Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Developmental and inherited disorders affecting the liver typically present in childhood but may affect individuals of any age. The effect of inherited metabolic disorders on the liver may be considered primary , caused by the accumulation of a metabolite resulting from an enzyme defect (e.g. sphingomyelin in Niemann–Pick [NP] disease), or secondary , when the major changes in the liver are the result of a primarily extrahepatic defect (e.g. steatosis of the liver secondary to pancreatic insufficiency in Shwachman syndrome). Likewise, as the list of identified inherited metabolic disorders continues to expand, many have significant but indirect relevance to the liver. For example, liver histology is usually normal in primary hyperoxaluria while the kidneys and other organs may be irreparably damaged; however, despite medical management, dialysis and repetitive kidney transplants, cure is obtained only with a liver transplant. In other inherited disorders, the liver disease may remain asymptomatic until precipitous acute liver failure develops; the classic example is Wilson disease (WD). This similarly occurs with developmental disorders; choledochal cyst may be diagnosed by prenatal sonography, or it may not become evident until late in childhood. In many cases the clinical diagnosis of an inherited disorder is not clinically evident, and consequently, diagnosis becomes the responsibility of the histopathologist, who may save not only the life of the patient but that of other family members as well. Advances in molecular genetics constantly improve our understanding of the biological basis of many metabolic diseases. Prenatal diagnosis is available for more families. Whole-exome sequencing carried out on 19 adult patients with liver disease of unknown aetiology showed that 5 were affected by four monogenic disorders including partial lipodystrophy, MDR3 deficiency, a mitochondriopathy and a hypobetalipoproteinaemia.
Many metabolic disorders manifest similar morphological findings despite having diverse aetiopathogenesis. Numerous diverse disorders are characterized neonatally by cholestasis and giant cell transformation of liver cells, such as α1-antitrypsin (α1-AT) deficiency, NP disease type C and bile salt export pump (BSEP) deficiency, also known as progressive familial intrahepatic cholestasis type 2 (PFIC2). Steatosis is one of the most frequent abnormalities, either alone (as in the urea cycle disorders, homocystinuria, lipoprotein disorders, the mitochondrial cytopathies and Shwachman syndrome) or in combination with other changes such as cholestasis, pseudogland formation and fibrosis (e.g. in galactosaemia, hereditary tyrosinaemia type 1 [HT1] and hereditary fructose intolerance [HFI]). Furthermore, neutral lipid may be stored in combination with other metabolites such as cholesterol (e.g. in lysosomal acid lipase deficiency manifesting as either Wolman disease or cholesterol ester storage disease) or glycogen (as found in glycogen storage disease [GSD] types I and III). Many disorders of lipid metabolism are expressed morphologically by ‘foam’ cells (as in NP disease types A and B and the gangliosidoses). The ultimate diagnosis depends on the clinical and laboratory data and identification of the specific enzyme defect. Histological changes may not lead to a specific diagnosis, but characteristic findings add to the phenotypic description of individual disorders and guide the selection of further investigations, in particular enzymatic assays and genomic studies. Accordingly, the following discussion includes the major clinical, laboratory and genetic features—the context in which histological changes should be evaluated.
The histopathologist has access to many special stains and techniques, both at the light microscopic and ultrastructural levels. The best all-round fixative for light microscopy remains 10% buffered formalin; however, some metabolic diseases (e.g. the mucopolysaccharidoses, cystinosis and the GSDs) require special fixatives to prevent leaching of water-soluble metabolites. Special stains for lipid, cholesterol and sphingomyelin must be performed on frozen sections cut from formalin-fixed or fresh material since routine processing will extract the lipid material from the cells. The histopathologist must be familiar with the many special stains that can be used on formalin-fixed and routinely processed material: uroporphyrin, haemosiderin, copper, copper-binding protein, bile, lipofuscin, lipomelanin (the pigment in the Dubin–Johnson syndrome), carbohydrates, mucopolysaccharides and other substances. Immunohistochemical stains are useful in a number of diseases, such as for identifying catalase in patients with peroxisomal diseases or the presence of α1-AT, α1-antichymotrypsin (α1-ACT) or fibrinogen in eosinophilic globules in various storage diseases. The repertoire of specific antibodies working on paraffin sections is continuously broadening, including antibodies reacting with various bile canalicular transporters such as the BSEP and multidrug-resistance protein 3 (MDR3); such antibodies can be commercially sourced and are readily available in specialist centres. Special microscopy should be utilized whenever necessary. The porphyrins can be demonstrated by their autofluorescence in frozen sections made from unfixed hepatic biopsy or autopsy material. Polarizing microscopy is especially useful for the identification of various crystals such as cholesterol, cystine, calcium oxalate, uroporphyrin and protoporphyrin.
Transmission electron microscopy (TEM) is very important in the diagnosis of many inherited metabolic diseases. According to Phillips et al., the findings are diagnostic in α1-AT deficiency, Farber disease, GSD types II and IV, HFI, Gaucher disease, metachromatic leucodystrophy (MLD), the gangliosidoses, Dubin–Johnson syndrome, erythropoietic protoporphyria (EPP), WD, Zellweger syndrome and infantile Refsum disease. In other disorders, TEM, although not diagnostic, can help to categorize the disease (e.g. as a glycogenosis, phospholipidosis or oligosaccharidosis) or to suggest the correct diagnosis, as in familial intrahepatic cholestasis type 1 (formerly known as PFIC1, now FIC1 deficiency), arteriohepatic dysplasia and lysosomal acid lipase deficiency (cholesterol ester storage disease). The role of scanning electron microscopy (SEM) is much more limited. Whether TEM will continue to serve a critical role in histological diagnosis of genetic/metabolic liver diseases, given the wide-ranging and cost-effective advances in immunohistochemical and molecular methods, is currently an area of debate.
In summary, histopathological studies contribute significantly to the diagnosis of metabolic disorders. Selection of appropriate pathological studies must be made before liver biopsy, to ensure appropriate fixation and handling of samples. Prebiopsy consultation with a histopathologist, and at times with laboratory staff of a centre dealing with highly specialized techniques, may preclude the loss of diagnostic information. In general, a portion of the biopsy core at least 5 mm in length should be snap-frozen using an optimal cutting temperature (OCT) compound, which does not interfere with most enzyme assays and allows subsequent frozen sectioning for microscopy. Ideally, a few bits (each 1 mm ) should be immersed in a fixative appropriate for electron microscopy (EM). Tissue freezing and EM fixative, in addition to usual fixation and paraffin processing, should be applied to all biopsy specimens taken from children or older patients in whom a metabolic disorder is suspected.
Many different disorders manifest with conjugated hyperbilirubinaemia in neonates ( Table 3.1 ). Some are caused by direct hepatocellular injury, and others by defects in hepatocyte bile formation or physical obstruction to bile flow. The distinction between primarily hepatocellular versus primarily hepatobiliary injury is critical for clinical management. The primary histological differential diagnosis for infantile conjugated bilirubinaemia disorders is therefore between obstructive and nonobstructive causes.
| Categories | Specific diseases/causes | Comments | Liver histology | Diagnostic investigations |
|---|---|---|---|---|
| Infection | Toxoplasmosis (congenital) | NSH (calcifications) | Maternal infection/IgM-specific Abs | |
| PCR on amniotic fluid | ||||
| Rubella (congenital) | NSH | IgM-specific Abs | ||
| Cytomegalovirus (CMV) (congenital) | NSH ‘owl-eye’ nuclear inclusions | Urine for viral culture, IgM Abs, PCR | ||
| Herpes simplex virus (HSV) (congenital) | Acute-pattern neonatal liver failure | Necrotizing hepatitis/viral inclusions (IHC) | Liver biopsyViral culture (scrapings from skin vesicles) | |
| Syphilis (congenital) | Diffusely fibrosing hepatitis | Standard test, VDRL, fluorescent treponema Abs | ||
| Human herpesvirus 6 (HHV-6) | Acute-pattern neonatal liver failure | Necrotizing hepatitis/viral inclusions (rare) | Serology, PCR | |
| Herpes zoster virus (HZV) | NSH | Serology, PCR | ||
| Hepatitis B virus (HBV) (mainly vertical) | Acute-pattern neonatal liver failure | Severe hepatitis | Mother’s serum eAg positive (or negative due to precore mutant) | |
| Hepatitis C virus (HCV) (mainly vertical) | Rarely cause of NHS | Screening of infants born to HCV+ mothers by RT-PCR | ||
| Human immunodeficiency virus (HIV) (vertical) | Rarely cause of NHS | NSH (opportunistic infections, in particular CMV) | Anti-HIV, CD4 count | |
| Parvovirus 19 infection | Chronic-pattern neonatal liver failure | NSH, marked haemopoiesis, siderosis, perisinusoidal fibrosis, few GC | Severe anaemiaIgM Abs, PCR | |
| Syncytial giant cell hepatitis (?paramyxovirus) | NSH, prominent syncytial GC (EM paramyxovirus-like inclusions) | Liver ultrastructure (no supportive serology) | ||
| Enteric viral sepsis (echoviruses, coxsackieviruses, adenoviruses) | Acute-pattern neonatal liver failure | NSH GC/cholestasis | Appropriate serology, viral culture or direct fluorescent assay | |
| Bacterial infection (extrahepatic or sepsis) | Acute-pattern neonatal liver failure | Nonspecific hepatitis/cholestasis (ductular bile casts) | Blood, urine or CNS culture | |
| Listeriosis | NSH, focal necrosis granulomas | Listeria isolation from blood, CSF or liver | ||
| Tuberculosis | Caseating granulomas (acid-fast bacilli) | High index of suspicionMantoux test | ||
| Structural | Biliary atresia (BA) | Biliary features: loose portal fibroplasia, ductular reaction and cholestasis including ductular bile plugs/GC (15%); DPM-like (20%) | Acholic stools/liver histologyNo excretion on hepatobiliary scanERCPLaparotomy | |
| Choledochal cyst | Differentiate from BA | Biliary features | Ultrasound, cholangiography | |
| Caroli disease/syndrome | Biliary features/DPM | Ultrasound, cholangiography | ||
| Choledocholithiasis | Differentiate from BA | Biliary features | Ultrasound, cholangiography | |
| Neonatal sclerosing cholangitis | Biliary features/periductal fibrosis inconstant | Cholangiography | ||
| Extrahepatic biliary hypoplasia (‘hair-like bile duct syndrome’) | Differentiate from BA | Biliary features | Cholangiography | |
| Spontaneous perforation of common bile duct | Differentiate from BA | Biliary features | Imaging | |
| Bile-stained ascites (paracentesis) | ||||
| Nonsyndromic duct paucity (idiopathic) | Paucity of intrahepatic bile ducts | Liver biopsyExtrahepatic syndromic featuresHigh serum cholesterolLiver biopsy JAG1 mutations (20p) or Notch2 (1p13) | ||
| Cholestasis | ||||
| Alagille syndrome | Differentiate from BA | Paucity of intrahepatic bile ducts Cholestasis |
||
| Identifiable bile ducts ± mild ductular reaction occasionally seen in early biopsy | ||||
| Metabolic genetic | α1-Antitrypsin deficiency | Differentiate from BA | Variable; biliary features mimicking BA, duct paucity or NSH (DPAS not diagnostic before 8–12 weeks of age); GC rare; periportal steatosis | Serum α1-antitrypsin concentration α1-Antitrypsin phenotype (PI type) |
| Cystic fibrosis | Differentiate from BA | Steatosis, cholestasis, biliary features (focal fibrosis) Cholangiolar eosinophilic casts |
Sweat chloride, extrahepatic complicationsGene mutation (7q31.2–CFTR protein) | |
| Galactosaemia | Acute- or chronic-pattern neonatal liver failure | Steatosis, biliary features, fibrosis Severe parenchymal damage and loss Later cirrhosis (now rare) |
Galactose-1–6-phosphate uridyltransferase assay Erythrocyte galactose-1-phosphate level |
|
| Tyrosinaemia, type 1 | Acute- or chronic-pattern neonatal liver failure | Severe parenchymal injury and loss, steatosis, GC, regenerative nodules cell dysplasia; fibrosis, cirrhosis | Elevated serum tyrosine, phenylalanine, methionine/elevated succinylacetone in urine FAA activity in fibroblasts or lymphocytes |
|
| Gene mutation (15q23–25) | ||||
| Hereditary fructosaemia | Chronic-pattern neonatal liver failure | Steatosis, biliary rosettes, GC, fibrosis, later cirrhosis | Liver biopsy (enzyme analysis)Gene mutation (9q22) | |
| Glycogen storage disease, type IV | Early perinatal variant, very rare | Eosinophilic (‘ground-glass’) cytoplasmic inclusions, PAS-positive, diastase resistant (DPAS+) (amylopectin-like) | Liver biopsyBrancher enzyme in liver, white blood cells or cultured fibroblasts | |
| Niemann–Pick, type A | Hepatocyte and macrophage storage (lipidic, microvesicular/foamy) | Sphingomyelinase assay (peripheral blood cells or liver) | ||
| Niemann–Pick, type C | Chronic-pattern neonatal liver failure | Hepatocytic/macrophage storage as type A, but few cells; biliary features | Storage cells in bone marrow aspirateCultured fibroblasts, cholesterol esterification studies | |
| Wolman disease | Cholesterol ester stored mainly in macrophages (cholesterol crystals), neutral lipids in hepatocytes | Liver biopsyLysosomal acid lipase activity | ||
| Gaucher disease | Macrophage storage (foamy striated cytoplasm), variable perisinusoidal fibrosis | Liver biopsyAcid β-glucosidase activity (white blood cells or cultured fibroblasts) | ||
| FIC-1 deficiency (PFIC type 1) | Bland cholestasis, mild disease | Low-GGT cholestatic syndromeLiver biopsy (EM)Gene mutation (18q21–22) | ||
| EM: granular bile (‘Byler bile’) | ||||
| Bile salt export pump (BSEP) deficiency (PFIC type 2) | Severe parenchymal injury, ballooned (cholate-static) hepatocytes, GC, absent canalicular BSEP (IHC staining) | Low-GGT cholestatic syndromeLiver biopsyGene mutation (2q24) | ||
| Multidrug-resistant protein 3 (MDR3) deficiency (PFIC type 3) | Biliary features; progressive fibrosis; absent canalicular MDR3 (IHC staining) | High-GGT cholestatic syndromeHistology, IHCGene mutation (7q21) | ||
| North American Indian childhood cirrhosis | Bland cholestasisLater progressive fibrosis | Gene mutation (16q22/cirhin) | ||
| Aagenaes syndrome | Very rare | Cholestasis | Cholestatic syndrome | |
| Lymphoedema (lower limb) | ||||
| Primary disorders of bile acid synthesis: | Resemble PFIC type 2, but canalicular | Low-GGT cholestatic syndromeUrine and plasma bile acids | ||
| BSEP present (IHC) | ||||
| 3β-hydroxy-Δ 5 -C 27 -steroid dehydrogenase/isomerase deficiency | ||||
| Δ 4 −3-oxosteroid 5β-reductase deficiency | ||||
| Arthrogryposis, renal dysfunction and cholestasis (ARC) | Variable cholestasis | Ichthyosis, recurrent infection, failure to thriveFanconi-like renal tubular dysfunction; arthrogryposis may not be evident | ||
| Gene mutation ( VPS33B on 15q26) | ||||
| Peroxisomal disorders (e.g. Zellweger syndrome) | VariableCholestasis, fibrosis, haemosiderosisAbsence of peroxisomes on EM | Dysmorphic featuresVery-long-chain fatty acid study/red cell plasmalogens | ||
| X-linked adrenoleucodystrophy | Chronic-pattern neonatal liver failure? | Absent or reduced peroxisomes on EM | Dysmorphic features (less striking than Zellweger) | |
| Perinatal haemochromatosis | Chronic-pattern neonatal liver failure | Severe parenchymal injury and loss; GC, haemosiderin pigment in hepatocytes | High serum ferritin, TIBCIron accumulation in heart and/or pancreas on computed tomography or magnetic resonance imaging | |
| Liver biopsy/lip biopsy for extrahepatic iron storage (accessory salivary glands) | ||||
| Mitochondrial DNA depletion syndrome | Chronic-pattern neonatal liver failure | Microvesicular steatosis, oxyphilic cells, siderosis, cirrhosis | Lactic acidosis hypoglycaemiaAbnormally low ratio of mtDNA/nDNA in tissue | |
| Mitochondrial anomalies (EM) | ||||
| Citrullinaemia, type II | Mainly Asian descent | Cholestasis, steatosis, siderosis | Gene mutation ( SLC25A13 ) | |
| Adenosine deaminase deficiency | Very rare | |||
| Panhypopituitarism (septo-optic dysplasia) | NS hepatitis, no distinctive features | Low cortisol, TSH and T4 | ||
| Hypothyroidism | NSH, cholestasis | High TSH titre, low T4, free T4 and T3 | ||
| Genetic (gross chromosomal abnormalities) | Trisomy 18 | Associated with biliary atresia | Biliary features | Karyotype |
| Cat-eye syndrome | Associated with biliary atresia | Biliary features | Karyotype | |
| Trisomy 21 | Chronic-pattern neonatal liver failure (rare); associated biliary atresia (occasional) | Fibrosing hepatitis with leukaemoid cell infiltration | Karyotype | |
| Kabuki syndrome a , b | Rarely associated with biliary atresia | Biliary features | Congenital anomalies, mental retardation | |
| Neoplasia (see Chapter 15 ) | Neonatal leukaemia | Acute-pattern neonatal liver failure | Leukaemic infiltration | Peripheral blood, bone marrow aspirate |
| Neuroblastoma | Acute-pattern neonatal liver failure | Small cell tumour, rosettes | ImagingUrinary vanilmandelic and homovanillic acid | |
| IHC, EM | ||||
| Langerhans cell histiocytosis | Biliary features similar to sclerosing cholangitis; Langerhans cells (CD1a) inconstant | Involvement of other systems (skin, bone, lung) | ||
| Haemophagocytic lymphohistiocytosis | Chronic-pattern neonatal liver failure | Haemophagocytic activity | High level of macrophage-derived cytokines in serum | |
| Elevated ferritin; elevated triglycerides | ||||
| Toxic | Total parenteral nutrition-associated cholestasis | Differentiate from BA | Biliary features, cholestasis | |
| Drug-induced (via breast milk or other) | ||||
| Vascular | Budd–Chiari syndrome | Rare | Features of venous outflow block | |
| Severe congestive heart failure | Perivenular cell dropout/congestion | |||
| Perinatal/neonatal asphyxia | Differentiate from BA | Ischaemic necrosis | ||
| Immune | Inspissated bile syndrome associated with ABO incompatibility | Differentiate from BA | Biliary features | Coombs test |
| Neonatal lupus erythematosus | NSHAnti-Ro, anti-La in liver tissue (IHC) | Maternal history, congenital heart block, skin rash (discoid lupus)Anti-Ro and/or anti-LA Abs | ||
| Gestational alloimmune liver disease (GALD) | See perinatal hemochromatosis (above) | |||
| Neonatal hepatitis with autoimmune haemolytic anaemia | Severe acute or chronic liver disease | Prominent syncytial GC, necrosis, inflammation, fibrosis | Coombs-positive haemolytic anaemia | |
| Idiopathic | Idiopathic neonatal hepatitis (seronegative) | Differentiate from biliary atresia | GC, parenchymal loss with stromal collapse of variable severity | Liver biopsy |
| ‘Le foie vide’ (infantile hepatic nonregenerative disorder) | Chronic-pattern neonatal liver failure | Total parenchymal cell dropout, scant ductular reaction | Liver biopsy | |
| Hardikar syndrome c | Differentiate from biliary atresia, Alagille syndrome | Biliary features | Associated with cleft palate, pigmentary retinitis, hydronephrosis |
a Selicorni A, Colombo C, Bonato S, et al. Biliary atresia and Kabuki syndrome: another case with long-term follow-up (letter). Am J Med Genet. 2001;100:251.
b Van Haelst MM, Brooks AS, Hoogeboom J, et al. Unexpected life-threatening complications in Kabuki syndrome. Am J Med Genet. 2000;94:170–173.
c Nydegger A, Van Dyck M, Fisher RA, et al. Hardikar syndrome: long term outcome of a rare genetic disorder. Am J Med Genet. 2008;146A:2468–2472. Note: Acute-pattern neonatal liver failure denotes metabolic instability, coagulopathy and extremely elevated serum aminotransferases in a neonate; chronic-pattern neonatal liver failure denotes metabolic instability, coagulopathy, hypoalbuminaemia, near-normal serum aminotransferases in a neonate; conditions which require specific consideration vis-à-vis biliary atresia are denoted ‘Differentiate from biliary atresia’. Abs , Antibodies; CFTR , cystic fibrosis transmembrane regulator; CNS , central nervous system; CSF , cerebrospinal fluid; PASD , Periodic acid-Schiff diastase-resistant; DPM , ductal plate malformation; EM , electron microscopy; ERCP , endoscopic retrograde cholangiopancreatography; FAA , fumarylacetoacetase; GC , multinucleated giant cells; GGT , γ-glutamyltransferase; HIV , human immunodeficiency virus; IgM , immunoglobulin M; IHC , immunohistochemistry; mtDNA , mitochondrial DNA; nDNA , nuclear DNA; NSH , nonspecific hepatitis; PAS , periodic acid-Schiff stain; PFIC , progressive familial intrahepatic cholestasis; RT-PCR , reverse-transcriptase polymerase chain reaction; T3 , triiodothyronine; T4 , thyroxine; TIBC , total iron-binding capacity; TSH , thyroid-stimulating hormone; VDRL , Venereal Disease Research Laboratories.
Obstructive causes include biliary atresia (BA), choledochal cysts, neonatal sclerosing cholangitis (NSC), spontaneous perforation of the common bile duct (CBD), the inspissated bile syndrome specifically associated with cystic fibrosis (CF) and Langerhans cell histiocytosis. A survey at King’s College Hospital of 1086 consecutive cases of neonatal conjugated hyperbilirubinaemia showed that BA constituted approximately one-third of all cases. Destruction and obliteration of the extrahepatic biliary tree of variable extent occurring early in embryonal development or perinatally are the causes of BA. Surgery is the only treatment option at present. Clinically, jaundice due to conjugated hyperbilirubinaemia, acholic stools, ‘nondraining’ hepatobiliary scan and ultrasonographic abnormalities of the biliary tree support a diagnosis of BA.
Portal expansion associated with oedema and fibroplasia, a variable mixed inflammatory cell infiltrate along with a ductular reaction and cholangiolar bile plugs are the histological hallmarks of an obstructive pattern. In the lobule, giant cell transformation with multinucleation of hepatocytes and canalicular bile plugs are also present. These portal changes are typically seen at about 6 weeks of life in infants with BA. These may be very mild or absent in biopsies taken earlier than 6 weeks. Biopsies from neonates 2–4 days old have shown minimal portal changes, suggesting that the portal changes observed later are the result of the perinatal bile flow causing periductal leakage and consequent inflammation and fibrosis.
The typical obstructive pattern observed in BA can be simulated by other disorders, such as α1-AT deficiency, CF, MDR3 deficiency, total parenteral nutrition (TPN)-associated cholestasis, sepsis, the so-called inspissated bile syndrome associated with ABO incompatibility and NSC. Steatosis often accompanies the biliary changes in both α1-AT deficiency and CF. Diastase-resistant periodic acid-Schiff (PAS)-positive globules are usually not evident in the first few months of life but may be demonstrated by immunohistochemistry (IHC) for α1-AT. If present, eosinophilic cholangiolar casts are a classic finding in liver biopsies from patients with CF. MDR3 deficiency is caused by a mutation of the ABCB4 gene, which encodes the MDR3 protein, critical for the transport of phospholipids, and in particular phosphatidylcholine, into the bile. Without phosphatidylcholine, bile salts are not incorporated into micelles. The bile remains hydrophobic and damages the biliary epithelium, causing a cholangiopathy, without gross architectural changes to the bile ducts, which may nevertheless manifest histologically with an obstructive pattern.
Nonobstructive causes include diverse disorders causing hepatocellular injury. The portal changes previously described are not observed. The histological picture is dominated by the lobular changes, usually described with the term ‘neonatal hepatitis’. These consist of a combination of hepatocellular changes such as swelling, multinucleation, apoptosis, cholestasis, lobular and portal inflammation, extramedullary haematopoiesis and fibrosis. These features are described in more detail in the section on neonatal hepatitis. Hepatocellular injury can be very severe in some patients, resulting in confluent hepatocellular loss and parenchymal micronodular transformation or even massive hepatic necrosis. The clinical scenario is that of acute liver failure occurring in a neonate. There may be clues in the clinical presentation, biochemistry and/or histology pointing to a metabolic aetiology or even to a specific metabolic disorder.
A defect of bile acid metabolism including inborn errors of bile acid synthesis, and certain defects in bile formation due to defective bile canalicular transporters, previously called ‘progressive familial intrahepatic cholestasis’ (PFIC), caused by mutations of ATP8B1 (FIC1) or ABCB11 (BSEP) are usually suspected when conjugated hyperbilirubinaemia is associated with normal or near-normal serum levels of γ-glutamyltransferase (GGT). Inborn errors of bile acid synthesis and BSEP deficiency are associated with marked hepatocyte disarray, hepatocyte multinucleation and a tendency to progressive fibrosis. FIC1 deficiency caused by ATP8B1 mutations manifests histologically more mildly with bland cholestasis, in the form of pale canalicular bile plugs not accompanied by hepatocellular disarray or giant cell transformation. Other forms of low-GGT cholestasis exist, and many have been described recently. Severe low-GGT cholestasis has been described in patients with mutations in the tight junction protein 2 gene ( TJP2 ).
Steatosis associated with features of neonatal hepatitis is seen in galactosaemia, HT1, fructosaemia, mitochondrial disorders and citrullinaemia type 2 (citrin deficiency). Severe forms manifest with the clinical picture of neonatal liver failure, in which the hepatocellular injury progress to confluent multilobular hepatocellular loss.
Siderosis in residual hepatocytes, pancreas, heart and salivary glands (demonstrated with a lip biopsy)—rather than in reticuloendothelial cells—of infants with neonatal liver failure due to massive hepatocyte damage and loss characterizes the syndrome of neonatal (or perinatal) haemochromatosis. It is currently regarded for most, but not all, cases as a form of alloimmune injury to the fetal liver (gestational alloimmune liver disease [GALD]). A similar clinical presentation occurs with ‘le foie vide’, characterized by massive loss of hepatic plates, but not cirrhosis. Its pathogenesis remains uncertain, apparently a failure of regenerative capacity.
Ground-glass inclusions are associated with the uncommon early manifestation of GSD type IV. Accumulation of lipidic substances in hepatocytes and portal or sinusoidal macrophages suggests a storage disorder such as NP disease type A or B, Wolman disease and Gaucher disease. Histological features of neonatal and early infantile liver disease must be evaluated in the context of normal histological features at birth. These include two-cell-thick hepatic plates, extramedullary haematopoiesis, periportal siderosis and accumulation of copper and copper-binding protein. Portal tracts may be lacking interlobular bile ducts. Biliary development appears to progress from the hilum through to the periphery, and the biliary tree may not be fully developed at birth. Degree of hepatobiliary maturation needs to be taken into consideration in the differential diagnosis of cholestatic conditions.
The clinical and histological manifestations of inherited and metabolic liver disorders in older children and adults are heterogeneous. They constitute a wide spectrum, which includes acute, subacute or chronic liver or multiorgan dysfunction, organomegaly and systemic metabolic abnormalities such as hypoglycaemia, protein intolerance or metabolic acidosis. An external factor can exacerbate their course and make them manifest acutely. They can mimic other metabolic disorders, disorders of various other aetiologies or even the effect of medical treatment. WD is a typical example. Its histological picture ranges from steatosis resembling nonalcoholic fatty liver disease (NAFLD) to a chronic hepatitis similar to autoimmune hepatitis. The clarified appearance of hepatocytes in GSD is identical to that of poorly controlled diabetes in Mauriac syndrome.
Histological interpretation is usually required in patients with a known disorder (to confirm the diagnosis and estimate the extent of liver damage) or in patients in whom the clinical diagnosis is uncertain. A systematic approach ensures a comprehensive histological assessment. It can be divided into the following components: (1) assessment of structural changes caused by acute parenchymal injury or chronic disease and fibrosis; (2) changes affecting hepatocytes; (3) presence and pattern of cholestasis and (4) changes suggestive of reticuloendothelial storage. These four components are described in more detail next. Of note, in some metabolic inherited disorders, there may be minimal or no significant histological changes, sometimes in relation to metabolic fluctuations. With urea cycle defects, for example, the histological appearance of the liver parenchyma ranges from near-normal to steatosis or cytoplasmic clarification simulating GSD when ammonia levels are high. Liver tumours may be the first manifestation or may complicate the course of many metabolic and inherited disorders (e.g. α1-AT deficiency, GSD). Cystic transformation is a characteristic of some of the ductal plate malformation (DPM) (ciliopathy) disorders.
Since metabolic and inherited disorders can present acutely, subacutely or chronically, their histological manifestations can be in the form of acute parenchymal injury or fibrosis, whose pattern, development and rate of progression may vary among different diseases, single-disease variants or individual patients. In some cases the lobular architecture can be entirely normal. For example, HT1, α1-AT deficiency, mitochondrial disorders, inherited disorders associated with intrahepatic cholestasis and NP disease type C may progress rapidly to advanced fibrosis. In CF and MDR3 deficiency the pattern of fibrosis is biliary, although these are highly disparate disorders. Type IV GSD progresses rapidly to cirrhosis, whereas certain other types of GSD do not. Bands of fibrous tissue associated with irregularly shaped parenchymal islands and containing bile ducts reminiscent of the ductal plate and scant portal vein branches are typical of congenital hepatic fibrosis (CHF).
Despite the presence of fibrosis, some metabolic disorders (e.g. WD, HT1) may present with acute liver failure. External factors such as infections or drugs may act as triggers. They manifest histologically with confluent parenchymal collapse, in some cases resulting in multiacinar loss or even massive hepatic necrosis, which may be superimposed to underlying fibrosis. Liver biopsy has a limited role in these cases because of its risks and proven limited contribution to diagnosis and clinical management. Mitochondrial disorders may present with neonatal liver failure or severe liver dysfunction later in infancy. Liver biopsy may play a role in confirming the diagnosis, noteworthy because multisystemic mitochondriopathies are a contraindication for liver transplantation (LT).
Hepatocyte morphology is affected in many metabolic and inherited disorders. Changes can be evident on standard haematoxylin and eosin (H&E)-stained sections or manifest as cytoplasmic accumulations demonstrated by histochemical stains, IHC or EM. In some disorders, the hepatocyte morphology can be entirely normal.
Steatosis is a common change, caused by primary or secondary abnormalities of pathways for metabolism of sugars, fats and amino acids. Macrovesicular steatosis , defined as a single, large droplet displacing the hepatocyte nucleus, is seen in galactosaemia, fructosaemia, citrullinaemia type 2 (citrin deficiency), hypertriglyceridaemia, urea cycle defects and WD. Paediatric obesity and associated fatty liver disease may act as a confounding factor. WD should always be considered, even in obese children and in the absence of histochemically demonstrable copper or copper-binding protein. Poor nutrition (as in CF) or a metabolic crisis can contribute to the development, severity and distribution of macrovesicular steatosis. Microvesicular steatosis , defined as fine cytoplasmic vacuolation around a centrally placed nucleus, is typically observed in fatty acid oxidation defects and other mitochondrial disorders. Histochemical techniques on frozen tissue or EM may be necessary to confirm that the changes noted are caused by accumulation of lipid. Micro- and macrovesicular steatosis can coexist and also can be associated with other hepatocyte changes, such as oncocytosis (mitochondriopathies), or clarification of the hepatocyte cytoplasm due to glycogen accumulation (GSD). Reye syndrome, first described in the 1960s and now very rare, was characterized clinically by encephalopathy and liver dysfunction after a viral illness and exposure to salicylates and histologically by microvesicular steatosis. Reye syndrome could be part of an idiosyncratic reaction to acute illness in individuals harbouring an underlying metabolic disorder.
Accumulation of glycogen in the hepatocyte cytoplasm results in a plant cell-like appearance. It is typically seen in the context of GSD. It simulates microvesicular steatosis, lysosomal storage and urea cycle disorders and drug-induced expansion of the smooth endoplasmic reticulum (ER). EM can help in the differential diagnosis. It is indistinguishable from the clarification of hepatocellular cytoplasm observed in patients with poorly controlled diabetes (Mauriac syndrome).
Ground-glass cytoplasmic inclusions are observed in a number of metabolic conditions, including GSD type IV, myoclonic epilepsy (Lafora disease), immunodeficiency and autoinflammation due to variants in RBCK1 , hypofibrinogenaemia, as well as in drug-induced hepatocellular injury and chronic hepatitis B. In adults, fibrinogen-associated ground-glass changes do not always indicate genetic disorder of fibrinogen production, as some cases result from the reactive accumulation of mixed proteins in cytoplasmic inclusions, which include but are not exclusively fibrinogen.
Diastase-resistant PAS-positive (D-PAS+) globules in periportal hepatocytes usually indicate α1-AT deficiency, and typically patients with the PI*Z allele, due to retention of the polymerized abnormal enzyme within the rough ER (RER). These globules may represent an acute-phase phenomenon in patients with PI*M phenotype or when heterozygosity for a defective M variant has not been detected. They are seen in the liver of patients with PI*MZ but not with PI*S or PI*MS. Finer D-PAS+ inclusions have been described in association with α1-ACT deficiency, and D-PAS-negative/α1-AT-negative globules may be found with antithrombin III deficiency.
Copper accumulation may be observed. Periportal copper and copper-binding protein deposits (rhodanine and orcein or analogue stains) and siderosis (Perls stain) are physiological up to approximately 4 months after birth. Accumulation of copper or copper-binding protein suggests a chronic cholestatic disorder, WD or copper toxicosis. In the early stage of WD, hepatocellular copper is intracytoplasmic rather than lysosomal and therefore not routinely demonstrable histochemically.
Iron accumulation may be localized in hepatocytes or nonparenchymal cells. Siderosis of Kupffer and endothelial cells indicates secondary iron overload or ferroportin disease. Hepatocellular siderosis is a feature of genetic HFE haemochromatosis. (See Chapter 4 for full discussions of inheritable iron disorders.) Children with genetic haemochromatosis are asymptomatic. Juvenile haemochromatosis, associated with mutations in the gene encoding hemojuvelin ( HJV ) or encoding hepcidin antimicrobial protein (HAMP), usually presents clinically in the second decade of life, mainly as a cardiac disorder. Siderosis may also be observed in children with peroxisome disorders (e.g. Zellweger syndrome).
Canalicular cholestasis in the absence of portal changes of large bile duct obstruction, cholate stasis or ductopenia is called ‘bland cholestasis’. It may be the only histological change in children, adolescents or adults presenting with pruritus with or without jaundice and with or without an external trigger such as an infection or hormonal imbalance (e.g. oral contraceptives). These symptoms may be caused by mutations of the same genes that cause bile canalicular transport defects ( ATP8B1 and ABCB11 ) in neonates, but in a milder form. IHC for canalicular enzymes, mutation analysis and exclusion of other causes (e.g. drugs, lymphoma) is part of the diagnostic workup. Lobular cholestasis accompanied by progressive bile duct loss and periportal deposits of copper and copper-binding protein is the histological picture of Alagille syndrome (ALGS). A ductular reaction may be present at an early stage. A sclerosing cholangitis-type pattern is observed in MDR3 deficiency. ‘Cholate stasis’ refers to the changes affecting periportal hepatocytes, usually as a result of a chronic biliary process. Hepatocytes contain granules of copper and copper-binding protein and may be swollen with clarification of their cytoplasm. Cholestasis is not seen in uncomplicated CHF, although septal ducts may contain bile. Histological changes are usually minimal in Gilbert syndrome and may consist simply of accumulation of lipofuscin. Dubin–Johnson syndrome is characterized by black Fontana-positive pigment in the hepatocyte cytoplasm, particularly in the perivenular region. Deficient expression of canalicular multispecific organic anion transporter (cMOAT, MRP2) is diagnostic.
Deficiency of enzymes (typically single-enzyme deficiency) involved in lipid or glycoprotein metabolism results in the accumulation of substances usually in the lysosomes of one or more cell types and one or more organs. These disorders are rare and often fatal in infancy, but some may remain undetected through adulthood.
The combination of cell type and organ involvement helps in identifying a specific disorder. For example, cholesterol ester storage disease (lysosomal acid lipase [LAL] deficiency) affects the liver, Gaucher disease affects liver and reticuloendothelial organs, and mucopolysaccharidosis (MPS) affects multiple organs including the central nervous system (CNS). The affected cell type in the liver is also an important clue to the underlying disorder. Both hepatocytes and reticuloendothelial cells, including Kupffer cells, portal macrophages and endothelial cells, are affected in LAL deficiency (Wolman disease and cholesterol ester storage disease). Sphingomyelin accumulation in Kupffer cells and hepatocytes is characteristic of NP disease types A and B.
In contrast, other disorders affect reticuloendothelial cells only and spare hepatocytes. In NP disease type C, accumulation of esterified cholesterol and other lipids affects very few Kupffer cells, often on a hepatitic fibrotic background. They can be easily overlooked as ceroid-laden macrophages in the context of a nonspecific hepatitic condition. In Gaucher disease, pale PAS-positive portal macrophages and Kupffer cells show a typical ‘crinkled paper’ appearance. In Fabry disease, Kupffer cells, portal macrophages and endothelial cells contain D-PAS+ globotriaosylceramide. Other lysosomal storage disorders associated with predominant accumulation in Kupffer cells include gangliosidoses, MLD, Farber lipogranulomatosis and cystinosis. D-PAS and IHC for CD68 help in evaluating Kupffer cells. Again, portal or sinusoidal storage cells can easily be missed or interpreted as scavenging ceroid-laden macrophages, particularly in disorders associated with hepatitis and fibrosis.
‘Neonatal hepatitis’ is a term that was originally coined in the early 1950s for presumed viral infections of the liver in early infancy. It has subsequently become evident that disorders demonstrating neonatal hepatitis are by no means exclusively viral, or even infectious, in aetiology. Moreover, disease processes affecting mainly the infantile biliary tree can display parenchymal inflammation. In effect, neonatal hepatitis represents a clinical pattern of neonatal liver disease; thus the designation ‘neonatal hepatitis syndrome’ has merit despite the inherent vagueness of the term ‘syndrome’. Other diseases, such as galactosaemia, HFI, CF and the conditions discussed in relation to BA and paucity of the intrahepatic bile ducts, may also present with pathological changes in the liver resembling an infectious process. Giant cell transformation, a frequent histological component of neonatal hepatitis, can be been seen in all cholestatic conditions in infancy, including pure haemolytic anaemias and endotoxic injury. Although clinical jaundice is not present in every case of neonatal hepatitis syndrome, conjugated hyperbilirubinaemia is invariably present. Therefore the entire spectrum of these diseases might best be called ‘infantile conjugated hyperbilirubinaemia disorders’, a term which avoids the inherent disadvantages of each of the component terms of ‘neonatal hepatitis syndrome’. Table 3.1 shows a classification of infantile conjugated hyperbilirubinaemia disorders (neonatal hepatitis syndrome). In the past 25–30 years the proportion of cases with no known aetiology has fallen substantially, from 50–60% to approximately 30% or less. Many of the disorders dissected out of the ‘idiopathic’ category are inherited metabolic diseases. The advent of high-throughput methodologies for gene identification (e.g. whole-exome and whole-genome sequencing) is accelerating the discovery of these disorders presenting in infancy with elevated serum conjugated bilirubin.
