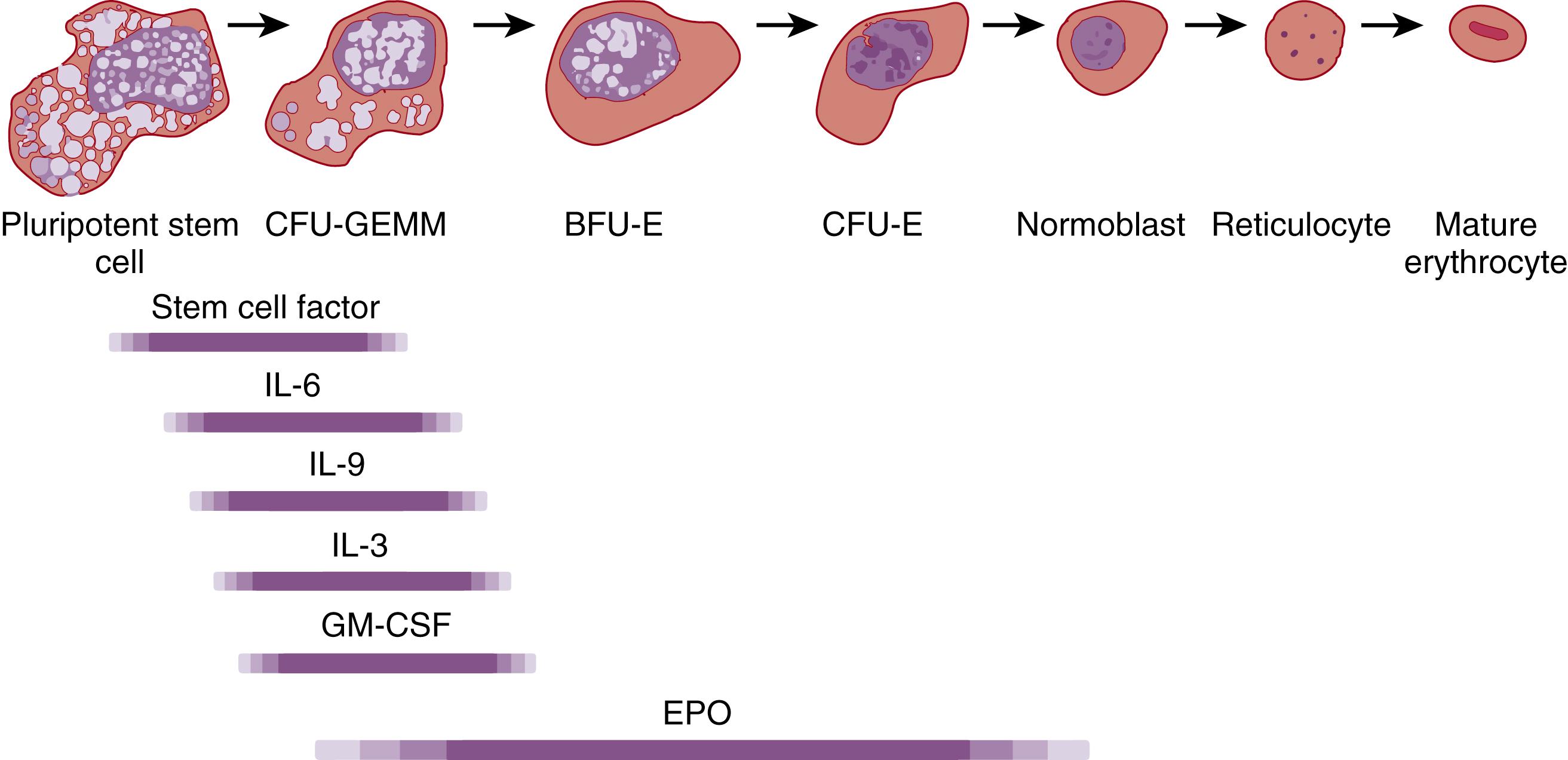
The ability of an organ to produce red blood cells is based on the number of progenitor cells it contains, as well as the growth factors stimulating those cells to proliferate. Determination of erythroid progenitor numbers obtained from cell suspensions of liver, marrow, spleen, and blood of second-trimester human fetuses ( Table 109.2 ) showed twice the number of multipotent progenitors and erythroid progenitors in the fetal liver compared with marrow. , , Erythroid progenitors from fetal liver also appeared to be more sensitive to EPO (the primary erythroid growth factor) than were progenitors from fetal marrow. Progenitor cell concentrations in the spleen were nearly identical to concentrations in the circulation. Whether the progenitors isolated from liver and marrow represent different subpopulations of progenitors (e.g., populations that express different erythroid growth factor receptors, different receptor numbers, different receptor affinity, or different cycling rates) is not known.
