Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
This chapter discusses the changing morphology of the developing fetal, neonatal, and postnatal pulmonary vascular bed. Studies have focused on structural and functional alterations in endothelial cells during postnatal development and have addressed the phenotypic heterogeneity of the vascular smooth muscle cells (SMCs) in the perinatal period. There are new insights into mechanisms regulating endothelial migration and angiogenesis, SMC proliferation, hypertrophy, and migration. These studies have also provided novel therapeutic targets whereby progression of pulmonary vascular disease may be slowed or prevented and regression induced.
Primitive pulmonary vessels arise from the sixth aortic arch in the 5-week human embryo to supply the upper poles of the right and left lung, and a pair of intersegmental arteries arising from the dorsal aorta penetrate upward through the diaphragm to supply the lower poles. At this stage, the parenchymal blood vessels of the developing lung are largely localized to the interlobular septa. Between 5 and 8 weeks of gestation it is likely that numerous paired dorsal intersegmental arteries supply the emerging blood vessels developing alongside the branching bronchi in the lung parenchyma. Once the true central pulmonary arteries form from the aortopulmonary trunk and anastomose with the intrapulmonary arteries, the primitive pulmonary arteries arising from the aorta and the primitive intersegmental arteries involute. By 9 weeks of gestation, the developing bronchial system is observed as one or two small vessels extending from the dorsal aorta, subsequently extending to the lung periphery as the airways increase in size. At each airway generation, the airway acquires an accompanying artery, in addition to numerous supernumerary arteries, branching at frequent but irregular intervals to enter the lung parenchyma from the