The nomenclature for neonatal liver disease is very problematic. The simplest term ‘neonatal jaundice’ may be confused with physiological jaundice in the newborn. The term ‘neonatal cholestasis’ is imprecise because in the first 3–4 months of life, every infant has some degree of cholestasis physiologically. This physiological cholestasis occurs because mechanisms for uptake of bile acids and other organic anions by hepatocytes are immature and thus inefficient, leading to high concentrations of bile acids in blood. In addition, hepatocellular pathways for bile acid conjugation and biliary secretion are also immature, in part because bile canalicular transporters are also regulated developmentally. The circulating bile acid pool is contracted, and ileal uptake of bile acids is underdeveloped. The term ‘neonatal hepatitis’ is obviously imprecise because hepatic inflammation is not a feature of every condition but remains widely used. ‘Neonatal hepatitis syndrome’, a term applied by Mowat in 1974, emphasizes the uniformity of the clinical presentation and similarity of pathological findings, as well as the broad spectrum of causative disease processes. The more recent term ‘infantile conjugated hyperbilirubinaemia disorders’ (ICHBRDs) not only identifies the definitional finding but it also unifies a broad spectrum of infectious, metabolic, structural and other aetiologies; however, it suffers from being linguistically unwieldy.
Recent developments and current usage draw attention regarding many of these disorders as ‘neonatal cholestasis’, and thus by implication nonphysiological. It emphasizes the complex hepatocellular mechanisms by which the liver accomplishes elaboration of bile. Some structural problems pertain to inadequate development of the biliary tree, resulting in actual or operational ductopenia, and any structural blockage to bile flow. Importantly, genetic disorders of intrahepatic cholestasis represent a diverse assortment of disorders including abnormalities in production of bile acids, expression of bile canalicular transporters in the composition of the bile canalicular membrane and in the myriad of protein networks which support bile canalicular function. Classifications which separate these interlocking parts may need to be reconstructed as additional disorders are identified. Classification is further complicated by some overlap of categories: for example, α1-AT deficiency can rarely be complicated by hypoplasia of the extrahepatic bile duct. Transient neonatal cholestasis may be associated with heterozygosity for a bile canalicular transporter defect. Narrow application of this term emphasizes the connection to intrahepatic cholestasis.
Numerous infections, usually congenital, are implicated in neonatal hepatitis syndrome, including cytomegalovirus (CMV), rubella virus, hepatitis B virus (HBV), herpes simplex virus (HSV), herpes zoster virus, coxsackievirus, echovirus, paramyxovirus, Toxoplasma and Treponema pallidum . An unusually high incidence of CMV infection (49%) was reported in a series of 45 cases from Taiwan. HSV, enteroviruses, adenovirus and HBV may cause neonatal liver failure characterized by an acute pattern of liver disease, with extremely elevated serum aminotransferases. In addition to genetic metabolic disorders, endocrine disorders may cause neonatal hepatitis syndrome. An association with hypopituitarism was reported in two infants by Herman et al., and later Sheehan et al., and confirmed in a subsequent large pathology case series of neonatal hepatitis with prominent giant cells. Immunological disorders may cause neonatal hepatitis syndrome; neonatal lupus erythematosus (NLE) is most common. Most infants with the rare disorder known as ‘neonatal (or perinatal) haemochromatosis’ have an immunological disorder which results in neonatal liver failure with a chronic pattern (near-normal serum aminotransferases, profound coagulopathy, subnormal serum albumin), called ‘gestational alloimmune liver disease’ (GALD).
Rarely, a Coombs-positive haemolytic anaemia defines a severe form of giant cell hepatitis which rapidly progresses to cirrhosis or death. It may present in the neonatal period; however, affected infants may present later in the first 1–2 years of life. Early and sustained immunosuppressive therapy may control the disease in some patients. The liver lesion has been shown to recur in the allograft of the few cases transplanted. , Transient neonatal cholestasis was initially identified in infants with birth asphyxia who developed severe neonatal hepatitis syndrome. It may be caused by an ischaemic hepatitis. With this ‘transient neonatal cholestasis’, conjugated hyperbilirubinaemia typically occurs at 1 week of age, lasts 3–4 months, and the hepatomegaly and liver tests return to normal by 1 year of age. Disease definition has been refined to include specific risk factors: preterm birth (<34 weeks gestation), small for gestational age, receiving TPN >7 days and abdominal or thoracic surgery. Within this definitional framework, transient neonatal cholestasis is being identified more often. Various hepatobiliary structural abnormalities, most importantly BA, are associated with the neonatal hepatitis syndrome. Furthermore, some endocrine problems and certain chromosomal defects are associated with neonatal hepatitis syndrome. Clinically, a methodical approach to diagnosis is necessary. Targeted next-generation sequencing and other genomic diagnostic strategies may be informative.
In approximately 30% of infants with conjugated hyperbilirubinaemia, no aetiology is found. The prognosis for this so-called idiopathic neonatal hepatitis is generally good, with mortality of 13–25%. , In the study of Dick and Mowat, 2 of 29 patients with idiopathic neonatal hepatitis died, and only two others had signs of persisting liver disease. Overall, predictors of poor prognosis include persisting severe jaundice, acholic stools, prominent hepatomegaly, severe inflammation on liver biopsy and familial occurrence. Numerous inherited disorders causing the neonatal hepatitis syndrome have recently been described in terms of gene defect, such as the bile canalicular transporter disorders and bile acid synthesis disorders, and these had previously been classified as idiopathic neonatal hepatitis. A hereditary form with giant cell transformation and lymphoedema resulting from abnormal deep lymphatics has been reported, but the gene abnormality has not yet been determined. , Another identified aetiology is adenosine deaminase deficiency , with recovery after enzyme replacement. Citrullinaemia type 2 due to deficiency of citrin can cause neonatal hepatitis syndrome (then also known as NICCD, neonatal intrahepatic cholestasis caused by citrin deficiency ); it may occur in ethnicities other than East Asian. ‘Le foie vide’, of which multiple cases have been identified, describes a severe neonatal liver disorder characterized by failure of hepatocellular regeneration, also manifested as chronic-pattern neonatal liver failure. , , Undoubtedly, other such disorders remain to be defined. Cases of transient neonatal hepatitis without identifiable risk factors have been investigated via next-generation sequencing leading to the identification of a genetic disorder in many (not all) cases. Thus the ‘idiopathic neonatal hepatitis’ component of the neonatal hepatitis syndrome continues to diminish.
Liver biopsy specimens are characterized by varying degrees of cholestasis (with or without pseudoglandular structures), giant cell transformation, ballooning, apoptotic bodies, extramedullary haemopoiesis, lobular and portal inflammation and progressive fibrosis in some cases. Unusually severe inflammation and hepatocellular damage may be found in α1-AT deficiency, HT1, NP disease type C, syncytial giant cell hepatitis, citrullinaemia type 2, primary disorders of bile acid synthesis (mainly Δ 4 –3-oxosteroid-5β-reductase deficiency), BSEP deficiency (PFIC2) and idiopathic neonatal hepatitis. Associated macrovesicular steatosis favours a metabolic disorder. Confluent hepatocyte necrosis or loss with bridging collapse is seen in the rare patients presenting with acute-pattern neonatal liver failure or having a subacute clinical course associated with perinatal haemochromatosis, non-Wilsonian copper toxicosis or other metabolic disorders such as HT1. It may also occur occasionally with viral hepatitis, especially in infants born to mothers carrying the precore mutant of hepatitis B, and idiopathic neonatal hepatitis.
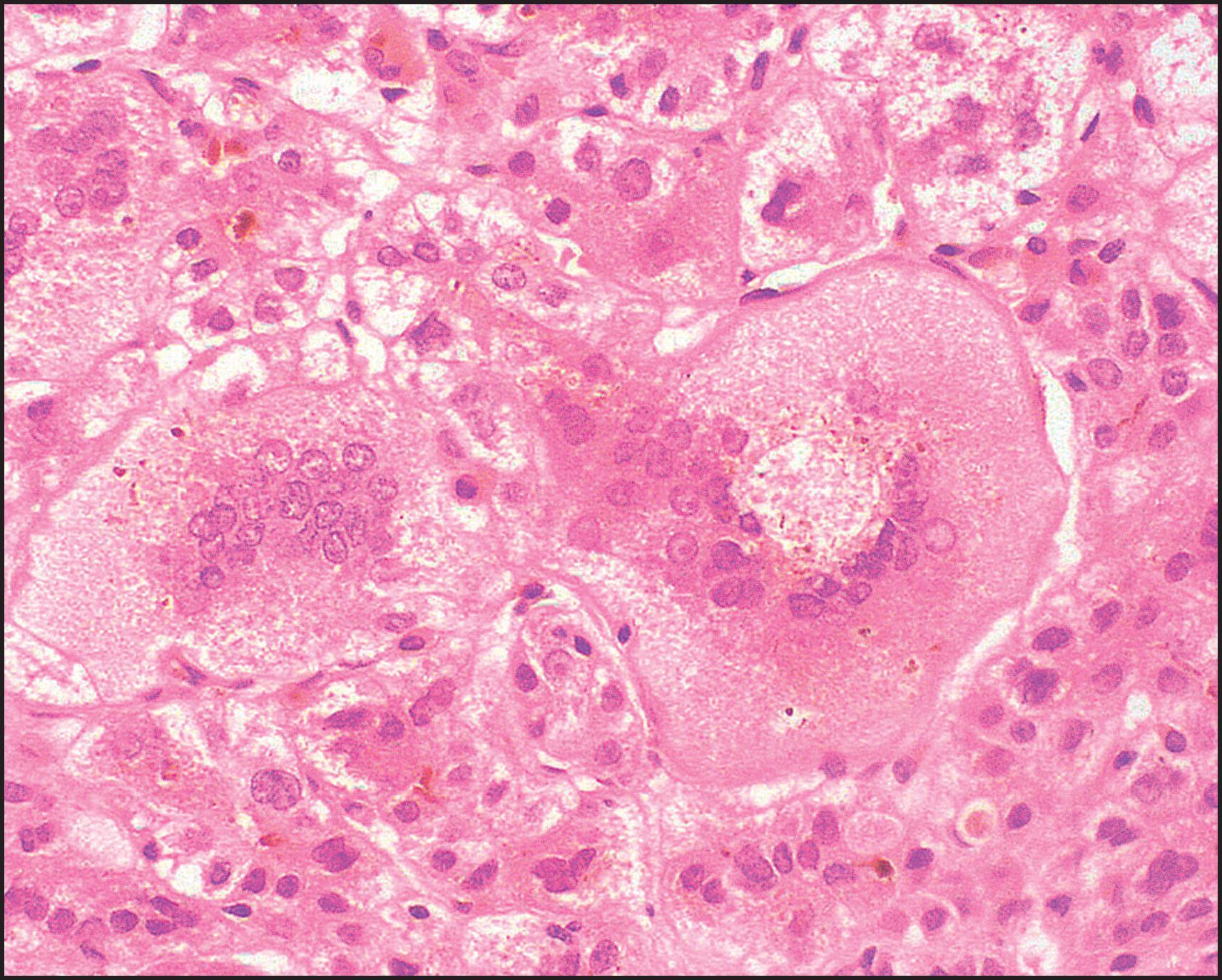
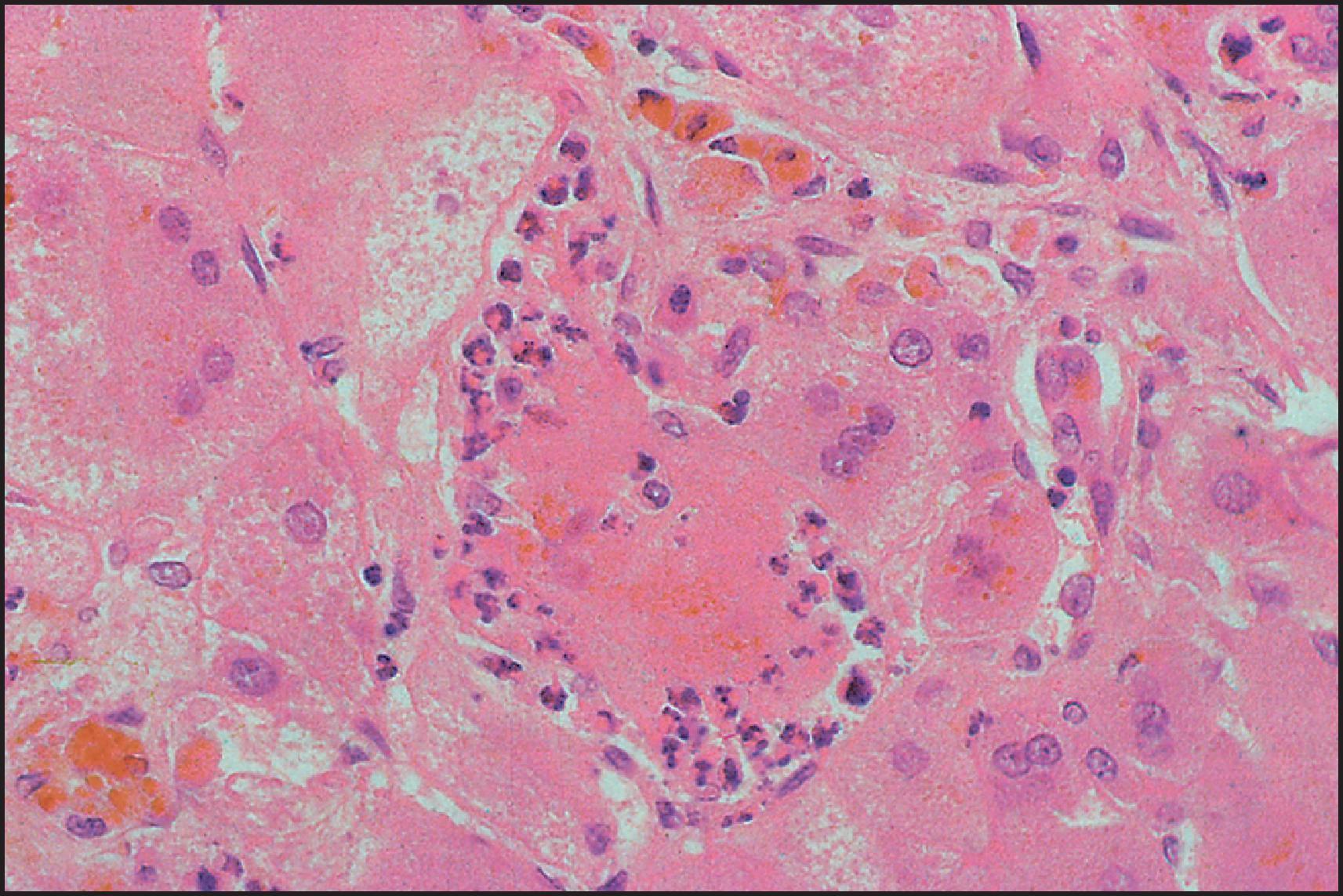
The pathological aspects of giant cell transformation, a frequent and often dominant finding in neonatal hepatitis, have been reviewed extensively. The change is seen throughout the parenchyma but is often more marked in the perivenular areas. The giant cells contain four or more nuclei, sometimes as many as 40 per cell, have poorly defined outlines and may be detached from other cells in the hepatic plate ( Figs 3.1 and 3.2 ). The cytoplasm of some giant cells may contain remnants of cell membranes. It is partially rarefied and often contains bile and/or haemosiderin. The cells may have more glycogen than normal hepatocytes and a greater activity of a variety of enzymes, such as glucose-6-phosphatase, acid phosphatase and succinic dehydrogenase. Death of the giant cells is associated with a neutrophilic inflammatory response ( Fig. 3.3 ). In severe forms, extensive bridging cell loss may divide the parenchyma into micronodules which are highlighted by a reticulin stain ( Fig. 3.4A, B ). The number of giant cells decreases as patients grow older and they are rare after age 1 year. Formation of giant cells is considered to be a characteristic change resulting from mitotic inhibition of the young, growing liver tissue by a number of agents, such as viruses, drugs and hereditary abnormalities. Negative nuclear staining for cell proliferation markers and the demonstration of canalicular remnants using carcinoembryonic antigen (CEA) immunostaining support a fusion of rosette-forming hepatocytes as the likely mechanism of giant cell formation. Phillips et al. used the term ‘syncytial giant cell hepatitis’ to describe 10 patients (age range, 5 months to 41 years) with hepatitis characterized by giant multinucleated hepatocytes, usually containing up to 30 nuclei within a single large cell body. These large cells conformed to the reticular framework of the hepatic cords but in some cases crossed the sinusoids to form syncytial masses with adjacent cords. The authors concluded that these large cells were the product of fusion of hepatocytes (thus the term ‘syncytial’), most likely caused by paramyxovirus infection.
![Figure 3.1, Neonatal hepatitis, idiopathic, with giant cell transformation. (Haematoxylin and eosin [H&E] stain.) Figure 3.1, Neonatal hepatitis, idiopathic, with giant cell transformation. (Haematoxylin and eosin [H&E] stain.)](https://storage.googleapis.com/dl.dentistrykey.com/clinical/DevelopmentalandInheritedLiverDisease/0_3s20B978070208228300003X.jpg)
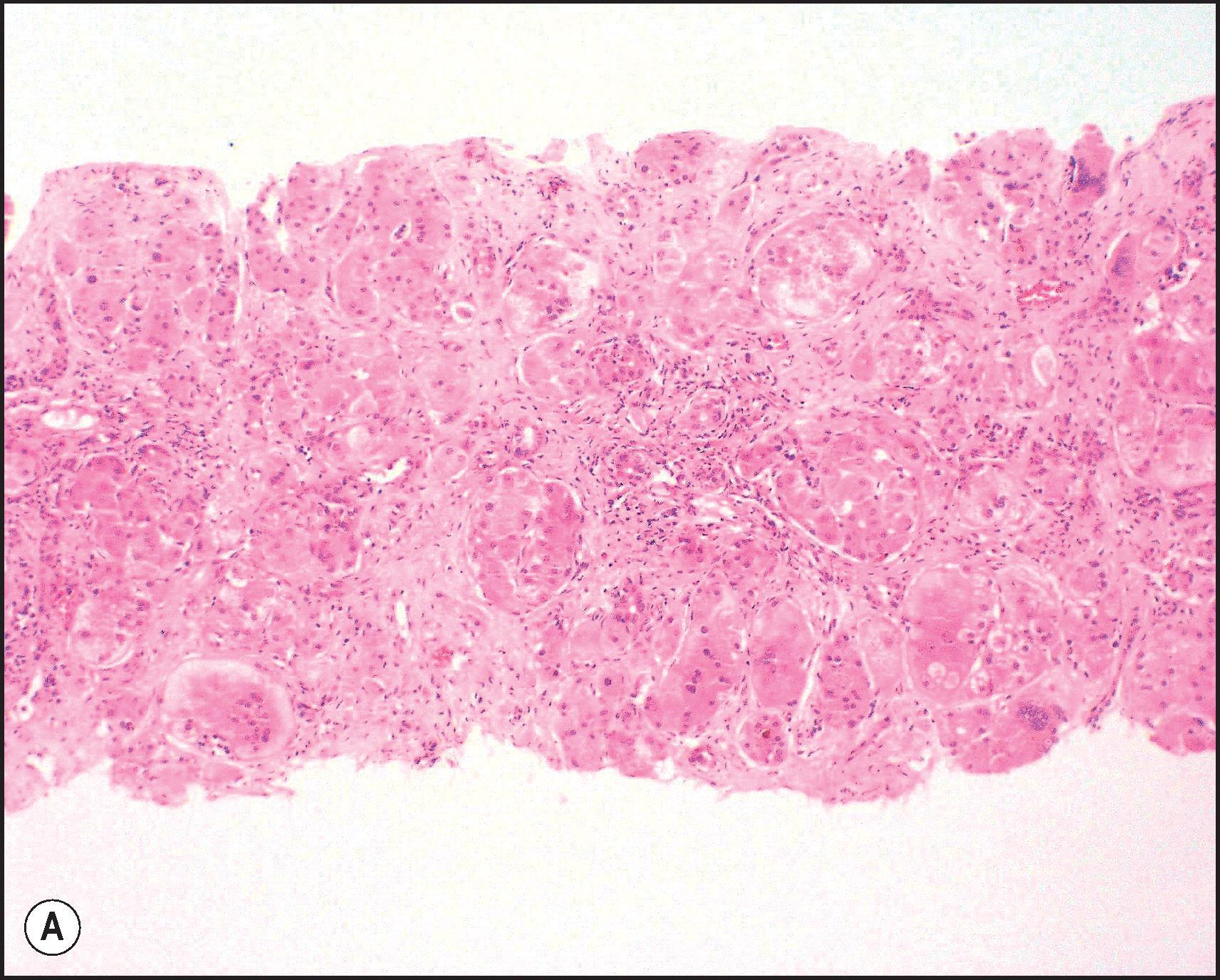
BA is one of the most important causes of severe neonatal liver disease and must be investigated expeditiously as a top priority in any infant with conjugated hyperbilirubinaemia. It is the major indication for LT in young children. Initially, the extrahepatic biliary tree is affected, evidenced as an obstructive picture both clinically and histopathologically; this is the defining lesion of this disorder. Biliary cirrhosis develops early in life. The children who survive infancy because of a successful Kasai portoenterostomy continue to have intrahepatic bile duct damage, which leads eventually to profound loss of small intrahepatic bile ducts and recurrent cholestasis due to bile duct paucity. Accordingly, this hepatobiliary disorder is now called simply biliary atresia , without specifying an extrahepatic (or intrahepatic) location. The term ‘biliary atresia’ (BA) is used in this section interchangeably with the former term ‘extrahepatic biliary atresia’ (EHBA). The term ‘intrahepatic biliary atresia’ to denote intrahepatic bile duct paucity has been obsolete for many years and should be abandoned. Additionally, biliary ‘atresia’ must be distinguished from biliary ‘agenesis’.
Approximately 30% of infants presenting with conjugated hyperbilirubinaemia in the neonatal period have BA, the overall incidence being approximately 1 in 6000 to 1 in 19,000 live births. There is no clear-cut racial predilection, although some ethnicities appear to have a higher incidence: Black Americans and Polynesians compared with White infants. BA is more common in girls than boys. , Seasonal variation in the occurrence of this disease has been suggested in North American studies, , although this pattern has not been confirmed in Europe or Japan. Unquestionably, multiple disease mechanisms can produce BA because it may occur as an isolated lesion or in association with various types of congenital structural abnormalities or with specific chromosomal abnormalities. It can be conceptually useful to classify BA in two general patterns: ‘embryonic’, occurring early in development and accounting for 15–35% of cases, and ‘perinatal/acquired’, comprising 65–85% of cases. This aetiopathogenic heterogeneity of BA was first delineated by a study of 237 children by Silveira et al. Forty-seven of the children (20%) had associated congenital anomalies (28 cardiovascular, 22 digestive and 19 splenic). The splenic malformations included 13 with polysplenia syndrome and two with asplenia. Karyotypic abnormalities were found in two of eight children studied. The investigators divided BA into four distinct subgroups, three involving a congenital form that could arise through a malformation, a disruption or a chromosomal abnormality, with the fourth attributable to agents active in the perinatal period (the acquired form).
Most infants have this perinatal/acquired pattern; their apparently normal biliary system has been subjected to a fibrosing inflammatory process late in gestation or shortly after birth. Discordance for BA in monozygotic twins supports a postnatal event and epigenetic factors as being of primary importance in the pathogenesis of perinatal/acquired BA. By contrast, approximately 10–30% of infants with BA have extrahepatic congenital abnormalities such as polysplenia, left atrial isomerism, double-sided left lung, preduodenal portal vein, intestinal malrotation and/or congenital heart defects. , , Preduodenal portal vein is the result of a variation in the normal developmental pattern of the embryonic precursors of the portal vein, i.e. the right and left vitelline veins and their three anastomotic channels. These congenital defects are sometimes grouped as a ‘laterality complex’. Abnormalities of the spleen are not invariably present in infants who have other typical features of the laterality complex, and thus ‘splenic disorder’ does not define this association. The best currently available term for this category of BA is ‘biliary atresia with structural malformations’ (BASM). A small proportion of children with BA have a major congenital anomaly (typically, cardiac, genitourinary or gastrointestinal [GI]) without having the congruent features of BASM. Congenital cardiac abnormalities in infants with BA are diverse and may compromise overall survival.
A further subset of infants with BA, comprising 6–10% of patients overall, have damage to the biliary tree which produces cystic dilation ; these patients look as if they have a choledochal cyst. Exceptionally, the cystic change is confined to intrahepatic ducts. This type of BA, now termed ‘cystic’ BA (formerly called ‘correctable’ BA), may be identified on sonography of the fetus in approximately 40% of affected cases. It may represent yet another disease mechanism for BA, distinct from those represented by embryonic and perinatal BA, although cystic BA has been identified in patients with embryonic BA. Notably, some infants with BA have specific chromosomal abnormalities such as trisomy 17–18, Turner syndrome or cat-eye syndrome. BA has been reported with the Kabuki make-up syndrome, in one patient with Zimmermann–Laband syndrome and brachydactyly and in Martinas–Frias syndrome. Other genetic disorders may be associated with BA because familial occurrence has been reported, , and in one case a woman who previously had BA gave birth to a daughter with BA. A lethal autosomal recessive syndrome with intrauterine growth retardation, once called intra- and extrahepatic BA, and oesophageal and duodenal atresia was reported in one family; description of the findings suggests agenesis of the extra- and intrahepatic ducts.
Viral infection in combination with a genetic predisposition to a robust or disordered inflammatory response may play a role in the development of perinatal/acquired BA. Chance occurrence of a viral infection during a limited period of susceptibility would explain the rarity of BA. No consensus exists as to which viruses might be implicated in such an aetiopathogenesis. The proposed viruses form a heterogeneous group, including both DNA and RNA viruses. Some recent observations suggest that viral infection is a secondary phenomenon. CMV infection is found in a high proportion of children with BA. Accordingly, a recent classification of BA specifies CMV association not only in the perinatal form, but also in the embryonic form and cystic variant. Tarr et al. found evidence for viral infection in 5 of 23 patients with BA. The diagnosis was based on histopathological evidence of CMV infection, serology (IgM antibodies) or culture. The detection of CMV infection by polymerase chain reaction (PCR) is higher in neonatal hepatitis than in BA. Infants with BA and concurrent CMV infection may have a worse prognosis. CMV may elicit immune responses, which interfere with the action of regulatory T (Treg) cells. Reovirus 3 was suggested as a cause of BA and neonatal hepatitis on the basis of clinical and experimental studies, but this association was questioned by other investigators. , Tyler et al. provided more compelling evidence for an aetiological association between BA and reoviruses, detecting reovirus RNA from hepatobiliary tissues of 55% of patients with BA and 78% of patients with choledochal cysts. Riepenhoff-Talty et al. suggested a possible relationship between group C rotavirus and BA. Subsequently, however, no evidence of group A, B or C rotaviruses was detected by PCR in BA. Human papillomavirus (HPV) has been detected in neonatal hepatitis and BA by nested PCR for DNA, but the role, if any, of HPV needs to be clarified.
A toxic aetiopathogenesis has been proposed for perinatal/acquired BA. The implicated toxin is a plant isoflavonoid, called biliatresone, associated with an outbreak of BA in lambs. Damage to the biliary system typical of BA has been shown in zebrafish, murine cholangiocyte culture systems and mice. Biliatresone appears to operate through oxidative stress. A signalling pathway comprised of GSH, RhoU/Wrch1, Hey2 and Sox17 is operative upon GSH depletion and leads to bile duct damage. Downstream effects may involve alterations in the Notch signalling pathway.
Recent progress has focused on immune mechanisms and the role of inflammasome-related genes in the pathogenesis of BA, especially perinatal/acquired BA. , The envisioned disease mechanism is that during the perinatal period a viral infection occurs and targets the biliary epithelium and provokes an aberrant autoimmune injury to the bile ducts that persists long after the viral infection is gone. This proposed mechanism entails an active, ongoing immune response, which can be documented empirically, and it accounts for the conflicting reports of viral infection in BA as well as the absence of detectable ongoing viral infection in liver or biliary tissue from BA patients. Currently, it is seen as involving macrophages and dendritic cells primed as a result of the infection, followed by activation of natural killer (NK) cells, which then actuate biliary epithelial damage. Amplification of the immune response by T cells and proinflammatory cytokines then takes place. , Molecular mimicry may play a role. This general disease mechanism is based on findings in human tissue from BA patients and also on disease models of BA. The hepatic inflammatory infiltrate in BA was found remarkable for evidence of lymphocyte activation. Studies in liver tissue from infants with BA showed a T-helper cell type 1 (Th1) form of cytokine expression pattern with CD4+ and CD8+ lymphocytes, CD68+ macrophages in portal tracts and increased interleukin-2 (IL-2), IL-12, interferon-γ (IFN-γ) and tumour necrosis factor-alpha (TNF-α). Determinants on the activated T cells are typical of an oligoclonal expansion, consistent with being evoked by a specific antigen.
The rarity of BA raises the possibility of genetic susceptibility in perinatal/acquired BA, likely related to complex traits which are not yet fully characterized. Genomic studies of liver from infants with BA have shown upregulation of genes involved in regulating lymphocyte differentiation, mainly of those with Th1 commitment. An upregulated expression of IFN-γ and osteopontin was notable. Subsequently, upregulation of osteopontin expression in intrahepatic biliary epithelium was found to correlate with portal fibrosis and ductular reaction. A different genomic study, which included somewhat older patients, also found upregulation of genes involved in morphogenesis, cell signalling and regulation of gene transcription. A long noncoding RNA sequence has been implicated in the development of fibrosis and cholangiocyte proliferation in BA. Further studies suggested that the pattern of regulatory gene expression in perinatal/acquired BA is not equivalent to that in embryonic BA; however, these data also failed to show a pattern of gene expression relating to laterality genes in embryonic BA. Both forms of BA appear to induce a strong immunological response.
Genome-wide association studies have been used to identify possible susceptibility loci. In North American children, a locus at 2q37.3 was found to be GPC1 , , which encodes a regulator of Hedgehog signalling and inflammation. In Chinese children a locus at 10q24.2 was also identified as a possible susceptibility locus for BA; it proved to be ADD3 , and this finding was confirmed in a North American series. , A further study in North American children found a locus at 14q21.3, and this was identified as encoding ADP ribosylation factor-6 ( ARF6 ). These three proteins have been investigated in the zebrafish, where they play a role in biliary morphogenesis. Of note, GPC1 mediates fibroblast growth factor signalling, and ARF mediates epidermal growth factor (EGF) signalling. XPNPEP1 , which is also found in the 10q24 region, is expressed as X-prolyl aminopeptidase P1 on biliary epithelial cells. , XPNPEP1 is involved in the metabolism of inflammatory mediators, but whether XPNPEP1 is a susceptibility locus remains uncertain. More recently EFEMP1 has been proposed as a new candidate susceptibility locus. Further exome-sequencing studies focused on isolated (perinatal/acquired) BA suggest that genes ( STIP1 and REV1 ), whose gene products are associated with stress responses, may be abnormal. Taken altogether, these studies point to heterogeneous, paucigenic or oligogeneic mechanisms in BA.
Human leukocyte antigen (HLA) studies in BA might support a disease mechanism involving autoimmunity, but results to date are contradictory. One early study showed that infants with perinatal/acquired BA have a high prevalence of the HLA-B12 determinant compared both to normal controls and to infants with BA plus congenital anomalies ; haplotypes A9-B5 and A28-B35 were more common in infants with late-pattern (perinatal/acquired) BA. A subsequent study failed to confirm any characteristic HLA pattern in BA. However, additional studies have shown an association with HLA-DR2 and with HLA-B8 and HLA-DR3.
Further insight into the possible mechanism of perinatal/acquired BA has come from work in the Rhesus rotavirus (RRV) murine model of BA. It can be simulated in Balb/c-mice which have been infected with rotavirus. , This model shares many features with the human disease. IFN-γ plays an important role in bile duct damage: knockout mice not expressing IFN-γ failed to incur severe duct damage after infection with RRV despite a brief hepatitis, whereas wild-type animals did; administration of recombinant IFN-γ abrogated the protective effect of not being able to produce IFN-γ. Certain chemokines may also contribute to biliary damage ; IL-12 seems to play a lesser role ; TNF-α appears not to be involved. Recent observations implicate IL-17, which plays a role in various autoimmune disorders. In this mouse model, primed neonatal CD8 T cells appear capable of initiating damage to bile ducts. When T cells from RRV-disease mice were transferred into naive syngeneic severe combined immunodeficiency (SCID) mice, the recipients developed bile duct-specific inflammation without previous RRV infection. Some autoantibodies have been detected in this model (directed to α-enolase or vimentin). Thus the combination of observations in infants with BA and in this mouse model strongly suggests that a complex pattern of immune reactivity appears to be important in perinatal/acquired BA. Of interest, circulating markers of inflammation persist after surgical palliation with the Kasai portoenterostomy, although it is not clear why. In embryonic BA, other genes may play a more direct role.
Whereas the pathogenesis of perinatal/acquired BA probably involves immunogenetic susceptibility and exposure to an instigating factor, such as viral infection, during a limited period of susceptibility, the aetiopathogenesis of embryonic BA appears to be much more diverse. An important subset has BASM (BA with structural malformations). Extrahepatic congenital abnormalities such as polysplenia, congenital heart defects and disturbed rotation of the intestines suggest an extensive and early developmental abnormality. Some infants with BASM show features of DPMs on liver biopsy. This abnormal configuration of small bile ducts is attributed to disorganization in the fetal development of the biliary tree; failure of remodelling of the ductal plate leads to residual embryonic bile duct structures in this rather striking configuration. Finding the ductal plate lesion in extrahepatic BA is consistent with a destructive hepatobiliary process beginning early in gestation and has been linked to a higher risk for requiring LT. Abnormal cilia have been reported in children with polysplenia syndrome and BA. , Although the association with abnormal cilia is unclear, ciliary function appears to be important in left/right asymmetry. Rare, potentially pathogenic variants in the gene PKD1L1 (polycystic kidney disease 1 like 1) have been identified in a few infants with BASM. There is a pathogenic role for multiple defects in the laterality sequence. Early studies focused on the inversion ( inv ) gene, one of three genes that control left/right asymmetry in the mouse. Beginning in early embryonic development, the liver is a predominant site for this gene expression. A transgenic mouse with recessive deletion of inv develops situs inversus and jaundice; the early fetal lesion is a complete obstruction with cystic change of the biliary tree. Few of the various genes which have been found mutated in human laterality disorders ( ZIC3 , CFC1 , LEFTYA , ACVR2B , NODAL ) have been investigated in BA; however, mutations in CFC1 and ZIC3 have been found in infants with BA and major laterality defects. Three children were described with ultrastructural abnormalities of the canalicular microvilli and no expression of villin; phenotypically they had BA without laterality complex or DPM.
The extrahepatic biliary tree in BA may be totally atretic, or the atresia may involve only proximal or distal segments. The intrahepatic bile ducts are gradually destroyed with progression of the disease. Most infants with BA have conjugated hyperbilirubinaemia from an early age, but clinical jaundice is not always apparent or appreciated. Indeed, in many infants, jaundice is initially physiological and merges with the jaundice of advancing liver disease. Infants typically have dark urine and pale stools, but the stools may retain enough colour to be falsely reassuring. The infants look well and generally gain weight adequately. At clinical presentation they have hepatomegaly and usually some degree of splenomegaly, unless polysplenia is present. The infant who presents with congenital heart disease and conjugated hyperbilirubinaemia requires intensive evaluation because the leading hepatic diagnoses will be BA or Alagille syndrome. Untreated BA rapidly progresses to hepatic fibrosis and cirrhosis with all the complications of portal hypertension, in addition to malnutrition and fat-soluble vitamin deficiency. The median age of death is 12 months if BA is not diagnosed and treated. Early diagnosis and treatment may be promoted by the use of stool colour cards for all infants at discharge from the newborn nursery. ,
Clinically, the differential diagnosis is the broad spectrum of disorders constituting the neonatal hepatitis syndrome presenting with conjugated hyperbilirubinaemia (see Table 3.1 ). Congenital infection should be excluded, although CMV may be found along with BA. Systemic bacterial infection should be ruled out, including a silent urinary tract infection. Inherited metabolic diseases require specific attention, especially α1-AT deficiency, which can be associated with severe cholestasis and acholic stools and very rarely has been associated with BA. CF can generate a duct lesion indistinguishable clinically from BA. These two conditions as well as galactosaemia may produce a histological picture closely resembling that of BA. Structural abnormalities of the extrahepatic biliary tree cause the clinical presentation similar to BA: choledocholithiasis, idiopathic perforation of the biliary tract, true choledochal cyst and extrahepatic biliary hypoplasia or ‘hair-like’ bile duct syndrome. Some infants with Alagille syndrome show ductular reaction, rather than duct paucity, on liver biopsy taken early in the course of the disease. Whether some infants with perinatal/acquired BA have an unusually slow progression of liver disease, sometimes called ‘BA in evolution’, is disputed, but these patients pose a diagnostic challenge and require repeated assessment.
Preoperative diagnosis relies on demonstrating the presence or absence of bile secretion in the intestine. Hepatic sonography may reveal a dilated extrahepatic biliary tree, consistent with distal cystic atresia, but it is unusual to find dilated intrahepatic bile ducts. Hepatobiliary scanning, using a technetium-99m-labelled iminodiacetic acid derivative such as DISIDA or PIPIDA, fails to demonstrate passage of the radiolabelled substance into the intestinal tract over a 24-hour period. This is the ‘nondraining hepatobiliary scan’. Although hepatobiliary scanning has high sensitivity, scanning may appear normal if performed very early in the disease process in late-pattern BA. , Hepatobiliary scanning is informative if it shows that tracer, and thus bile, reaches the intestine; it is objective, recorded and can be quantified. A negative or nondraining scan does not mean that the disorder is necessarily BA, because nondraining hepatobiliary scans may be found with severe idiopathic neonatal hepatitis, small duct paucity syndromes (e.g. Alagille syndrome), severe α1-AT deficiency or TPN-associated cholestasis. The role of endoscopic retrograde cholangiopancreatography (ERCP) remains controversial: ERCP is technically feasible in infants and may be useful in select cases when performed by an experienced endoscopist. Percutaneous liver biopsy is extremely helpful and has high diagnostic specificity in the range of 60–95%, depending on the timing of the biopsy, adequacy of the specimen and expertise of the pathologist. In our experience, the majority of nondiagnostic biopsy specimens are taken within the first few weeks of life. In fact, incidental liver biopsy specimens taken at laparotomy for duodenal stricture within the first week of life in three infants with BA showed only trivial liver abnormalities. This may imply that although bile duct destruction is likely to have started in utero , the actual liver damage may not occur until the placenta no longer provides clearance of biliary products, in particular bile salts. Repeat diagnostic evaluation is necessary for an infant with persisting conjugated hyperbilirubinaemia and a nondiagnostic liver biopsy performed in the first weeks of life. Repeat hepatobiliary scanning may be informative. Repeat liver biopsy may be appropriate: it should be performed within an advantageous timeframe for possible portoenterostomy, namely at 6–8 weeks of life.