| CFU-MIX | BFU-E | |
|---|---|---|
| Liver | 12.7 ± 2.1 | 20.7 ± 3.1 |
| Marrow | 6.7 ± 1.4 a | 9.3 ± 2.7 a |
| Spleen | 4.2 ± 2.7 a | 5.9 ± 3.7 a |
| Blood | – | 8.0 ± 5.4 a |
Erythropoietic regulation in the human fetus differs markedly from that in the adult. In the adult, erythropoietic regulation primarily involves maintaining the red blood cell mass. In contrast, constant and dramatic changes characterize erythropoiesis in the embryo and fetus. The incredible rate of somatic growth and the resultant need to constantly increase the fetal red blood cell mass necessitate an extraordinary erythropoietic effort. Moreover, the relatively low oxygen tensions but high metabolic rates of fetal tissues require a system of oxygen delivery that differs significantly from the adult system.
The production of erythrocytes, from pluripotent stem cell to mature red blood cell, is governed by various growth factors. These erythropoietic growth factors are produced by accessory cells such as liver macrophages and marrow stromal cells, and they stimulate maturation, growth, and differentiation at various stages of red blood cell production. The progenitors involved in red blood cell production and the factors that stimulate their cellular maturation are depicted in Fig. 109.2 . Although these growth factors all facilitate production of red blood cells, none plays a more important regulatory role than EPO . EPO is a 30- to 39-kDa glycoprotein that binds to specific receptors on the surface of erythroid precursors and stimulates their differentiation and clonal maturation into mature erythrocytes. , The EPO gene (EPO) is located on chromosome band 7q21-22. During fetal erythropoiesis, EPO is produced principally by cells of monocyte/macrophage origin residing in the liver. Postnatally, EPO is produced almost exclusively by peritubular cells of the kidney.
Other growth factors besides EPO play a role in the differentiation and clonal expansion of erythroid progenitors. These include granulocyte-macrophage colony-stimulating factor (GM-CSF), stem cell factor (also known as c-kit ligand ), and interleukin (IL)-3, IL-6, IL-9, and IL-12. Thrombopoietin, a growth factor involved in platelet production whose receptor is similar in structure to the EPO receptor, also stimulates erythroid colony formation. IL-3 and GM-CSF were the first growth factors noted to have erythroid-stimulating properties, initially described as “burst-promoting activity.” In combination with EPO, these factors synergistically stimulate differentiation and proliferation of BFUs-E and CFUs-GEMM.
Stem cell factor is a multipotent growth factor that, in combination with other factors, supports clonal maturation of hematopoietic progenitors. Murine studies indicate that stem cell factor may be expressed during embryogenesis, thereby affecting embryonic and fetal erythropoiesis. In vitro studies show that stem cell factor alone stimulates clonal maturation of fetal (but not adult) multipotent progenitors. Erythroid progenitors isolated from term umbilical cord blood are more responsive to stem cell factor, alone and in synergism with GM-CSF and IL-3, than are adult marrow progenitors.
Term circulating erythroid progenitors are also more sensitive to IL-6 and IL-9 than are adult progenitors. IL-6 is a multifunctional, 22- to 26-kDa glycoprotein cytokine involved in B-cell stimulation and immunoglobulin production, acute-phase reactions, and induction of hematopoietic progenitors from a noncycling (G 0 ) phase into an active cycling (S) phase. IL-6 alone supports clonogenic maturation of newborn umbilical cord blood BFUs-E and CFUs-GEMM and induces progenitor cell cycling. IL-9 is also a multipotent cytokine and is similar to IL-6 in its ability to stimulate primitive erythroid progenitors and multipotent progenitors isolated from umbilical cord blood. Insulin-like growth factor 1 has been shown to cause clonal expansion of erythroid progenitors isolated from adult marrow , and on erythroid and mesenchymal-like cells in the fetal spleen. A recently identified growth factor termed regulator of human erythroid cell expansion is an erythroid growth factor that promotes formation of hemoglobinizing erythroblasts. It comprises a new EPO / EPO receptor target and regulator of human erythroid cell expansion that additionally acts to support late-stage erythroblast development.
Fetal erythroid progenitors respond in a slightly different fashion than do adult erythroid progenitors. In addition to the features noted earlier, fetal progenitors appear more sensitive to EPO than adult erythroid progenitors. Specifically, BFUs-E of fetal origin develop more rapidly into erythroid colonies, and the colonies generally contain significantly more normoblasts. In addition, BFUs-E from adult bone marrow require a combination of EPO plus another factor, such as IL-3 or GM-CSF, to mature clonally; however, many fetal BFUs-E mature in the presence of EPO alone. Studies have shown that fetal clones produce GM-CSF and IL-3, which may explain their unique capability for growth factor independence and auto-stimulation.