pulmonary arterial branches running alongside the bronchi. By 16 weeks of gestation, development of the preacinar airways and accompanying arteries is complete. Thereafter, as the acini (terminal and respiratory bronchioles, alveolar ducts, and alveoli) develop, so do the accompanying and supernumerary arteries. , Experimental evidence shows that branching morphogenesis of the large preacinar airways provides cues to regulate branching morphogenesis of the preacinar arterial tree, whereas the intraacinar vessels regulate the development of the alveoli (this is discussed later).
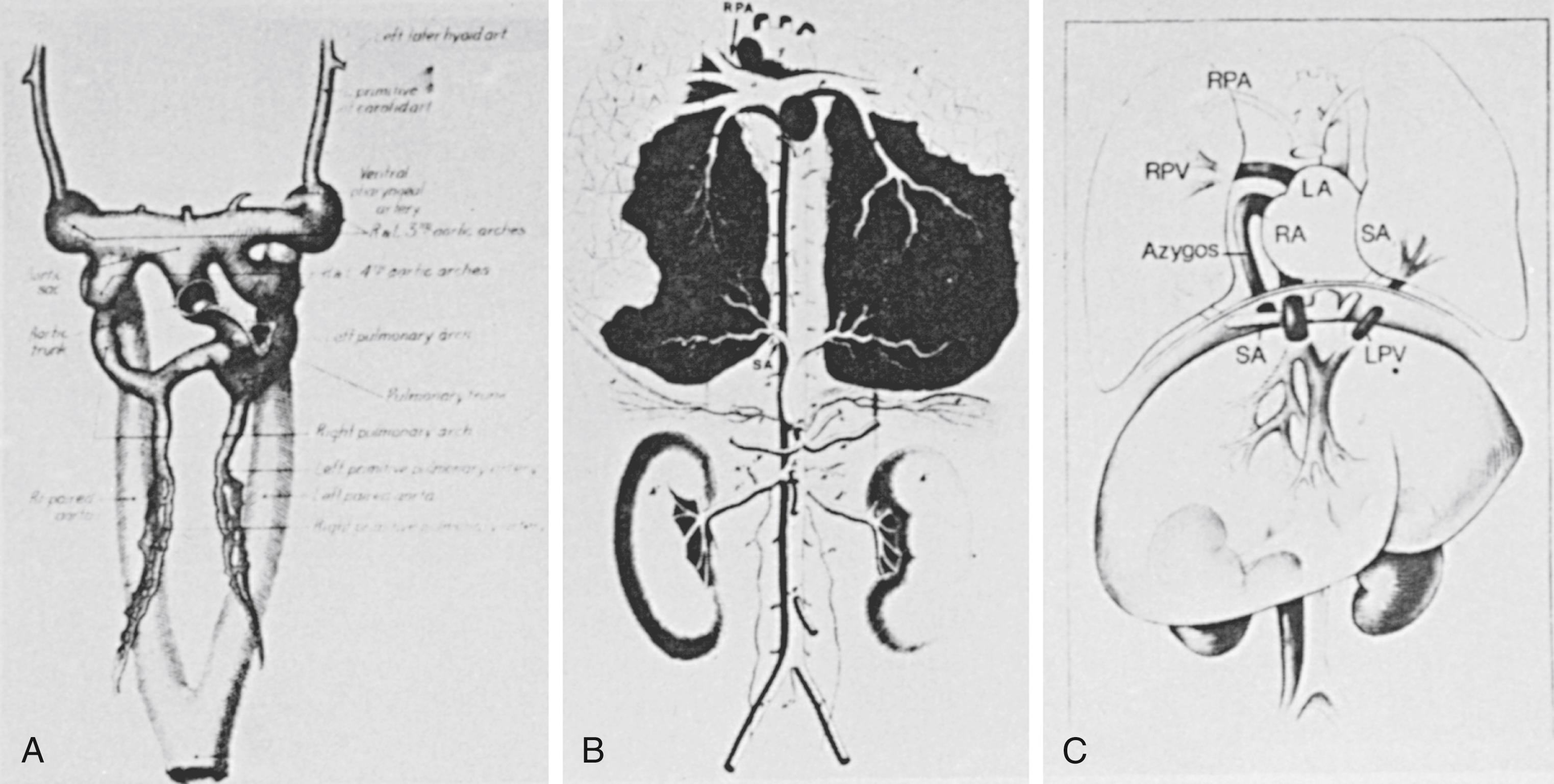
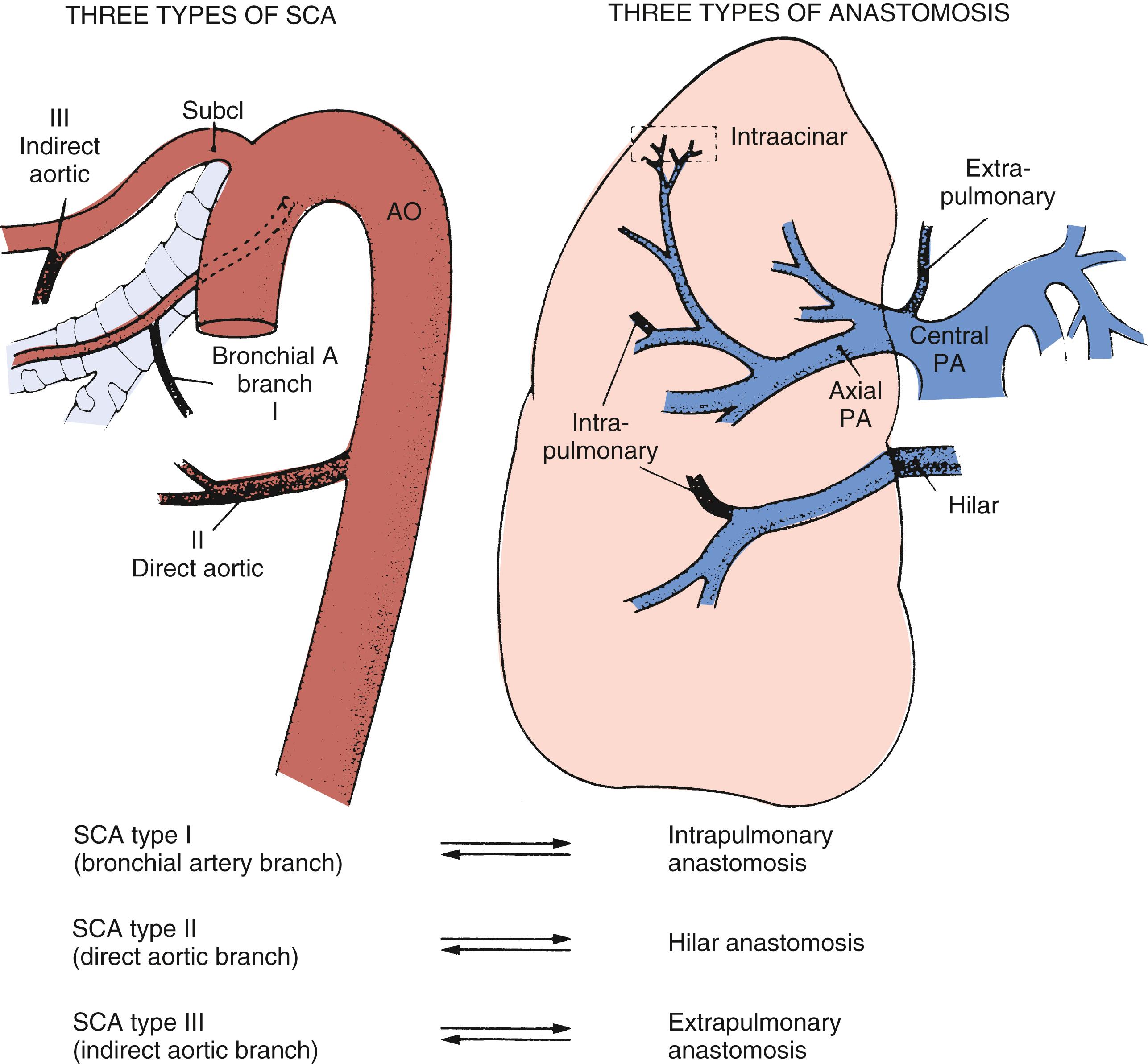
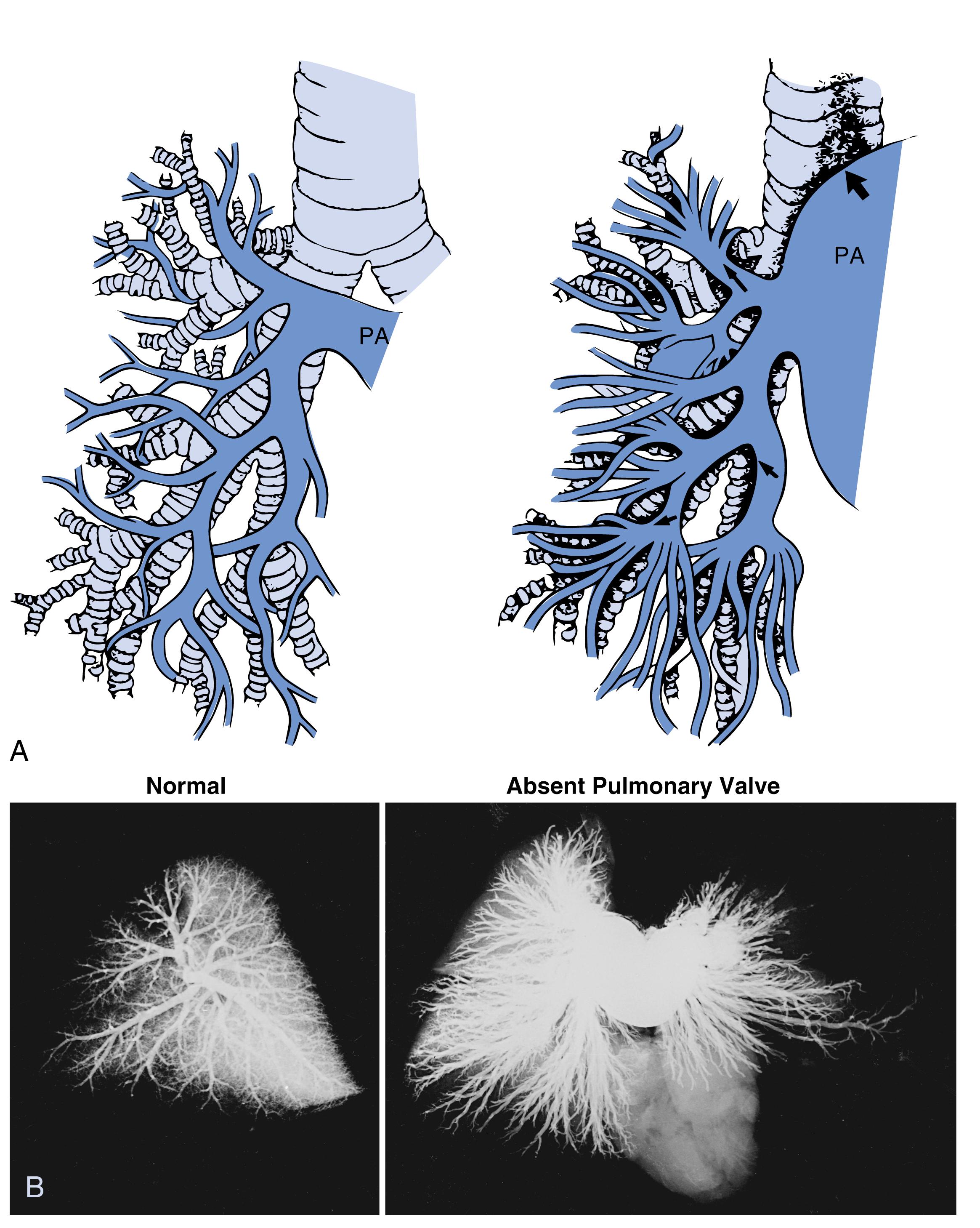
Antenatal disruption of pulmonary vascular development occurring as a result of congenital heart disease and other intrauterine factors may present with respiratory difficulties soon after birth. For example, persistent pulmonary hypertension of the neonate has been reported in association with pulmonary arterial maturational arrest at the fifth week of gestation ( Fig. 47.1 ). Infants with pulmonary atresia and ventricular septal defect frequently have persistence of the intersegmental arteries (aortopulmonary collaterals) that can serve as a source of blood supply to a lobe or lobar segment ( Fig. 47.2 ). Alternatively, a dual circulation with intersegmental arteries anastomosing to arteries can be traced back to a central pulmonary artery origin. In these infants there can also be multiple indirect aortopulmonary collaterals arising from branches of aortic branches (e.g., from subclavian, intercostal, and coronary arteries). Because these indirect collaterals can be observed in the absence of direct collaterals, they probably represent the sequelae of pulmonary atresia when it occurs later in development. Finally, in some cases of pulmonary atresia, there is supply by anastomotic vessels that arise from