Portoenterostomy (Kasai procedure) was introduced in 1959 and remains the only potentially corrective procedure for BA, other than LT. In this operation, the atretic biliary tree is resected, and bile drainage is re-established through a broad anastomosis at the end of an intestinal Roux-en-Y loop to the bare edge of the transected porta hepatis. The efficacy of the laparoscopic version of the Kasai portoenterostomy, introduced in the past 10 years, remains uncertain. Extensive retrospective studies have shown that the prognosis for a good long-term result from the conventional Kasai procedure depends primarily on operation before 60 days of age and the absence of cholangitis. , , The potential exists, however, for reasonable medium-term survival in about one-third of infants coming to primary corrective surgery at 100 days or older. Most centres continue to favour the Kasai procedure as the first therapeutic option, rather than subjecting patients to immediate LT simply on the basis of age. The lack of significant fibrosis at operation may play a role in a good long-term outcome. Computerized quantification of fibrosis on liver biopsy obtained at portoenterostomy may discriminate between negligible fibrosis and sufficient fibrosis to portend a poor prognosis. One group suggested that histological features on the initial liver biopsy specimen can predict the success of portoenterostomy. Another group reported that the extent of ductular reaction, based on keratin 7 (K7) immunostaining, found in the liver biopsy specimen obtained at the Kasai procedure was predictive of native liver survival over the following year. The outcome may also depend on the balance between active fibroplasia and re-epithelialization of bile ducts not already obliterated. Histological studies of the extrahepatic bile ducts removed at surgery have been performed by several groups of investigators. In a study of 98 cases, Gautier and Eliot classified the biliary remnants into three types. In the first type the duct is completely atretic, with few or no inflammatory cells in the surrounding connective tissue ( Fig. 3.5A ). In the second type the duct is present as a cleft-like lumen lined by occasional cuboidal or low columnar epithelium which is variably necrotic, hyperplastic or focally absent ( Fig. 3.5B, C ). The altered ducts are sometimes very numerous, usually having lumina of <50 µm; periluminal neutrophilic infiltration is characteristic; cellular debris, and less often bile, may be found in the lumen. Epithelial necrosis is evident in ducts with a diameter exceeding 300 µm. The third type of biliary remnants consists of altered bile ducts incompletely lined by columnar epithelium, in addition to numerous smaller epithelial structures ( Fig. 3.5D ). These histological types were evaluated by Gautier et al. at three levels: porta hepatis, junction of the cystic and common hepatic ducts and an intermediate level; completely atretic duct becomes increasingly more common from the porta hepatis to the junction of the hepatic duct and cystic ducts. This classification, which may help pathologists describe the changes observed in the biliary remnants removed at portoenterostomy, is of limited clinical significance because in individual cases, serial sectioning often shows atretic ducts alternating randomly with variably destroyed ducts. In addition, the numerous smaller structures intermingled with variably altered ducts are likely to represent anastomosing channels recruited from peribiliary glands, of which effectiveness in bypassing the atretic duct is uncertain; anastomoses between ramified peribiliary glands are well demonstrated in normal adult livers using injection techniques (see Chapter 9 ). Correlations between the size and number of residual ducts and establishment of bile flow after surgery have yielded conflicting results. Two groups of investigators believed that bile flow is most likely to occur when the diameter of the residual ducts exceeds 150 µm. , , However, another study of the extrahepatic biliary remnants of 204 cases of BA showed that the patterns of bile duct obliteration are not indicative of prognosis.
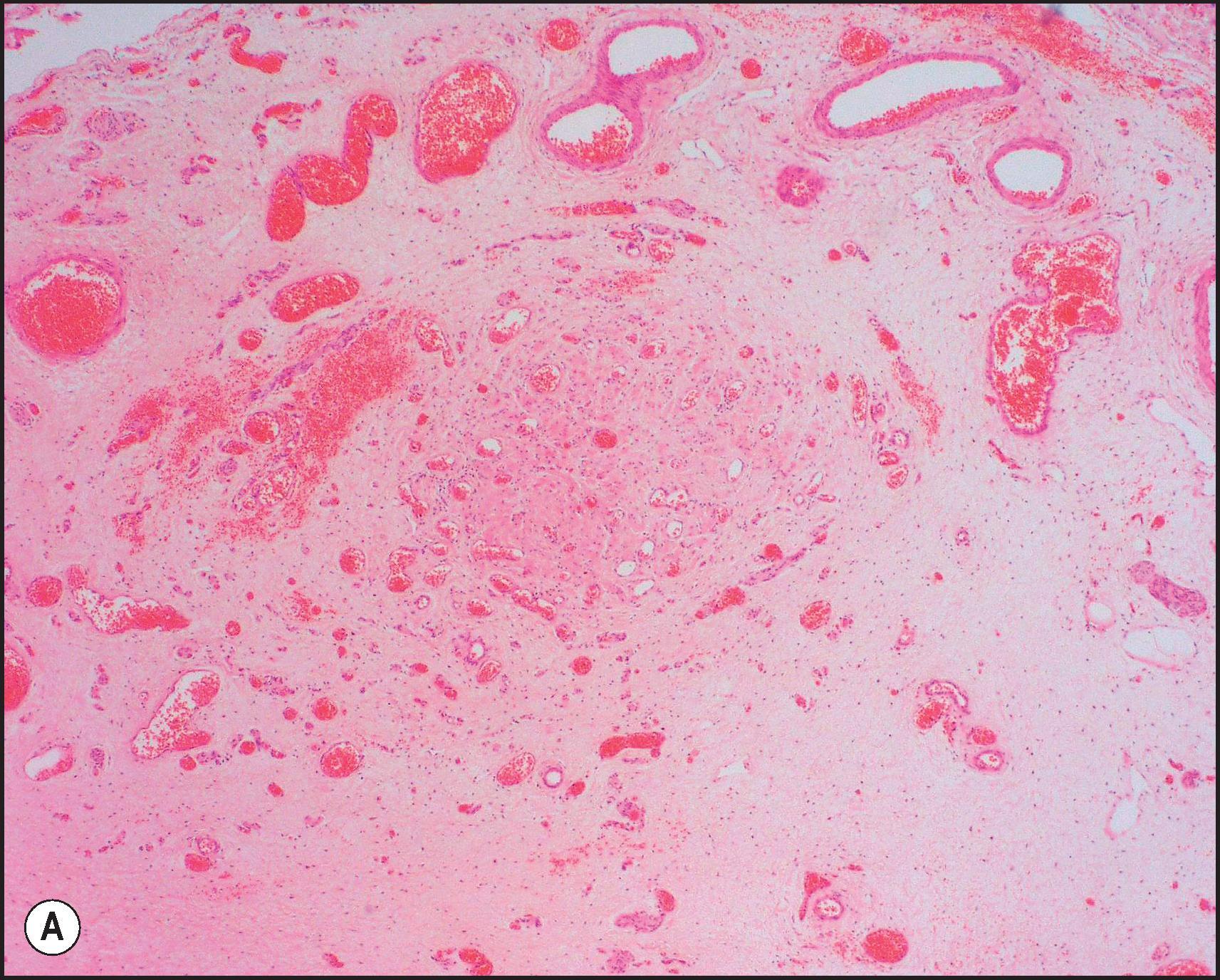
Although the Kasai procedure is essentially a palliative operation, many children enjoy prolonged good health afterward, and approximately 20–25% of patients who undergo portoenterostomy will survive into adulthood without LT. Approximately 30–35% of patients drain bile but develop complications of cirrhosis and require LT before age 10 years. For the remaining patients, bile flow is inadequate after portoenterostomy and cirrhosis rapidly develops. Survival in BA with a functioning Kasai portoenterostomy but without orthotopic LT is 10–20% by age 20 years. In a recent survey of 271 patients, 23% were alive with their native liver 20 years after surgery, all but two having signs of cirrhosis; after age 20, two patients died of liver failure and 14 underwent, or were waiting for, a liver transplant. A large French study on 1428 BA patients showed that 22% survive to the age of 30 years without transplantation. Women who are long-term survivors with BA and have not yet had a liver transplant may have a normal pregnancy, but in general such pregnancies must be treated as high risk because of complications from portal hypertension or hepatic decompensation. LT has become the treatment of choice for infants and children in whom the Kasai portoenterostomy has failed. The safety and results of LT with the use of livers from living-related donors and cadaveric donors are excellent. One-year survival is >90%, with better results obtained under elective conditions and in children who weigh more than 10 kg. , Recent data indicate that the overall survival at 10 years for all surgical treatment is 75–80% , ; general health 10 years after paediatric LT is good, although renal impairment and cardiovascular disease may develop.
The macroscopic appearance of the liver in BA varies according to the stage of the disease. At first the liver is enlarged and dark green in colour, becoming finely nodular as cirrhosis develops ( Fig. 3.6 ). In untreated cases, the cirrhosis may take between 1 and 6 months from birth to develop. Dilated bile ducts filled with inspissated bile may be seen in sections of large portal areas ( Fig. 3.7 ). The cystically dilated bile ducts may resemble Caroli disease. They occur only after age 3 months and are not amenable to surgical drainage procedures. There may be portal lymphadenopathy. The median maximum node dimension in six cases studied by Hübscher and Harrison was 14 mm. These lymph nodes are brown in colour and full of pigment-laden macrophages. Livers removed at transplantation after an apparently successful Kasai procedure (loss of jaundice), but with subsequent development of cirrhosis and portal hypertension, are often coarsely nodular with areas of macronodular hypertrophy and broad intervening or peripherally located scars resembling the gross appearance of focal nodular hyperplasia ( Fig. 3.8 ). Portal hypertension may be due to an obliterative portal venopathy rather than advanced fibrosis.
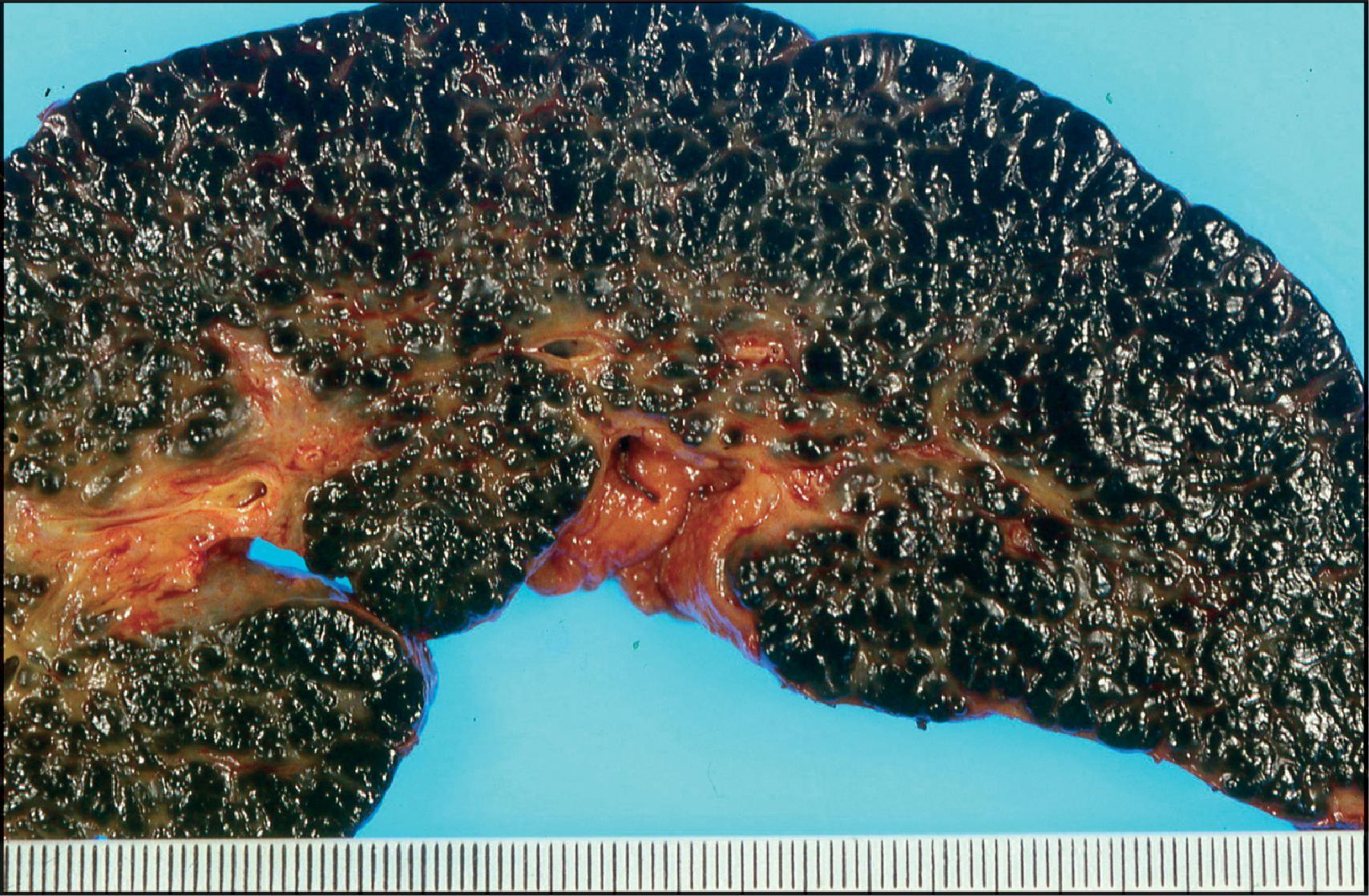
The histological features of BA include cholestasis, portal tract expansion by oedematous fibroplasia and periportal ductular reaction with the presence of bile plugs in dilated lumens of cholangioles that are distorted and often form an irregularly anastomosing network at the portal periphery ( Figs 3.9 and 3.10 ). Arterial branches are unusually prominent, and portal vein branches appear attenuated. Giant cell transformation of hepatocytes is seen in some cases ( Fig. 3.11 ) and may occasionally be prominent. Loose fibrosis is progressive and periportal/perilobular in location, with linkage of portal areas and eventual development of a secondary biliary cirrhosis ( Fig. 3.12 ). In a study of the extracellular and cellular components of the connective tissue matrix in BA, de Freitas et al. suggested that activation of a connective tissue cellular clone by the reactive ductules may be responsible for the portal fibroplasia. Ho et al. reported an arteriopathy (hyperplasia and hypertrophy) of the common hepatic artery and its peripheral branches supplying the entire biliary tree in 11 cases of BA. Thickening of the medial layer of small hepatic arteries may be present. Subsequent studies have indicated that the transcription factor HNF6 plays an important role in intrahepatic bile duct and arterial development. , HNF6 and HNF1ß appear necessary for ductal plate formation. Large perihilar bile ducts may show ulceration with loss of epithelial lining, bile impregnation of the wall and bile sludge formation in the lumen. In addition to the severe cholestasis, the cirrhotic stage of BA is characterized by marked pseudoxanthomatous transformation, the presence of bile lakes, Mallory–Denk bodies and variable accumulation of copper and copper-associated protein in liver cells ( Figs 3.13 and 3.14 ). In one study, copper concentrations were increased in more than two-thirds of liver samples obtained during portoenterostomy and decreased in some patients after successful biliary drainage. However, copper deposition in liver is elevated in the first 2 months of life, and periportal deposition of copper on liver biopsy specimens does not discriminate extrahepatic from intrahepatic causes of cholestasis in early infancy. Acute and chronic inflammation is noted in portal/periportal areas in BA in both precirrhotic and cirrhotic stages, and bile duct degeneration and inflammation may be evident ( Fig. 3.15 ). The mononuclear infiltrate in portal and lobular areas of livers with end-stage BA is similar to normal adult liver and very different from that associated with autoimmune hepatitis or chronic HBV infection. It is particularly prominent in cases associated with CMV infection. The growth of large, perihilar regenerative nodules, probably as a consequence of functioning intrahepatic ducts in this region, may be important for maintaining biliary drainage after Kasai procedure. Bile lakes occur after age 3 months, by which time irreversible hepatic damage has occurred.
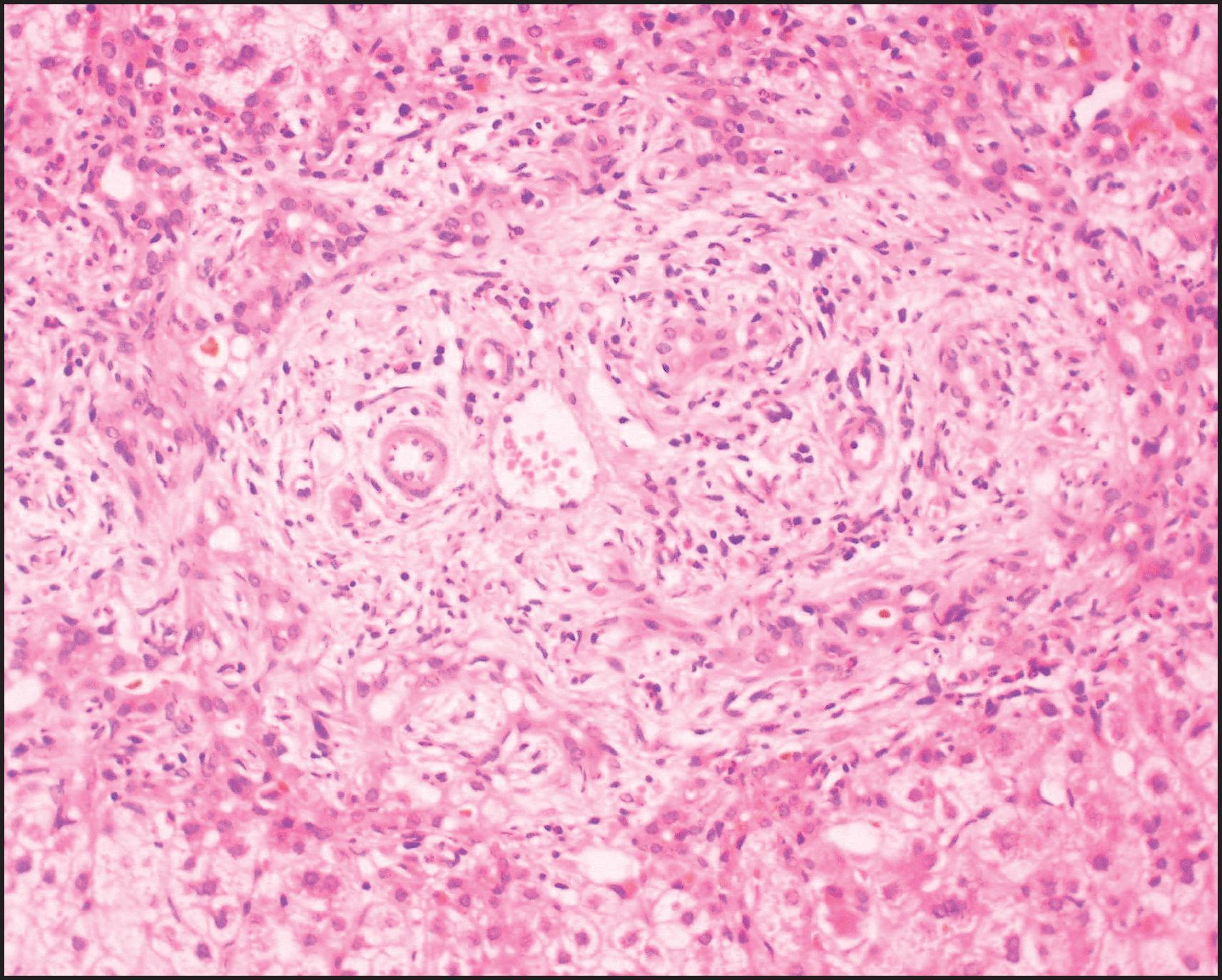
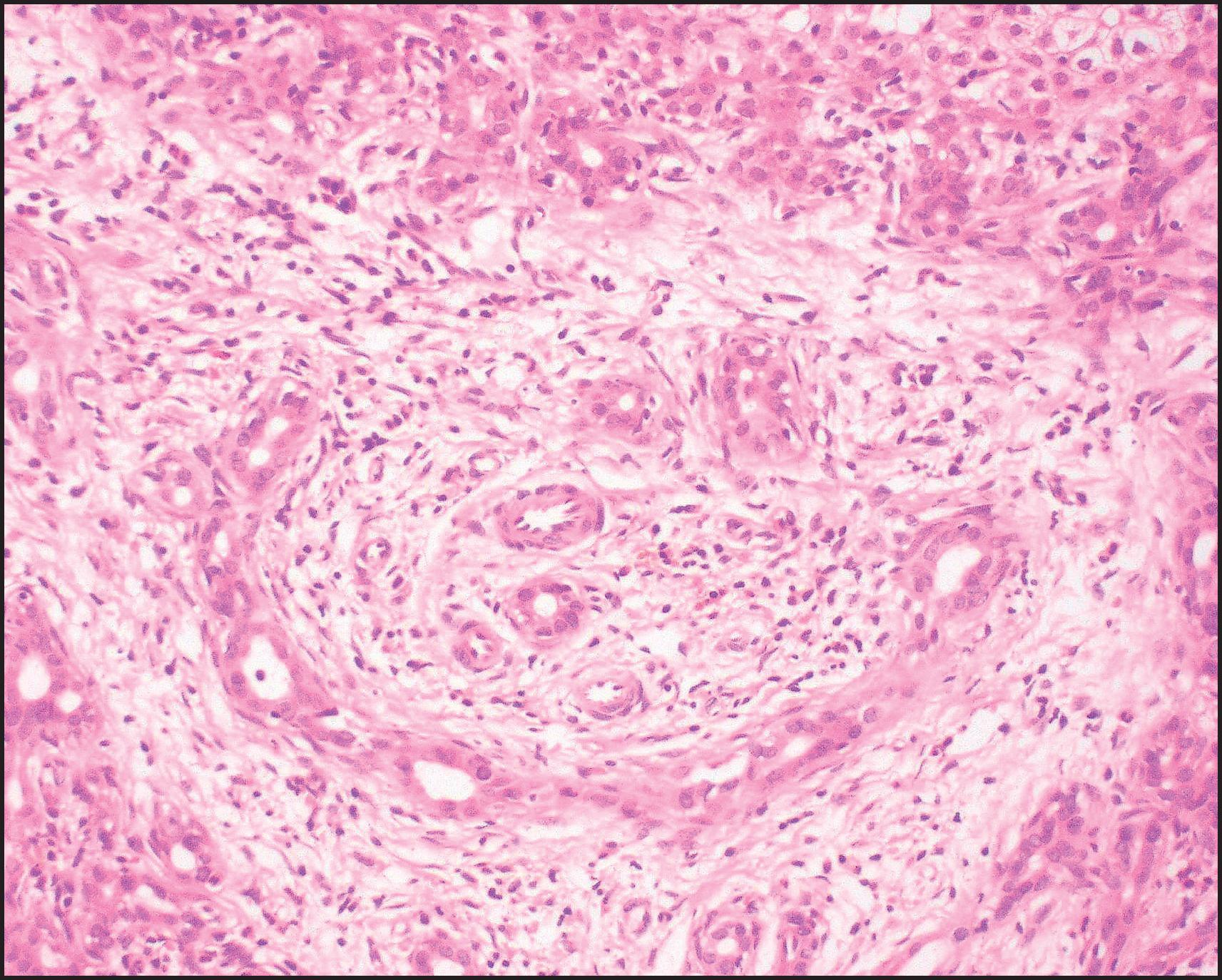
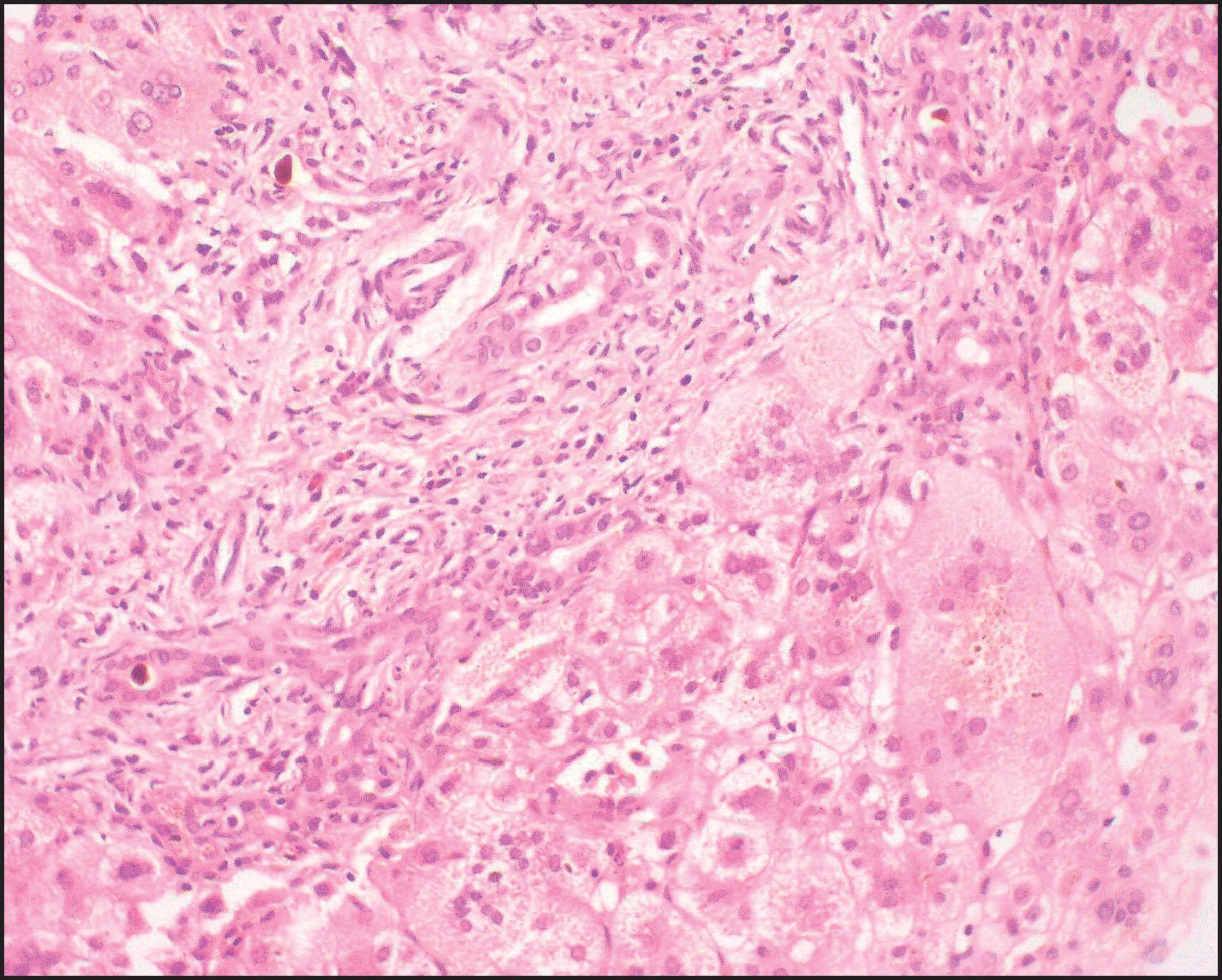
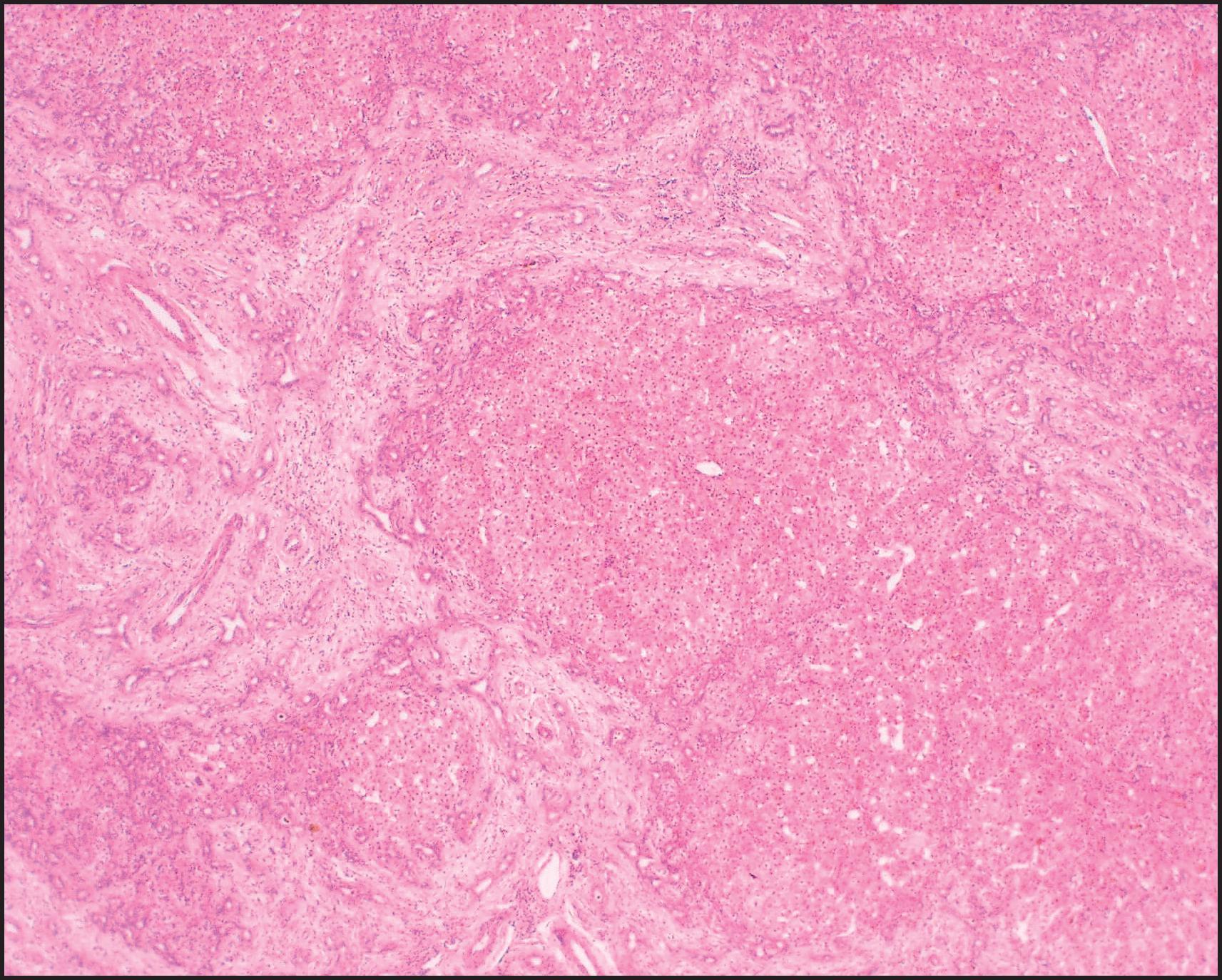
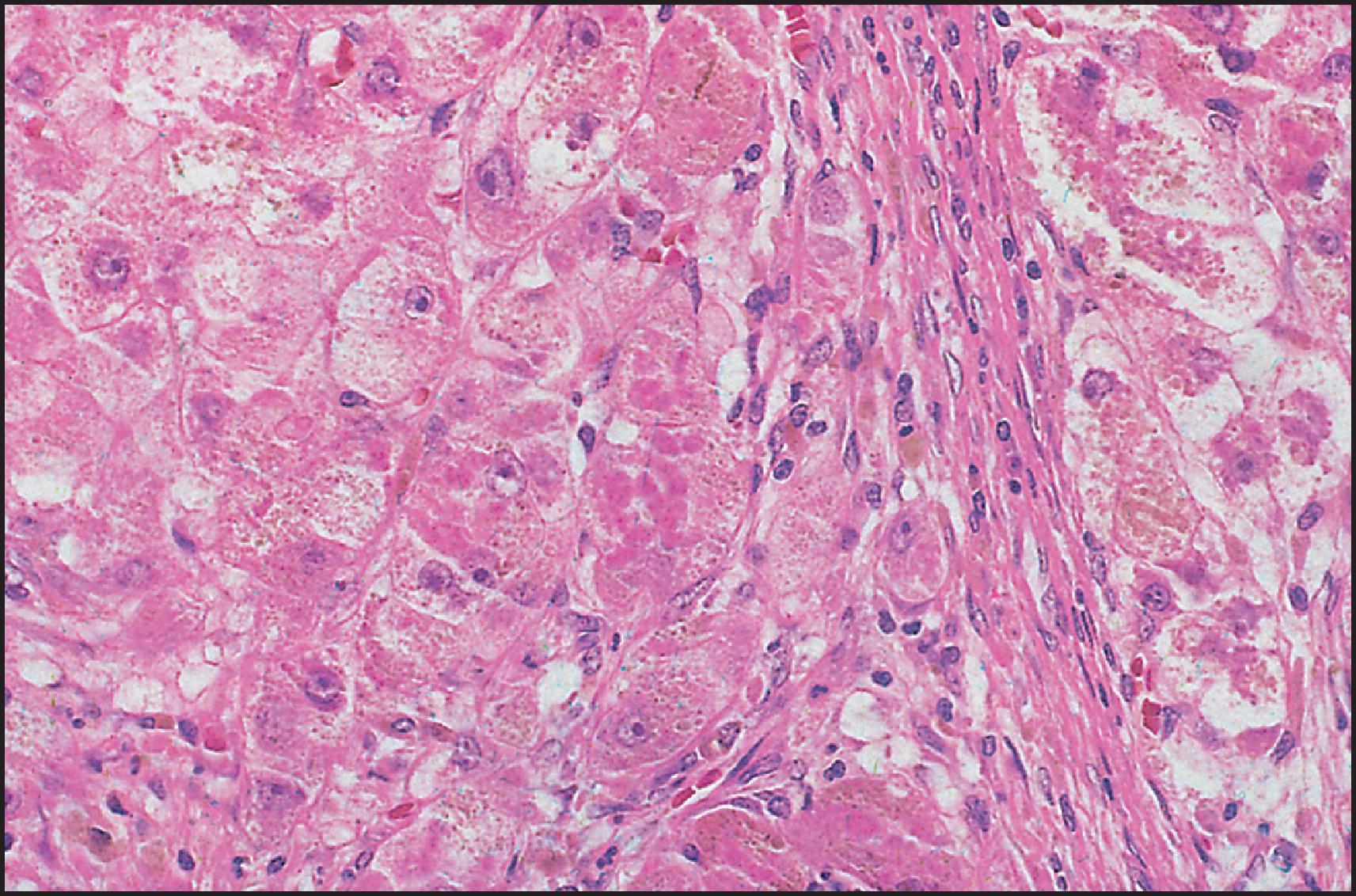
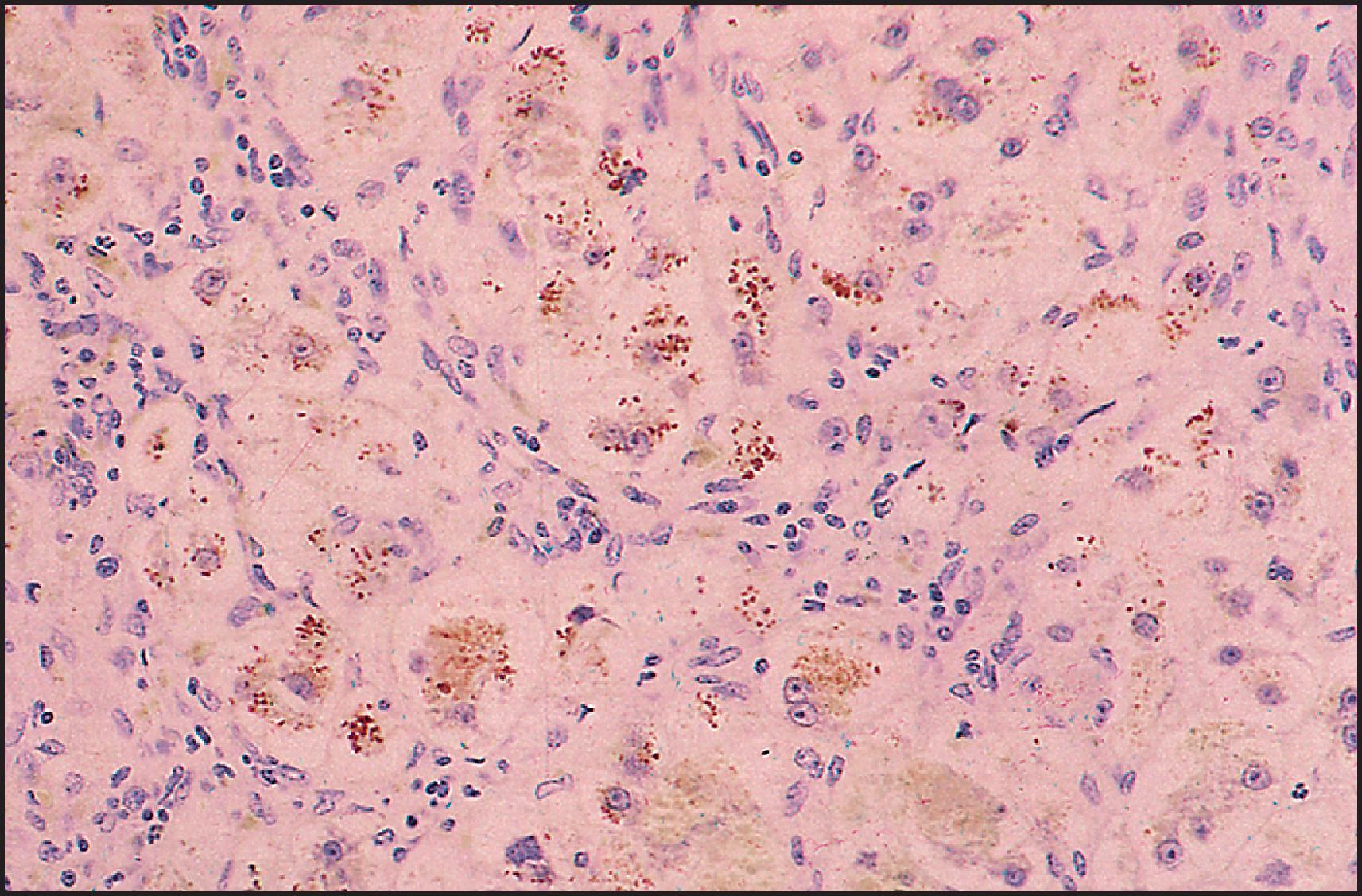
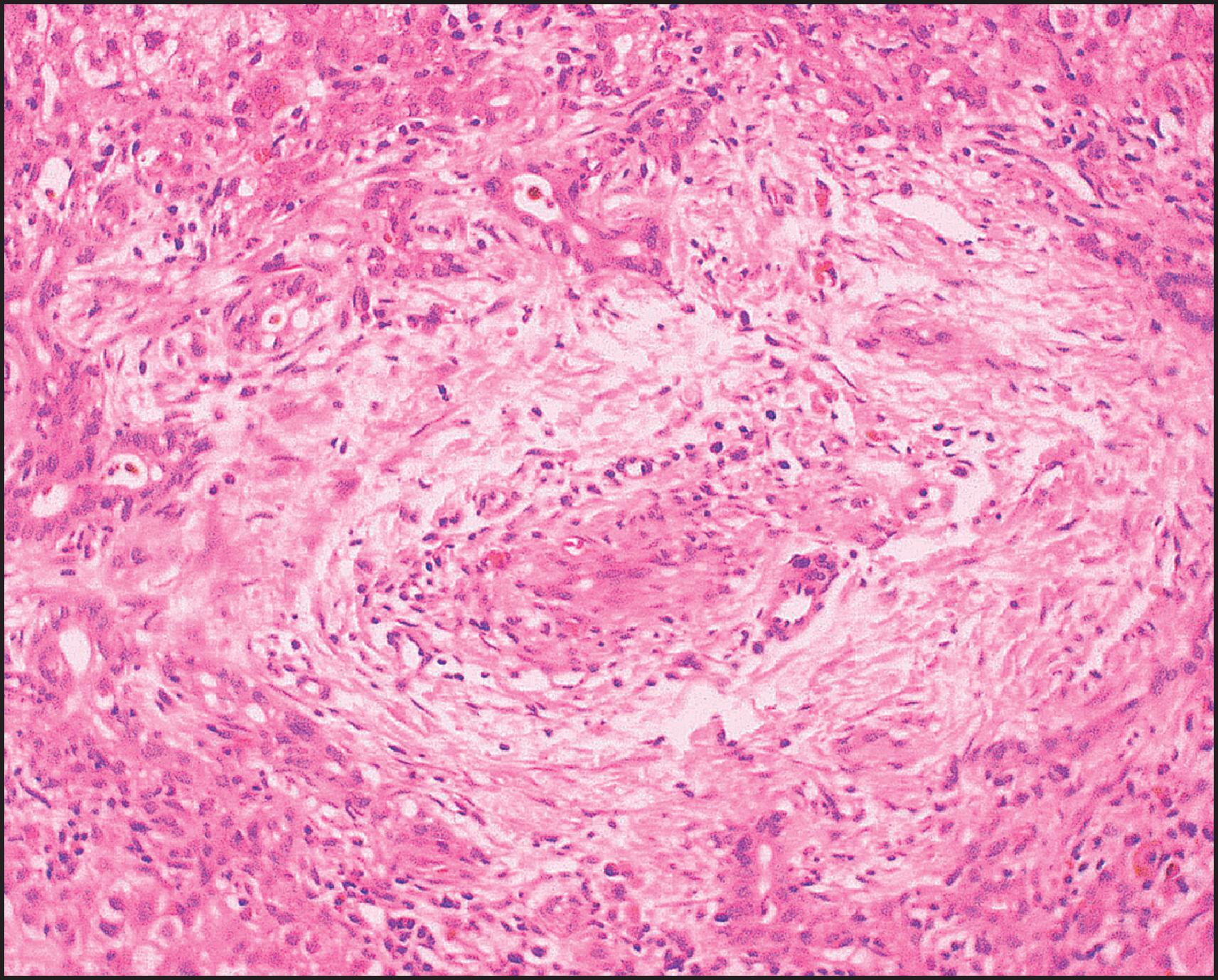
Interlobular bile ducts become few in number as early as the fourth or fifth month after birth ( Fig. 3.16 ), and advanced duct loss accompanies progressive fibrosis by age 8 or 9 months. Activated hepatic stellate cells are responsible for increased collagen production. , In addition to paucity of ducts, Raweily et al. identified concentric tubular ductal structures in 21.6% of cases of BA; these bore some resemblance to those seen in DPMs ( Fig. 3.17 ). Similar observations had been made earlier by Desmet and Callea, who hypothesized that this subgroup has more severe and rapidly progressive liver damage. Interestingly, children in this subgroup reveal a histopathological picture resembling that of CHF 4 or 5 years after portoenterostomy , ( Fig. 3.18 ; see also Fig. 3.17 ). The interlobular bile ducts continue to disappear, and cirrhosis can develop despite satisfactory bile drainage after portoenterostomy. The bile duct loss has been attributed to persistent interference with bile flow, recurrent cholangitis or continuation of the immune process causing the atresia. Detailed histopathological study of sequential liver specimens taken at Kasai operation, repeat laparotomy and/or transplantation has provided evidence that the bile duct loss is caused by an unpredictable and uneven obliteration of bile ducts in the porta hepatis during wound healing and scarring after portoenterostomy.