Erythropoiesis in utero is controlled by erythroid growth factors produced by the fetus, not the mother. EPO is the primary regulator of erythropoiesis in adults and appears to be the controlling factor for fetal erythropoiesis, especially during late gestation. EPO does not cross the placenta in humans, , monkeys, or sheep, although it has been reported to do so in mice. In the mouse, suppression of maternal erythropoiesis by hypertransfusion does not suppress fetal erythropoiesis. In humans, stimulation of maternal EPO production does not result in stimulation of fetal red blood cell production.
The expression of EPO is thought to be controlled by an oxygen-sensing mechanism in the liver and kidney. Both hypoxia and anemia stimulate erythropoiesis by stimulating messenger RNA (mRNA) transcription and EPO production. Two factors, hepatic nuclear factor 4 and hypoxia-inducible factor 1 (HIF-1), serve as transcription factors for EPO as well as other hypoxia-inducible genes. Hepatic nuclear factor 4 binds to the EPO promoter and enhancer regions of the gene. HIF-1 is a basic helix-loop-helix transcription factor composed of HIF-1α and HIF-1β subunits that bind to cis -acting hypoxia-response elements and induce EPO transcription. HIF-1 is expressed in many cells and is involved in up-regulating a variety of oxygen-regulated proteins, including vascular endothelial growth factor. HIF-1α appears to be constitutively expressed and rapidly degraded under normoxic conditions. RNA stability depends on the ubiquitin proteasome degradation system; inhibition of this system leads to increased levels of HIF-1 and EPO, even under normoxic conditions. Promoter and enhancer elements within the EPO gene are responsive to hypoxia, as well as to cobalt exposure in vitro. In animal models, the liver-sensing mechanism has a decreased sensitivity to hypoxia, producing one tenth the amount of EPO in response to comparable stimuli in the kidney. , The liver also appears to require more prolonged hypoxia to achieve an EPO response. , Other substances, including testosterone, estrogen, thyroid hormone, prostaglandins, vitamin E, and lipoproteins, have been shown to enhance EPO production or its effects, both in vivo and in vitro. ,
It is not known what factors regulate the switch of EPO production from the liver to the kidney. Renal production of EPO is not necessary for normal fetal erythropoiesis. The lack of a renal contribution to EPO production is illustrated by the normal serum EPO concentrations and normal hematocrits of anephric fetuses. In the sheep, EPO production in both the liver and the kidney is highest at 60 days’ gestation (term being 140 days). EPO production in the fetal liver decreases by 90 days’ gestation; however, increased renal production of EPO continues until 130 days’ gestation, when production falls to levels seen in adult sheep.
Studies in human fetal and neonatal kidney obtained from postmortem specimens also report measurable quantities of EPO mRNA, and quantitative mRNA studies in second-trimester human fetuses reveal that the fetal kidney produces approximately 5% of the amount of EPO mRNA that the fetal liver produces during the second trimester. Thus it appears that regulation of EPO gene transcription differs between the liver and the kidney in utero.
One mechanism by which gene expression can be differentially regulated is through methylation of promoter and enhancer regions of a gene, and another is through deacetylation of histones. Increased methylation generally results in decreased gene expression, whereas histone deacetylation causes relaxation of DNA structures and allows gene expression to occur. These two mechanisms may be involved in developmental EPO gene expression. For example, methylation of the enhancer region (leading to decreased expression) of the EPO gene in developing human kidney during the second trimester was much greater than in the liver.
EPO levels have been measured in umbilical cord blood during the third trimester, and these levels gradually increase throughout later development. From EPO measurements made in umbilical cord blood from infants of laboring and nonlaboring mothers and from infants undergoing labor stress, it seems likely that individual umbilical cord EPO levels primarily reflect hypoxic stress during labor and delivery. Serum EPO concentrations at birth normally range from 5 to 100 mU/mL. For comparison, serum EPO concentrations in anemic, nonuremic adults may be as high as 300 to 400 mU/mL. ,
Red blood cell indices change during gestation and continue to change through the first year of life. Circulating red blood cell concentrations gradually increase during the second trimester, from (2.85 ± 0.36) × 10 6 /μL at 18 to 21 weeks to (3.82 ± 0.64) × 10 6 /μL at 30 weeks ( Table 109.3 ). At term gestation, circulating red blood cell concentrations range from 5.0 × 10 6 to 5.5 × 10 6 /μL. In parallel with increasing red blood cell concentrations, hematocrits increase from 30% to 40% during the second trimester and continue to increase to term values over the latter part of the third trimester. Changes in hematocrit from 22 to 42 weeks’ gestation can be seen in Fig. 109.3 . Term hematocrits range from 50% to 63%, with some variability noted because of delayed clamping of the umbilical cord. Values are also dependent on the sampling site. Capillary hemoglobin concentrations are as much as 3.5 g/dL higher than venous hemoglobin concentrations.