true bronchial arteries. Pulmonary vascular abnormalities are also observed with absence of the pulmonary valve. In those patients who present with severe respiratory problems from birth, abnormal branching of the vessels has been observed with tufts of intersegmental pulmonary arteries arising in a weeping willow or squidlike fashion, encircling and compressing the intrapulmonary airways ( Fig. 47.3 ).
The normal morphologic development of the pulmonary circulation has also been studied at the microscopic level. In the fetus, the preacinar arteries and those at the level of the terminal bronchioles are muscular, whereas the intraacinar arteries (i.e., those accompanying respiratory bronchioles) are either partially muscular (surrounded by a spiral of muscle) or nonmuscular. Arteries at alveolar duct and alveolar wall levels are also nonmuscular. The preacinar arteries are thick walled and change little in wall thickness relative to external diameter throughout the fetal period. Experimental studies suggest that the immediate postnatal period is characterized by rapid recruitment of small alveolar duct and wall vessels that appear to be functionally and structurally closed in the prenatal period. There is also progressive dilation of muscular arteries. Within a few days the smallest muscular arteries (<250 μm) dilate, and their walls thin to adult levels; by 4 months of age this process has included the largest pulmonary arteries at the hilum. As intraacinar arteries increase in external diameter during maturation, muscularization extends peripherally. Nonmuscular arteries first become partially muscular and become fully muscularized with postnatal age. For example, alveolar duct arteries are still largely nonmuscular in infancy, but become partially muscularized in childhood, and can be fully muscularized in adulthood. In contrast, alveolar wall arteries remain largely nonmuscular, even in the adult.