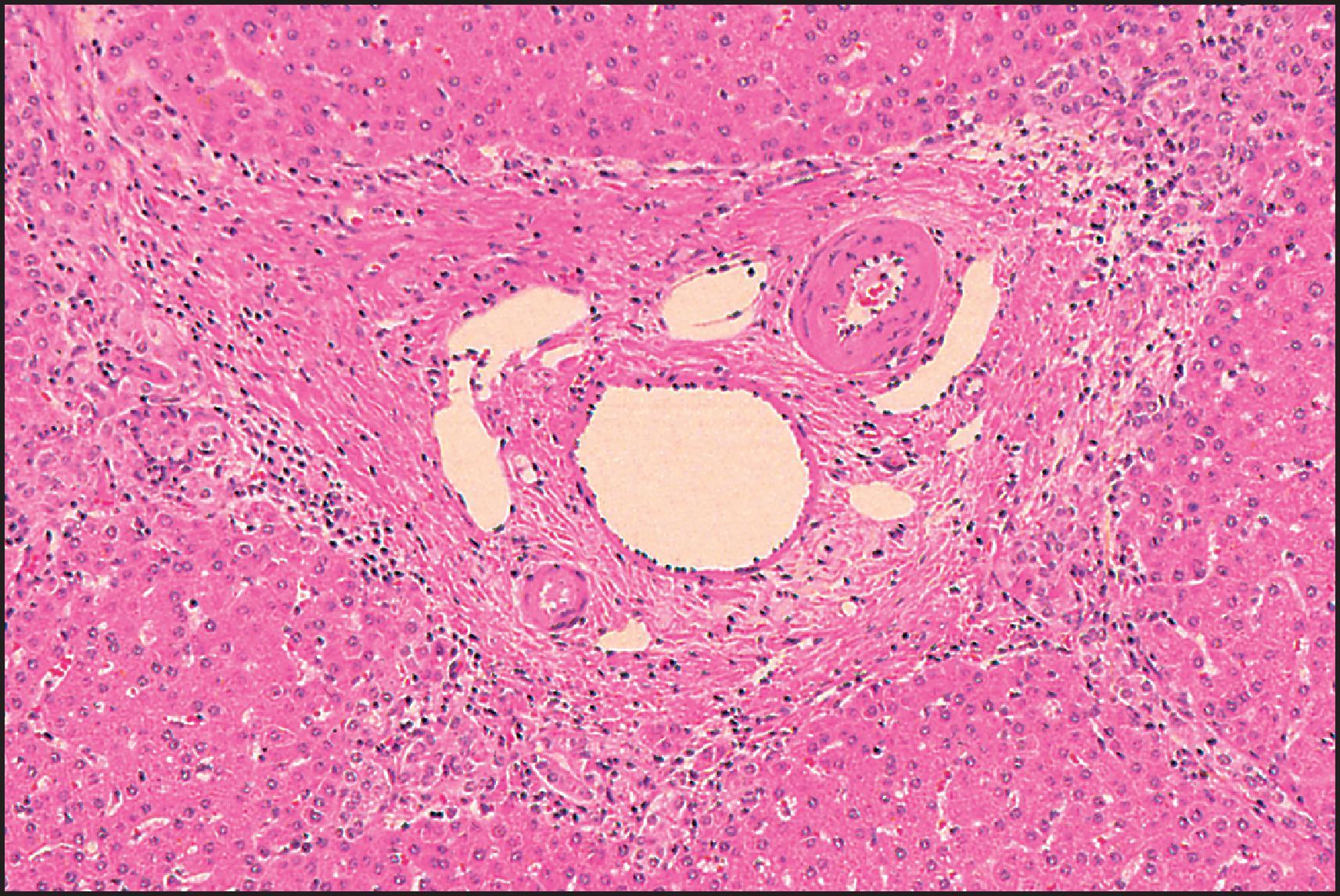
![Figure 3.17, Biliary atresia. Liver resection 1 year after portoenterostomy. Pattern of fibrosis and reactive small bile ducts and ductules are reminiscent of congenital hepatic fibrosis. There were only minimal chronic cholestatic features in this liver. (Periodic acid-Schiff [PAS] stain after diastase digestion.) Figure 3.17, Biliary atresia. Liver resection 1 year after portoenterostomy. Pattern of fibrosis and reactive small bile ducts and ductules are reminiscent of congenital hepatic fibrosis. There were only minimal chronic cholestatic features in this liver. (Periodic acid-Schiff [PAS] stain after diastase digestion.)](https://storage.googleapis.com/dl.dentistrykey.com/clinical/DevelopmentalandInheritedLiverDisease/16_3s20B978070208228300003X.jpg)
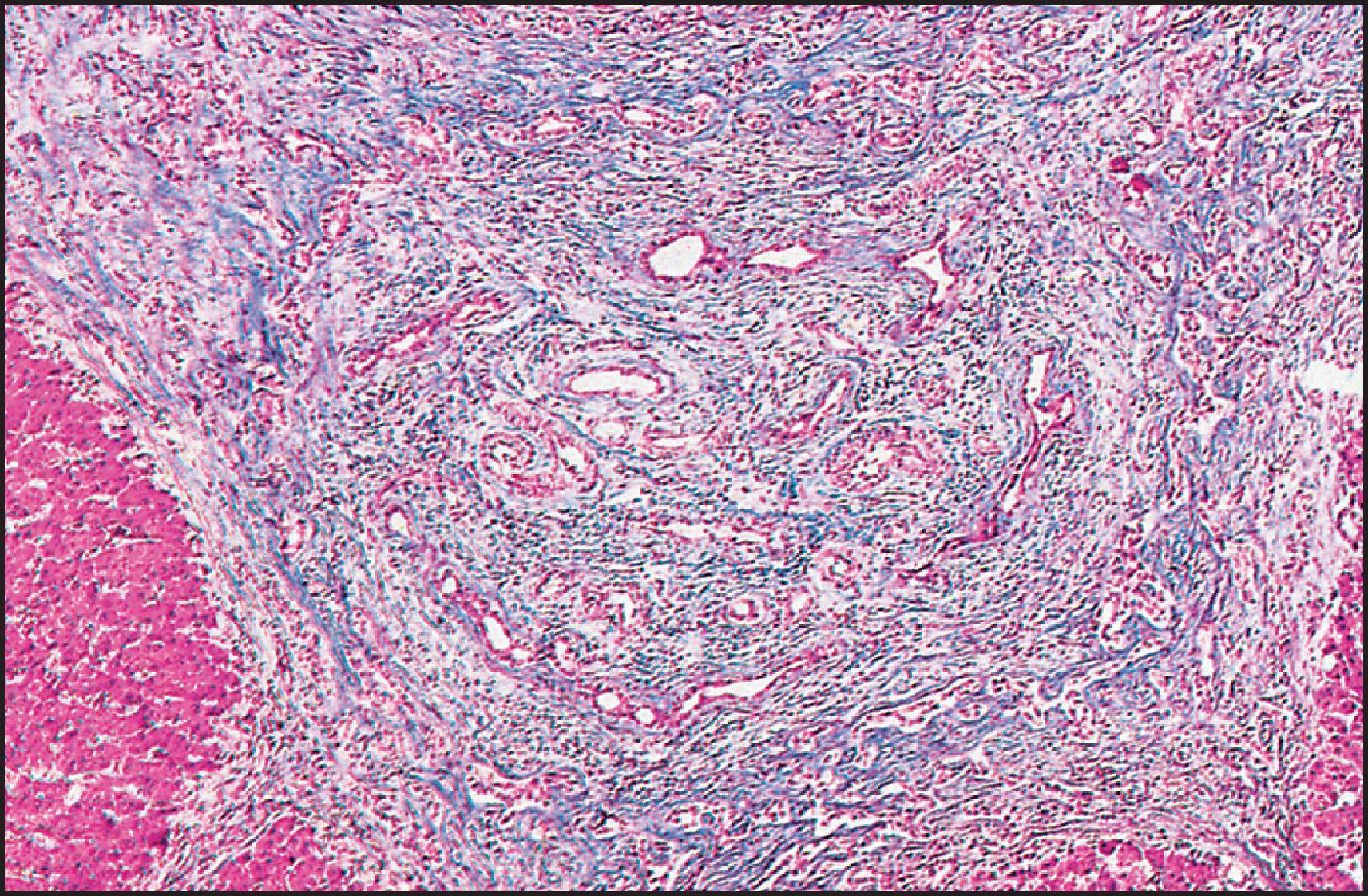
Ultrastructural degenerative changes affecting the intrahepatic bile ducts and ductules in BA have been described in detail by Ito et al., who found that the degree of obstruction of the lumen of these ducts appears to be an important determinant of prognosis following corrective surgery.
Malignant epithelial tumours of hepatobiliary origin rarely complicate biliary cirrhosis associated with BA, but both hepatocellular carcinoma (HCC) and cholangiocarcinoma have been reported. Focal nodular hyperplasia after portoenterostomy has been reported in a few children with BA. , Macroregenerative nodules may also develop.
Paucity of the intrahepatic bile ducts has been reported in many conditions, either congenital or acquired, affecting all age groups, especially infants and children. The relevant finding is a reduction in the number of interlobular bile ducts, that is, in the small bile ducts within portal tracts. The normal ratio of small bile ducts per portal tract in full-term infants, children and adults is 0.9–1.8 ducts per tract. In duct paucity syndromes, this ratio is <0.5, given an adequate number of portal tracts (at least 10) examined on biopsy. Herman et al. have proposed the use of IHC for K7 and EMA to help calculate the ratio between interlobular bile ducts and neocholangioles, with a low ratio indicating bile duct paucity. Premature infants have a reduced number of small bile ducts per portal tract, and if they have duct paucity with cholestasis, it may be physiological. In addition, early biopsy specimens in a few cases of clinically undisputed paucity of the intrahepatic bile ducts have shown not only identifiable ducts, but significant ductular reaction as well.
Liver disorders with paucity of the intrahepatic bile ducts are generally divided into two groups: ‘syndromic’, which refers to Alagille syndrome, and ‘nonsyndromic’, which refers to all the rest of these diseases. The nonsyndromic duct paucity conditions include numerous diseases in which portal small duct paucity is associated with another identifiable disease. These include infection (congenital rubella or CMV infection), immune abnormality (graft-versus-host disease, liver allograft chronic rejection) and hepatotoxicity (from carbamazepine or amoxicillin–clavulanic acid), the latter two groups being generally referred to as ductopenia , formerly ‘vanishing bile duct syndromes’ (see Chapters 9 and 14 ). Various inherited metabolic diseases such as Zellweger syndrome, α1-AT deficiency and inborn errors of bile acid metabolism may display paucity of the intrahepatic ducts. Chromosomal defects, such as 45,XO Turner syndrome, trisomy 17–18, trisomy 21 and prune-belly syndrome, may have duct paucity. More importantly, the term ‘nonsyndromic duct paucity’ may be used to refer to isolated, idiopathic paucity of interlobular bile ducts in infancy and childhood; this condition may be the same as idiopathic adulthood ductopenia. Finally, paucity of the intrahepatic ducts or ductopenia is frequently found as a late feature of certain chronic diseases, such as BA, primary sclerosing cholangitis, Langerhans cell histiocytosis and primary biliary cirrhosis (see Chapter 9 ).
Patients in the syndromic group have Alagille syndrome (ALGS), also known as arteriohepatic dysplasia. Comprehensive descriptions have been reported by Alagille and colleagues , and others. Two distinct genetic mechanisms are known to be responsible for ALGS. Most cases (ALGS1) are caused by mutations in JAGGED1 ( JAG1 , encoding the ligand for the Notch 1 receptor) on chromosome 20p12 , and may be associated with a macroscopic deletion of the short arm of chromosome 20 in some patients or microdeletions of 20p in others. The pattern of genetic transmission is autosomal dominant due to haploinsufficiency or dominant negative effect ; gene penetrance is high, but expression is extremely variable. The reported incidence of arteriohepatic dysplasia as 1 in 70,000 live births was an underestimate, and it is actually 1 in 30,000. Approximately 50–70% of patients have new mutations, rather than genetic transmission within the family. Crosnier et al. found mutations of the JAG1 gene in 69 of 109 patients (63%) with ALGS, and transmission analysis showed a high frequency of sporadic cases (70%). Numerous mutations have now been defined. The second genetic basis for ALGS (ALGS2) accounts for a very small proportion of cases. Children with mutations in the gene encoding Notch 2 (found on chromosome 1p13) have a clinical disorder similar to conventional ALGS, notably with intrahepatic duct paucity leading to cholestatic liver disease; however, the typical facies and skeletal abnormalities were much less common. The frequency of renal involvement is similar to that in ALGS1. Notch 2 appears to have an important role in bile duct development. , In a recent series on 401 ALGS patients, 13 (3.2%) did not have a pathogenetic variant in JAG1 or NOTCH2 , possibly due to undetected JAG1 variants or variants in other genes such as ATP8B1 , ABCB4 and NEK8 genes causing clinical manifestations overlapping with those of ALGS.
The common clinical findings in ALGS include cholestatic liver disease caused by paucity of the intrahepatic bile ducts (94%); congenital heart disease, usually peripheral pulmonary stenosis, although complex congenital heart disease, usually right sided, may occur (92%); a typical facies (91%); posterior embryotoxon in the eye (80–93%); and butterfly-shaped vertebrae (40–67%). The facies of ALGS consists of an inverted triangle shape, slight hypertelorism, deep-set eyes, broad and rather prominent forehead and beak-like nose. Although the specificity of the facies has been questioned, it is accepted as a typical finding, better appreciated in the actual clinical setting than in photographs. The facies is sometimes not evident in the first months of life, and in adults the facies is somewhat different from that described in children (longer face, rather coarse features, prominent forehead and comparatively small nose). Most patients have a systolic murmur related to stenosis of the pulmonary arterial system. More severe conditions include tetralogy of Fallot, pulmonary valve stenosis, aortic stenosis, ventricular septal defect, atrial septal defect, anomalous pulmonary venous return and complex problems involving a single right ventricle with pulmonary valve atresia. , Up to 15% of patients may have life-threatening cardiac complications. In addition to posterior embryotoxon or Axenfeld anomaly, abnormal retinal pigmentation may be found.
Strabismus, ectopic pupil and hypotrophic optic discs have also been reported. Optic disc drusen, which are calcified deposits in the extracellular space of the optic nerve head, often occur in ALGS. These can be found by ocular ultrasound examination and may facilitate the diagnosis. In addition to ‘butterfly vertebrae’ (vertebral sagittal cleft), other skeletal abnormalities include short distal phalanges and clinodactyly, and ALGS patients may be prone to bone fractures, beyond what might be attributed to chronic cholestatic liver disease.
Systems other than those in the cardinal criteria for the syndrome may be affected. Renal abnormalities may be prominent; renal dysplasia with or without cysts is most common. Renal tubular acidosis may be found, and vesicoureteral reflux is also a frequent finding. Renovascular hypertension may develop. Single horseshoe kidney has been reported. The most frequent histological finding is mesangiolipidosis, and other findings include tubulointerstitial nephropathy and membranous nephropathy. Nephrolithiasis may occur. Two children with ALGS and nephroblastoma were reported. Renal impairment in ALGS detected before LT does not improve spontaneously after liver transplant and mandates consideration of ‘renal-sparing’ immunosuppression. Systemic vascular disease appears to be more prevalent than originally appreciated. Several cases of moyamoya disease in association with ALGS have been reported. The propensity to intracranial bleeding found in the first few years of life in ALGS may be caused by abnormal intracranial vessels. Abnormalities in the large intra-abdominal vessels have been found, and these abnormalities may complicate LT. , Skin changes relate mainly to the formation of xanthomas, which regress after successful LT.
Abnormalities of the biliary tract include hypoplasia of the extrahepatic bile ducts, hypoplasia of the gallbladder and cholelithiasis. ERCP has demonstrated narrowing of the intrahepatic ducts, reduced arborization and focal areas of dilation, as well as narrowing of the extrahepatic ducts which may result in a clinical overlap with BA. , Portal venous disease may also be present, specifically hypoplasia of the portal vein.
Many patients develop growth retardation before adolescence, , especially if they have persisting clinical jaundice. Short stature is found in some affected children whose nutrition is normal. Although some display a degree of mental retardation, in general children with ALGS show a broad range of cognitive and intellectual ability. Almost all patients have pruritus, although it may be mild. They have elevated serum bile acids; cholic acid levels are greater than chenodeoxycholic acid levels. Variable hyperlipidaemia (sometimes severe, with xanthomas) is common. Treatments for the pruritus include cholestyramine, rifampicin or surgical diversion of bile flow. , Inhibition of the ileal bile acid transporter (IBAT) has been proposed for the treatment of ALGS.
In general, the prognosis is good for children whose jaundice resolves; however, approximately 25% of patients succumb in childhood to severe cardiac disease or progressive liver disease. The outcome in 92 patients in the series of Emerick et al. was as follows: the 20-year predicted life expectancy was 75% for all patients, 80% for those not requiring LT and 60% for those who required transplantation. LT was reserved for patients with chronic liver failure, intolerable pruritus unresponsive to medical treatment and severe growth failure and was necessary for hepatic decompensation in 19 of 92 cases (21%), with 79% alive 1 year after transplantation; mortality was 17%. In the Bicêtre series of 163 patients followed over 40 years, 102 of 132 patients presenting with cholestatic jaundice in infancy remained jaundiced at the study endpoint, and one-third required LT; cirrhosis was found in some children with ALGS, but no neonatal hepatitis syndrome, and none underwent transplantation (two succumbing to end-stage liver disease before the advent of transplantation). Overall survival in this series was 62% at 20 years and seemed unaffected by the availability of LT. Mortality is higher among patients who have more severe cardiac disease or intracranial bleeding or who had previously undergone portoenterostomy. , A retrospective study suggested that affected preschool-aged children with total serum bilirubin >111 μmol/L, serum conjugated bilirubin >77 μmol/L and serum cholesterol >13.5 mmol/L were likely to have a severe outcome, irrespective of actual JAG1 mutation. Liver transplantation is an option for those in end-stage liver failure, or with severe chronic cholestasis or intractable pruritus; however, the severity of cardiac and renal involvement, as well as intra-abdominal vascular abnormalities, must be evaluated as contributing to increased surgical risk.
Catch-up growth after transplantation is variable. , The long-term complications of ALGS not requiring transplantation have included renal failure and intracranial bleeding or stroke. , , HCC has been reported in children and adults with ALGS. Although rare, hepatic malignancy may occur in ALGS infants.
The histopathological aspects of ALGS are described in various reports and reviews. , , , The major finding is absence of bile ducts from portal areas ( Figs 3.19 and 3.20 ). The ratio of interlobular bile ducts to the number of portal areas is between 0.0 and 0.4, compared with 0.9–1.8 in normal children. A reduced number of portal areas has been noted. The loss of bile ducts is progressive from early infancy to childhood. , , Cholangiodestructive lesions have been observed in infants between 3 and 6 months of age. , The degree of cholestasis is variable in intensity and is especially prominent in the first 12 months of life. Immunohistochemical staining for endopeptidase (CD10) is typically absent from the canalicular surface in ALGS children. Endopeptidase, however, is not expressed physiologically until about 7 years of age. Its expression is lost during fibrosis progression in various liver disorders. Giant cell transformation of hepatocytes may be seen in early infancy. , , There is usually patchy pseudoxanthomatous change and accumulation of stainable copper and copper-associated protein in periportal hepatocytes. , , Copper accumulation has been demonstrated by quantitative methods in both syndromic and nonsyndromic types of paucity of the intrahepatic bile ducts. Periportal fibrosis is mild, and it may remain unchanged long-term, possibly due to the absence of ductular reaction, known to play a role in periportal fibrogenesis. Nonetheless, although progression to cirrhosis is rare, some patients do develop extensive fibrosis or cirrhosis ( Fig. 3.21 ). Large, often centrally located, regenerative nodules may also develop. Portal inflammation and periportal ductular reaction, when present, are seen mainly in early infancy and can suggest the presence of distal duct obstruction. , , In rare ALGS cases, the extrahepatic bile is narrowed; however, typically it is not. Misdiagnosis of ALGS as BA leading to portoenterostomy in the ALGS patient risks a failed portoenterostomy , and consequent early LT.
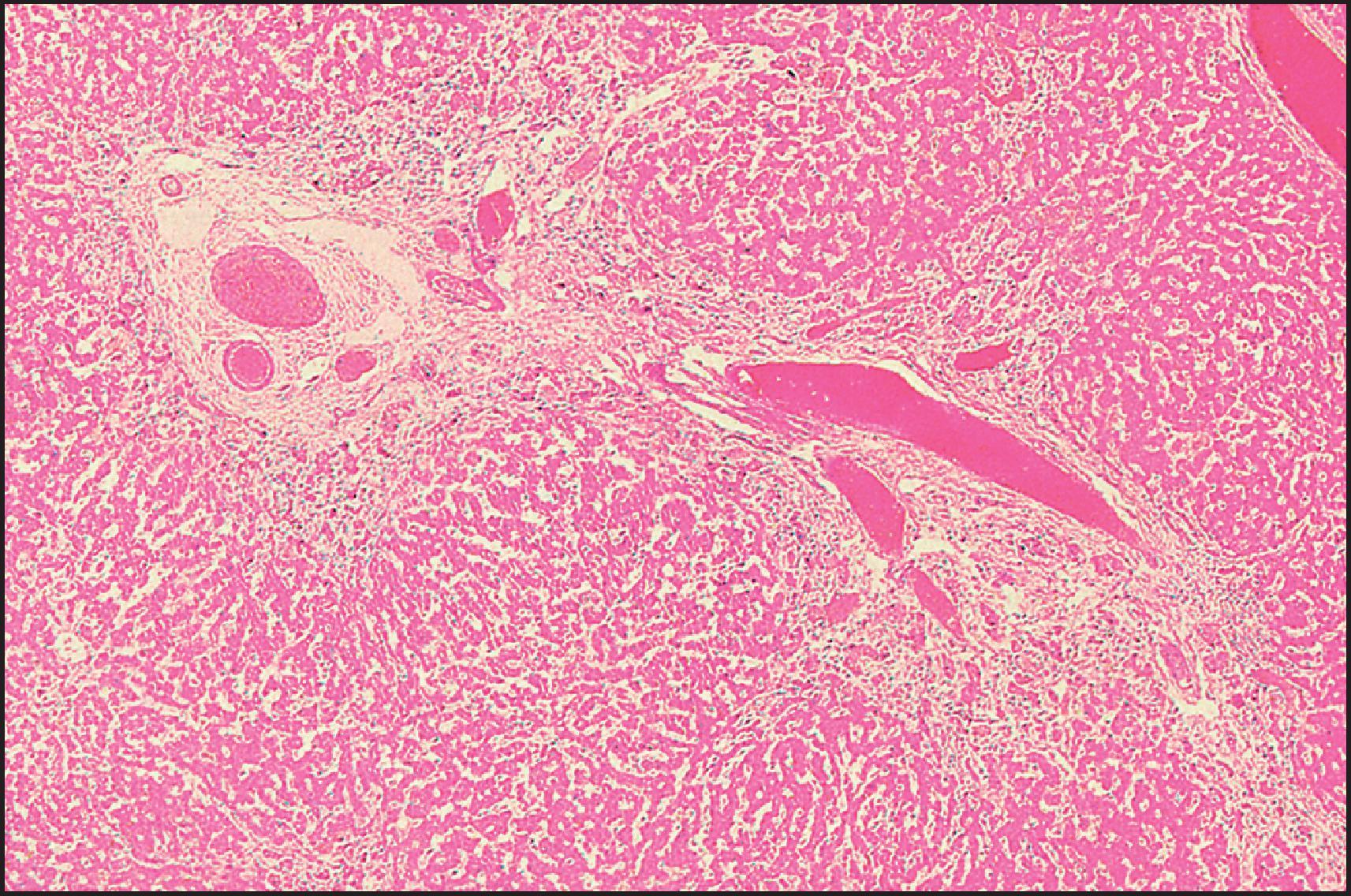
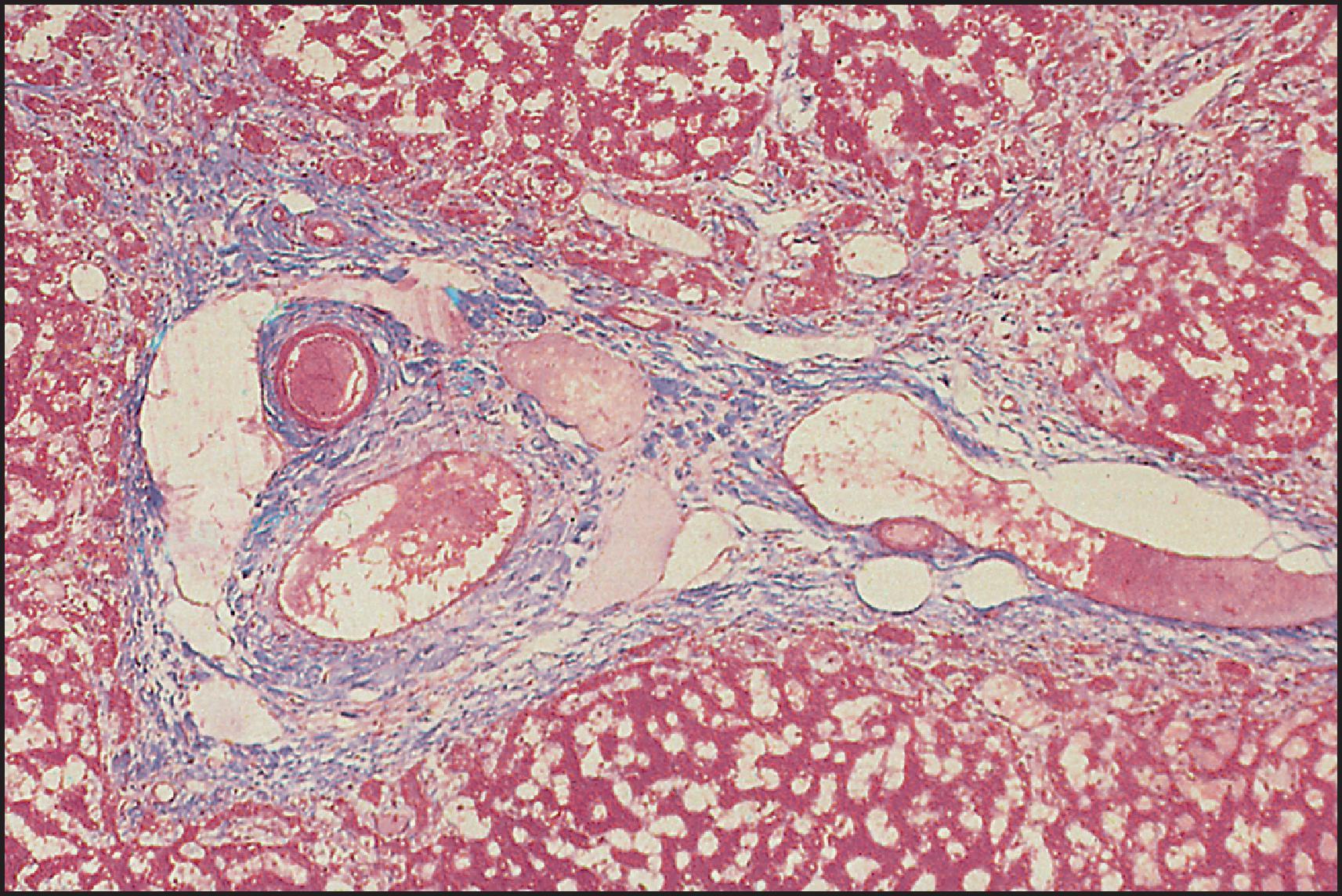
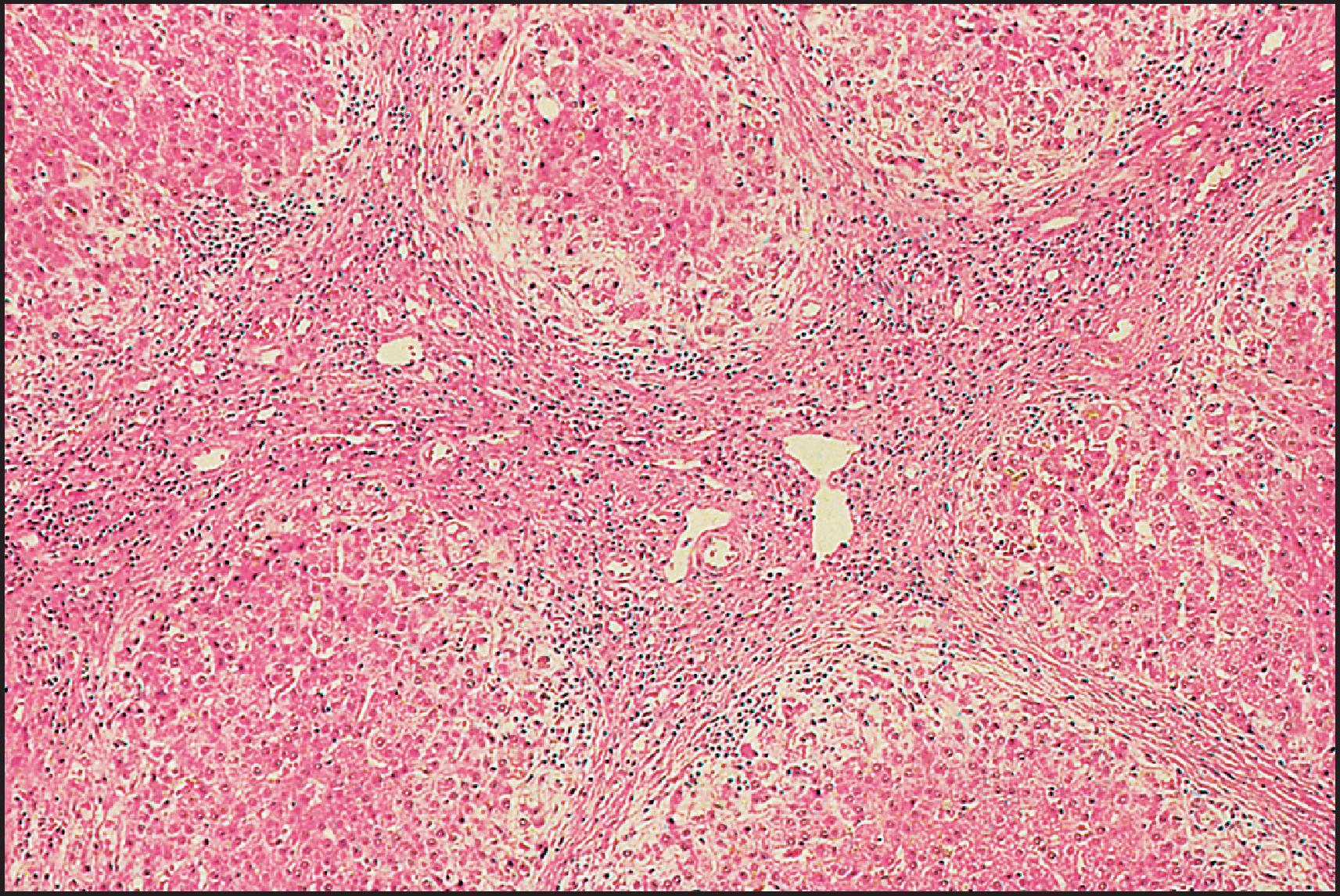
Ultrastructural studies of the intrahepatic bile ducts in ALGS have been reported. , , , In one study of 12 biopsies from 10 patients, distinctive ultrastructural changes were noted. Bile pigment retention was found in the cytoplasm of liver cells, especially in lysosomes and in vesicles of the cis -Golgi, but only rarely in bile canaliculi or the immediate pericanalicular region. It was suggested that the basic defect in ALGS involves the bile secretory apparatus. The aetiopathogenesis of the cholangiodestructive lesions described histopathologically and ultrastructurally remains to be elucidated, but the possibility of ‘disuse atrophy’ has been raised by two groups of investigators. , Recent studies suggest defective branching of intrahepatic bile ducts in the postnatal period.
ALGS is the first childhood disorder identified with a mutation in a ligand for a Notch protein. JAG1 encodes a ligand of Notch 1, one of a family of transmembrane proteins with EGF-like motifs. Notch proteins are highly conserved and have a role in determining cell fate during differentiation, especially in tissues where epithelial–mesenchymal interactions are important. Jagged/Notch interactions are known to be critical for the determination of cell fates in early development. Notch 4 expression during embryogenesis is seen in endothelial cells of vessels forming the dorsal aorta, intersegmental vessels, cephalic vessels, and the heart. The expression of Notch 1 and its ligand includes many of the organs potentially abnormal in ALGS. JAG1 plays an important role in embryogenesis of the heart, kidneys and blood vessels; in embryonic and fetal liver it is expressed in portal vascular tissue. Recent studies in mice indicate that Notch signalling regulates the remodelling of the ductal plate and bile duct morphogenesis and that Jagged 1 plays an important role in this complex process. Jagged 1 on ductal plate and Notch 3 on portal tract mesenchyme and hepatic arterial endothelium interact for ductal plate remodelling and development of intrahepatic bile ducts. ,
Mice with defects in murine Jagged 1 and Notch 2 expression have abnormalities similar to human ALGS; zebrafish with knockdowns of jagged ± notch genes have biliary, pancreatic, cardiac, renal and craniofacial developmental abnormalities; these studies suggest that Notch may promote biliary epithelial cell evolution from a bipotential precursor cells. Transforming growth factor-β (TGF-β) signalling drives hepatocyte transdifferentiation and de novo bile duct formation. In humans, mutations in JAG1 result in truncated and inactive proteins; since residual gene expression cannot compensate, there is haploinsufficiency. Dose of Notch ligands is critical and may contribute to the clinical diversity of ALGS. No clear relationship between genotype and phenotype has been found, but the Delta/Serrate/Lag-2 (DSL) domain in the JAG1 protein may influence the severity of liver disease.