| Gestational Age (wk) | White Blood Cells a (×10 9 /L) | Total White Blood Cells (×10 9 /L) | Platelets (×10 9 /L) | Red Blood Cells (×10 12 /L) | Hemoglobin (g/dL) | Hematocrit (%) | Mean Corpuscular Volume (fL) |
|---|---|---|---|---|---|---|---|
| 18–21 ( n = 760) | 4.68 ± 2.96 | 2.57 ± 0.42 | 234 ± 57 | 2.85 ± 0.36 | 11.69 ± 1.27 | 37.3 ± 4.3 | 131.1 ± 11.0 |
| 22–25 ( n = 1200) | 4.72 ± 2.82 | 3.73 ± 2.17 | 247 ± 59 | 3.09 ± 0.34 | 12.2 ± 1.6 | 38.6 ± 3.9 | 125.1 ± 7.8 |
| 26–29 ( n = 460) | 5.16 ± 2.53 | 4.08 ± 0.84 | 242 ± 69 | 3.46 ± 0.41 | 12.91 ± 1.38 | 40.9 ± 4.4 | 118.5 ± 8.0 |
| >30 ( n = 440) | 7.71 ± 4.99 | 6.40 ± 2.99 | 232 ± 87 | 3.82 ± 0.64 | 13.64 ± 2.21 | 43.6 ± 7.2 | 114.4 ± 9.3 |
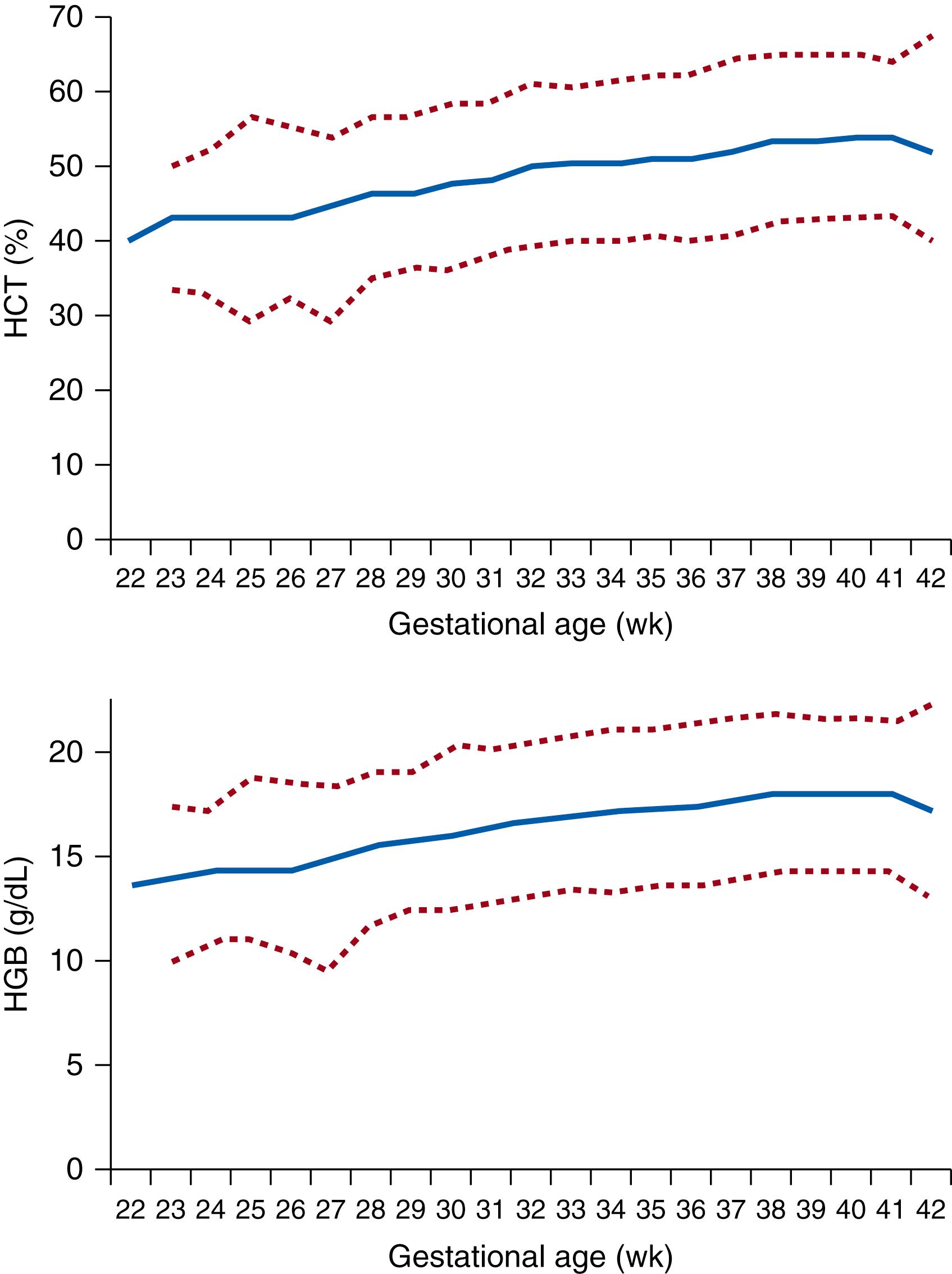
The hemoglobin concentration gradually rises during gestation. At 10 weeks gestation the average hemoglobin concentration is approximately 9 g/dL. By 22 to 24 weeks’ gestation, fetal hemoglobin values reach 11 to 12 g/dL, and by 30 weeks the hemoglobin concentrations are 13 to 14 g/dL. Premature male infants reach term umbilical cord hemoglobin values earlier than premature female infants, possibly because of the erythropoietic effects of testosterone. Hemoglobin concentrations are relatively constant during the last 6 to 8 weeks of gestation (see Fig. 109.3 ); at term the average hemoglobin concentration is approximately 16 to 17 g/dL. An increase in hemoglobin concentration by 2 hours of postnatal life occurs in most infants, resulting from a decrease in plasma volume. By 8 to 12 hours after birth, the hemoglobin concentration achieves a relatively constant level. Red blood cell production decreases significantly at birth, so that hemoglobin concentrations gradually decline by the end of the first postnatal week. The decrease in red blood cell production after birth is