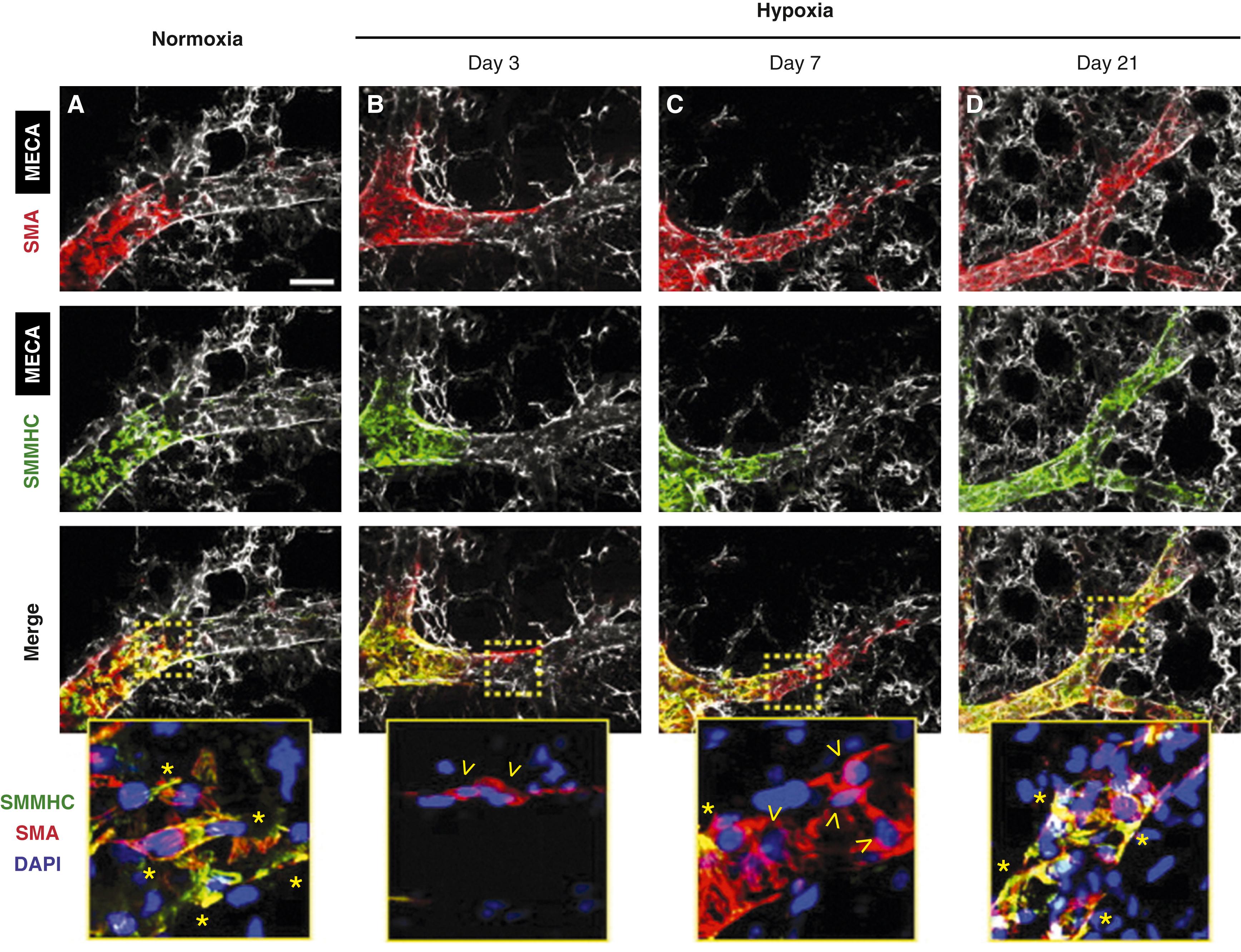
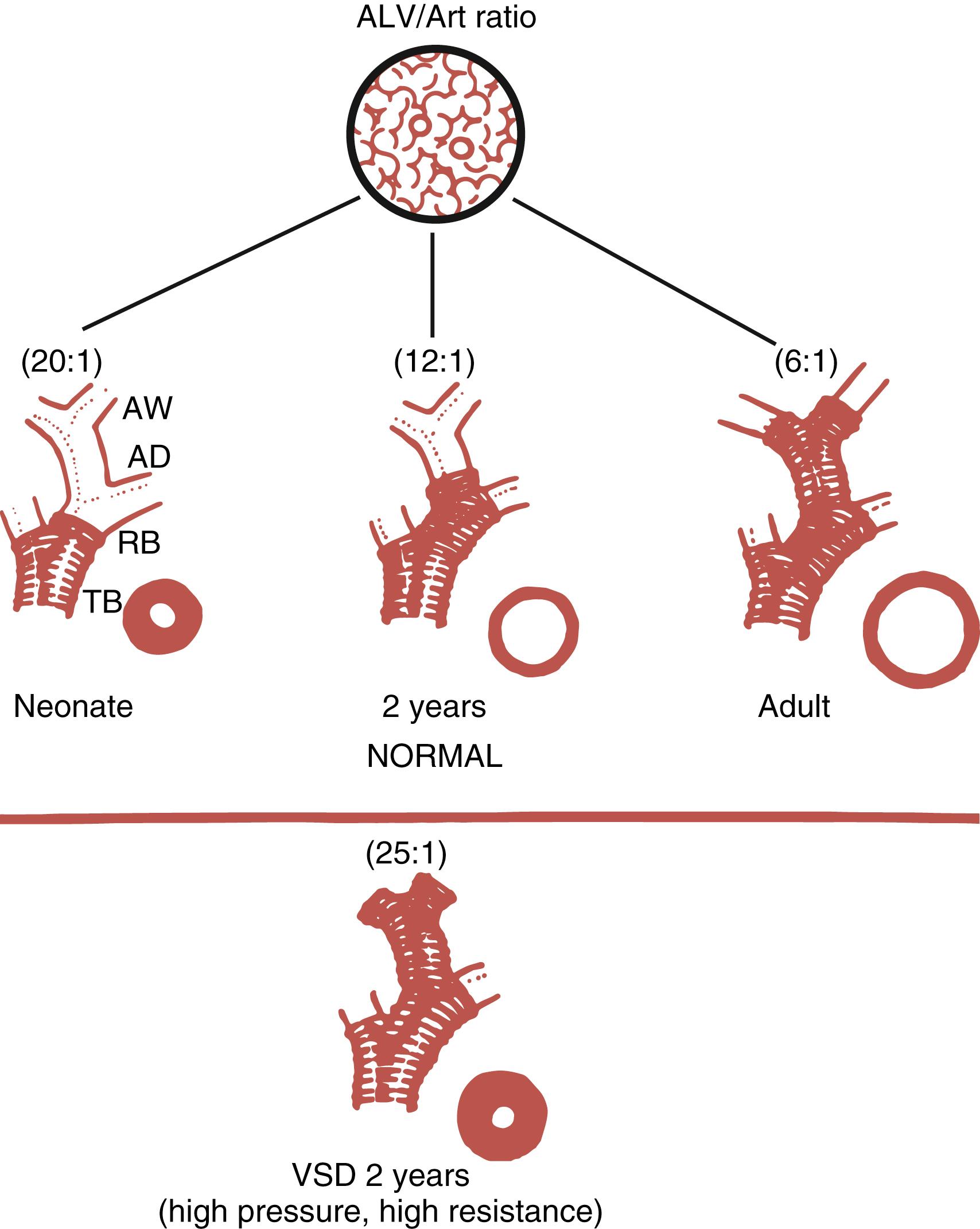
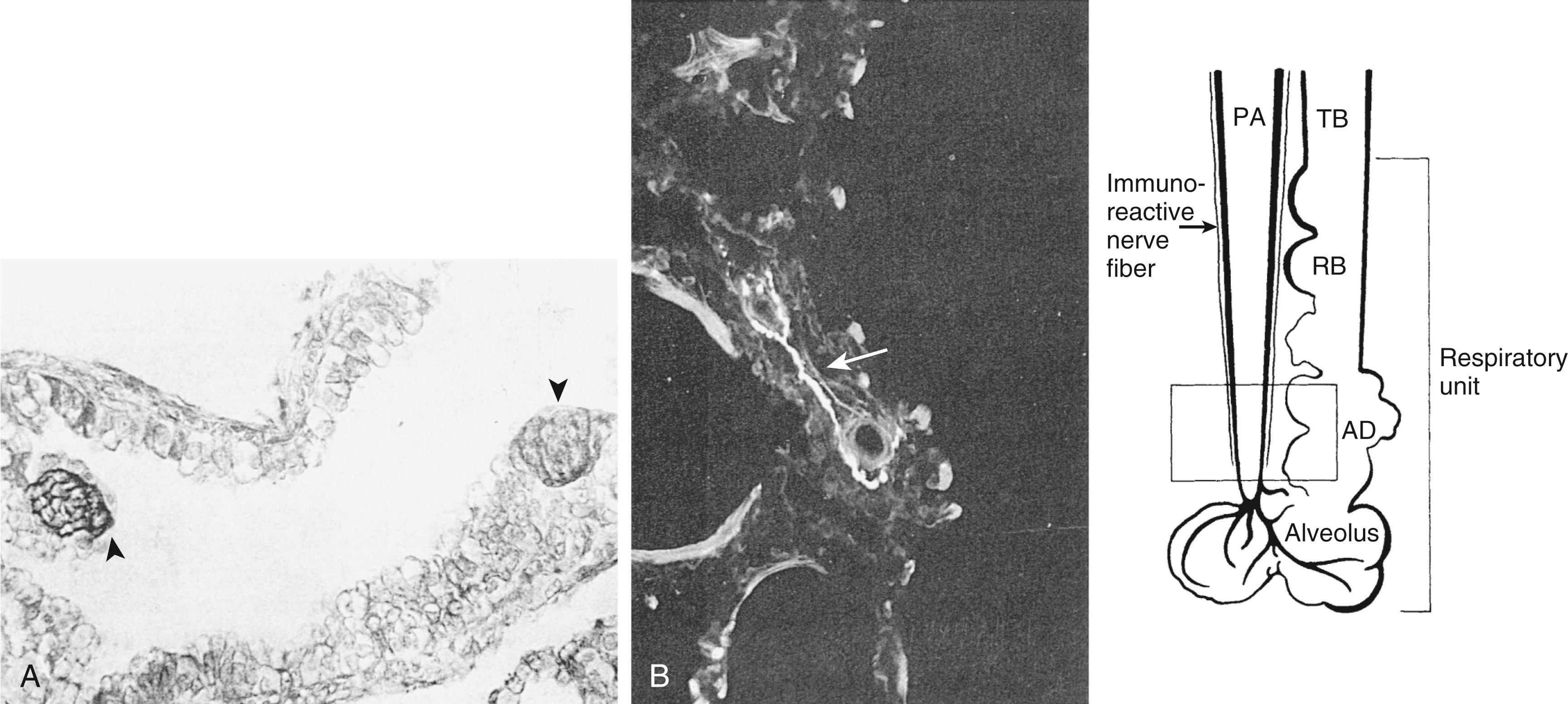
Clinical and experimental studies have suggested that the muscularization of peripheral pulmonary arteries involves the differentiation of pericytes and recruitment of fibroblasts. , However, more recent studies using fate mapping indicate that strategically located resident SMCs dedifferentiate and migrate to muscularize peripheral arteries ( Fig. 47.4 ). Arteries proliferate through early infancy, accompanying the proliferation of alveoli. Therefore, vessel density or the alveoli-to-artery ratio can be used as a measure of arterial growth, with the ratio decreasing from 20:1 in the newborn to 8:1 by early childhood ( Fig. 47.5 ). The growth and development of the pulmonary circulation may also be influenced by the trophic effects of neuropeptides with growth-factor-like properties released from neuroendocrine cells and neuroendocrine bodies associated with accompanying airways ( Fig. 47.6 ).
Experimental studies have indicated how changes in connective tissue, especially elastin and collagen, , and cellular arrangement modulate the normal adaptation to postnatal life. The endothelial cells in peripheral arteries increase elastin deposition to form an intact elastic lamina. Similar changes occur in the central pulmonary arteries, and elastin remodeling in the subendothelium is prominent in the early neonatal period. In contrast, elastin synthesis, as judged by messenger RNA (mRNA) levels, appears to be less prominent in the outer media and adventitia ( Fig. 47.7 ). Subpopulations of vascular SMCs with differing proliferative potentials and specialized functions, characterized by expression of specific cytoskeletal proteins, become more distinct during late fetal and early neonatal development. For example, metavinculin-positive SMCs are relatively resistant to proliferation, yet the high proliferative potential demonstrated in neonatal bovine pulmonary artery SMCs is reflective of a difference in activation of protein kinase C.