In contrast to patients with ALGS, those with nonsyndromic bile duct paucity do not have a constellation of extrahepatic features. Children with nonsyndromic bile duct paucity are supposed to have a less favourable outlook than children with ALGS. They present with persistent cholestasis and severe pruritus. Growth retardation is common. No associated aetiological agent, defined genetic factors or congenital anomalies have been found in this group, except for one study of 10 patients with a high rate of consanguinity. A chronic cholestatic disease with duct paucity, called ‘idiopathic adulthood ductopenia’, has been described in adults. Most of these patients are young adults, although patients over age 60 have been reported. Nonsyndromatic paucity of bile ducts in infancy and idiopathic adulthood ductopenia may be related diseases. The outlook for younger patients with idiopathic adulthood ductopenia is poor; approximately 50% succumb to progressive liver disease or require transplantation. HCC was reported in a woman with intrahepatic biliary hypoplasia, apparently distinct from ALGS.
A histopathological study of 17 children with nonsyndromic paucity of bile ducts was reported by Kahn et al. Before 90 days of age there was a paucity of ducts and periportal fibrosis as well as nonspecific parenchymal changes (cholestasis, giant cell transformation, perisinusoidal fibrosis and haematopoiesis). After 90 days, the duct paucity and fibrosis persisted but cholestasis was mild or no longer apparent. Kahn et al. suggested that the paucity in nonsyndromic cases may result from a primary ductal insult with destruction and disappearance of the ducts. The differential diagnosis of paucity of the intrahepatic bile ducts in children and adults has been reviewed by West and Chatila.
Congenital dilations of the bile ducts are classified into five types, both extrahepatic and intrahepatic :
Type I: a dilation of the common bile duct which may present three anatomical variations: (a) large saccular, (b) small localized and (c) diffuse fusiform
Type II: diverticulum of the common bile duct or the gallbladder
Type III: choledochocoele
Type IV: multiple intrahepatic and extrahepatic dilations (Caroli disease)
Type V: fusiform intrahepatic and extrahepatic dilations
Types I and IV account for the majority of reported cases, although types IV and V may prevail in the Far East, where the disease occurs more frequently. Type IV cysts are more frequent in adults than in children. Although this classification remains in common use, its value has been questioned by Visser et al., who consider the distinction between types I and IV arbitrary; they suggest that the term ‘choledochal cyst’ should be reserved for congenital dilation of the extrahepatic and intrahepatic bile ducts, with other forms referred to by their name, e.g. choledochocoele and bile duct diverticulum. Caroli disease, assimilated to type IV in the Hadad classification, is not clearly related to ‘choledochal cyst’, given its common association with both CHF and fibrocystic lesions in the kidney and its distinct morphological features (see next). Hukkinen et al. have observed a considerable increase in the incidence of cholechal malformation in Finland. Recently reports highlight problems with current classificatory schemes and propose modifications. ,
The classic clinical triad of pain, a mass in the right upper quadrant and jaundice occurs in less than a third of patients with a choledochal cyst. , In children, jaundice is the most common presentation, while in adults the signs and symptoms are those of ascending cholangitis. In the early years of life, cholestasis is usually associated with cystic dilation of the common bile duct (CBD) and accounts for 2% of infants presenting with cholestasis. Up to 60% of choledochal cysts are diagnosed before age 10 years, but diagnosis can be made at any age, and some cases may present for the first time at as late as the eighth decade. Several cases have been diagnosed antenatally. , About 80% of the patients are female. Differences in presentation between children and adults with choledochal cysts have been emphasized in two large series. The preoperative diagnosis can be made in the majority of patients by cholangiographic studies, ultrasonography (US) and isotope scanning. Dynamic magnetic resonance cholangiopancreatography (MRCP), including secretin stimulation, contributes to the understanding of the pathophysiology.
Complications include perforation, liver abscesses, stone formation, secondary biliary cirrhosis, pancreatitis, amyloidosis and carcinoma of the biliary tree. , Regression of biliary cirrhosis following drainage of a choledochal cyst has been reported. One patient presented with anaemia due to bleeding from erosions of the duodenal mucosa between the ampullary sphincter and the sphincters of the CBD and pancreatic duct. Biliary tract anomalies reported in association with a choledochal cyst include double CBD, double gallbladder, absent gallbladder, annular pancreas, BA or stenosis, stenoses of the intrahepatic bile ducts and, most frequently, anomalies of the pancreaticobiliary junction. In a series of 104 choledochal cysts from Japan, 25% of patients were found to have coexisting biliary anomalies. Differences between (1) isolated choledochal cysts and (2) choledochal cysts associated with BA have been noted by US. In the former the cysts are larger, intrahepatic ducts are dilated and the gallbladder is not atretic as compared to those with choledochal cysts and BA. In general, the apparent choledochal cyst associated with BA is actually proximal duct dilation associated with focal atresia of the distal CBD, so-called correctable or cystic atresia.
Maljunction of the pancreaticobiliary ductal system (common channel) remains the most plausible aetiopathogenic mechanism for choledochal cysts, which is supported by experimental studies. The lesion is defined as a junction of the pancreatic and bile ducts located outside the duodenal wall, usually forming an extremely long common channel. The anomaly allows pancreatobiliary regurgitation because hydrostatic pressure within the pancreatic duct is usually higher than that in the CBD. As a result, elevated pancreatic enzyme levels are found in the bile. Common channels may occur without bile duct dilation and lead to primarily gallbladder rather than biliary complications, including malignancy. In this situation, prophylactic cholecystectomy might be sufficient, whereas biliary complications and the risk of cholangiocarcinoma underpin the need for radical surgical resection in cases associated with choledochal cysts.
Reovirus-3 RNA sequences have been recovered from resected choledochal cysts, but the implication of reovirus infection in the aetiology of choledochal cyst is unclear. A single report of choledochal cysts in association with familial adenomatous polyposis raises the possibility of a genetic basis for the cysts. Duplications of 17q12 with consequent hepatocyte nuclear factor 1-β (HNF1B) overdosage has been described recently.
Treatment is surgical: complete cyst resection, cholecystectomy and Roux-en-Y hepaticojejunostomy as a preventive measure against the subsequent development of carcinoma. , Laparoscopic repair has been successful , against a morbidity risk of 24% and a mortality risk of 2% in a recent series from the Netherlands. Dilemmas may arise when the cysts involve the intrahepatic or intrapancreatic segments, requiring more extensive surgery, given the small risk of malignancy developing in cystic remnants at the anastomotic site or in the dilated intrahepatic bile duct of type IV or V cysts. , In a report of 48 Japanese patients treated by total or subtotal excision, no malignant change occurred after a mean follow-up of 9.1 years. Similar excellent results were reported from Finland with median follow-up of 4.8 years (range, 1.3–13.2). Choledochal cysts vary greatly in size, with some larger lesions containing 5–10 L of bile ( Figs 3.22 and 3.23 ). Histopathologically, the wall is usually thickened by inflammation and fibrosis and is bile stained. Smooth muscle fibres may be identified in the lower portion of the cyst but not in the narrow (intrapancreatic) portion. A recent study has suggested that the pattern of the smooth muscle bundles may predict postoperative complications. There is generally no epithelial lining, but islets of cylindrical or columnar epithelium may be preserved ( Fig. 3.24 ). Intestinal metaplasia with mucous gland proliferation has been reported, as well as the presence of goblet and Paneth cells and neuroendocrine differentiation. , According to Komi et al., the intestinal metaplasia increases with age and can be demonstrated in almost all cysts from patients over 15 years of age. Kusunoki et al. noted the absence of ganglion cells in the narrow portion of a choledochal cyst and suggested that the cyst could be the result of postganglionic neural dysfunction.
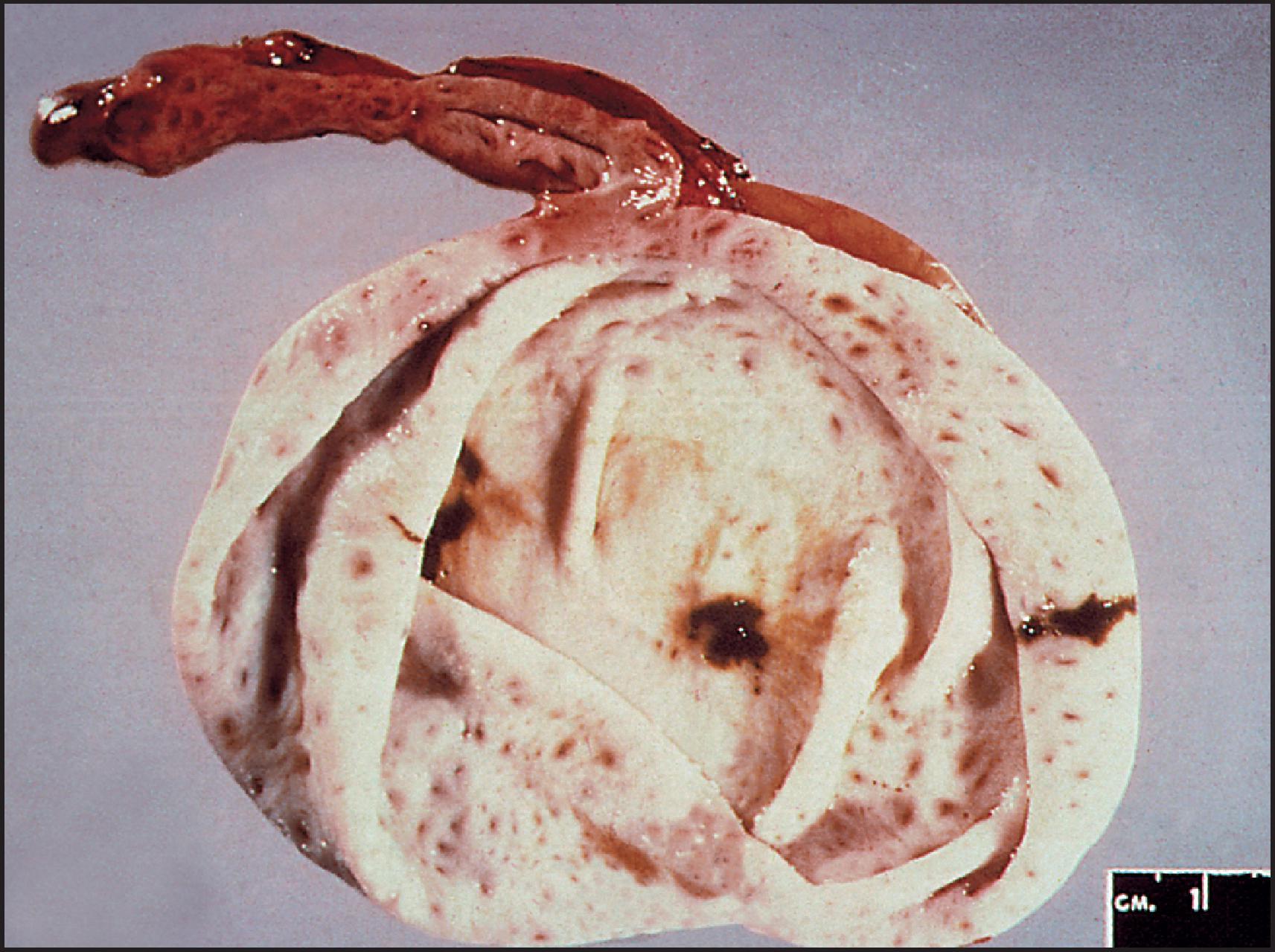
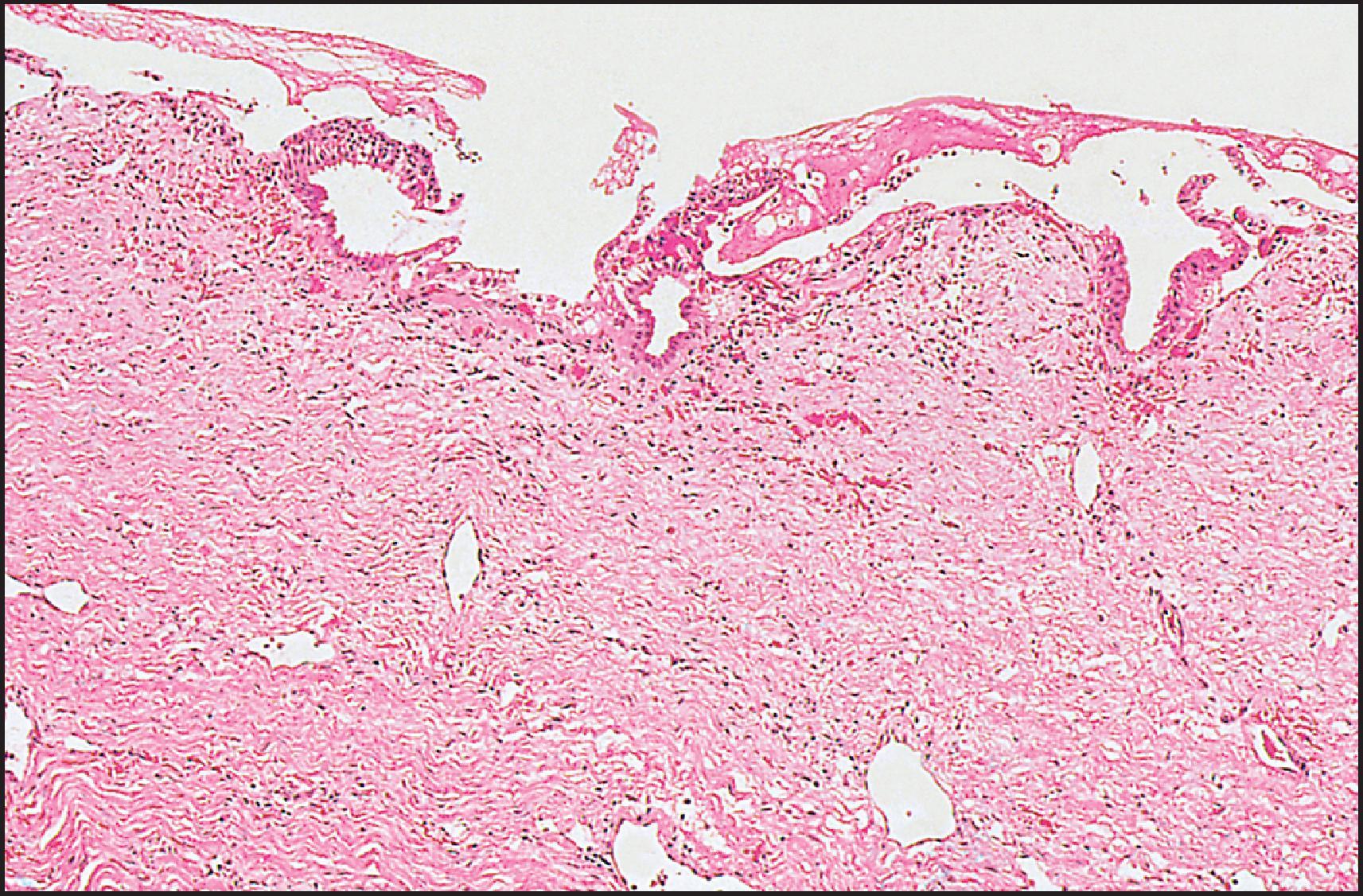
The majority of tumours arising in congenital cystic dilations of the bile ducts are adenocarcinomas, but some anaplastic and several squamous carcinomas have been reported, and one report mentioned sarcomatous changes. The overall incidence of carcinoma arising in all cystic dilations of the bile ducts is about 3%. The risk is age related, increasing from 0.7% in the first decade to 6.8% in the second decade to 14.3% in later decades. This finding has been confirmed in a recent series of 36 choledochal cysts describing a high rate of biliary intraepithelial neoplasia in approximately a third of cases and adenocarcinoma in about 15%. The complication is thus usually seen in adults; only three patients reviewed by Iwai et al. were under 18 years of age. An 11-year-old boy is the youngest reported to date. Reveille et al. found that stasis of bile in the choledochal cyst contributes to bacterial overgrowth and the generation of unconjugated secondary bile acids, a possible cause of biliary metaplasia and carcinoma. Interestingly, bile from congenital choledochal cyst patients is shown to promote the proliferation of human cholangiocarcinoma QBC939 cells. Certain bile acid fractions together with reflux of pancreatic enzymes may play a primary role, because pancreaticobiliary maljunction is associated with an increased risk of biliary tree malignancy irrespective of the presence of cysts.
The term ‘fibropolycystic diseases’ of the liver—not to be confused with cystic fibrosis—is used to describe a heterogeneous group of genetic disorders in which segmental dilations of the intrahepatic bile ducts and associated fibrosis can be interpreted as sequelae of persistence and aberrant remodelling of the embryonal ductal plate (see Chapter 1 ). They represent a spectrum of microscopic and macroscopic cystic lesions often associated with fibrocystic anomalies in the kidneys. The severity of the renal lesions may overshadow the liver disease, as in the early presentation of autosomal recessive polycystic kidney disease (ARPKD). Conversely, portal hypertension with a preserved liver function may dominate the picture later in life, as exemplified by CHF. Cholangitis may develop, especially when the cysts communicate with the biliary system. The microscopic abnormalities are classified as ‘ductal plate malformations’, , a term that refers to the histological changes of circumferentially disposed and variably ectatic bile ducts and ductules, often directly abutting the hepatocytic plates, which resemble an exuberant embryonal ductal plate. The main disorders, in particular ARPKD, the closely associated CHF and Caroli disease and autosomal dominant polycystic kidney disease (ADPKD), are discussed in detail. Rarer associated syndromes are briefly mentioned. Over the past decade, genes and encoded proteins for several of these disorders have been identified ( Table 3.2 ). Whole-genome sequencing analysis has suggested that the prevalence of ADPKD-related variants is higher than expected. The recognition that the ‘cystoproteins’, the mutation of which causes polycystic disease, have been localized in the primary cilia or basal bodies of tubular epithelial cells has led to a renewed interest in these forgotten structures. The clinicopathological discussion of specific disorders is therefore preceded by a short account on cilia and cystogenesis; more details and comprehensive references can be found in reviews. ,
| Disorder | Gene | Product | Localized in cilium |
|---|---|---|---|
| ARPKD | PKHD | Fibrocystin/polyductin | Yes |
| ADPKD | PKD1 | Polycystin 1 | Yes |
| PKD2 | Polycystin 2 | Yes | |
| PCLD | PRKCSH | β-subunit of glucosidase II | No |
| SEC63 | Component of translocon in endoplasmic reticulum | No | |
| SEC61B | Sec61 translocon β-subunit (SEC61β) | No | |
| LRP5 | Low-density lipoprotein receptor-related protein 5 | No | |
| GANAB | Glucosidase II subunit-α | No | |
| ALG8 | α–1,3-glucosyltransferase | No |
Most studies on the mechanisms of cyst development in hereditary polycystic disease have been conducted in the kidney, but there are strong suggestions that a number of pathways are common to the conversion of tubes into cysts in general, especially in the kidney tubules and hepatic bile ducts. Primary (or solitary) cilia arise from centrioles and form a finger-like extension of the cytoplasm covered with the cell membrane. In their axis, primary cilia contain a system of nine pairs of longitudinal microtubules arranged in a circle (the axoneme); in contrast, motile cilia (e.g. those in ciliated respiratory epithelium) contain a set of two centrally located microtubules within the circle of the nine pairs of longitudinal microtubules (‘9+2 pattern’). The pattern typical of, and defining, primary cilia is the 9+0 pattern. The microtubules secure the circulation of non-membrane-bound macromolecules, intraflagellar transport, which is essential for the assembly and maintenance of cilia and also acts as a sensory process, in that the particles and associated peptides are changed in the cilium and carry a message back to the cell body. The solitary cilium is at present considered to represent a sensory antenna, functioning through the polycystin complex as a mechanotransducer. Loss of function of the complex results in perturbation of normal intracellular calcium ion (Ca 2+ ) concentration, which underlies a multitude of pathological reactions. Severe alterations in structure and function of tubular or ductal cells consequently develop: they involve cell–cell contact, actin cytoskeleton organization, cell–extracellular matrix interactions, cell proliferation and apoptosis.
An enticing finding of recent years has been the demonstration that most proteins mutated in experimental polycystic liver disease (PCLD) have been localized in the primary cilia or basal bodies of tubular epithelial cells. They include those responsible for human disease, in particular polycystin 1 and polycystin 2 and fibrocystin/polyductin, but not the cystoproteins of isolated PCLD: hepatocystin and SEC63. Mutational analysis best studied in ADPKD suggests that both germline and somatic mutations may be involved in a two-hit model, as either a prerequisite initiating event or a later somatic event needed for cyst expansion and progression. Although the ciliary model is most attractive, it may represent only one of several parallel pathways that control essential cellular functions, such as proliferation, apoptosis, adhesion and differentiation.
Cyst epithelial lining cells show profound pathological alterations. An increased proliferation has been observed in ADPKD, comprising the formation of papillary projections and overexpression of Ki-67 and proliferating cell nuclear antigen (PCNA), a loss of their ‘planar or tissue polarity’ (the ability to sense their position and orientation relative to the overall orientation of the epithelial sheet) and a switch from an absorptive to a secretory cell type, resulting in fluid secretion that is responsible (together with epithelial proliferation) for further cyst expansion. Although studies of cyst development have been mainly performed in kidney, primary cilia have been explored in cholangiocytes, and evidence showed that, at least to some extent, cystogenesis in bile ducts and ductules may be similar to that in the kidney. In ADPKD, PKD1 and PKD2 are co-localized in bile duct epithelial cells and the two-hit model of germline plus somatic mutations of PKD1 in focal liver cyst development appears applicable, as in the kidney cysts. As with kidney cyst, biliary cyst expansion occurs by secretion (as well as proliferation) of the cyst lining epithelial cells. Autocrine and paracrine factors, including IL-8, epithelial neutrophil attractant 78, IL-6 and vascular endothelial growth factor (VEGF), secreted into the cystic lumen modulate the rate of errant hepatic cyst growth. In ARPKD, findings suggestive of similarity with renal cystogenesis include the localization of PKHD1 in the cilia of cholangiocytes in the PCK rat, which is a model for Caroli syndrome, and evidence that HNF1β localized in bile duct epithelium regulates expression of PKHD1, together with other ‘cystoproteins’ that co-localize into the cilium. A variant in DNAJB11, a cofactor to the chaperone protein BiP, has been associated with a clinical overlap of autosomal dominant polycystic liver disease (ADPLD) with autosomal dominant tubulointerstitial kidney diseases (ADTKDs).
Differences in cystogenetic pathways between liver and kidney in ARPKD are indicated in animal models of the disease. The mouse cpk mutation is the most extensively characterized murine model, closely resembling human ARPKD, with the exception that the B6- cpk/cpk homozygotes do not express the lesion of DPM. However, homozygous mutants from outcrosses to some other strains express the DPM. Genetic analysis supports a loss-of-function model (two-hit model) for biliary cysts developing in an age-dependent fashion. There is no correlation between the severity of the DPM and the renal cystic disease. The expression of the biliary lesion is modulated by genetic background, and the specific biliary phenotype (DPM predominant or cyst predominant) is determined by whether loss of function of the cpk gene occurs as a germline or a somatic event.
In the mouse model generated by targeted mutation of Pkdh1 , the animals develop severe malformation of their intrahepatic bile ducts but develop normal kidneys. The cholangiocytes maintain a proliferative state secreting TGF-β1 that continuously stimulates mesenchymal cells to synthesize and put down extracellular matrix, resulting in fibrosis. The findings suggest that fibrocystin/polyductin, already expressed in the embryonic ductal plate stage, acts as a matrix sensor and signal receptor during intrahepatic bile duct development, and that its mutation results in a CHF-like picture. Its roles in liver and kidney appear to be functionally divergent, because protein domains essential for bile duct development do not affect nephrogenesis in this model.
This PKD is inherited in an autosomal recessive manner. The incidence is estimated to be between 1:10,000 and 1:60,000 live births. The gene for this disorder, PKHD1 (polycystic kidney and hepatic disease 1), was mapped to chromosome 6p 21.2–p12 , ; both the severe and the mild forms of ARPKD map to the same locus. , The gene encodes a 4074-amino acid protein, called either fibrocystin or polyductin ; this large transmembrane polypeptide, also known to localize to the cilia, may be a receptor that acts in the differentiation of renal collecting duct and bile duct. An animal model is widely used. In humans, incidence in males and females is equal. Depending on the age at presentation and the degree of renal involvement, Blyth and Ockenden divided ARPKD into four types: perinatal, neonatal, infantile and juvenile. Subsequent experience has found a spectrum of severity such that not all cases fit into these sharply defined subgroups. ARPKD has been reviewed by a number of authors. , ,
The majority of ARPKD patients present in the perinatal period with enlarged kidneys and signs of respiratory distress related to pulmonary hypoplasia. Those with extensive renal cystic change affecting at least 90% of renal parenchyma typically die in the perinatal period. Some ARPKD patients present later in childhood with increasing renal insufficiency and portal hypertension. In one series of 42 children with ARPKD from 20 sibships, 12 patients presented in the perinatal period, 9 in the neonatal period, 13 in the infantile period, and 8 in the juvenile period; the presentation and course of the disease were disparate in over 50% of patients. The organ predominantly affected may vary within the same family. Routine liver enzymes are usually normal, although alkaline phosphatase (ALP) levels may be increased.
The liver in ARPKD does not appear abnormal macroscopically, although it may be enlarged and firm. About 70% of patients have enlargement of the left lobe on imaging.
Histologically, the changes range from a persistent, circular ductal plate ( Fig. 3.25 ) to a striking increase in the number of biliary channels which arise in portal areas and extend irregularly and deeply into the parenchyma ( Fig. 3.26A ). They appear to branch or ‘anastomose’ and often show polypoid projections ( Fig. 3.26B ). Normal interlobular ducts with corresponding arteries are not seen. According to Witzleben, the biliary channels are in continuity with the rest of the biliary system, similar to the cystic spaces of Caroli disease (‘communicating’ cystic disease), in contrast to the noncommunicating ADPKD. The supporting connective tissue is very scanty and, in the intralobular extensions, the basement membrane of the epithelium appears to be in direct contact with the liver cell plates. The epithelial lining consists of a single layer of low columnar to cuboidal cells. Cyst formation is uncommon. The dilated channels may contain a small quantity of a pink or orange-coloured material or rarely pus. Reconstruction studies by Adams et al. have shown irregularly dilated ducts running longitudinally at the periphery of the portal tract and anastomosing so extensively that they formed a single annular channel; no main interlobular duct could be identified in that portal tract or in several others from the same liver. A stereological study of 10 cases by Jörgensen showed ductal structures that could be divided into two groups: one consisted of irregular tubular structures shaped like circular cylinders, and the other of elliptical cylinders (the ‘ductal plates’). These were dilated but cysts were rare. In patients who survive for months or years, there is marked fibrosis in the liver as well as kidney lesions which appear to have been progressive lesions.
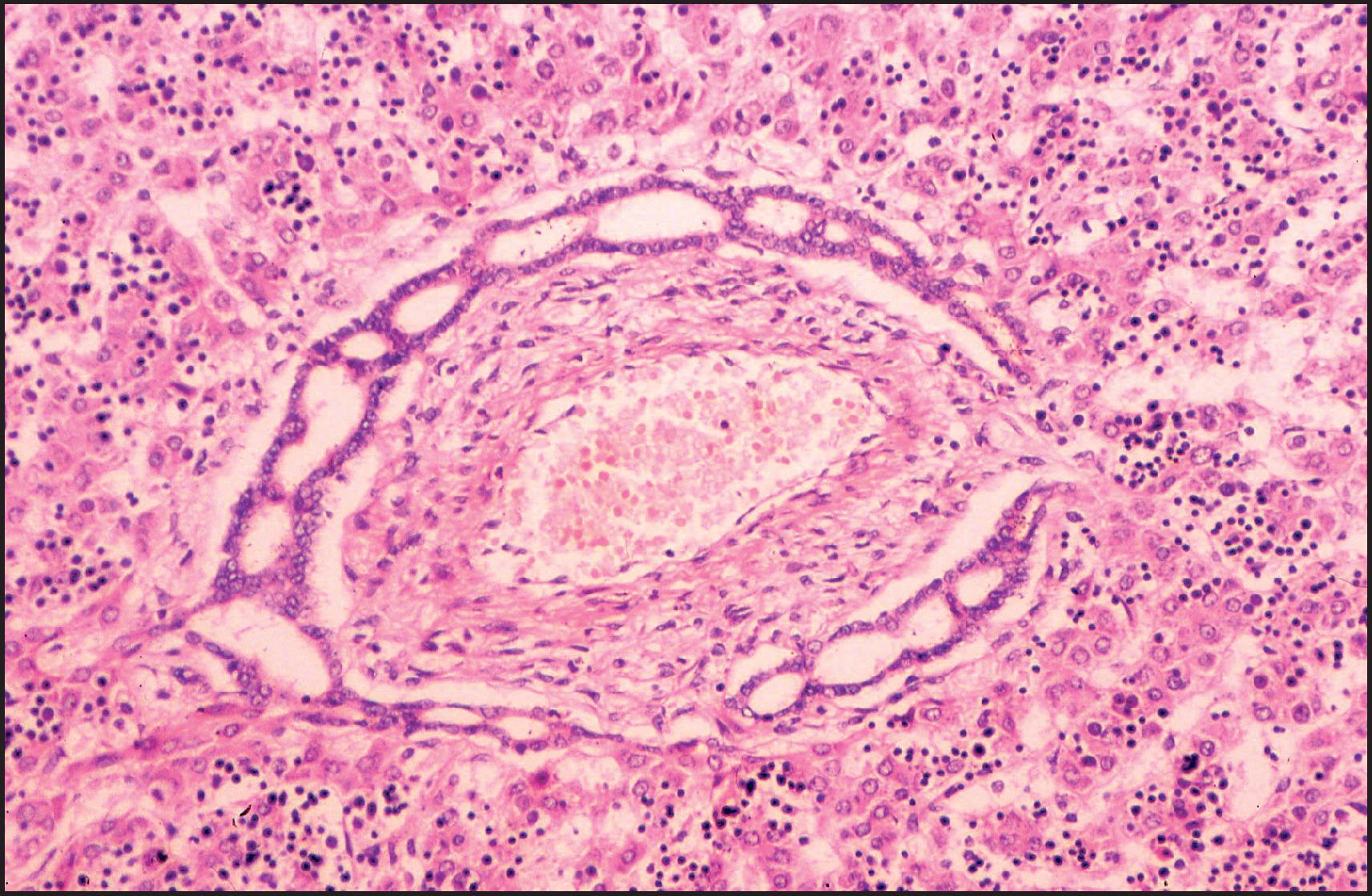
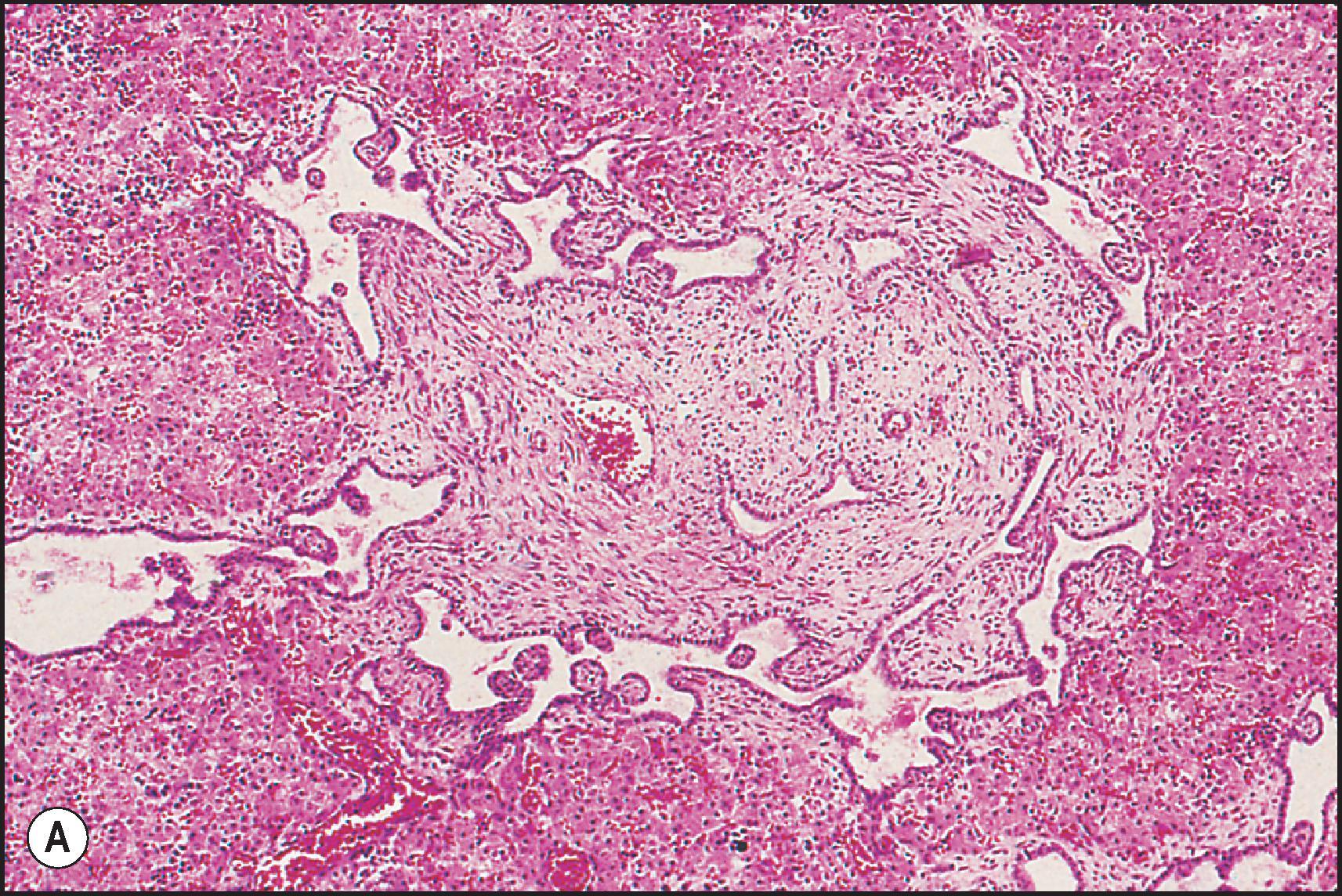
CHF is considered a variant of ARPKD affecting predominantly children and adolescents, some cases being identical to the ‘juvenile’ form. The inheritance pattern is not a simple autosomal recessive one. PKHD1 missense mutation has been identified in CHF patients with minimal kidney involvement. It may be associated with dilation of the intra- or extrahepatic bile ducts, , so-called Caroli syndrome (see next section), the intrahepatic cysts being detectable by US or magnetic resonance cholangiography (MRC). Infants present with abdominal distension from enlarged organs, respiratory distress and systemic hypertension. Older patients come to medical attention because of hepatosplenomegaly or bleeding from oesophageal varices, but asymptomatic cases have been reported. Cholangitis as a manifestation of CHF has been emphasized by Fauvert and Benhamou, who recognized four clinical forms: portal hypertensive, cholangitic, mixed portal hypertensive-cholangitic and latent. The pure cholangitic form is rare. In the mixed form, patients have recurrent bouts of cholangitis, with or without jaundice, in addition to the manifestations of portal hypertension. When present, the usual renal disease in CHF is medullary tubular ectasia, a fusiform or cystic dilation of tubules (particularly the collecting ducts). Occasionally, patients have cystic kidneys typical of adult-type polycystic disease (ADPKD), an autosomal dominant disease, and others may have nephronophthisis.
The prognosis in patients surviving beyond the neonatal period is generally good and depends on the extent of hepatic and renal disease. Death related to renal failure or uncontrollable variceal bleed is now the exception. The combination of a patent portal vein and well-preserved liver function makes patients with CHF ideal candidates for standard portosystemic shunt surgery. Follow-up examination of 16 patients who underwent portosystemic shunt surgery revealed no impairment of liver function or hepatic encephalopathy. The development of cholangiocarcinoma may have been a chance occurrence in an adult case not associated with Caroli disease. ,
Pathological descriptions of CHF have been published by many authors. , , Grossly, the liver is enlarged, has a firm to hard consistency and shows a fine reticular pattern of fibrosis; no cysts are visible to the naked eye. Although the entire liver is usually involved, occasional lobar cases of CHF are described.
Microscopically, there is diffuse periportal fibrosis, the bands of fibrous tissue varying in thickness. Irregularly shaped islands of hepatic tissue, some incorporating several lobules, may be seen ( Fig. 3.27 ). When the bands of fibrous tissue are thick, hepatic venules may be encroached on and become incorporated within the fibrous tissue; thus portal hypertension in this condition may not always be presinusoidal. The fibrous bands may encircle single or groups of lobules; occasionally, a small islet of hepatic tissue becomes separated from an acinus and encircled by fibrous tissue. Numerous uniform and generally small bile ducts are scattered in the fibrous tissue ( Fig. 3.28 ). An interrupted circular arrangement of the ducts (DPM) is often recognizable ( Fig. 3.29 ). The ducts are lined by cuboidal to low columnar epithelium and may contain bile or traces of mucin. They may be slightly dilated and irregular in outline. Cholestasis is not a feature of the uncomplicated case of CHF. Reduction in the number of portal vein branches is often apparent, the likely cause of the portal hypertension. There is generally minimal inflammation in CHF except in cases associated with cholangitis, when numerous neutrophils infiltrate ducts, ductules and surrounding connective tissue ( Fig. 3.30 ); rupture of the ducts can result in microabscess formation. Histopathologically, the latter cases may be difficult to differentiate from extrahepatic biliary obstruction with ascending infection, particularly since there may be an associated tissue cholestasis. The correct diagnosis must be based on the history, clinical findings, and the results of imaging studies. Cholestasis is particularly prominent in those cases associated with microscopic dilation of the intrahepatic bile ducts, too subtle to be seen on imaging (‘microscopical Caroli’). Recurrent cholangitis may lead to progressive fibrosis with functional impairment similar to cirrhosis.