predominantly the result of increased availability of oxygen in the extrauterine environment, which greatly reduces EPO production and endogenous erythropoiesis. The continued fall in hemoglobin concentration over the next several weeks results from (1) decreased red blood cell production, (2) a shortened red blood cell life span of the fetal/neonatal erythrocyte, and (3) plasma dilution and an increase in blood volume related to growth. Multiple groups have also hypothesized that a process termed neocytolysis (the selective lysis of young erythrocytes) contributes to excessive hemolysis after birth, and thus a rapid decrease in hematocrit as the neonate adapts to its new, relatively hyperoxic environment.
The nadir of hemoglobin concentration in term infants occurs at approximately 8 weeks, with an average hemoglobin concentration of 11.2 g/dL. , Hemoglobin values gradually rise such that, by 6 months, the average term infant has a hemoglobin concentration of 12.1 g/dL. Altitude may have a modest effect on the postnatal changes in hemoglobin concentration; infants living at 1600 m had higher hemoglobin concentrations (by 0.4 g/dL) by 6 months of age than infants living at sea level. ,
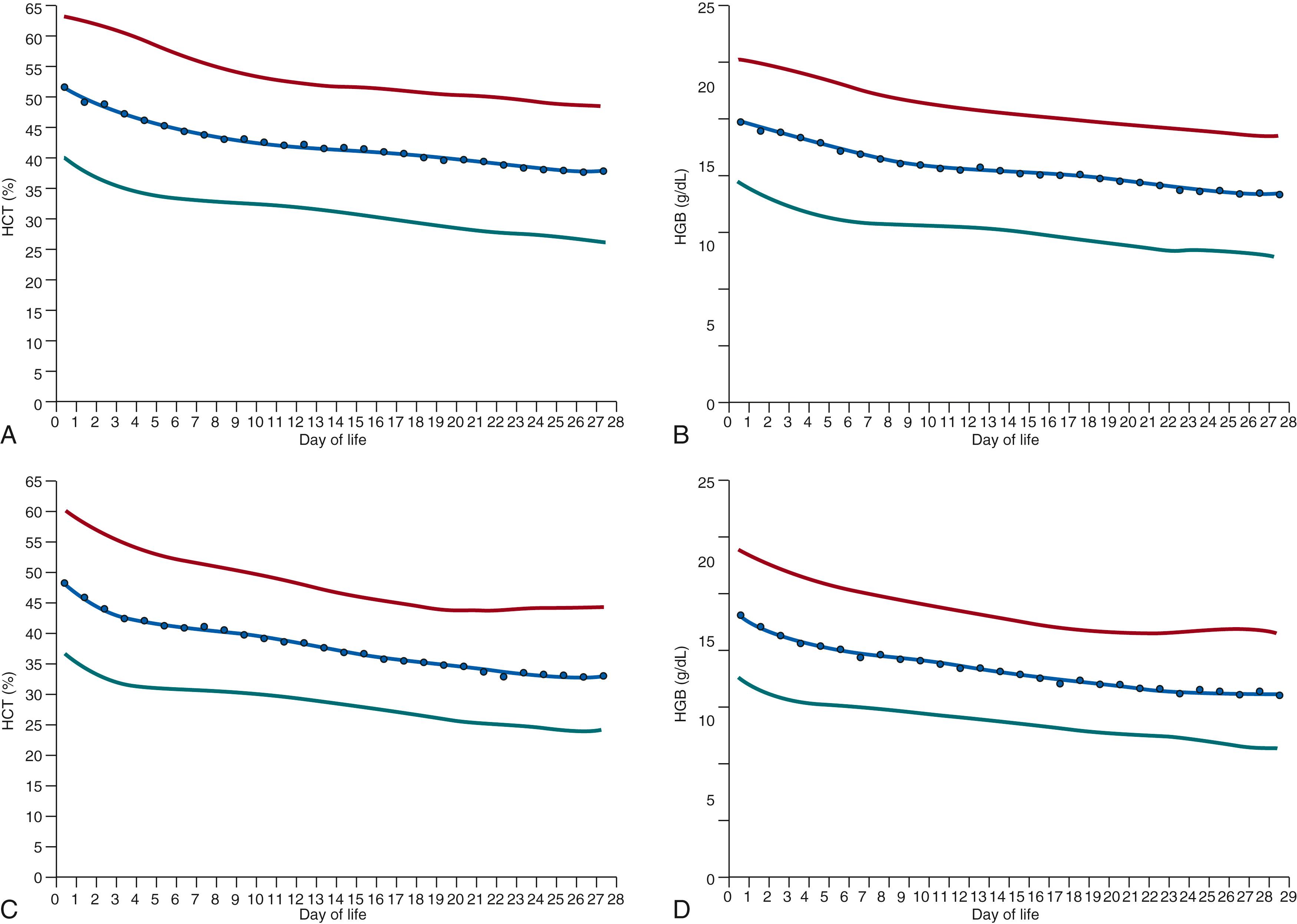
The average decline in the hemoglobin concentration of preterm very low-birth-weight infants (<1500 g) is remarkably different from that of term infants, in part due to phlebotomy losses that invariably occur in preterm infants, as well as the effects of transfusions on endogenous erythropoiesis. Very low-birth-weight infants reach a nadir of hemoglobin concentration of 8 g/dL at 4 to 8 weeks of age. Fig. 109.4 and Table 109.4 demonstrate relationships among birth weight, chronologic age, and red blood cell indices in term and preterm infants. , , ,
| Birth Weight (g) | Age (wk) | ||||