The cellular and molecular mechanisms that regulate growth and development of the fetal vasculature have been extensively studied. A summary of key molecules that influence pulmonary vascular growth and remodeling is presented in Table 47.1 . Cell adhesion molecules, such as vascular cell adhesion molecule 1 (VCAM-1) and platelet cell adhesion molecule (PECAM) ( Fig. 47.8 ), and the β 1 and β 3 families of integrin receptors, particularly α v β 3 direct interactions between endothelial and SMCs and the extracellular matrix. Perturbations of those interactions may lead to malformation of the pulmonary and systemic arteries. The β 1 integrins bind fibronectin; the β 3 family binds fibronectin, as well as a host of matrix molecules, but especially tenascin, osteopontin, and vitronectin, many of which govern vascular cell migration (e.g., fibronectin) and proliferation (e.g., tenascin).
| Molecule | Function |
|---|---|
| Cell Adhesion Molecules | |
| VCAM-1 | Binds α 4 β 1 integrins. Deletion results in embryonic lethality with defective placental vascularization and cardiac development. |
| PECAM | Promotes EC-EC and EC-matrix adhesion. |
| β 1 and β 3 integrins | Allows binding to fibronectin and other matrix components (e.g., tenascin, osteopontin, and vitronectin). Regulates vascular cell migration and proliferation. |
| Growth Factors, Chemokines, and Vasoactive Peptides | |
| VEGF-A | Signals through receptor FLT-1 and FLK-1 to promote EC survival and proliferation. Deletion in mice results in embryonic lethality in association with complete absence of blood vessel formation. |
| Angiopoetin-1 | Ligand for TIE2. Promotes blood vessel maturity and stability. Deletion in mice results in embryonic lethality in association with impaired vascular remodeling and complexity. |
| FGF-2 | Promotes autocrine expression of VEGF by EC and increases expression of alpha and beta-integrins. |
| HGF | Angiocrine factor produced by EC that promotes development of primary septae. |
| MMP-14 | Endothelial derived factor that drives expansion of epithelial progenitors by liberating ligands for the EGF receptor. |
| SDF-1α | Induced by VEGF and FGF. Chemoattractant for EC expressing CXCR4. Pro-angiogenic. |
| WNT7b | Activates canonical Wnt signaling to promote lung branching and vascular smooth muscle cell differentiation and survival. |
| BMP4 | Regulates proximal-distal patterning of the lung. Promotes EC and VSMC migration and proliferation. |
| IGF-1 | Promotes elastin expression and EC survival. Stabilizes nascent blood vessels. |