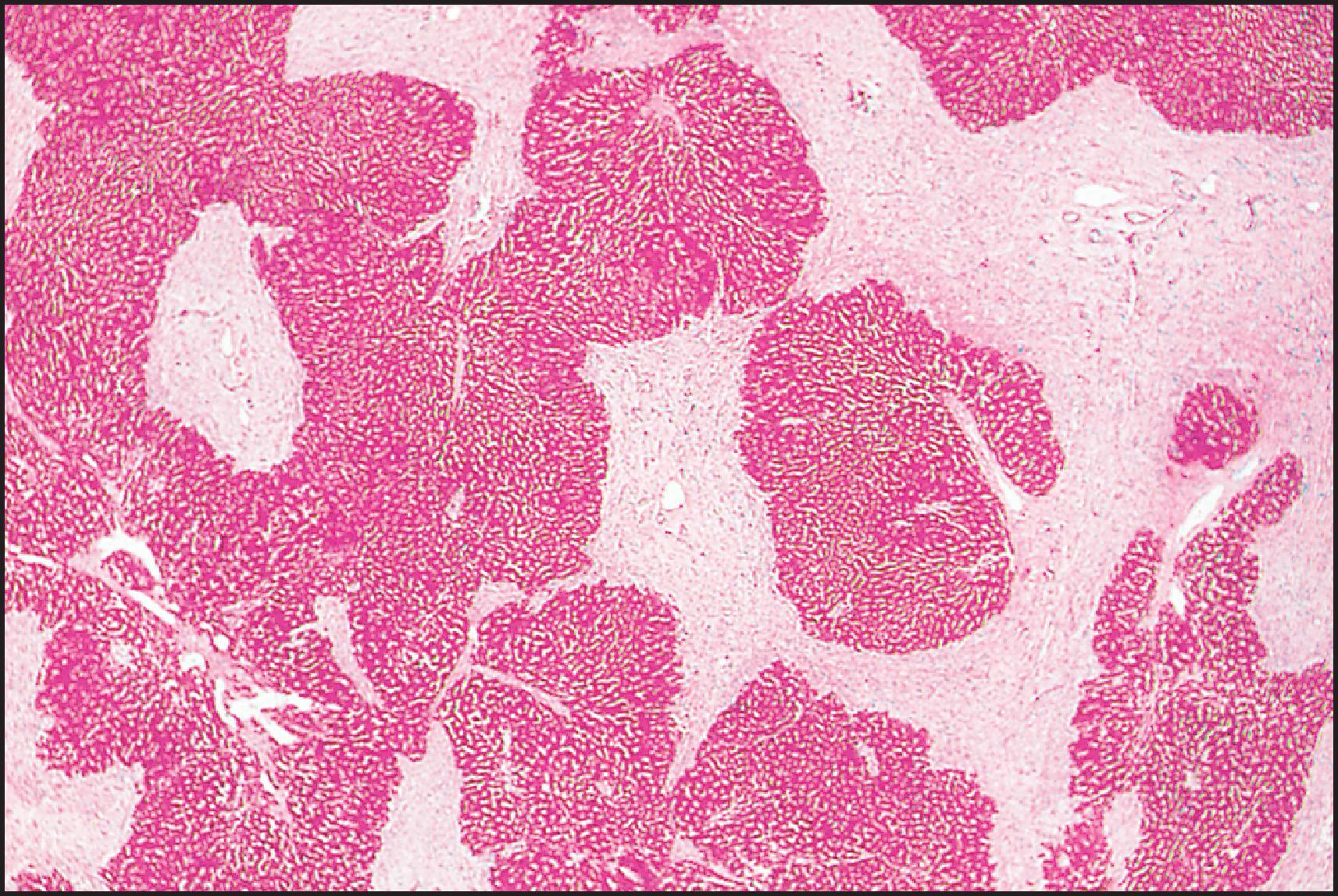
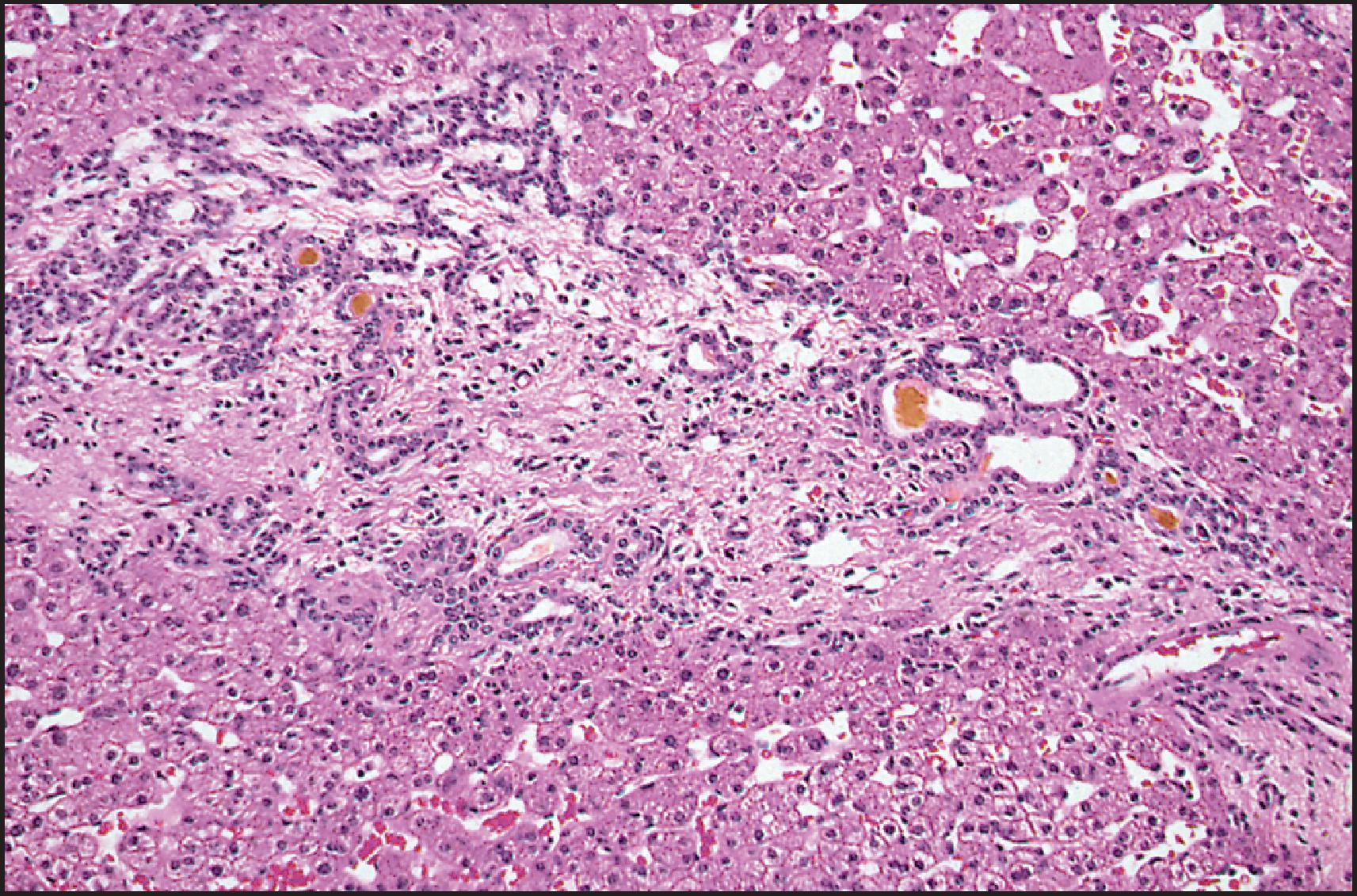
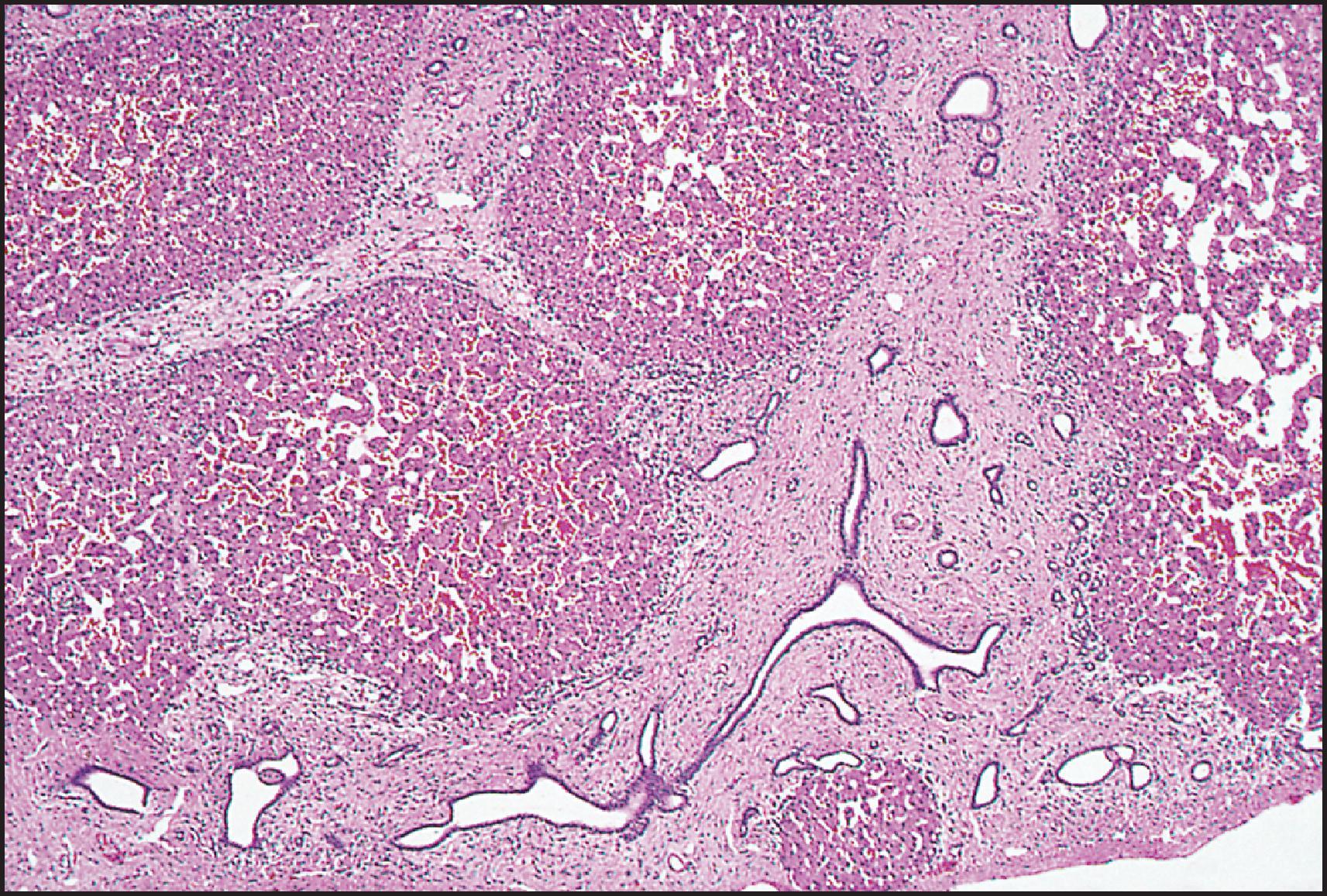
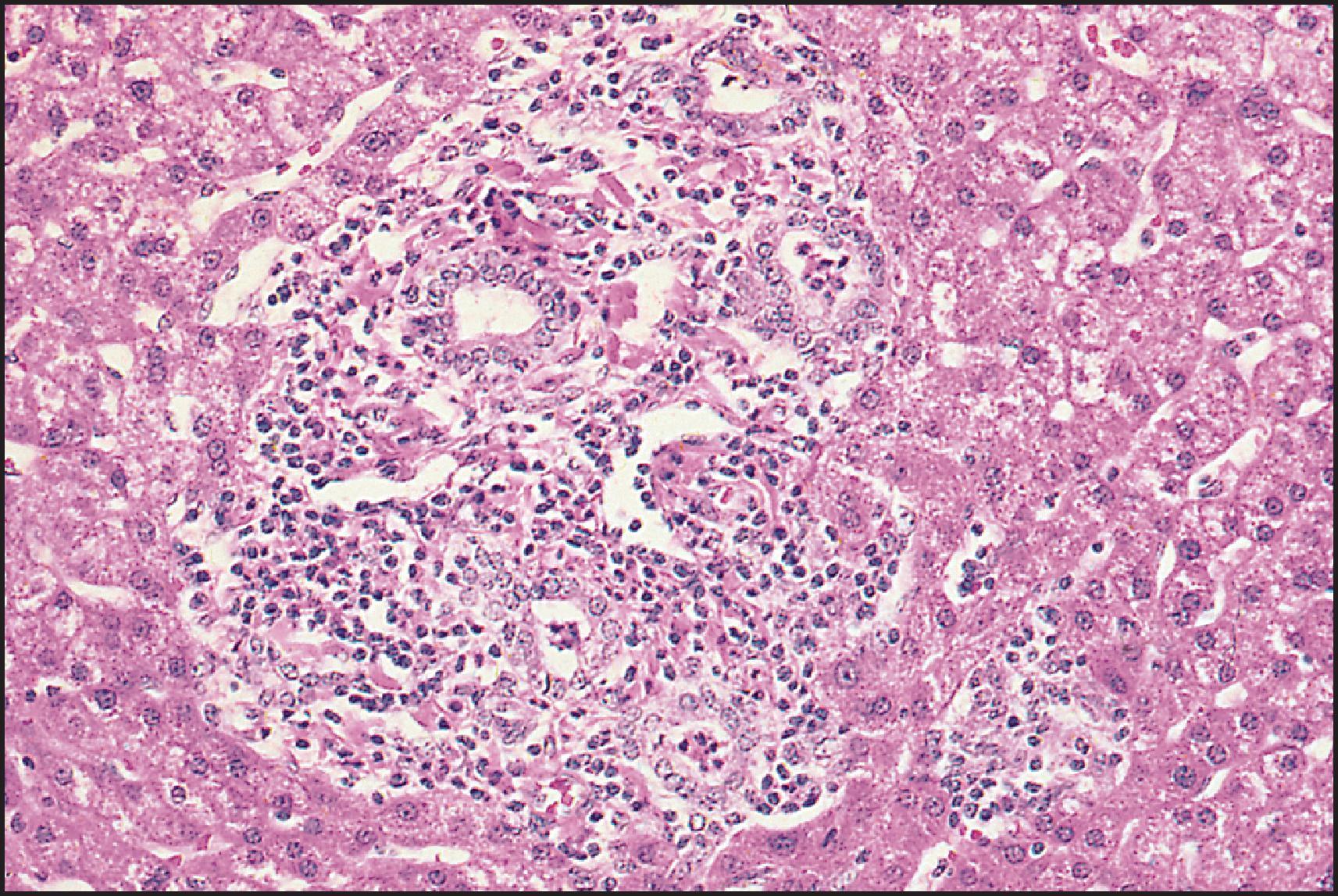
A number of malformation syndromes, characterized by hepatic morphological changes that resemble those of CHF (DPM), can be differentiated by the associated findings. Most of these are recognized as being ‘ciliopathies’ in that the defective protein is part of the ciliary apparatus. They include:
Medullary cystic kidney disease 1 (MCKD1), which has an autosomal dominant inheritance, has been mapped to chromosome 1q21.
Medullary cystic kidney disease 2 , via uromodulin (UMOD2), also autosomal dominant, mapped to chromosome 16p12.
Nephronophthisis – CHF , a recessive disorder whose gene NPHP1 has been mapped to chromosome 16p12.
Meckel syndrome , with encephalocoele, polydactyly and cystic kidneys.
Renal–hepatic–pancreatic dysplasia, with polycystic kidney disease, DPM in liver and pancreatic dysplasia, incompatible with postnatal survival; candidate genetic locus on chromosome 3.
Asplenia with cystic liver, kidney and pancreas , which represents Ivemark syndrome with variants, possibly in the same spectrum as renal–hepatic–pancreatic dysplasia.
Ellis–van Creveld syndrome or chondroectodermal dysplasia , with polydactyly, short limbs, short ribs, postaxial polydactyly and dysplastic nails and feet; mapped to chromosome 4p16.
Sensenbrenner syndrome ( cranioectodermal dysplasia) , with dwarfism and progressive tubulointerstitial nephritis.
Asphyxiating thoracic dystrophy (Jeune syndrome) , with skeletal dysplasia, pulmonary hypoplasia and retinal lesions.
Congenital disorder of glycosylation, type Ib (phosphomannose isomerase deficiency, MPI-CDG), , sometimes with protein-losing enteropathy.
Joubert syndrome, with cerebellar vermis hypoplasia/aplasia, colobomata and psychomotor retardation ; sometimes defined by the acronym COACH syndrome ( c erebellar vermis hypoplasia, o ligophrenia, a taxia, c oloboma and h epatic fibrosis), shown to carry mutation in a Meckel syndrome gene ( MKS3 ). ,
Vaginal atresia syndrome.
Tuberous sclerosis.
Caroli disease generally involves the entire liver, but it may be segmental or lobar. The inheritance is autosomal recessive. By 1982, some 99 cases had been reported; they were evaluated together with 10 personally studied cases by Mercadier et al. Since then, many other case reports, as well as small series, have been reported. , Clinically, patients suffer from bouts of recurrent fever and pain. Jaundice occurs only when sludge or stones block the CBD. Liver enzymes are generally normal except during episodes of biliary obstruction. The diagnosis is established by a variety of imaging modalities (e.g. ERCP, sonography, computed tomography [CT] and MRCP).
The complications of Caroli disease resemble those of choledochal cyst and include recurrent cholangitis, abscess formation, septicaemia, intrahepatic lithiasis and amyloidosis. Spontaneous rupture of a bile duct was reported by Chalasani et al. Adenocarcinomas, including some arising in cases with a lobar distribution, have also been reported, , with an incidence of 7%. HCC occurs rarely. Medical treatment consists of symptomatic or prophylactic treatment of cholangitis and promotion of bile flow with ursodeoxycholic acid. Surgical treatment includes internal or external drainage procedures. Transhepatic decompression has been advocated. Segmental or lobar forms of Caroli disease can be treated by partial hepatectomy. , Extracorporeal shock wave lithotripsy has been used for disintegration of intrahepatic stones. Liver or combined liver and kidney transplantation has been successfully performed in patients with mono- and multilobar disease with portal hypertension, with 96 and 8 receiving liver or combined liver and kidney grafts, respectively, from 1987 to 2006 in the United States ; the authors propose an algorithm for evaluation and treatment of Caroli disease. More recent experience has been reported. ,
Macroscopically, the intrahepatic cystic dilations are round or lanceolate, 1.0–4.5 cm in diameter, and may be separated by stretches of essentially normal duct ( Fig. 3.31 ). Transluminal fibrovascular bridges are reminiscent of the periportal location of the cysts (ductal plate) and explain in part the central dot sign observed on CT. Inspissated bile or soft and friable bilirubin calculi may be found in the lumen. Microscopically, the dilated ducts usually show severe chronic inflammation, with or without superimposed acute inflammation, and varying degrees of fibrosis ( Fig. 3.32 ). The epithelium may appear normal (cuboidal to tall columnar), partly or completely ulcerated or focally hyperplastic; all these changes can be found in different ducts in the same liver ( Fig. 3.33 ). Mucous glands (sometimes in abundance) may be present in the fibrotic and inflamed wall. Areas of severe epithelial dysplasia are seen rarely. The lumen contains admixtures of inspissated mucin and bile, calcareous material or frank pus during bouts of acute cholangitis ( Fig. 3.34 ). Caroli disease is frequently associated with CHF (in which case it is termed Caroli syndrome ), rarely with infantile polycystic disease (ARPKD) and even adult polycystic disease (ADPKD).
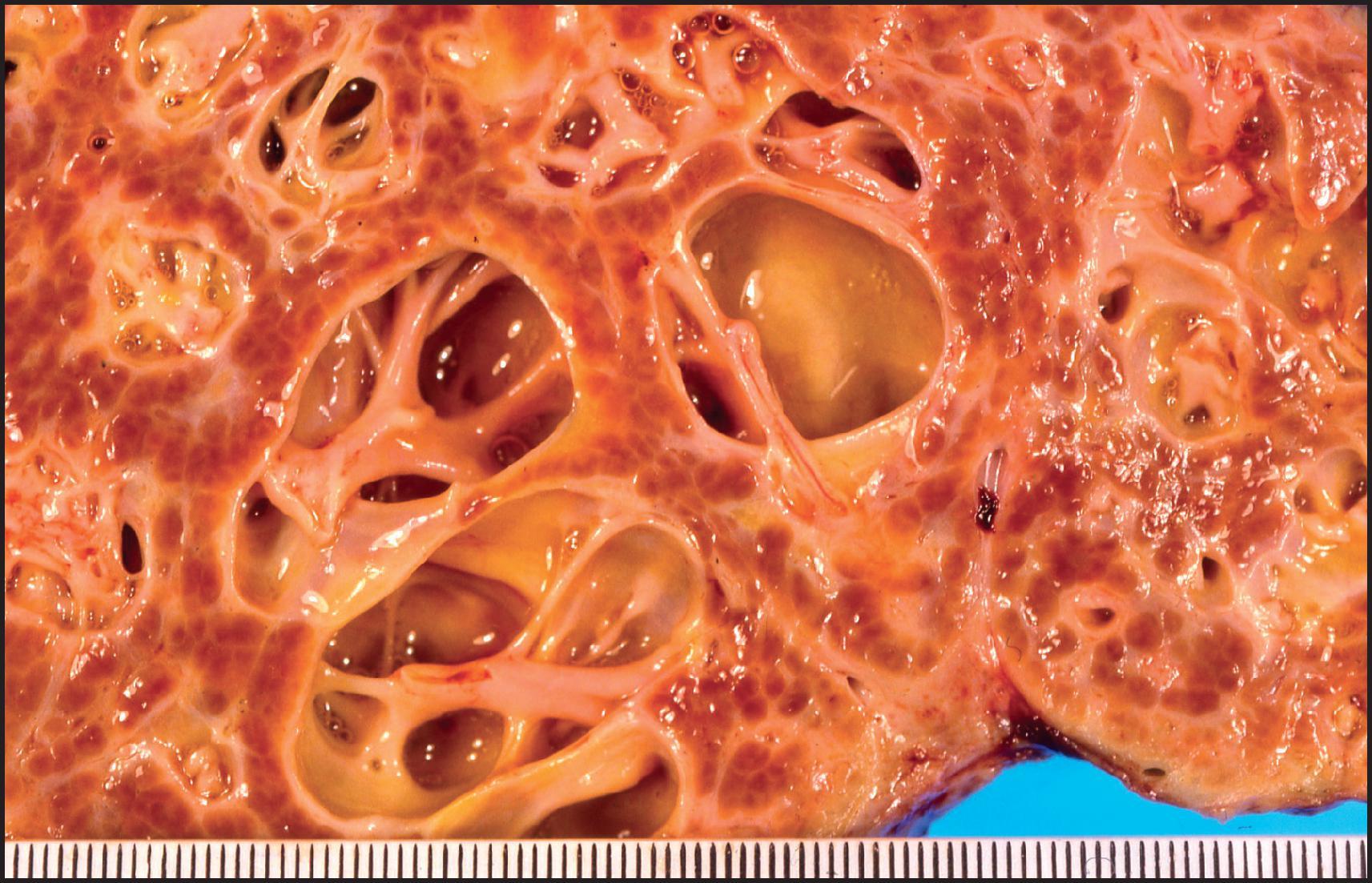
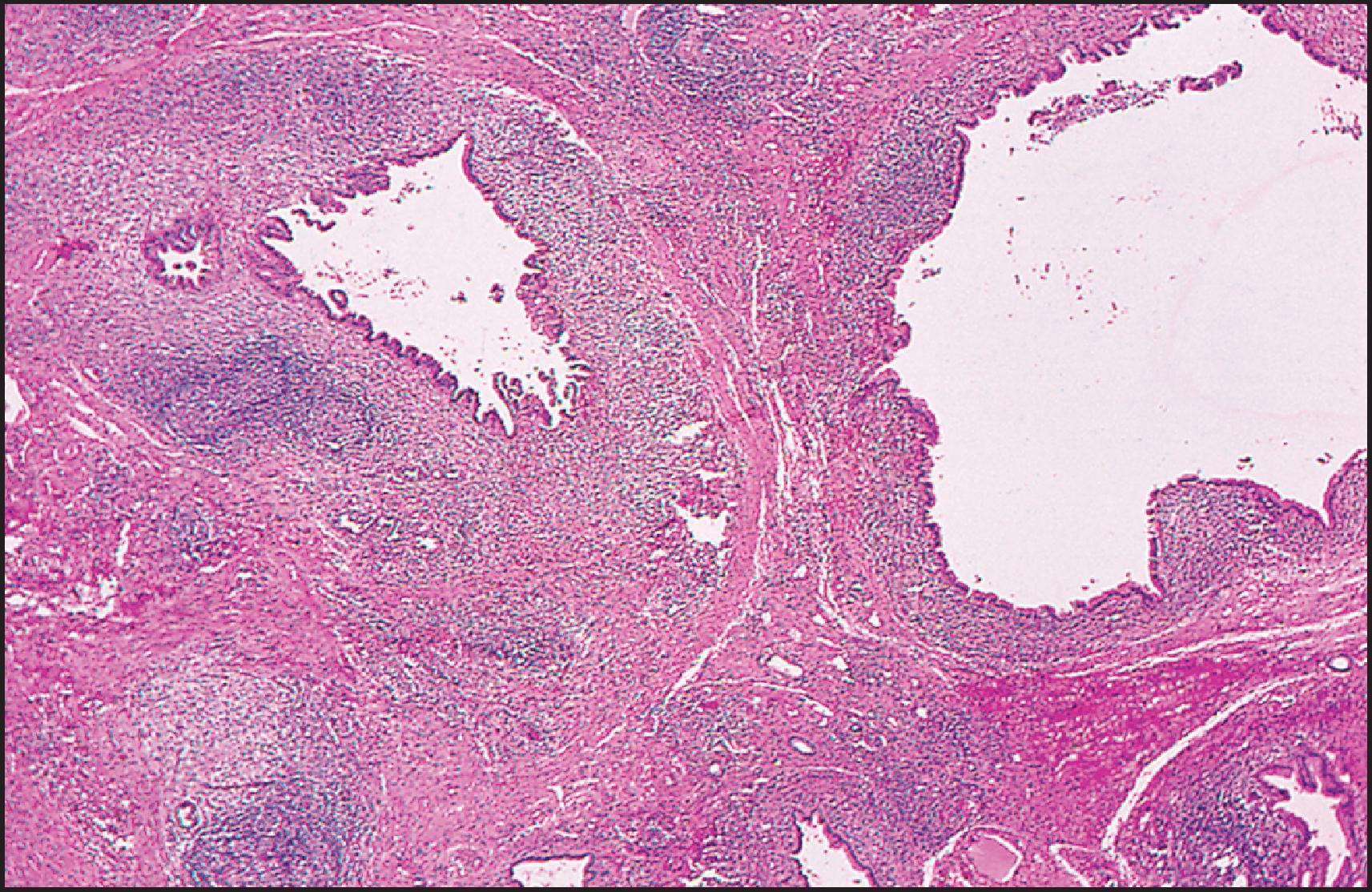
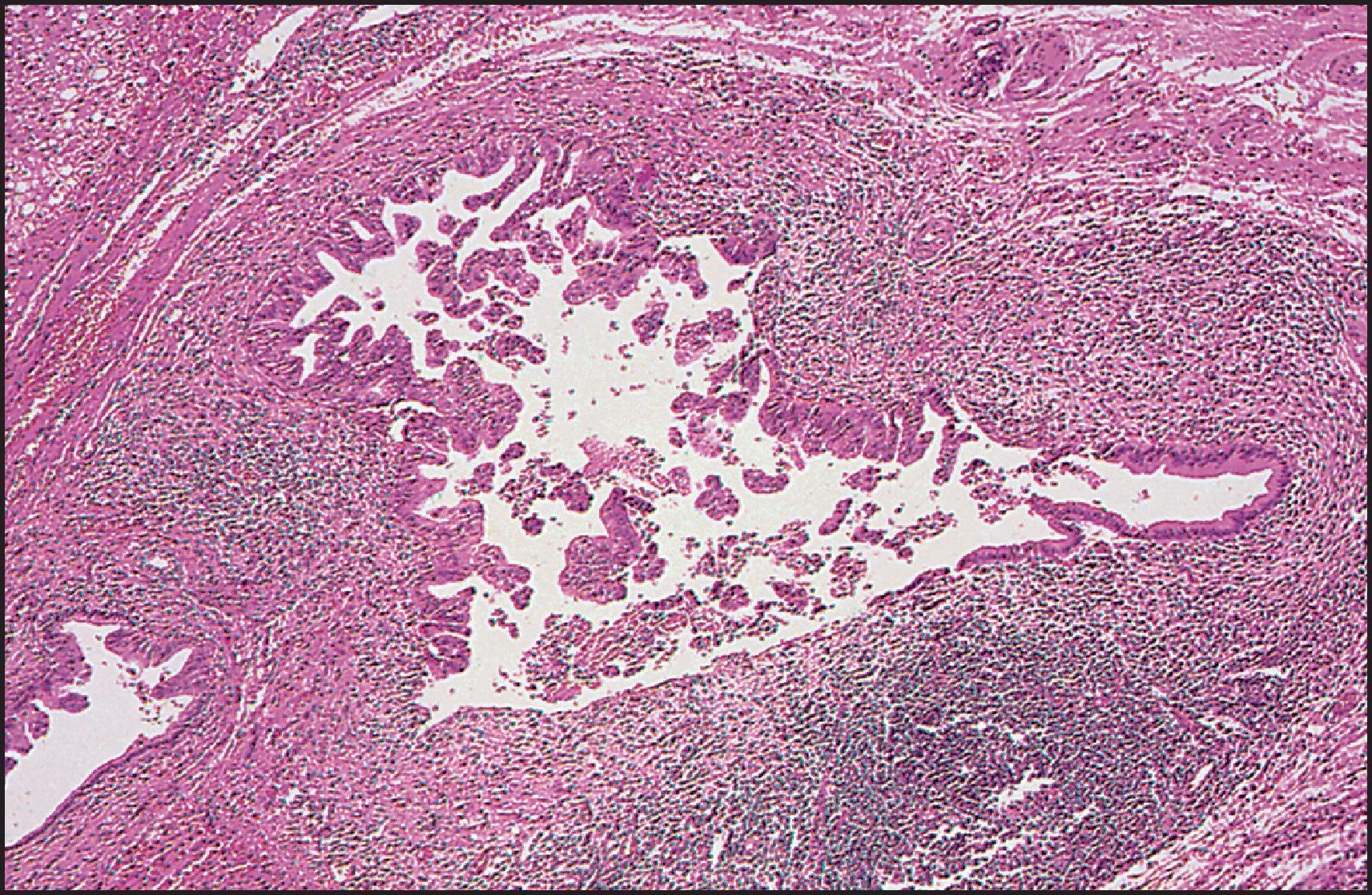
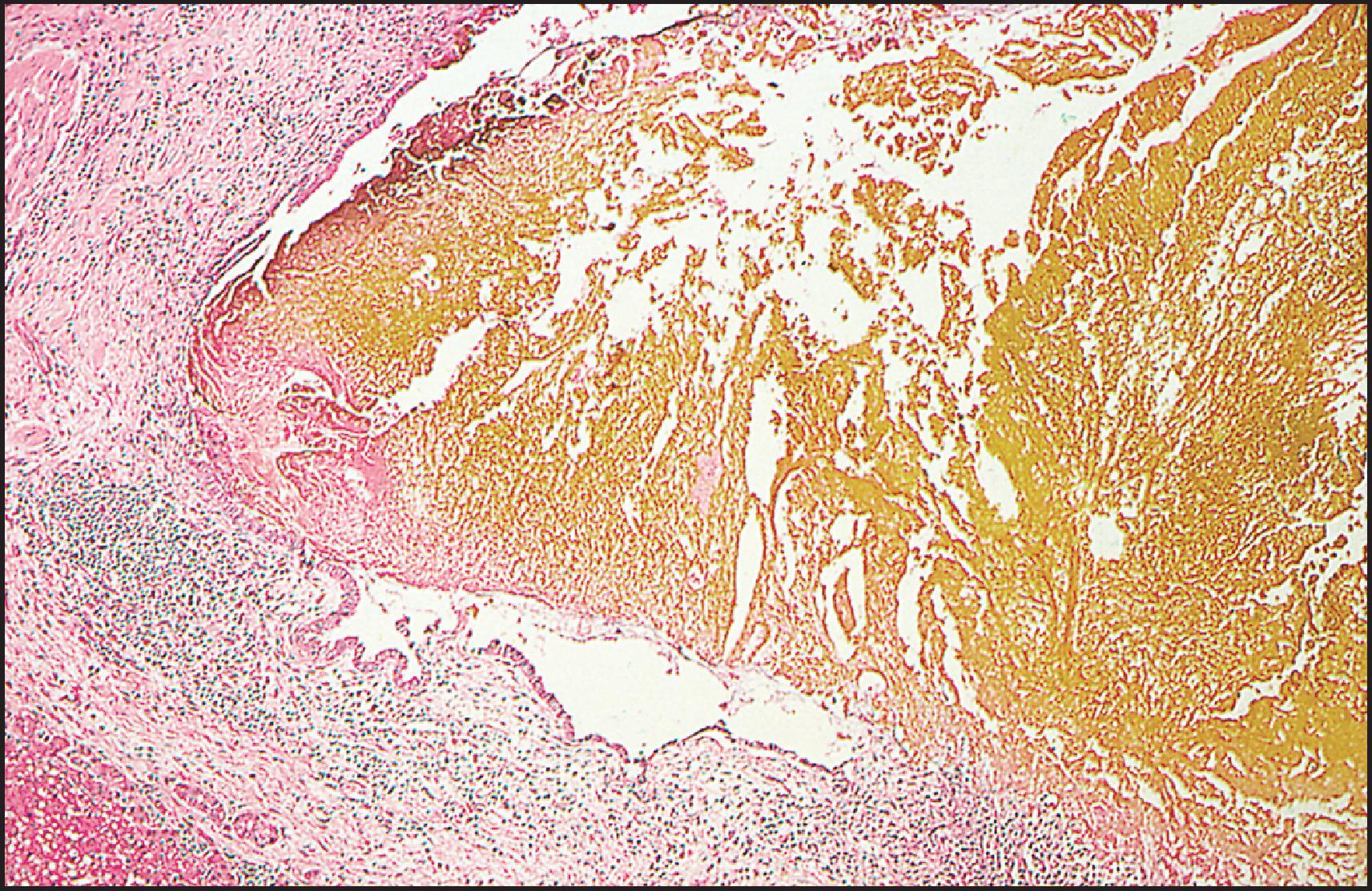
There is a single case described of ‘Marfan syndrome with diffuse ectasia of the biliary tree’. The authors suggested that the defect of connective tissue in that disease could have led to weakness of the wall of the bile ducts with resultant ectasia.
Mutations mainly in two different genes, PKD1 and PKD2 , can lead to ADPKD, one of the most common genetic disorders worldwide. The PKD1 gene lies on the short arm of chromosome 16 (16p 13.3), immediately adjacent to the TSC2 , a gene responsible for approximately 50% of tuberous sclerosis. , The incidence of ADPKD due to mutations in PKD1 is 1:1000 live births, comprising 90% of cases. The PKD1 gene encodes a protein called ‘polycystin’. It is present in plasma membranes of renal tubular cells, bile ductules, pancreatic ducts, hepatocytes and cells of large bile ducts. In the kidney, localization at the contact points (only between neighbouring cells) indicates that its main function is cell-to-cell interaction, such that loss of function contributes to cyst formation. The PKD2 gene is located on chromosome 4 (4q 21–23) and encodes a 100-kilodalton (kD) protein, polycystin 2, with sequence homology to α-subunits of voltage-activated calcium channels. As mentioned earlier, localization of polycystin 1 and polycystin 2 to primary cilia in cultured renal epithelial cells and demonstration that the proteins function as a ciliary flow-sensitive mechanosensors implicate defects in ciliary structure and function as an important mechanism of cyst formation. There is at least one unmapped locus, which accounts for 5% of the disease population. Although PKD2 is clinically milder than PKD1, it does have a deleterious impact on overall life expectancy and cannot be regarded as a benign disorder. The mean age to death or end-stage renal disease is 53 years in PKD1-associated disease, 69 years in PKD2-associated disease and 78 years in controls. PKD2 patients are less likely to have systemic hypertension, urinary tract infections and haematuria. Cerebral aneurysms are found in 10–15% of patients with PKD1 and represent a significant risk of subarachnoidal haemorrhage causing death and disability. Familial clustering of bleeding from intracranial aneurysms is recorded. In an earlier mutational analysis of 58 ADPKD families with vascular complications, 51 were PKD1 (88%) and 7 were PKD2 (12%). The authors found that the position of the mutation in PKD1 is predictive for the development of intracranial aneurysms (59 mutations are more commonly associated with vascular disease) and is therefore of prognostic importance. A de novo WT1 splice site variant was identified in addition to a PKD1 missense variant in a patient with a maternal history of ADPKD.
ADPKD is a multisystem disease with cysts and connective tissue abnormalities involving multiple organs. Associated conditions include colonic diverticula (70%), cardiac valve complications (25%), ovarian cysts (40%), inguinal hernia (15%) and intracranial aneurysms (10%), suggesting a diffusely abnormal matrix. Mitral valve prolapse was found in 12% of affected children. ADPKD is the cause of end-stage renal disease in 8–10% of adults; the number of cysts is age related. The renal disease can be present at birth, but hepatic manifestations are rare before adolescence.
The typical hepatic disorder in ADPKD is polycystic liver disease. The average age at first admission for liver-related problems was 52.8 years, with an average duration of symptoms of 3 years. The symptoms included a gradually enlarging abdominal mass, upper abdominal pain or discomfort and rare episodes of severe pain with or without nausea, vomiting and occasionally fever. The most frequent physical finding is hepatomegaly, which can be massive. Liver tests are often normal. Jaundice is unusual. , Portal hypertension is rare but may occur from hepatic outflow obstruction. , There is an increased risk of gallstones in patients with hepatic cysts. Rarely, treatment is needed by excision or a combination of excision and fenestration. Additionally, it has become evident that CHF may occur with ADPKD; it becomes clinically evident in childhood or adolescence and may lead to portal hypertension. Liver or combined liver and renal transplantation has been performed successfully in patients with ADPKD.
The incidence of liver involvement and its complications has been reviewed in a large series of 132 patients receiving and 120 patients not receiving haemodialysis. Liver cysts were found by noninvasive imaging procedures in 85 of 124 patients on dialysis; gender distribution was equal. In contrast, the nondialysed population demonstrated a 75% incidence of liver cysts in females and 44% incidence in males, the peak incidence occurring 10 years earlier in females. The cysts were larger and greater in number in the nondialysed females, and there was a correlation with the number of pregnancies. Nineteen autopsies were reported in which five deaths were liver related. Risk factors for the development of hepatic cysts in ADPKD were also examined in 39 patients and 189 unaffected family members by Gabow. The hepatic expression of the disease was found to be modulated by age, female gender, pregnancy and severity of the renal lesion and functional impairment. When compared with patients with PCLD without kidney abnormalities, young ADPKD female patients showed a more severe phenotype expressed as height-adjusted total liver volume. Oestrogen treatment of postmenopausal women with ADPKD is associated with selective liver enlargement and abdominal symptoms.