|---|---|---|---|---|---|
| 2 | 4 | 6 | 8 | 10 | |
| 800–1000 | 16.0 (14.8–17.2) | 10.0 (6.8–13.2) | 8.7 (7.0–10.2) | 8.0 (7.1–9.8) | 8.0 (6.9–10.2) |
| 1001–1200 | 16.4 (14.1–18.7) | 12.8 (7.8–15.3) | 10.5 (7.2–12.3) | 9.1 (7.8–10.4) | 8.5 (7.0–10.0) |
| 1201–1400 | 16.2 (13.6–18.8) | 13.4 (8.8–16.2) | 10.9 (8.5–13.3) | 9.9 (8.0–11.8) | 9.8 (8.4–11.3) |
| 1401–1500 | 15.6 (13.4–17.8) | 11.7 (9.7–13.7) | 10.5 (9.1–11.9) | 9.8 (8.4–12.0) | 9.9 (8.4–11.4) |
| 1501–2000 | 15.6 (13.5–17.7) | 11.0 (9.6–14.0) | 9.6 (8.8–11.5) | 9.8 (8.4–12.1) | 10.1 (8.6–11.8) |
Red blood cell indices may differ from normal ranges in infants born small for their gestational age, in whom placental insufficiency and secondary polycythemia are common. Infants of diabetic mothers, infants of mothers who smoke, and infants born at higher altitudes also tend to have higher hemoglobin concentrations at birth. , In growth-restricted infants born to hypertensive mothers, the blood supply to the placenta and the capacity to deliver oxygen to the fetus are diminished. Accelerated erythropoiesis is likely part of a compensating mechanism that raises oxygen-carrying capacity, maintaining an adequate supply to the fetus. In infants of diabetic mothers, increased metabolic demands of the fetus (resulting from increased glucose availability) may account for higher fetal oxygen needs and compensatory increase in hemoglobin concentration by the fetus. The increased red blood cell mass in infants of diabetic mothers does not result from higher maternal levels of hemoglobin A 1C (a high-affinity hemoglobin capable of decreasing the oxygen transferred to the fetus). In mothers who smoke, the increase in fetal carbon monoxide and the subsequent decrease in available oxygen are the likely causes of the compensatory increase in hemoglobin levels in the fetus.