| NO | Promotes pulmonary vasodilation during the fetal-neonatal transition. Promotes angiogenesis downstream from VEGF. |
| Endothelin-1 | Induces vasoconstriction. Promotes VSMC proliferation and may lead to pulmonary vascular remodeling. |
| Transcription Factors | |
| TAL1 | Expressed by endothelial progenitor cells during vasculogenesis. |
| Sox17 | Required for normal pulmonary vascular morphogenesis. Deletion is mesenchymal cells results in dilation of macrovasculature but diminished complexity of microvasculature. |
| NFκB | Promotes pulmonary EC survival, proliferation, and migration during alveolarization. |
| Prdx1 | Promotes VSMC differentiation by regulating ECM stiffness and TGFβ signaling. |
| Matrix Components, Proteinases, Elastases | |
| Elastin | Prevents excessive VSMC proliferation. |
| Endogenous vascular elastase | Release FGF-2 from the ECM to promote VSMC proliferation. |
| Plasmin | Serine proteinase that cleaves ECM proteins. Generates the antiangiogenic protein angiostatin. |
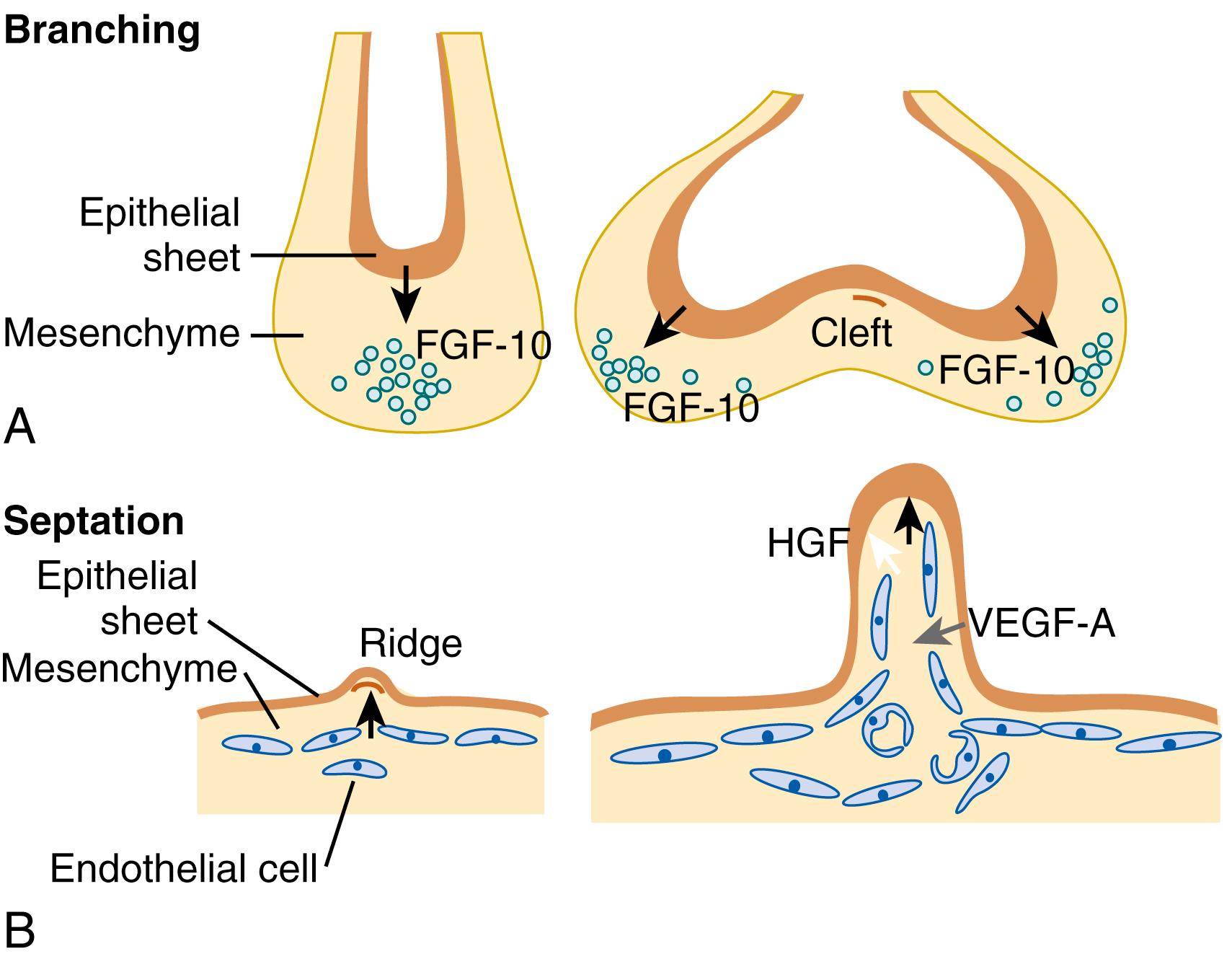
In addition, a variety of growth factors also appear to be responsible for the orderly growth and branching morphogenesis of the lung and blood vessels. These include vascular endothelial growth factor (VEGF), particularly VEGF-A, and angiopoietin-1, as well as acidic and basic fibroblast growth factor (FGF-1 and FGF-2). Epithelial production of VEGF is essential for angiogenesis, and blockade of VEGF-A mediated angiogenesis represses epithelial proliferation, suggesting that blood vessel formation promotes alveolar formation. The endothelium also appears to influence alveolar development via the production of angiocrine signals such as hepatocyte growth factor ( Fig. 47.9 ). In an experimental model of neoalveolarization induced by unilateral pneumonectomy, endothelial derived matrix metalloproteinase-14 drives expansion of epithelial progenitors by increasing the availability of ligands for the epidermal growth factor (EGF) receptor. In addition, endothelial nitric oxide synthase (eNOS) regulates distal vascular and alveolar growth, based on studies in transgenic mice with eNOS deleted. Growth factors also promote vascularization by inducing the chemokine stromal-derived factor-1, and also its receptor CXCR4 on endothelial cells. Interactions between members of the Wingless (Wnts) and bone morphogenetic protein families (BMPs) regulate lung branching morphogenesis, as well as endothelial and SMC survival, proliferation, and migration. Wnt7b has specifically been implicated in vascular development, particularly in regulating the investment of SMCs around the endothelial framework ( Fig. 47.10 ).