Although the leading complication in ADPKD is infection of the liver cysts, cholangiocarcinoma is the second most common complication. A study examining hepatic cyst infection suggested that the incidence increases from 1% to 3% during end-stage renal failure. Enterobacteriaceae were cultured from the infected cysts in 9 of 12 patients. In the case of Ikei et al., Pseudomonas aeruginosa was cultured from the infected cysts. Positron emission tomography (PET) scan contributes to making diagnosis of cyst infections more readily and more accurately. This may allow early treatment with appropriately selected antibiotics; the timely drainage of large infected cysts remains an option.
Hepatic cysts are rarely detected before puberty and increase with age (ultimately to 75%) in individuals over 70. However, cysts have been found in early childhood and even in the first year of life. , In the absence of molecular genetic diagnosis, the criteria for identification of this disorder in children include a positive paternal history, cysts in any portion of the renal tubule or Bowman space, macroscopic cysts in the liver and cerebral aneurysms. Molecular diagnosis is useful in individuals in whom the diagnosis of ADPKD is uncertain due to a lack of family history or equivocal imaging results and in younger at-risk individuals who are being evaluated as living-related kidney donors.
Grossly, the liver in ADPKD is enlarged and diffusely cystic, the cysts varying from <1 mm to 12 cm or more in diameter ( Fig. 3.35 ). One liver reported by Kwok and Lewin weighed 7.7 kg. Occasionally, only one lobe, usually the left, is affected. Diffuse dilation of the intra- and extrahepatic bile ducts has been reported in some cases. The cysts contain a clear, colourless or yellow fluid. Analysis of cyst fluid in one case disclosed similarities to the ‘bile salt-independent’ fraction of human bile, suggesting that such cysts are lined by a functioning secretory bile duct epithelium.
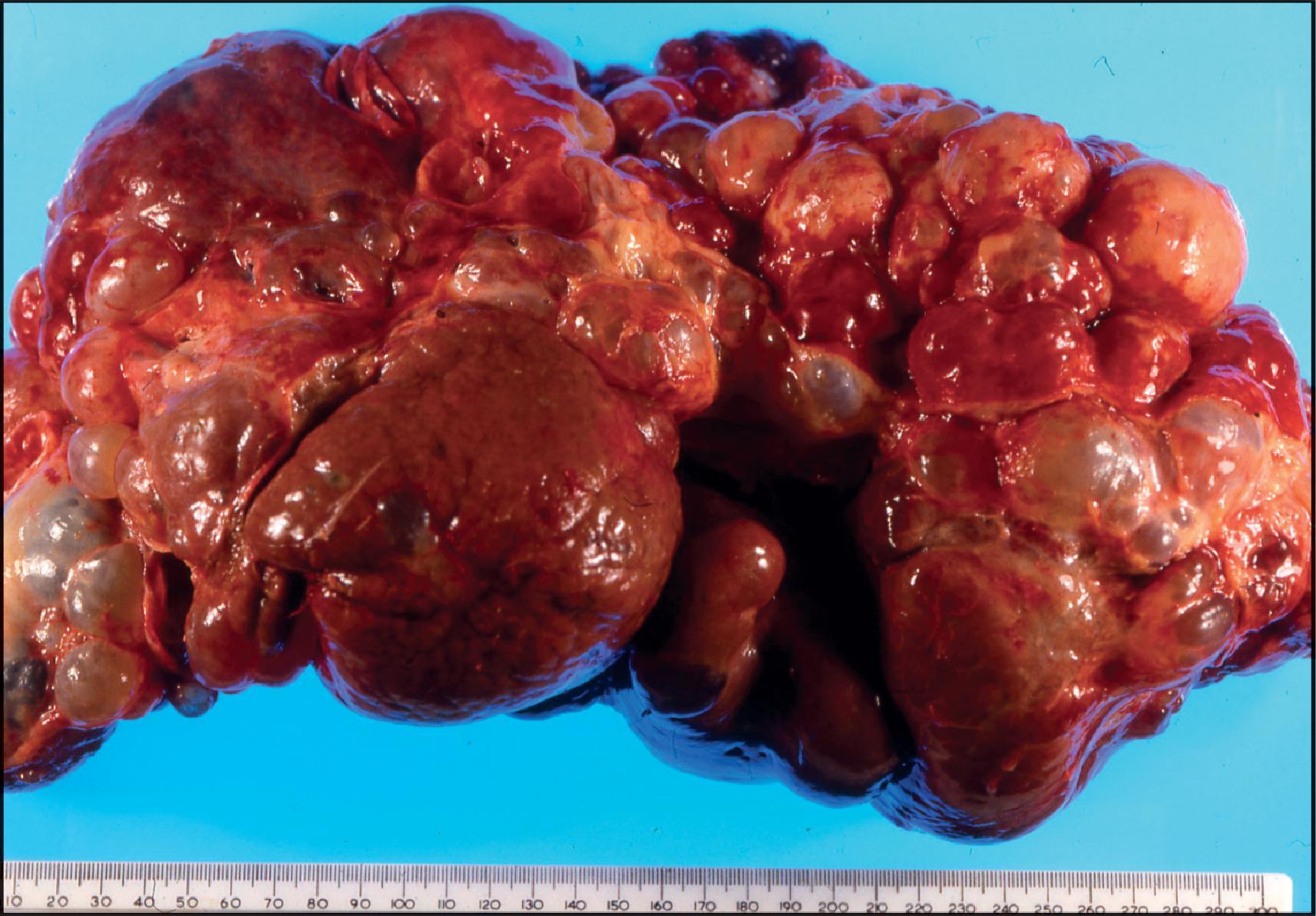
Microscopically, the cysts are lined by columnar or cuboidal epithelium, but the larger cysts have a flat epithelium ( Fig. 3.36 ). Collapsed cysts resemble corpora atretica of the ovary ( Fig. 3.37 ). The supporting connective tissue is scanty except in relation to von Meyenburg complexes (see Fig. 3.36 ), a frequently associated lesion, where it may be dense and hyalinized. A small number of inflammatory cells, usually lymphocytes, may infiltrate the supporting stroma. Infected cysts contain pus and may rupture ( Fig. 3.38 ). Calcification of the wall of hepatic (and renal) cysts in ADPKD has been reported.
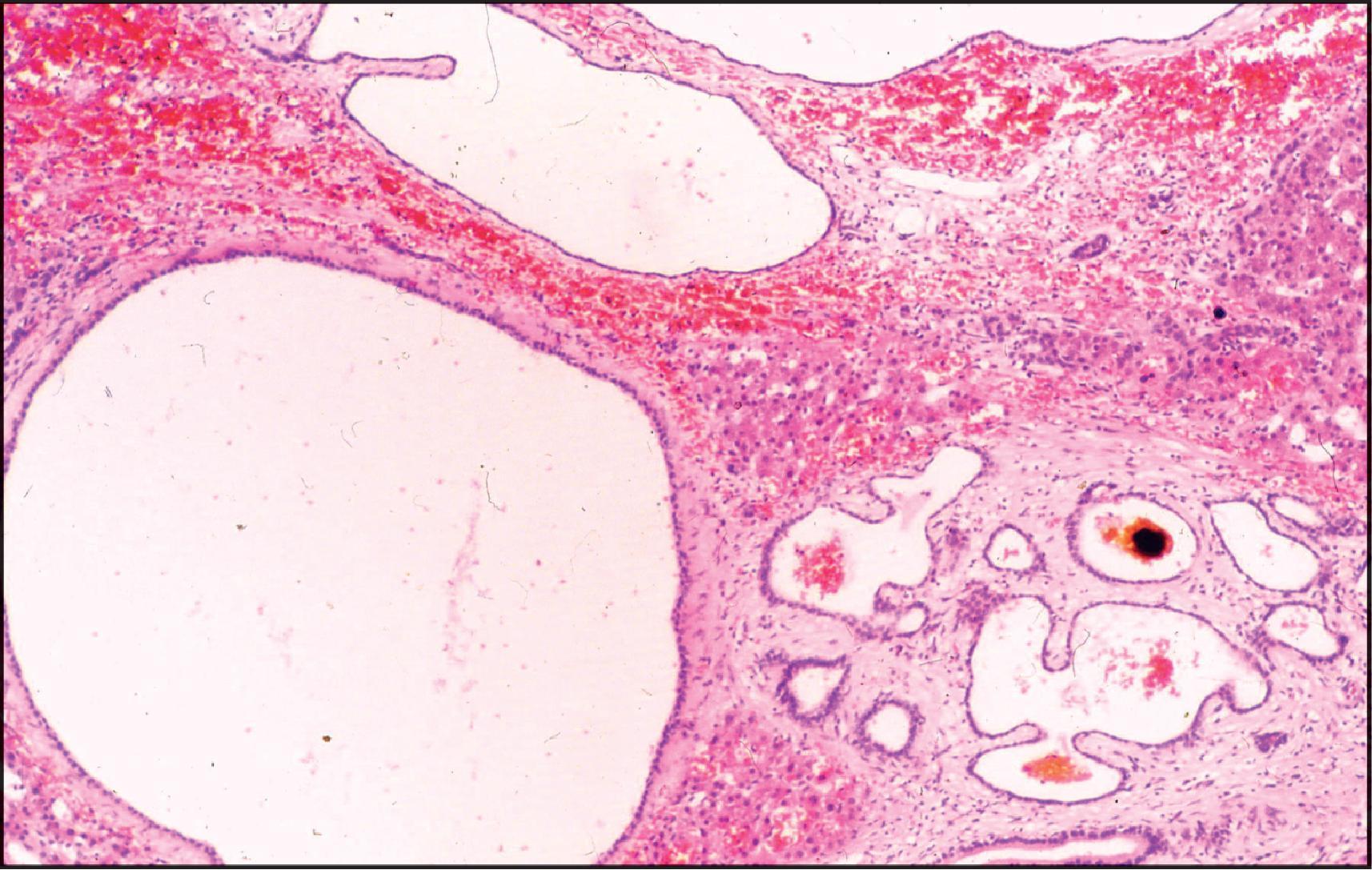
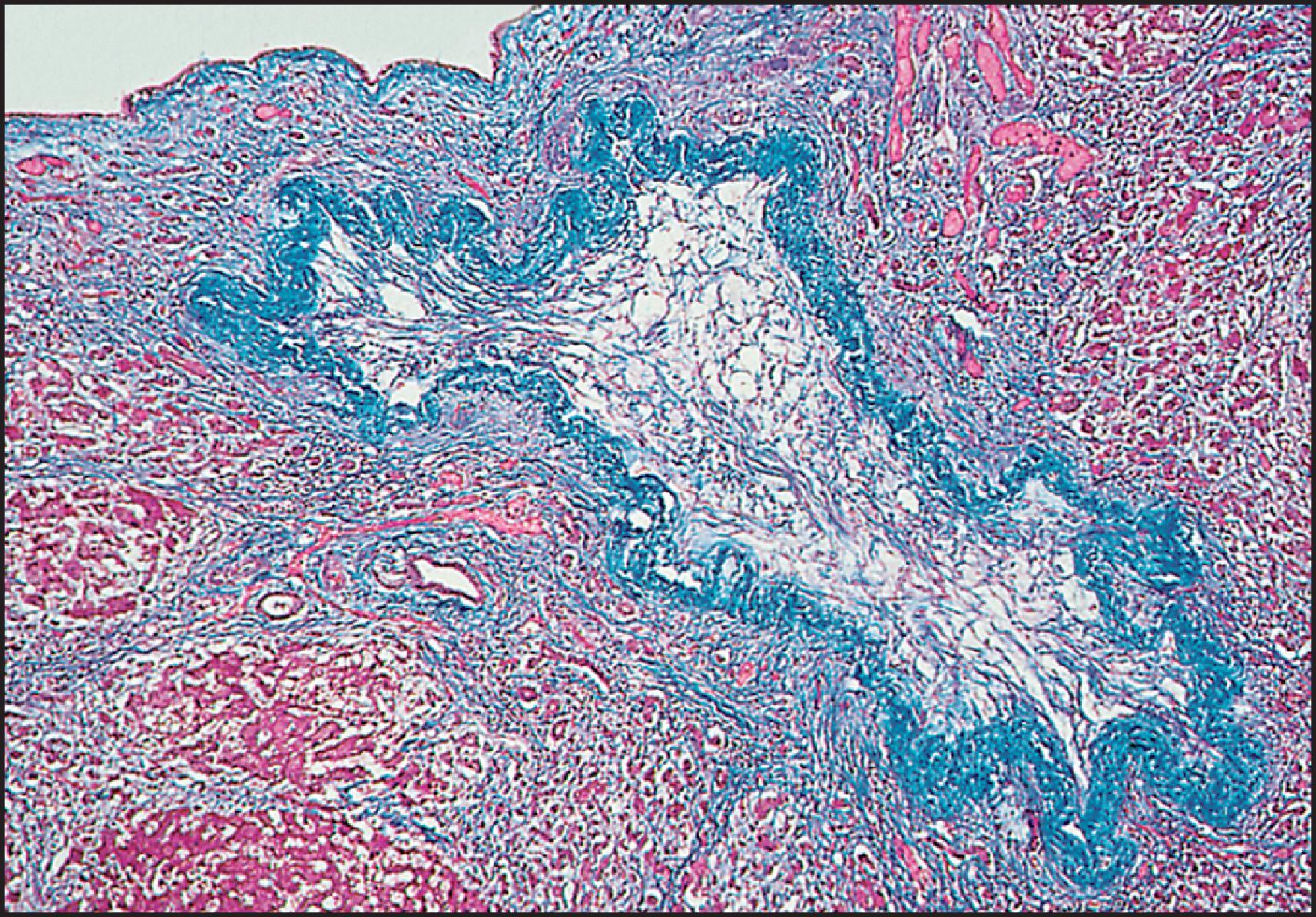
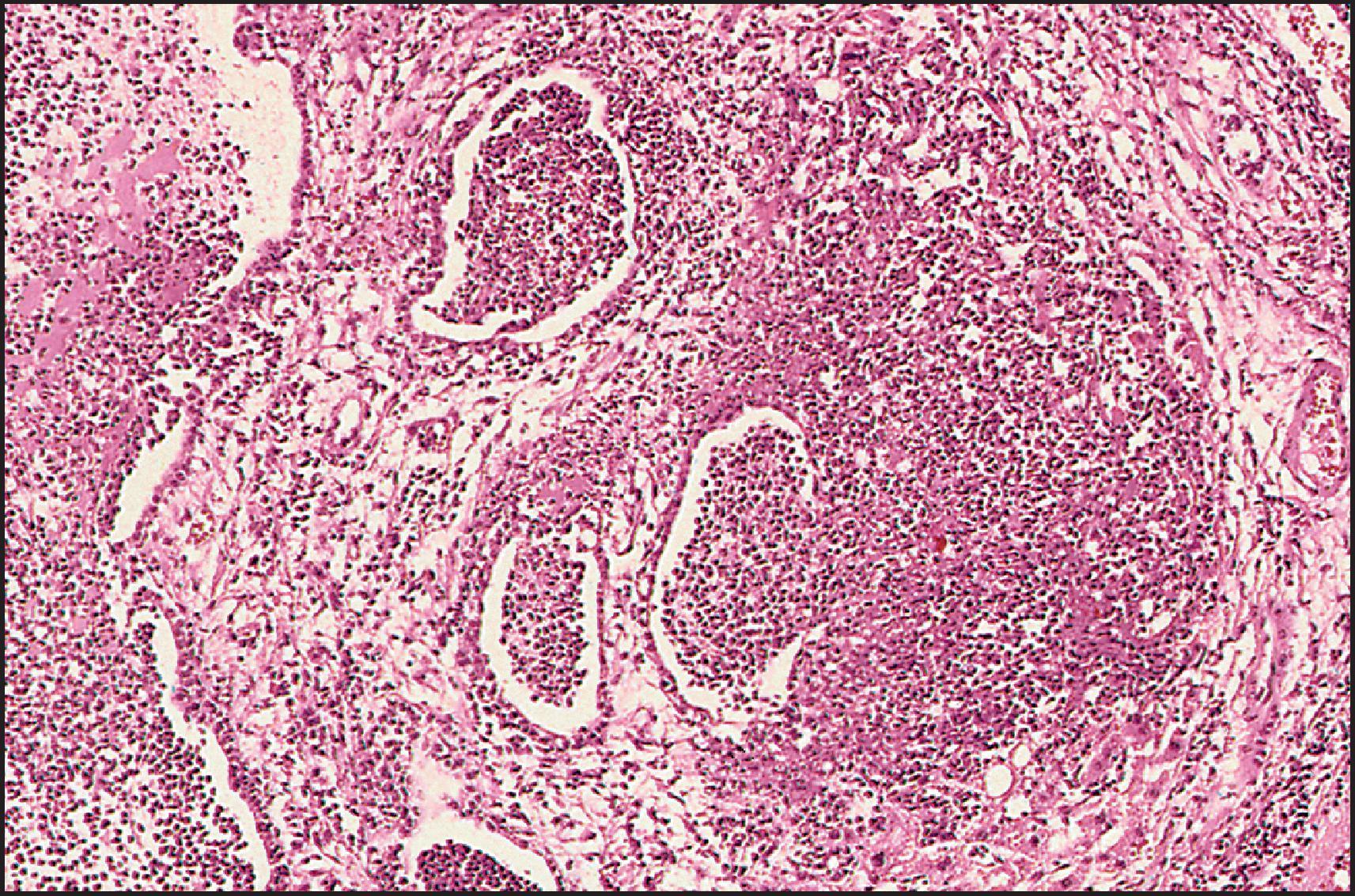
Von Meyenburg complexes are considered part of the spectrum of adult polycystic disease, and Melnick believed that polycystic disease of the liver develops progressively over years by gradual cystic dilation of these complexes. This view is supported by a histomorphometric and clinicopathological study of 28 cases of ADPKD reported by Ramos et al. Kida et al. have suggested that cystic dilation of peribiliary glands also may lead to formation of the cysts in ADPKD. Von Meyenburg complexes are small (<0.5 cm in diameter), greyish white or green and usually scattered in both lobes. They have an abnormal vascular pattern in angiographic studies and are occasionally associated with cavernous haemangiomas. Microscopically, the lesions are discrete, round to irregular in shape and typically periportal in location. The constituent ducts or ductules are embedded in a collagenous stroma, are round or irregular in shape and have a slightly dilated lumen ( Fig. 3.39 ) (see Chapter 13 ). They are lined by low columnar or cuboidal epithelium and contain pink amorphous material that may be bile stained or actual bile ( Fig. 3.40 ). CHF has been found in some cases. , Cholangiocarcinomas have been reported in association with von Meyenburg complexes, , as well as with multiple hepatic cysts considered part of the spectrum of ADPKD. , Intraductal papillary-mucinous neoplasm of the pancreas has been reported.
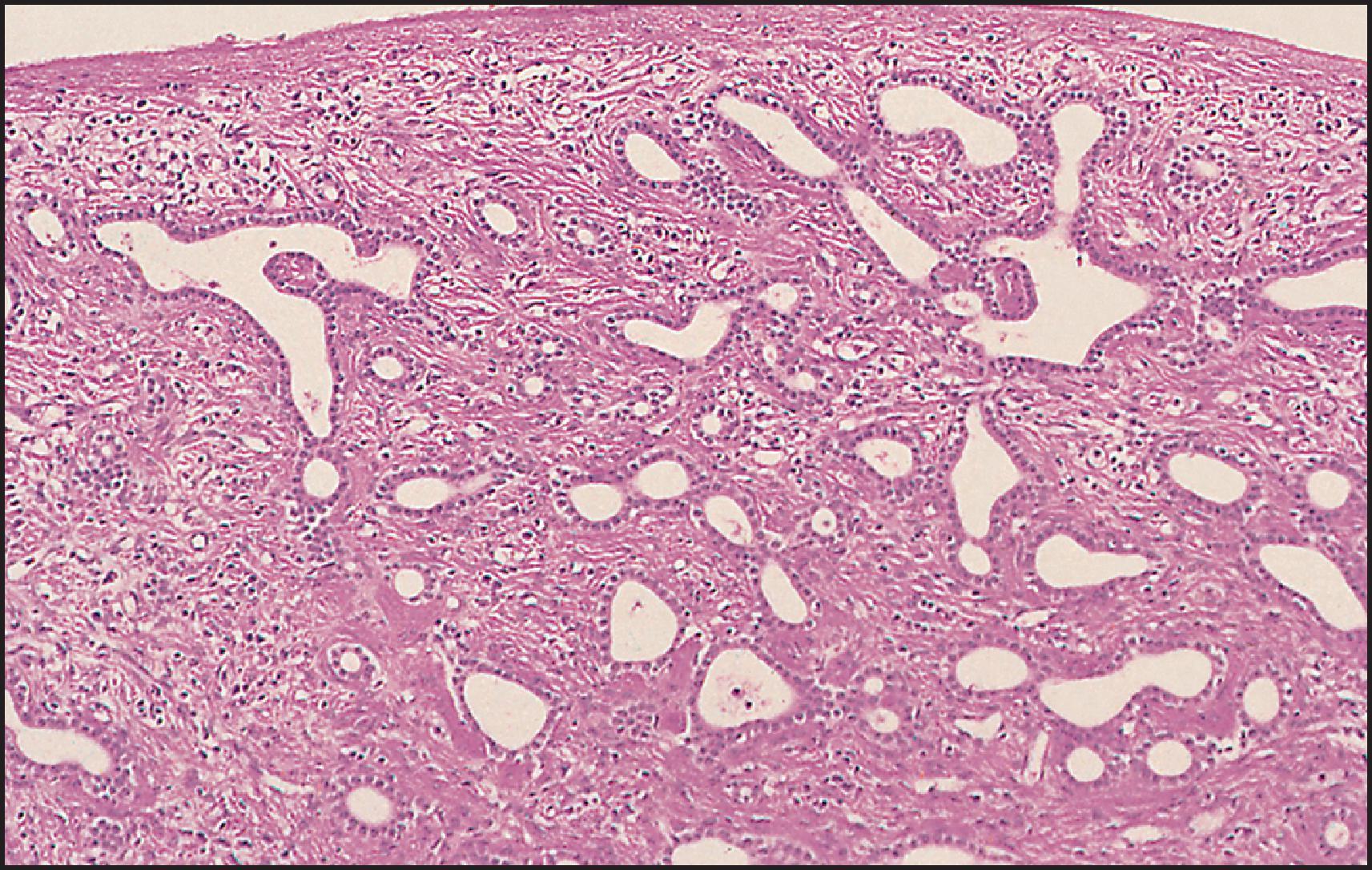
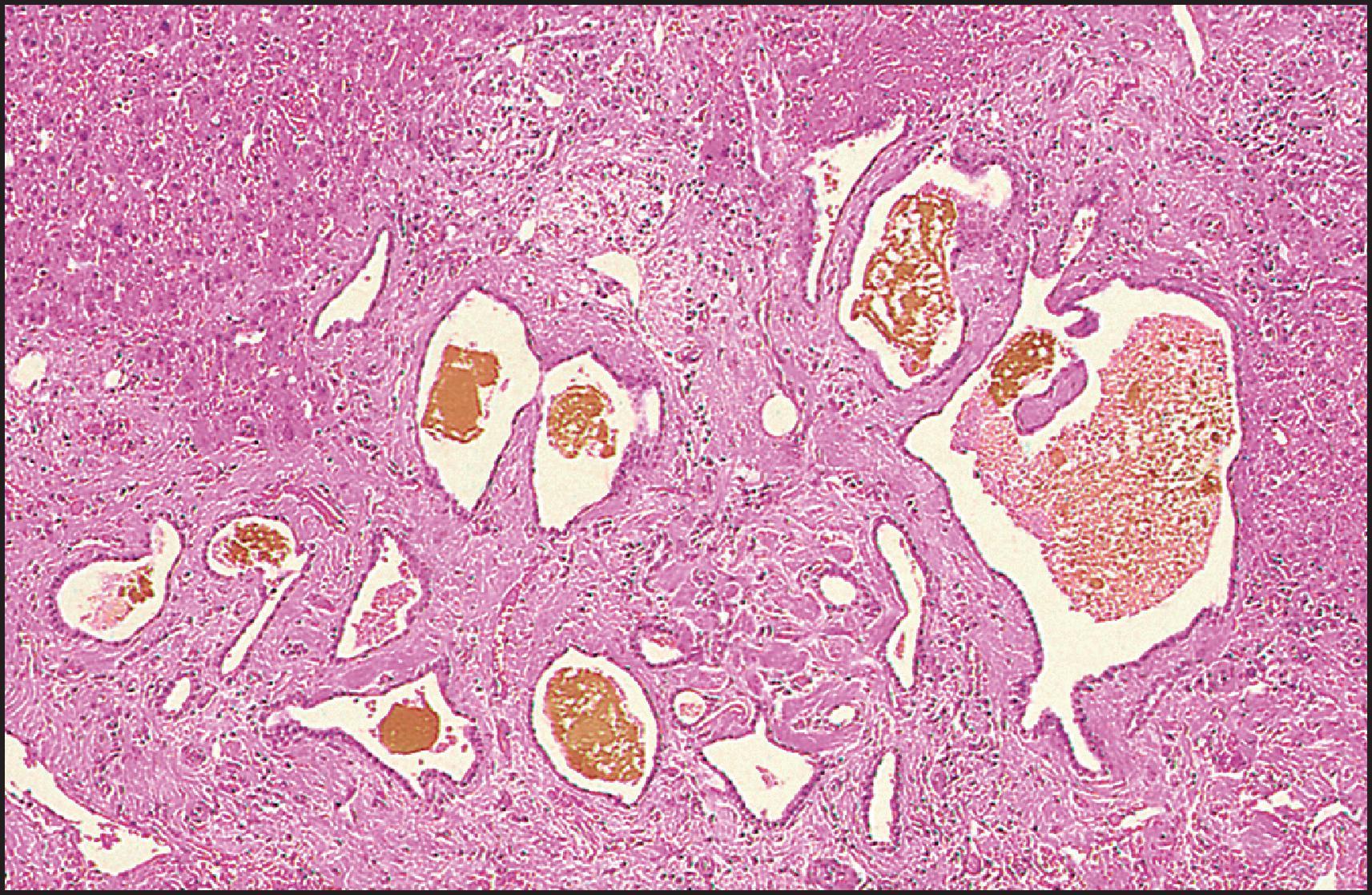
Isolated PCLD, not linked to PKD1 or PKD2 , has been reported. Germline mutations in protein kinase C substrate 80K-H gene ( PRKCSH ), a known gene encoding for a previously described human protein, ‘noncatalytic β-subunit of glucosidase II’ (GIIβ), have been associated with ADPKD. This protein, now renamed hepatocystin , segregates in some families with PCLD. The protein is widely distributed in tissues and predicted to be an ER luminal protein that recycles from the Golgi complex. The mutations found in PCLD are all predicted to cause premature chain termination, rendering loss-of-function changes most likely. The two-hit hypothesis for cyst formation in ADPKD appears applicable to PCLD. Glucosidase II plays a major role in the regulation of proper folding and maturation of glycoproteins. Polycystin 1, polycystin 2 and fibrocystin/polyductin are all glycoproteins. Mutations could compromise the processing of the N-linked oligosaccharide chains of the newly synthesized glycoproteins. Improper association and trafficking of the polycystins due to defective glycosylation by mutant GIIβ may link PCLD to the ADPKD pathway. This would be consistent with the marked similarity in clinical liver disease in both conditions. The second gene involved in PCLD, SEC63 , encodes a protein that is part of the multicomponent translocon of the ER (SEC63p), which is required in both the post-translational and the co-translational (or signal recognition particle-dependent) targeting pathway and may explain a functional link between the two genes known to be associated with PCLD. Respective lack of expression of hepatocystin and SEC63p in PCLD cyst lining appears to correlate with mutational results. Immunohistochemical analysis of cyst lining suggests that the disease involves overexpression of growth factor receptors and loss of adhesion, but proliferation or deregulated apoptosis does not seem to be implicated in PCLD. Differential findings for PRKCSH - and SEC63 -PCLD suggest a divergent mechanism for cystogenesis in the two groups. A recent study has identified mutations in the low-density lipoprotein (LDL) receptor-related protein 5 ( LRP5 ) gene in patients with PCLD. Other genes associated with PCLD are GANAB , ALG8 and SEC61B .
Solitary bile duct cyst is defined as a unilocular cyst lined by a single layer of columnar or low cuboidal epithelium resting on a basement membrane and a layer of fibrous tissue. The cysts occur at all ages, although the majority present in the fourth to sixth decade. They are rare in the paediatric age group; in the Boston Children’s Hospital series, 31 solitary nonparasitic cysts (26 unilocular, 5 multilocular) were diagnosed in 63 years. The female/male ratio is 4:1. Cysts smaller than 8–10 cm rarely cause symptoms. When present, symptoms include fullness or an upper abdominal mass, nausea and occasional vomiting. Rapid enlargement has been reported in infancy. Jaundice occurs infrequently. An acute abdominal crisis may result from torsion, strangulation, haemorrhage into the cyst or rupture. Diagnosis is usually established by sonography, CT or other imaging modalities. Solitary bile duct cysts involve the right lobe twice as often as the left. Rarely, they arise in the falciform ligament. They are usually round and rarely are pedunculated; the lining is typically smooth ( Fig. 3.41 ). The larger ones may contain one to several litres of fluid which is usually clear, but may be mucoid, purulent (if the cyst is infected), haemorrhagic or rarely bile-stained.
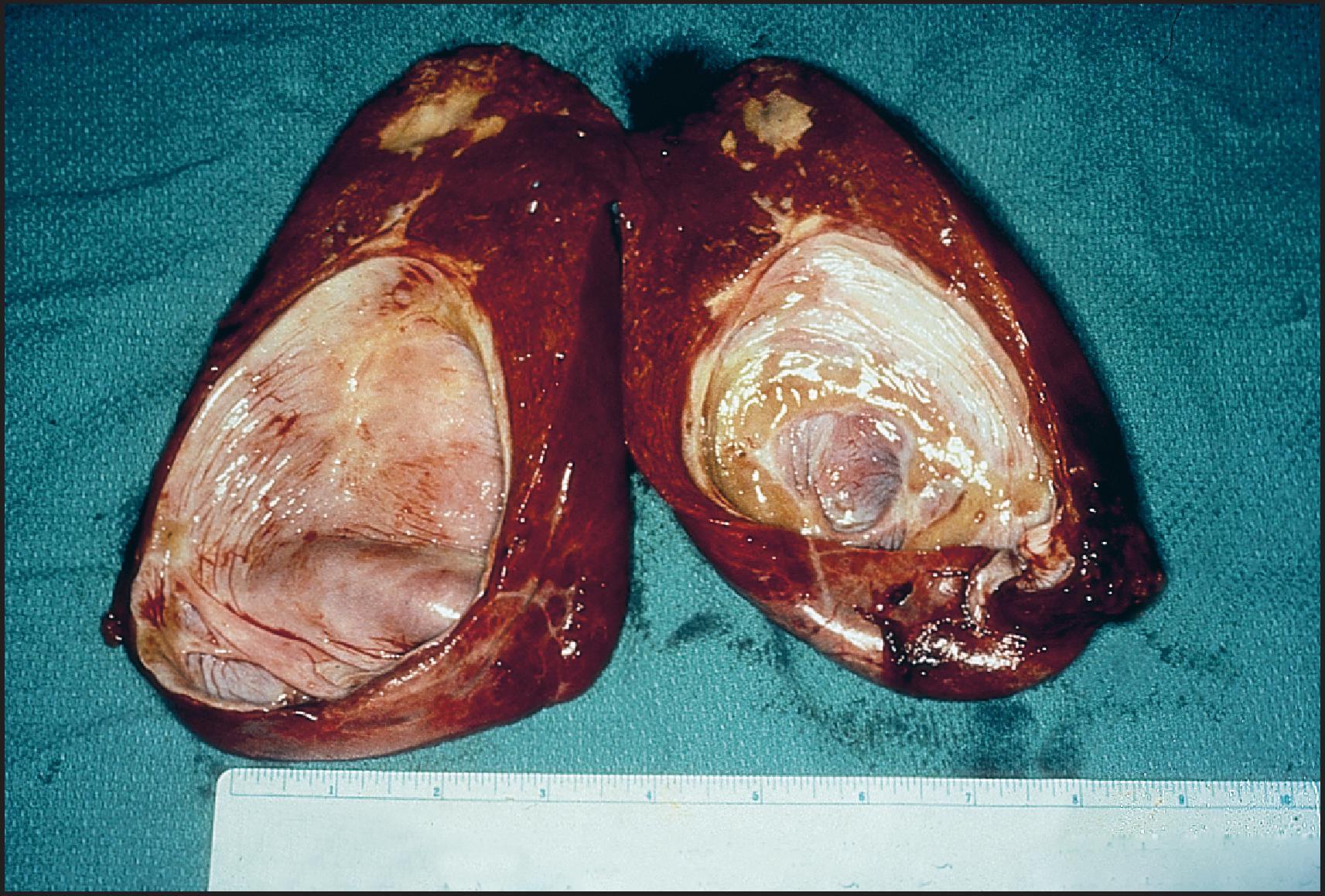
Microscopically, the cyst lining usually consists of a single layer of columnar, cuboidal or flat epithelium ( Fig. 3.42 ). The epithelium rests on a basement membrane that in turn is supported by a layer of fibrous tissue. Adenocarcinomas may arise in the cyst. Other malignancies reported to arise in solitary cysts include squamous cell carcinoma, carcinosarcoma and carcinoid tumour.
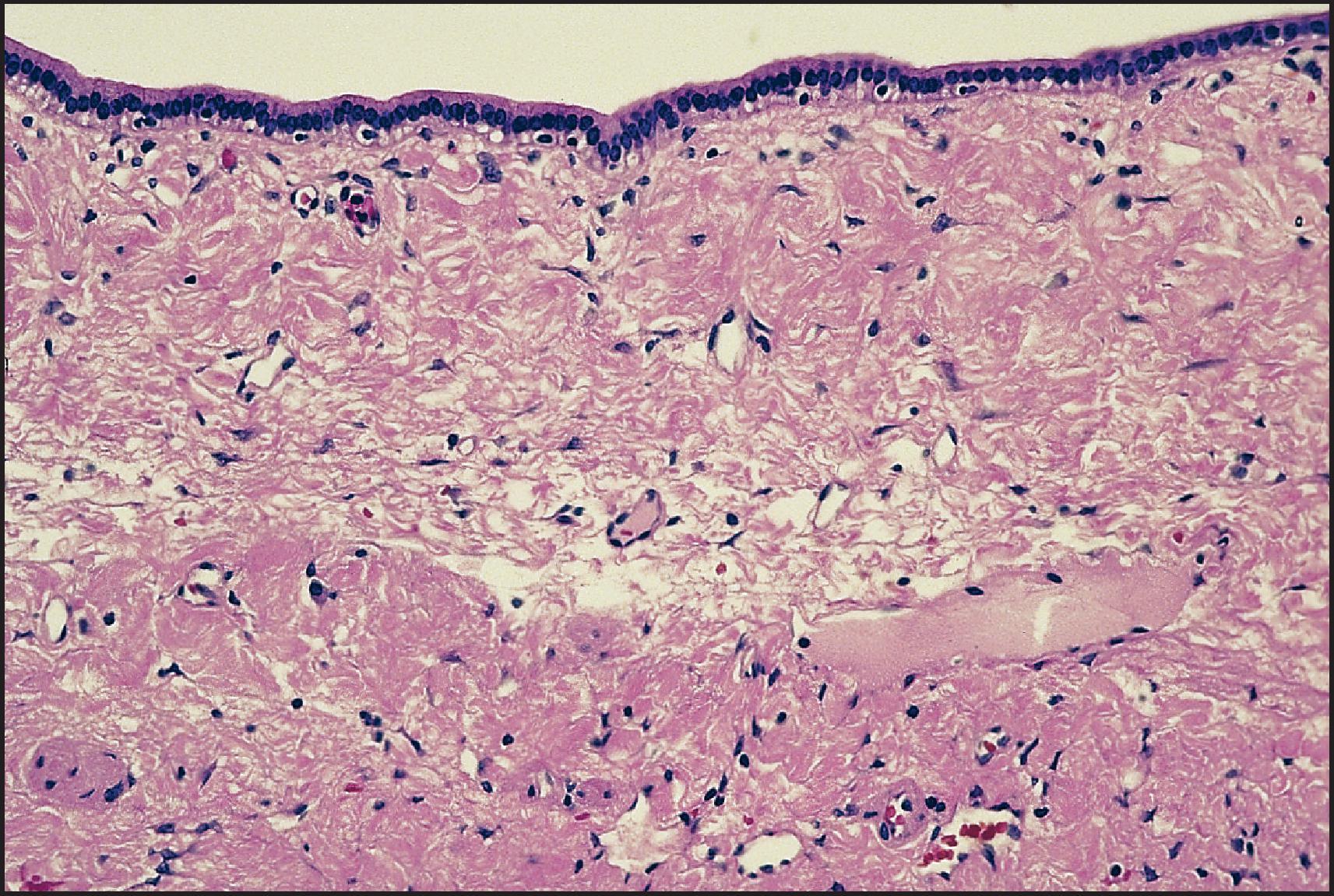
The pathogenesis of solitary bile duct cyst is unknown. A congenital origin is supported by the occurrence of the cysts in fetuses and newborns, by a case presenting as a congenital diaphragmatic hernia and the association of another case with the Peutz–Jeghers syndrome.
In the past, the treatment of choice of solitary cysts was excision. This has been supplanted by aspiration and sclerotherapy, , however, or laparoscopic fenestration. ,
CF, one of the most common autosomal recessive disorders, with an estimated incidence of approximately 1 in 2500 live births in White populations, has a worldwide distribution. CF was first recognized as a pancreatic disease distinct from coeliac disease in 1938. Originally known as ‘CF of the pancreas’ (mucoviscidosis), the disease was soon labelled simply ‘cystic fibrosis’, with the recognition that not only the exocrine pancreas but also the bronchial glands (obstructive bronchopulmonary disease), the intestinal glands (meconium ileus), the sweat glands (high sweat electrolytes) and the biliary tree (focal biliary cirrhosis) may be affected by the disease.
The gene was localized at chromosome 7q31.2 in 1989. It encodes a large protein named cystic fibrosis transmembrane regulator (CFTR), whose key role is in maintaining the fluid balance across epithelial cells. CFTR belongs to the ABC family of adenosine triphosphate (ATP)-dependent channels and transporters. It functions mainly as a cyclic adenosine monophosphate (cAMP)-dependent chloride channel in the apical membrane of secretory epithelial cells of many tissues, where it promotes transmembrane efflux of chloride ions. The protein is abundantly present in the branching ducts of the pancreas, intestinal epithelium and testes, and to a lesser degree in respiratory tissue. CFTR is expressed at the apical membranes of the epithelium of branching hepatic bile ducts and the gallbladder, but not on cells of the common hepatic bile duct or hepatocytes. , The CF secretory defect results in the inability to maintain the luminal hydration of ducts, leading to physicochemical abnormalities of secretions and duct obstruction. More than 2000 different disease-causing mutations have been identified. They are distinguished in terms of severity on the basis of the residual activity of the codified protein. The most common mutation is ΔF508, a three-base pair (bp) deletion in CFTR , resulting in loss of phenylalanine from the protein with subsequent retention in the ER.