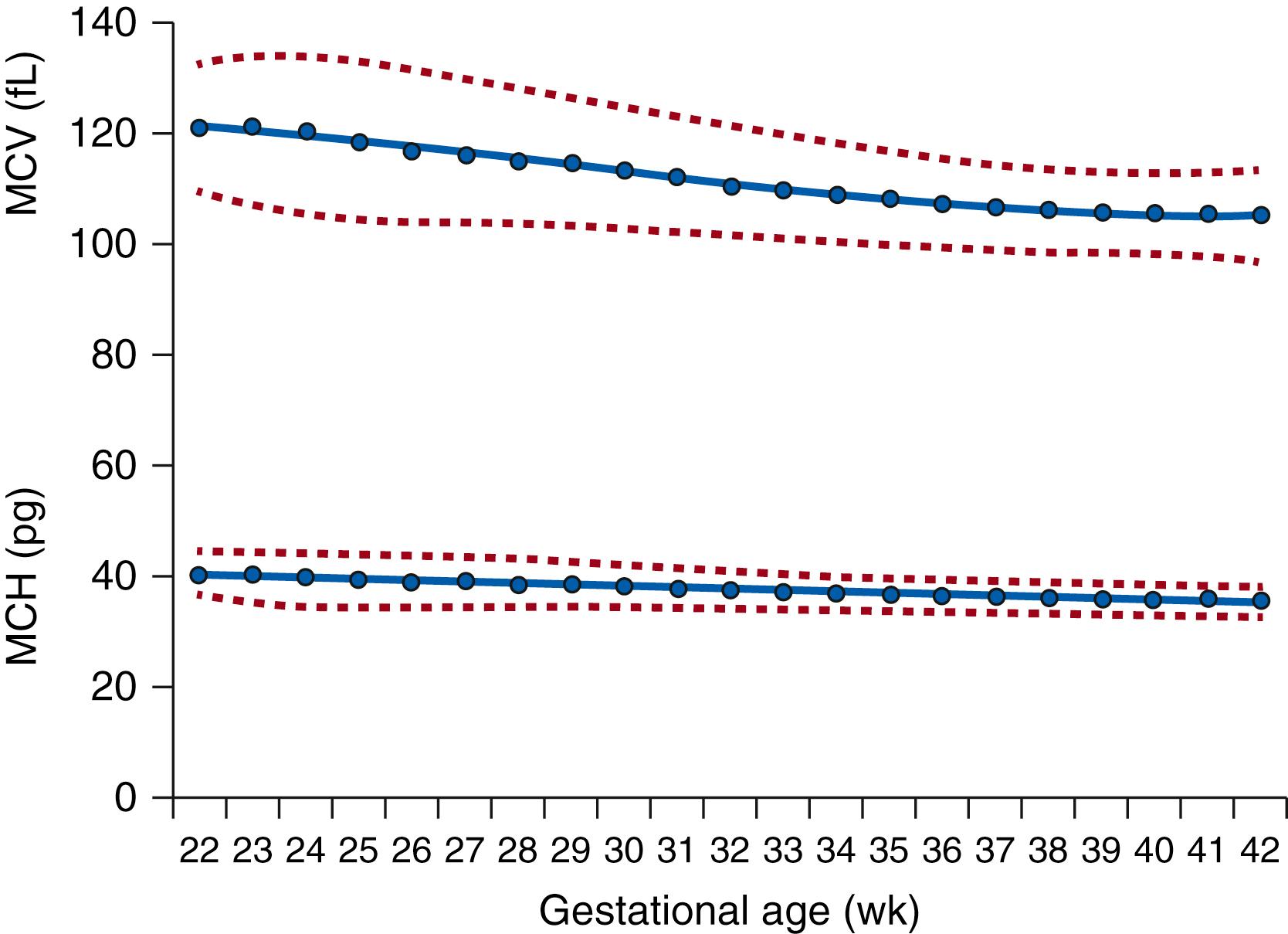
The size of the red blood cell gradually decreases during development. The MCV is more than 180 fL in the embryo, falls to 130 fL by midgestation, and decreases to 115 fL by the end of pregnancy ( Fig. 109.5 ). By 1 year of age, the MCV reaches an average of 82 fL. Similar to cells of other organs in infants born prematurely, the red blood cell MCV declines quickly after birth, and the postpartum changes in MCV appear to be related to chronologic age rather than postmenstrual age. The mean corpuscular hemoglobin concentrations remain relatively constant, and the mean corpuscular hemoglobin decreases slightly.