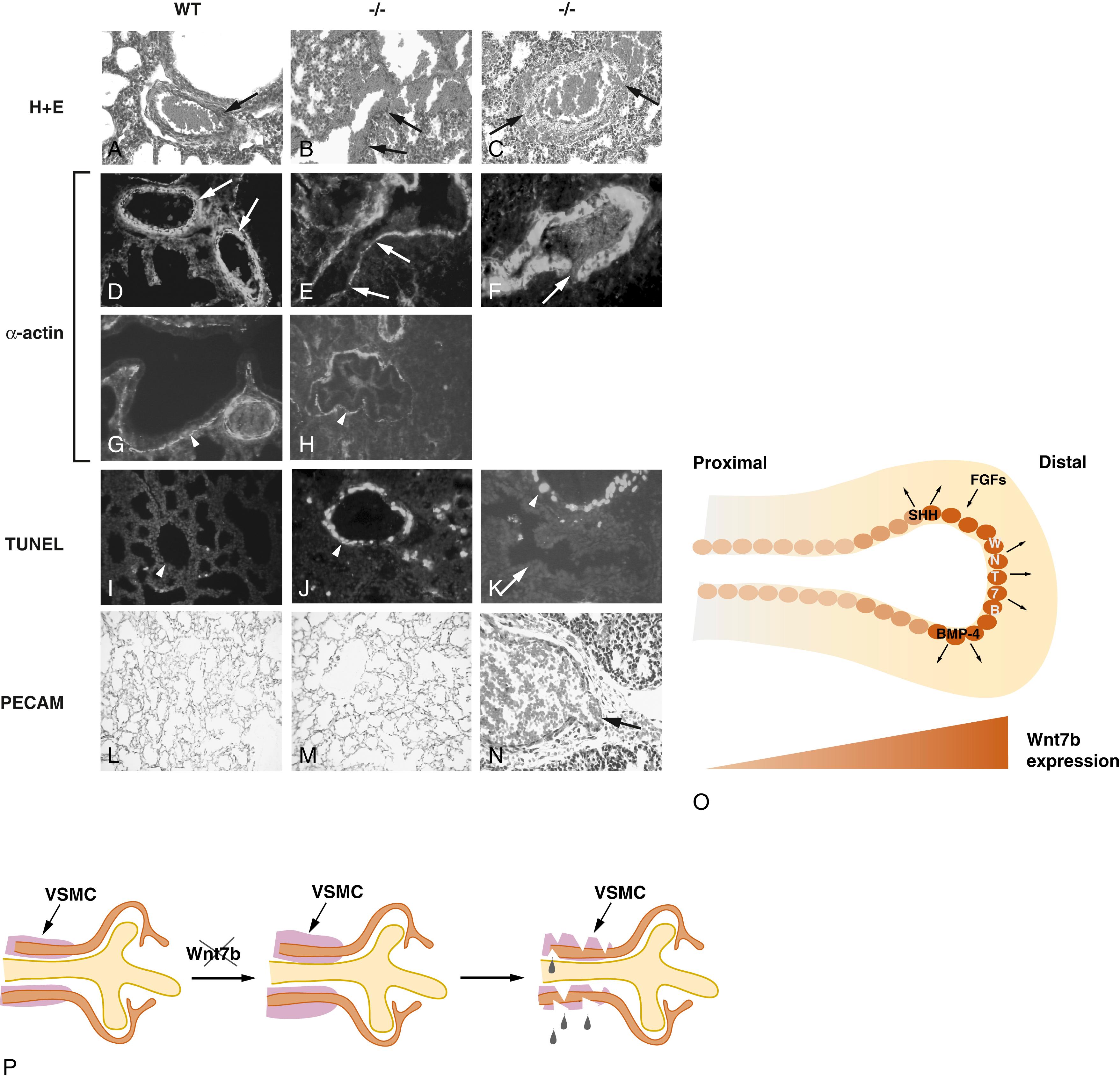
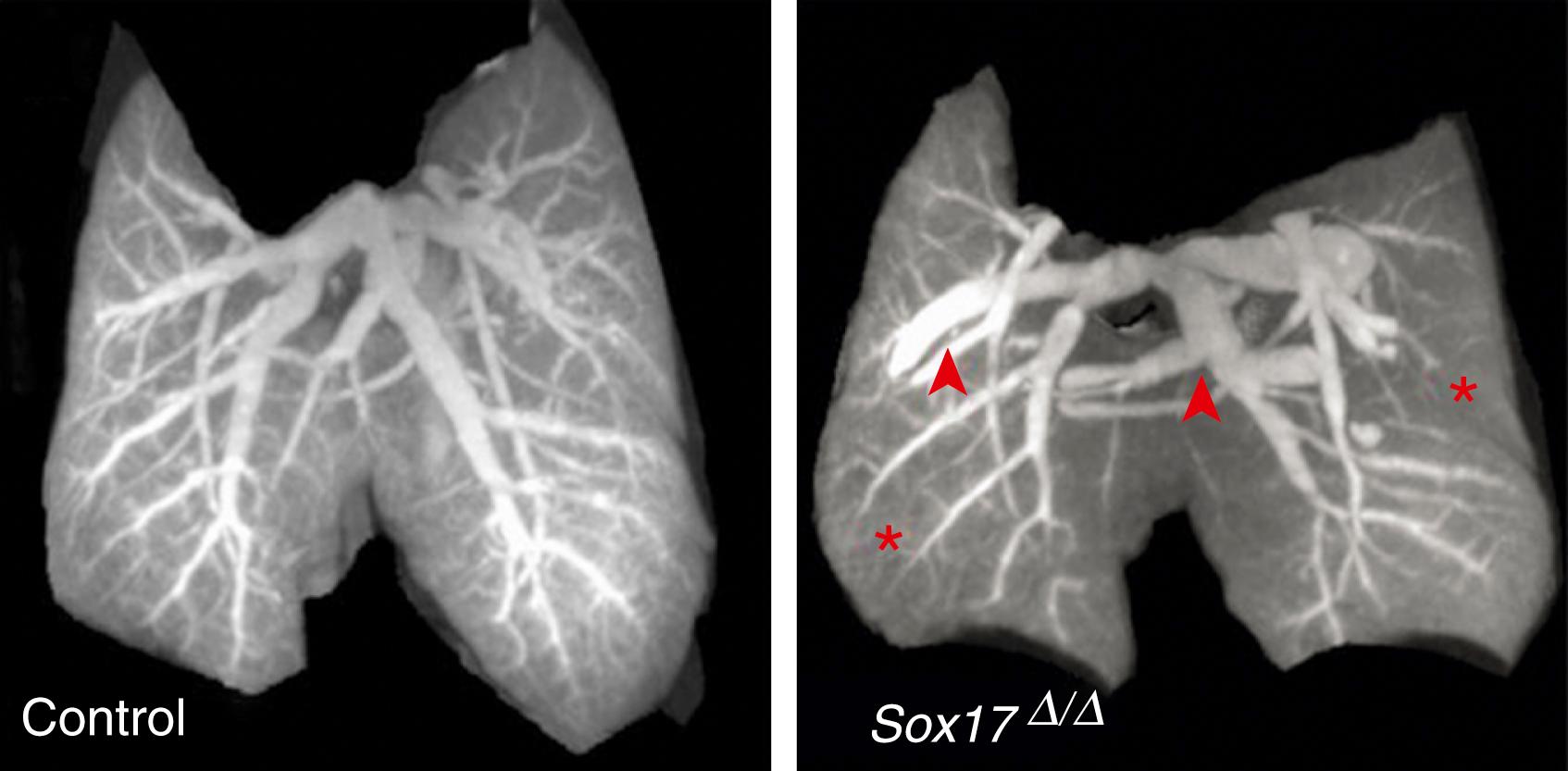
Downstream effects of growth factors are mediated through specific tyrosine kinase receptors, such as FLT1, FLK1, TEK, and TIE, as well as the activin receptor-like kinase (ALK1) and numerous BMP receptors. ALK-1 has been implicated in the late vasculogenesis, and mutations in ALK1 are associated with hereditary hemorrhagic telangiectasia, whereas BMP receptor ( BMPR ) 2 mutations are associated with primary pulmonary hypertension. Using a murine Flk1 reporter construct, Schachtner and colleagues found lung vascular development occurred at all stages of development and corresponded with overall lung growth. Notch and Jagged1 interaction are associated with early stages of lung vascular development. The transcription factor TAL1 is implicated in vasculogenesis from precursor cells of the hematopoietic lineage. Sox17 was identified as a major requirement for normal pulmonary vascular morphogenesis ( Figs. 47.10 and 47.11 ); NFκB is critical in neonatal angiogenesis and control of alveolar growth ; and Prx1 is critical to extracellular matrix production and vascular smooth muscle investment of endothelial cells during embryonic lung growth.
Matrix components (especially elastin and collagen) are regulated by insulin-like growth factor-I , and transforming growth factor-β (TGF-β). Insulin-like growth factor-1 and its receptor act in concert with VEGF to stimulate fetal vascular growth through the same intracellular signaling pathways. Lack of elastin leads to extensive proliferation of SMCs in pulmonary arteries. The balance between proteases and anti-proteinases also regulates growth factor interactions with cell surface molecules. Plasmin, thrombin, , and elastases, including leukocyte and endogenous vascular elastase (EVE), release active growth factors from storage sites in the extracellular matrix. Also, numerous endogenously expressed inhibitors of proteinases and elastases, such as plasminogen activator inhibitor and elafin, control growth and development, and other classes of molecules are known to control angiogenesis, such as angiostatin, a molecule derived from plasminogen.
The expression of vasoactive peptides in the lung during development also may play a role in the development of the pulmonary vasculature. Endothelin has been associated with cell proliferation and nitric oxide (NO) with the suppression of SMC growth. Early expression of NOS likely regulates vasculogenesis in addition to vascular tone.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here