The placenta and umbilical cord contain 75 to 125 mL of blood at term, or approximately one fourth to one third of the fetal blood volume. Umbilical arteries constrict shortly after birth, but the umbilical vein remains dilated, and blood flows in the direction of gravity. Infants held at or below the level of the placenta can receive half of the placental blood volume (30 to 50 mL) in 1 minute. Blood can also travel from the neonate into the placenta if there is a significant gradient, resulting in 20 to 30 mL of blood loss per minute. Studies evaluating the benefits of delayed cord clamping or cord milking showed improvements in blood volume, hematocrit, decreased transfusions, decreased intraventricular hemorrhage (IVH), decreased late onset sepsis, and improved iron stores.
The blood volume of infants with early umbilical cord clamping averages 72 mL/kg, while the blood volume of infants with delayed umbilical cord clamping averages 93 mL/kg. Preterm infants have slightly larger blood volumes (89 to 105 mL/kg), owing to an increased plasma volume. By 1 month of age, blood volumes in term infants average 73 to 77 mL/kg.
Though nucleated red blood cells (NRBCs) are rarely observed in healthy adults and children, they are often observed in term and preterm neonates. Multiple studies have hypothesized that high concentrations of NRBCs at birth (or within the first hours to days of life) are a marker of in utero hypoxia. Indeed, a high concentration of NRBCs at birth has been associated with an increased risk of developing IVH and/or retinopathy of prematurity. Placental abruption, intraamniotic infection, and intrauterine growth restriction have also been associated with significantly higher NRBCs at birth. NRBCs are measured as either NRBC/μL or NRBC per 100 white blood cells (WBC; NRBC/100 WBC). The normative patterns of NRBCs are similar, regardless of units used. The number of NRBCs on the day of birth decreases from a median of ∼25 NBRC/100 WBC for a neonate born at 23 to 28 weeks gestation to a median of 7 NRBC/100 WBC for a term neonate ( Fig. 109.6 ).
![Fig. 109.6, Reference ranges for blood concentrations of nucleated red blood cell (NRBC) on the day of birth by gestational age. The lower and upper lines represent the 5th and 95th percentile limits. The middle line represents the mean value. (A) Data expressed as NRBC/100 white blood cell (WBC) . (B) Data expressed as NRBC/μL. (From Christensen RD, Henry E, Andres RL, et al. Reference ranges for blood concentrations of nucleated red blood cells in neonates. Neonatology . 2011;99[4]:289–294.) Fig. 109.6, Reference ranges for blood concentrations of nucleated red blood cell (NRBC) on the day of birth by gestational age. The lower and upper lines represent the 5th and 95th percentile limits. The middle line represents the mean value. (A) Data expressed as NRBC/100 white blood cell (WBC) . (B) Data expressed as NRBC/μL. (From Christensen RD, Henry E, Andres RL, et al. Reference ranges for blood concentrations of nucleated red blood cells in neonates. Neonatology . 2011;99[4]:289–294.)](https://storage.googleapis.com/dl.dentistrykey.com/clinical/DevelopmentalErythropoiesis/5_3s20B9780323712842001099.jpg)
Erythrocytes are released from the marrow into the blood as reticulocytes. The cytoplasmic organelles (e.g., ribosomes, mitochondria, Golgi apparatus) persist for 1 to 2 days after release before involution. Special staining detects elements of the residual organelles and allows for a manual reticulocyte count. Automated hematology counters that utilize flow cytometry have made more accurate reticulocyte enumeration possible because much larger samples of cells are evaluated than in a manual count. These analyzers also make it possible to identify subpopulations of reticulocytes that appear to be clinically helpful.
The reticulocyte count reflects the rate of effective erythropoiesis. Because EPO production and endogenous erythropoiesis falls dramatically after birth, the number of reticulocytes in circulation also falls. In an otherwise well neonate, the low number of reticulocytes in circulation persists through the first 90 days of life until the physiologic nadir of hemoglobin concentration is reached.
The immature reticulocyte fraction (IRF) quantifies the proportion of reticulocytes that are particularly high in RNA as a ratio to total reticulocytes, and is an early and sensitive index of marrow erythropoietic activity. Because flow cytometric gating on automated hematology analyzers is not standardized, the IRF may vary among analyzer manufacturers for any given sample.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here