Since liver disease in CF is often subclinical, its prevalence is uncertain and to some extent depends on definition, type of screening and age. Clinical diagnostic criteria vary and have been reviewed recently by Sakiani et al. As overall life expectancy has improved, liver disease has become the third most frequent cause of death in CF patients. , A large recent longitudinal study in the UK has shown that the prevalence of liver disease in CF is increasing. The availability of new therapies targeting the function of CFTR that could prevent or reverse CF liver disease constitutes an additional rationale for identifying CF patients at risk of developing liver impairment. Children with a heterogeneous pattern on liver ultrasound are at risk of developing advanced CF-related liver disease. Based on prospective studies, significant clinical liver disease is seen in 4–6% of CF patients, but biochemical evidence of liver involvement without clinical symptoms occurs in 20–50%. Autopsy series in the 1950s found an incidence of multifocal biliary fibrosis (or ‘cirrhosis’) of 22–25%. , The liver disease usually becomes apparent in the first decade, with a sharp decline in incidence thereafter. , The mode of presentation of the liver disease is variable. There may be transient neonatal conjugated hyperbilirubinaemia. In one series the majority of such cholestatic infants had meconium ileus, 28% had mucus plugs and 11% had focal biliary fibrosis. Paucity of the intrahepatic bile ducts has been reported in one patient who presented with neonatal conjugated hyperbilirubinaemia. Obstruction by pancreatic fibrosis has been reported but is much more common beyond infancy. Bile duct obstruction due to a rubbery concretion of abnormal bile has been reported in a few infants. In late childhood, intermittent elevation of serum aminotransferases and ALP, asymptomatic hepatomegaly, hepatosplenomegaly, hypersplenism or variceal haemorrhage might be the first indications of liver involvement. The unique liver lesion, termed ‘focal biliary fibrosis’ (or ‘cirrhosis’), is found in over 70% of adults who die after 24 years of age. A prospective study of 183 CF patients followed for a mean of 10 years revealed an incidence of liver disease of 17%. Clinically relevant disease suggests an incidence of 1.4–2.7%, with a peak in adolescence. Rare and unusual manifestations of liver disease in CF include CBD involvement proximal to the pancreas, obstruction by pancreatic fibrosis and nodular regenerative hyperplasia (NRH) of the liver in CF-associated colitis with fibrosing colonopathy. At times, ERCP and liver biopsy findings may mimic those of primary sclerosing cholangitis. Irrespective of relatively rare chronic progressive liver disease, macrovesicular steatosis is the most common pathological finding in the liver and is present in more than 50% of autopsy cases. The cause of the steatosis is not entirely evident, although it has been attributed to malnutrition and essential fatty acid deficiency. Death from liver disease occurs in 3.4% of patients, whereas lung disease accounts for 78% of CF-related deaths.
Factors that contribute to the variability in incidence and severity of liver disease are unknown. Some studies have found significant association with meconium ileus, , male gender, meconium ileus and severe genotype or meconium ileus and pancreatic insufficiency, but such risk factors were not identified in a Swedish cohort. There is no close correlation between particular CFTR mutations and liver disease. , Kinnman et al. examined the expression of CFTR and its relationship to histopathological changes in CF. They found impaired processing of ΔF508 CFTR protein in intrahepatic biliary epithelium. Possible pathogenic mechanisms could be related to its role in regulating bile secretion, innate immunity and dysregulation of the gut–liver axis. Generally, there is discordance for liver disease within sibships, , but not always. These observations have intensified the search for modifier genes which may in part explain the lack of genotypic/phenotypic correlations. The mannose-binding lectin gene, a relation with HLA-DQ6 in males, and the PI*Z allele of α1-AT have already been proposed as potential candidates. Among these, the PI*Z allele has been shown to increase the risk of developing severe liver disease in CF patients. , The development of the sweat chloride test followed the observations of salt depletion through sweat during summer heat waves. Values >60 mmol/L are diagnostic of CF. Recent consensus guidelines have provided a detailed diagnostic algorithm including the use of genetic and physiologic testing in patients with an intermediate sweat chloride value (30–59 mmol/L) or within the normal range.
Most investigations have shown improved liver enzymes, lipid profiles and vitamin A levels with ursodeoxycholic acid (UDCA) therapy. , One report documents normalization of liver function tests (LFTs) and improvement in the portal tract inflammation on pathological examination. The same authors also noted improvement in ultrastructural abnormalities of bile ducts. Importantly, in CF patients, UDCA is not effective for gallstone disease, the incidence of which may be 12% in older patients. Although the stones are often radiolucent, they contain calcium bilirubinate instead of increased cholesterol. Recently, the use of UDCA has been questioned in a very large longitudinal study from France, based on their observation that early institution of UDCA did not change the incidence of severe CFLD. New medications have been developed to correct the dysfunctional CFTR associated with certain specific genotypes.
A transjugular intrahepatic portosystemic shunt (TIPS) procedure is only rarely used in patients with CF liver disease because either endoscopic band ligation therapy or sclerotherapy has been reasonably successful for treating bleeding oesophageal varices. , Because of possible recurrent bleeding, some surgeons advocate portosystemic shunts for patients with good synthetic function. , LT has been successful, but recent analyses suggest that the outcome is inferior to that of LT for other causes. Postoperative death has resulted mainly from lung complications. A few combined liver–heart–lung, liver–pancreas and liver–intestine transplants have been reported.
Pathological studies of the liver have been described in a number of reports. , , The changes include steatosis, periportal fibrosis with ductular reaction and dilation (with or without inspissation of secretions) ( Fig. 3.43 ), focal biliary fibrosis (‘cirrhosis’) and multinodular biliary fibrosis (‘cirrhosis’). Steatosis is probably the most frequent lesion, present in 121 of 198 cases (61%) reported by Craig et al. ( Fig. 3.43 ). The fat was located most frequently in periportal hepatocytes but was unevenly distributed throughout when severe. No positive correlations could be made between the presence of fat in the liver and the patient’s general nutritional status. An incidental finding in infant livers is the deposition of haemosiderin in periportal hepatocytes. This finding is presumably related to the increased iron transport from the gut in pancreatic insufficiency.
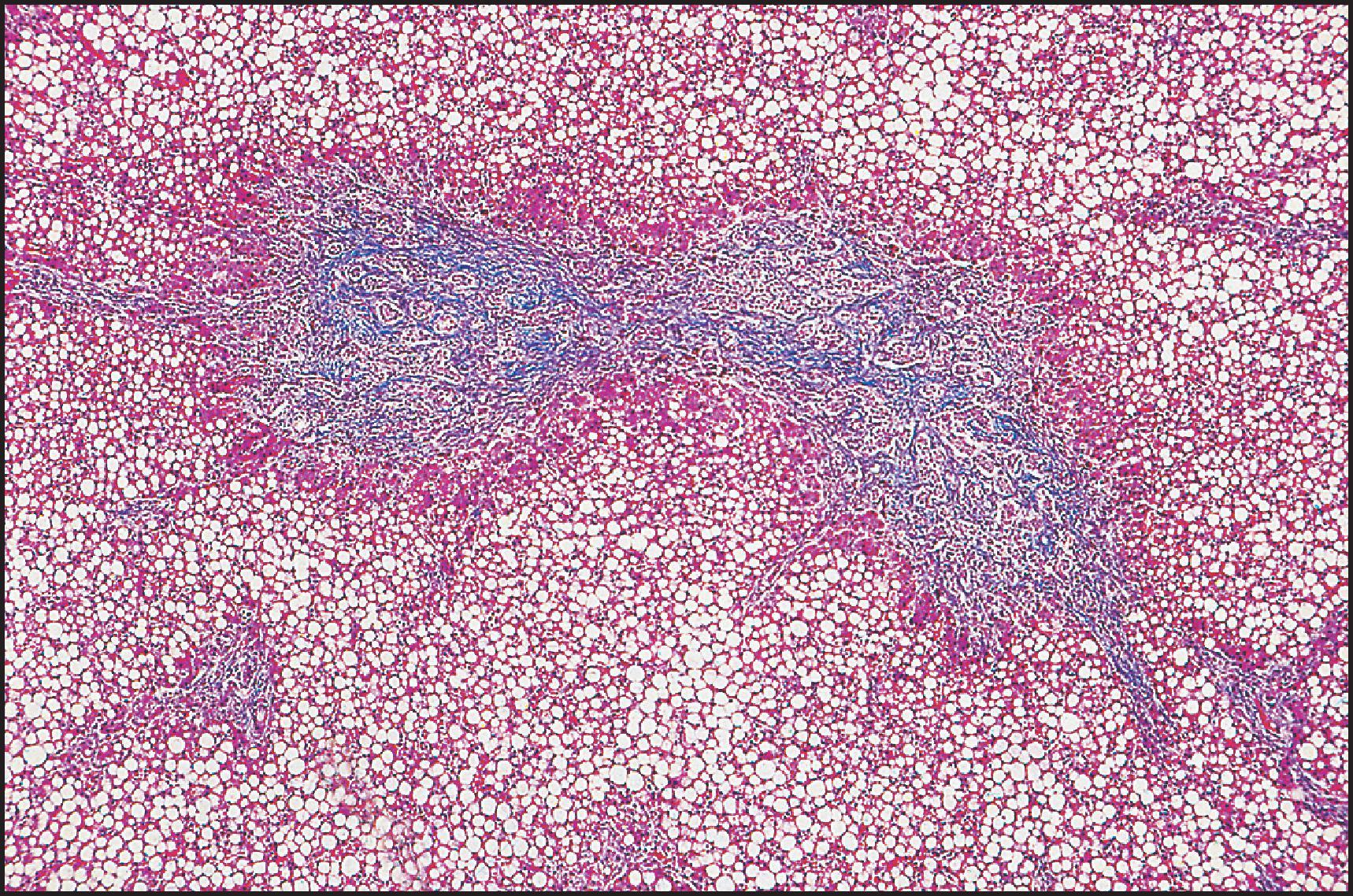
The characteristic, if not pathognomonic, lesion is focal biliary fibrosis, found in 22–33% of patients in three early series. , , An increasing frequency with duration of survival was noted. Thus it was found at postmortem examination in 10.6% of infants younger than 3 months, in 15.6% of infants from 3 to 12 months and in 26.9% of children older than 1 year.
Macroscopically, the liver with focal biliary fibrosis shows multiple, depressed, greyish white scars that are triangular or stellate in shape. , As already noted, there is variable ductular reaction and periportal fibrosis. The ductules are generally dilated and show varying degrees of atrophy of the lining cells. The lumen contains the pathognomonic pink to light-orange, rounded masses (concretions) or amorphous material ( Figs 3.44 and 3.45 ), first described by Farber in 1944. The inspissated secretions in the cholangioles stain intensely with PAS and resist diastase digestion; they do not stain positively with mucicarmine or Alcian blue, but there may be some mucin in the interlobular bile ducts. Some greatly dilated ductules eventually rupture with extrusion of their contents and induction of an acute inflammatory response. The cholangitis is thus ‘chemical’ but may be complicated by bacterial infection. Chronic inflammatory cells may also be present in the fibrous tissue. It is important to emphasize that periportal fibrosis may occur in the absence of ductular reaction and inspissation of secretion , ; this nonspecific change has been found only in infants less than 3 months of age.
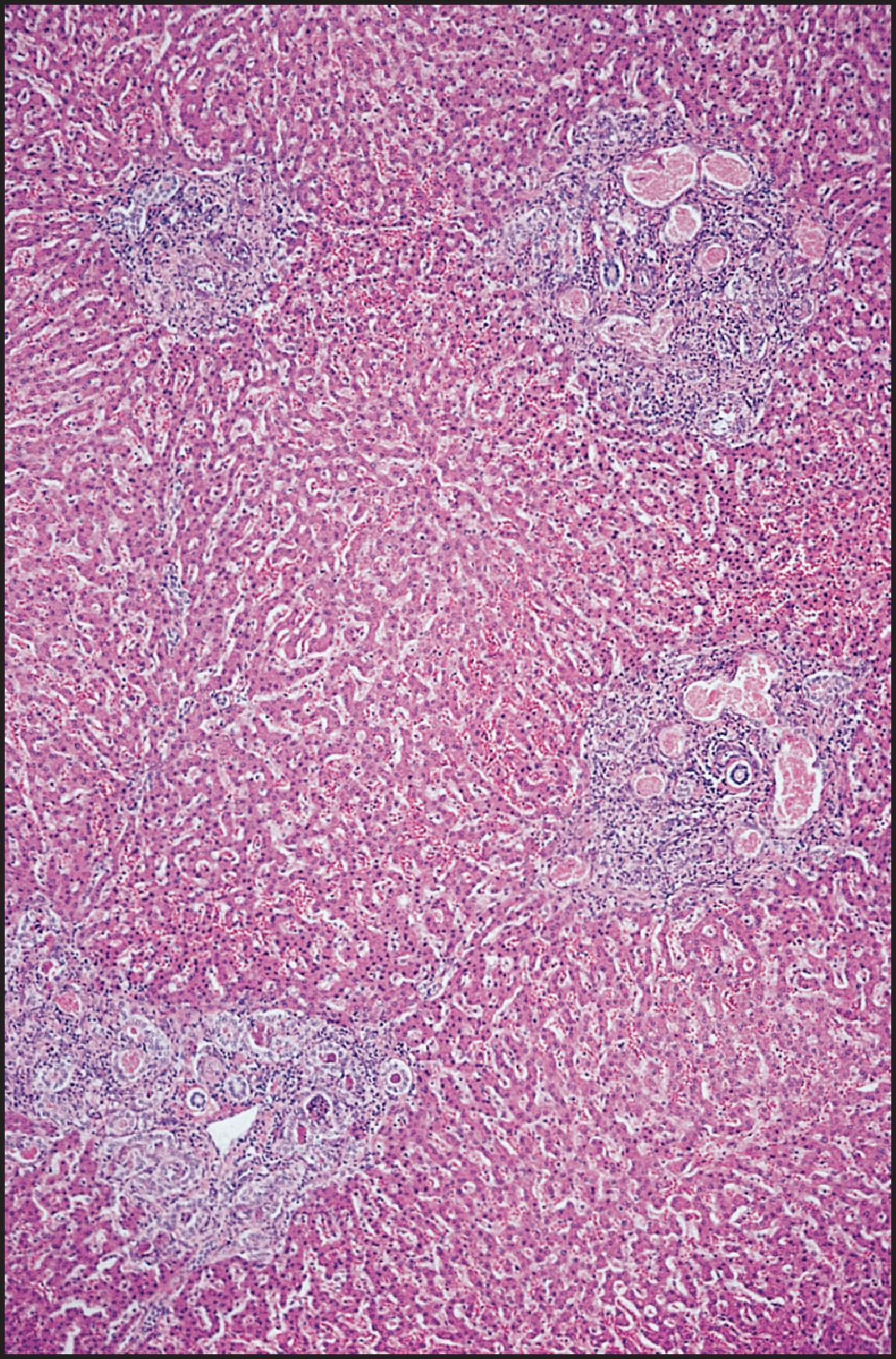
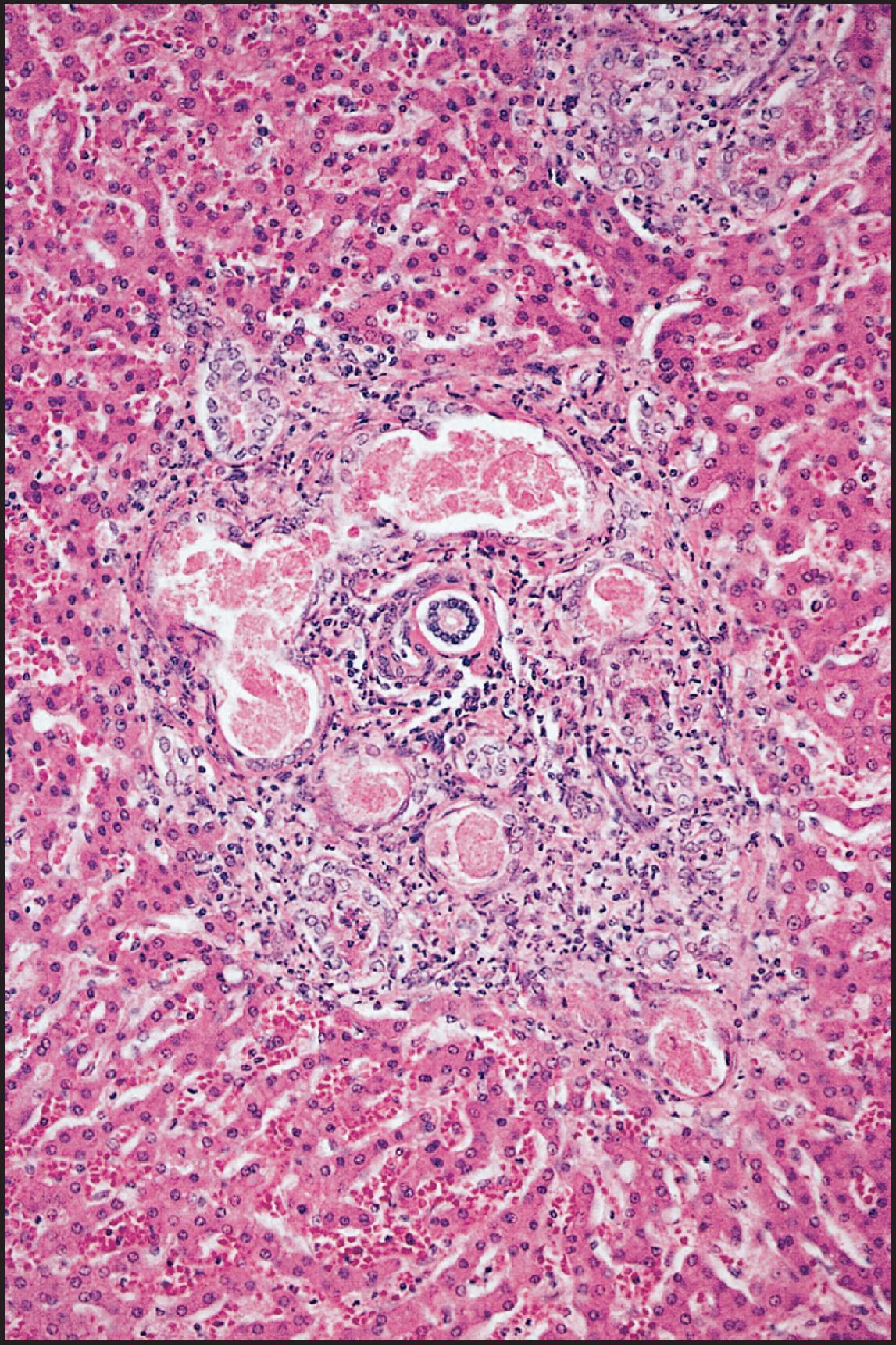
Over time the focal biliary lesions may coalesce, with extension of fibrosis, atrophy of the intervening parenchyma and entrapment and encirclement of groups of hepatic lobules. This type of lesion, referred to as ‘multilobular biliary cirrhosis with concretions’ or ‘multilobular cirrhosis’, occurs in 6% of patients older than 1 year. Large irregular nodules are produced, and the deep clefts between the nodules may resemble hepar lobatum. Liver biopsy has a role in predicting an individual’s risk of liver fibrosis or development of portal hypertension. A significant number of cases of noncirrhotic portal hypertension in children with CF have been found. ,
In addition to the presence of excessive mucus in extrahepatic bile ducts in occasional patients, there may be excessive mucus accumulation in intrahepatic bile ducts, particularly those adjacent to the porta hepatis. This change occurs in 23.4% of infants under 3 months of age and in 12.5% of patients from 3 to 12 months. In two-thirds of the patients less than 3 months old with mucus in their ducts, histological cholestasis is seen, and a history of jaundice is noted. The gallbladder in up to 30% of patients is hypoplastic and contains mucoid material and a small amount of viscid bile. Mucous cysts are seen in the wall, and stones may be present in the lumen.
Liver biopsy material from 11 patients with CF was examined ultrastructurally by Arends et al. A finding unique to this disease was the presence of filamentous material, with an average diameter of 15 nm, in the lumen of bile ductules and ducts. Bile granules were scattered between the filaments. A reaction product of carbohydrates was found in the filaments by histochemical techniques. It was suggested that the typical mucus deposits in bile ducts are built up from these filaments. Similar ultrastructural findings were noted by others. , Bradford et al. studied the ultrastructure of liver cells in detail and described the presence of membrane-bound deposits of electron-lucent material containing electron-dense cores resembling mucus. Bile duct cells with irregular shapes protruding into the lumen and the presence of necrotic cells have been observed. An increase in the number of hepatic stellate cells around portal areas, with deposition of collagen around bile ducts and ductules, has been noted, , and bile duct destruction, rather than cholestasis, is suggested as the inducer of collagen deposition in CF-associated fibrosis and cirrhosis. A definitive role for hepatic stellate cells has been shown and TGF-β1, produced by bile duct epithelial cells, has been demonstrated to be an inducer of stellate cell collagen gene expression.
Patients with bile acid synthetic defects present clinically with conjugated hyperbilirubinaemia within the spectrum of the neonatal hepatitis syndrome. Age-related levels of GGT are normal; otherwise, there are no distinguishing features among routine liver tests. In contrast to infants with other low-GGT cholestatic disorders, those with congenital bile acid synthetic defects typically are not pruritic. Total serum bile acids measured by a 3β-hydroxysteroid dehydrogenase assay are absent or else found at very low concentrations, clearly disproportionately low to the degree of jaundice, except when there is amidation defect present. Definitive diagnosis is by determining which bile acids are present in urine or bile using electrospray-ionization tandem mass spectrometry (ESI-MS/MS). Analysis can also be performed by fast-atom-bombardment mass spectrometry (FAB-MS) or gas–liquid chromatography (GLC). Determination of urinary cholanoids by ESI-MS/MS-MS is probably most practical, and accumulating experience argues for this investigation in any infant with neonatal hepatitis syndrome. Genes encoding for the more common types of bile acid synthesis disorders can be included in next-generation sequencing panels designed for neonatal cholestasis or neonatal hepatitis syndrome.
Most of the entities in this rapidly developing sector of genetic liver disease are rare, having been identified in only a few patients, but they may be more common than is apparent. Affected enzymes may be microsomal, cytoplasmic or peroxisomal. Importantly, infants with severe liver disease may acquire defects in bile acid synthesis, usually Δ 4 -3-oxosteroid-5β-reductase, and they must be distinguished from those with an inherited disorder of bile acid synthesis, since treatment may be different. Gene sequencing may be required to confirm or disconfirm the diagnosis in such cases. Clinical experience shows that patients with genetic disorders of bile acid synthesis often respond well to oral bile acid supplementation. Table 3.3 shows currently identified primary disorders of bile acid synthesis. The discussion here focuses on defects in (1) 3β-hydroxy-Δ 5 -C 27 -steroid dehydrogenase/isomerase (3βHSD), (2) Δ 4 –3-oxosteroid-5β-reductase, (3) oxysterol 7α-hydroxylase and (4) sterol 27-hydroxylase (CTX). Some rare defects causing infantile conjugated hyperbilirubinaemia are also discussed, as well as amidation disorders that may present with transient, apparently idiopathic, neonatal hepatitis syndrome or later in childhood with growth failure and fat-soluble vitamin deficiency. Bile acid physiology, synthesis, conjugation and transport are discussed in Chapter 9 .
| Deficiency | Associated disorders |
|---|---|
| 3β-Hydroxy-Δ 5 -C 27 -steroid dehydrogenase/isomerase deficiency | Neonatal hepatitis syndrome |
| Δ 4 –3-Oxosteroid-5β-reductase (5β-reductase) deficiency | Neonatal hepatitis syndrome |
| Oxysterol 7α-hydroxylase deficiency | Neonatal hepatitis syndrome |
| Sterol 27-hydroxylase deficiency (cerebrotendinous xanthomatosis, CTX) | Neonatal hepatitis syndrome, chronic diarrhoea in infancy, neurological symptoms, juvenile cataract, tuberous xanthomas (e.g. Achilles tendon) |
| α-Methylacyl-CoA racemase (AMACR) deficiency | Neonatal hepatitis syndrome, fat-soluble vitamin deficiency, adult-onset neuropathy |
| Bile acid conjugation defects (BAAT) | Neonatal hepatitis syndrome, fat-soluble vitamin deficiency |
| Zellweger syndrome | Peroxisomal biogenesis disorder (see page 209) Bile acid synthesis is completed in peroxisomes. |
This enzyme, 3βHSD, is the second step in the pathway for synthesizing bile acids. It is involved in the conversion of 7α-hydroxy-cholesterol to 7α-hydroxy-4-cholesten-3-one and then into the pathways producing the primary bile acids. Specifically, it can proceed directly into the pathway for synthesizing chenodeoxycholic acid, or it can be 12-hydroxylated and enter the pathway for producing cholic acid. Among the primary bile acid synthetic defects, 3βHSD deficiency is most common. The first patient reported was a 3-month-old infant with cholestasis. Early biopsies of the liver in the patient and siblings demonstrated giant cell hepatitis. Primary bile acids were not detected; however, the serum concentration of the natural substrate, unesterified 7α-hydroxy-cholesterol, was elevated. Fibroblasts from this patient demonstrated complete absence of the enzyme; the parents’ fibroblasts demonstrated reduced 3βHSD activity. The patient responded to chenodeoxycholic acid, including relief from pruritus. The presentation during infancy/childhood may be quite variable. Five patients aged 4–46 months had hepatomegaly, four of whom had jaundice and two with fatty stools; none was pruritic. Microcysts were found in the kidney in two patients. All patients responded to UDCA. The challenge here is to distinguish 3βHSD (and 5α-reductase) deficiency from bile canalicular transporter disorders, especially when the presence or absence of pruritus does not provide a clinical distinction. In both of these bile acid synthetic disorders, the abnormal bile acids that are formed inhibit the canalicular ATP-dependent bile acid transporter. In another consanguineous family, manifestations of 3βHSD deficiency were extremely variable: the proband died from end-stage liver disease at age 24 years, but one sister was asymptomatic at age 32 despite homozygosity for a null allele of the HSDRB7 gene encoding for 3βHSD.
Hepatic histopathological changes in 3βHSD deficiency depend on the age of the patient and rate of progression of the liver disease. Two of the three patients reported by Clayton et al. who were biopsied at 6 weeks and 18 months of age had giant cell hepatitis and giant cell hepatitis with bridging fibrosis, respectively. Similarly, Jacquemin et al. found giant cell hepatitis in young patients (4 and 6 months of age) and portal and perilobular fibrosis in older patients (36–46 months). Three patients studied by Bove et al. had portal and intralobular fibrosis with ‘pseudoductular metaplasia’ and cholangiolar proliferation (ductular reaction); there was no paucity of interlobular bile ducts. An adult with cirrhosis of undetermined aetiology has been described as having 3βHSD deficiency.
The responsible enzyme is found only in the liver , and not in fibroblasts. It is the enzymatic step in the primary pathway from cholesterol to cholic and chenodeoxycholic acid, involved in the saturation of the steroid ring. A deficiency would result in the decreased formation of these primary bile acids with an elevation of cholenoic bile acids. Δ 4 -3-Oxosteroid-5β-reductase was not detectable in the original patients presenting with neonatal hepatitis who were evaluated in follow-up after bile acid therapy. Enzyme analysis of the liver in a subsequent patient did not verify that this is a specific genetic defect, i.e. no mutation was present. Notably, this enzyme is somewhat fragile and can cease to function in the presence of severe liver disease.
A tentative diagnosis of 5β-reductase deficiency is presently based on >70% of the urinary bile acids being of the 3-oxo-Δ 4 type and significant detection of allo-(5α-hydroxy) bile acids. , The gene encoding Δ 4 -3-oxosteroid-5β-reductase is AKR1D1 (also known as SRD5B1 ); mutation analysis can confirm the diagnosis. Elevation of primary bile acids should raise doubts concerning a diagnosis of a bile acid synthetic defect, and other aetiologies should be sought. Why these patients might respond to UDCA is unclear, except that treatment may decrease the levels of the offending bile acids. If clinical response is insufficient, improvement subsequently can be observed with chenodeoxycholic acid and cholic acid therapy. More recent experience indicates that response to supplementation with chenodeoxycholic plus cholic acid is better in patients whose disease is not advanced.
Light microscopic and ultrastructural findings in 5β-reductase deficiency (and other bile acid synthetic defects) are discussed in detail by Bove et al. Light microscopic changes in most reported cases resemble nonspecific neonatal hepatitis, with prominent cholestasis, giant cell transformation and erythropoiesis. Interlobular bile ducts are consistently normal. Ultrastructurally, injury patterns common to other forms of cholestasis are seen, as well as features that may be specific for the defect. Thus there is a nonuniform mosaic of normal and abnormal canaliculi, often in adjacent clusters of hepatocytes, but there is no canalicular dilation. However, some canaliculi show diverticula, and junctional complexes are extraordinarily convoluted, often enclosing pockets of dense granular material presumed to be bile residue.
Deficiency of oxysterol 7α-hydroxylase, the third enzyme in the ‘acidic’ pathway that can generate chenodeoxycholic acid, can produce severe infantile liver disease. The deficiency results in the accumulation of 3β-hydrocholenoic and 3β-hydroxy-5-cholestenoic acids. One infant has been described who presented with conjugated hyperbilirubinaemia and low GGT and whose liver disease had already advanced to cirrhosis. Serum levels of 27-hydroxycholesterol were greater than 4500 times normal. Histopathologically, the liver showed giant cell transformation, ductular reaction and canalicular and bile duct plugging; no specific ultrastructural changes were noted. Both parents had an exon 5 premature terminated codon (R388*) as a heterozygous defect. There was no response to either UDCA or cholic acid, either by clinical indicators or by alterations in the measured bile acids. This patient underwent a liver transplant but died of complications relating to disseminated post-transplant lymphoproliferative disease. A second patient also succumbed to severe cholestatic liver disease in the first year of life. Two patients have been reported subsequently: one died at age 11 months before LT, and the other had successful living-related donor transplant from an obligate heterozygote. Both patients had cirrhosis with multinucleated giant cells on liver biopsy. A single case of successful treatment with chenodeoxycholic acid has been reported. Patients with oxysterol 7α-hydroxylase have normal serum GGT and, in general, rapidly progressive liver disease. Diagnosis based on finding elevated serum and urine 3β-monohydroxy -Δ 5 -C 24 bile acids may generate a false-positive diagnosis of oxysterol 7α-hydroxylase deficiency. Such an infant was found to have 3βHSD deficiency, not entirely surprising because 3βHSD serves as the next enzyme in that same ‘acidic’ pathway, as well as early in the ‘neutral’ pathway. Molecular genetic diagnosis may be critically important in doubtful cases.
Since bile acid metabolism represents a complex pathway, it seems reasonable to expect other rare defects in bile acid metabolism. Alternate pathways may be more prominent in the fetus and newborn. The effects of developmental changes in the intestinal microbiome may be relevant.
Rare defects in bile acid synthesis may account for what has been regarded as ‘idiopathic neonatal hepatitis’. For instance, a 25-hydroxylation pathway exists which may normally contribute in a limited fashion to cholic acid production. A deficiency in this hydroxylase was reported in a family where one child died at age 13 months after lifelong jaundice, and another was stillborn. The third child developed conjugated hyperbilirubinaemia with hepatomegaly at 7 days old ; his serum GGT was normal, and his liver biopsy showed giant cell hepatitis. On treatment with chenodeoxycholic acid, he later developed pruritus, which gradually improved when cholic acid was added to the treatment regimen and chenodeoxycholic acid was stopped. Although clinically well, including improvement in pruritus and normal LFTs and physical examination, this patient developed cirrhosis, which appeared to stabilize.
Amidation defects cause failure to conjugate a bile acid with glycine or taurine. This is the last step in bile acid synthesis. The mechanism is somewhat complicated: a coenzyme A (CoA) thioester of the bile acid is formed by the enzyme bile acid–CoA ligase (BACL, encoded by SLC27A5 ), and glycine or taurine is conjugated by bile acid–CoA:amino acid N -acyltransferase (BAAT, encoded by BAAT ). Some patients with defective amidation present in early infancy with conjugated hyperbilirubinaemia, occasionally severe enough to resemble BA. Liver biopsies in those patients showed lobular cholestasis, giant cell transformation of hepatocytes and mild to severe ductular reaction with bridging fibrosis but no ductular bile plugs. Other patients are never noted to be jaundiced but have growth retardation and severe deficiency of fat-soluble vitamins resulting in rickets or coagulation abnormalities. Infants who do manifest jaundice also have prominent clinical features of fat-soluble vitamin deficiency. Based on limited observations, this seems to be a low-GGT disorder. Detailed analysis of serum bile acids shows a striking lack of conjugated bile acids, but total bile acid concentrations may be elevated. Histological analysis may show a giant cell hepatitis or ductopenia. Immunostaining for BAAT is depleted or absent. Similar clinical disease appears to occur with BACL mutations. In one reported case where the amidation defect was associated with BACL deficiency, the infant was homozygous for a missense mutation in ABCB11 (thus compromising BSEP); however, other cases exclusively with BACL defects have been identified. These infants may be at greater risk for neonatal hepatitis syndrome if born premature and requiring TPN.
Cerebrotendinous xanthomatosis (CTX) is a rare autosomal recessive metabolic disease caused by deficiency of mitochondrial C 27 -steroid-26-hydroxylase on the inner mitochondrial membrane, which can be demonstrated in liver and cultured fibroblasts, leading to tissue accumulation of cholestanol. The defective gene, for sterol 27-hydroxylase, localized to chromosome 2q33, has been cloned, and mutations associated with the disease have been described. ,
The symptoms of this disease often begin with chronic diarrhoea in infancy. Neonatal hepatitis has been recorded in a few patients. Juvenile cataracts develop during childhood, as do tuberous xanthomas (particularly over the Achilles tendons). The neurological manifestations vary from mental retardation developing during adolescence to normal intelligence throughout adult life. , Spasticity and ataxia are usually detected in the second and third decades. Progression results in dementia, spinal cord paresis and peripheral neuropathy. Less frequent findings include seizures, gallstones and osteoporosis. Death in the fourth to sixth decade is usually related to neurological deterioration; however, some patients develop early heart disease and/or atheroma.
The 5α-dihydro derivative of cholesterol, cholestanol (but not cholesterol), is elevated in serum. There is excessive deposit of cholestanol and cholesterol in tissues. Bile acid concentrations are low in bile, with high bile alcohol levels in bile and urine. Heterozygotes can be detected by measurement of urinary bile alcohol levels after administration of a bile salt-binding agent, cholestyramine.
Liver specimens from two patients revealed intracellular inclusions that appeared either as amorphous pigment or in crystalloid form. The pigment is usually found in association with the smooth ER (SER) but occasionally lies free in the cytosol. An ultrastructural study of four cases revealed perisinusoidal fibrosis, bile canalicular changes (dilation, distortion and loss of microvilli, increase in microfilaments), fatty change, proliferation of SER, accumulation of lipofuscin, focal cytoplasmic degeneration, proliferation of microbodies and prominent mitochondrial changes. One patient had crystalloid cores in microbodies that disappeared after therapy. The accumulated material may indicate the presence of unmetabolizable bile alcohols resulting from a defect of bile acid synthesis.
In general, treatment is by bile acid supplementation to bypass or offset the synthetic defect. UDCA may serve as initial treatment, but this may not be curative. It is preferable to treat patients with primary bile acids: cholic acid or chenodeoxycholic acid, separately or in combination. However, these bile acids are not widely available commercially. Chronic oral cholic acid treatment for patients with 3βHSD or 5β-reductase deficiency appears to be safe and effective. With a median follow-up of 21 years, Gonzales et al. found that serum aminotransferases normalized and liver histology improved, including in three patients with apparent regression of cirrhosis. The recently reported American long-term experience was also favourable.
For CTX, a combination of chenodeoxycholic acid and pravastatin (an inhibitor of 3-hydroxy-3-methylglutaryl CoA, HMG-CoA reductase) has reduced serum cholestanol and prevented an increase of total cholesterol. The therapy has stopped disease progression, but has not reversed clinical manifestations. LDL apheresis has been added to the regimen in a few patients.
LT has been performed for oxysterol 7α-hydroxylase deficiency and occasionally for other of the bile acid synthesis disorders when liver disease is far advanced and unresponsive to medical treatment.
Two comprehensive reviews of diseases of bile acid synthesis are recommended for further reading. ,
PFIC was originally described in 1965 in a Pennsylvanian Amish kindred named Byler, thus called Byler disease/syndrome. Now PFIC is recognized as occurring worldwide. Although widely referred to as PFIC types 1, 2 and 3, these terms are confusing and should be avoided. The disorders have been reclassified according to molecular basis, with designation based on the protein that is deficient. Since genetic testing is not always immediately available, a clinical subdivision is helpful: diseases manifesting normal (or low) levels of serum GGT and those with elevated levels. Two low-GGT forms of PFIC are now termed ‘FIC1 deficiency’ and ‘BSEP deficiency’. The predominant form with a high GGT is termed ‘MDR3 deficiency’. All three are autosomal recessive disorders and involve different genes. The neonatal presentations of these three conditions are the best described: infantile presentation with rapidly progressive liver disease is most typical of FIC1 and BSEP deficiencies, with debilitating pruritus. Additionally, there are less severe and later-presenting forms of all three of these bile canalicular transporter defects, including but not limited to ‘benign recurrent intrahepatic cholestasis’ (BRIC). Later-onset disease may not be recognized as such, unless features indicating similarity to neonatal disease are noted. This applies particularly to the histological features. The list of genes associated with infantile cholestatic liver disease is increasing, in part due to the availability of whole-exome and whole-genome sequencing, and the classification of these disorders is moving from the historical clinical basis to a more physiopathological and genetic approach. Accordingly, these disorders are grouped either as bile canalicular transporter disorders or as inherited disorders causing intrahepatic cholestasis.
Previously known as ‘progressive’ familial intrahepatic cholestasis type 1 (PFIC1), FIC1 deficiency is caused by mutations in ATP8B1 . , Most recognized patients present in the first 6 months of life with conjugated hyperbilirubinaemia, normal serum GGT and itching. In FIC1 deficiency, extrahepatic manifestations of disease are clinically important. Chronic diarrhoea may develop; growth failure due to maldigestion as well as fat-soluble vitamin deficiency is a major complication. Sensorineural deafness requiring hearing aids develops in 30% of patients. Recurrent respiratory infections may occur. There is a slow progression to cirrhosis.
Liver biopsy shows a bland cholestasis ( Fig. 3.46 ), without significant giant cell transformation or cellular infiltrate. , Canaliculi filled with pale-appearing bile may be identified. In 1972, Linarelli et al. suggested that the ultrastructure of canalicular bile was coarsely granular in PFIC and called it ‘Byler’s bile’ ( Fig. 3.47 ). Subsequently, this type of bile was found to be characteristic of FIC1 deficiency, from which some members of the Amish Byler family suffer. ,
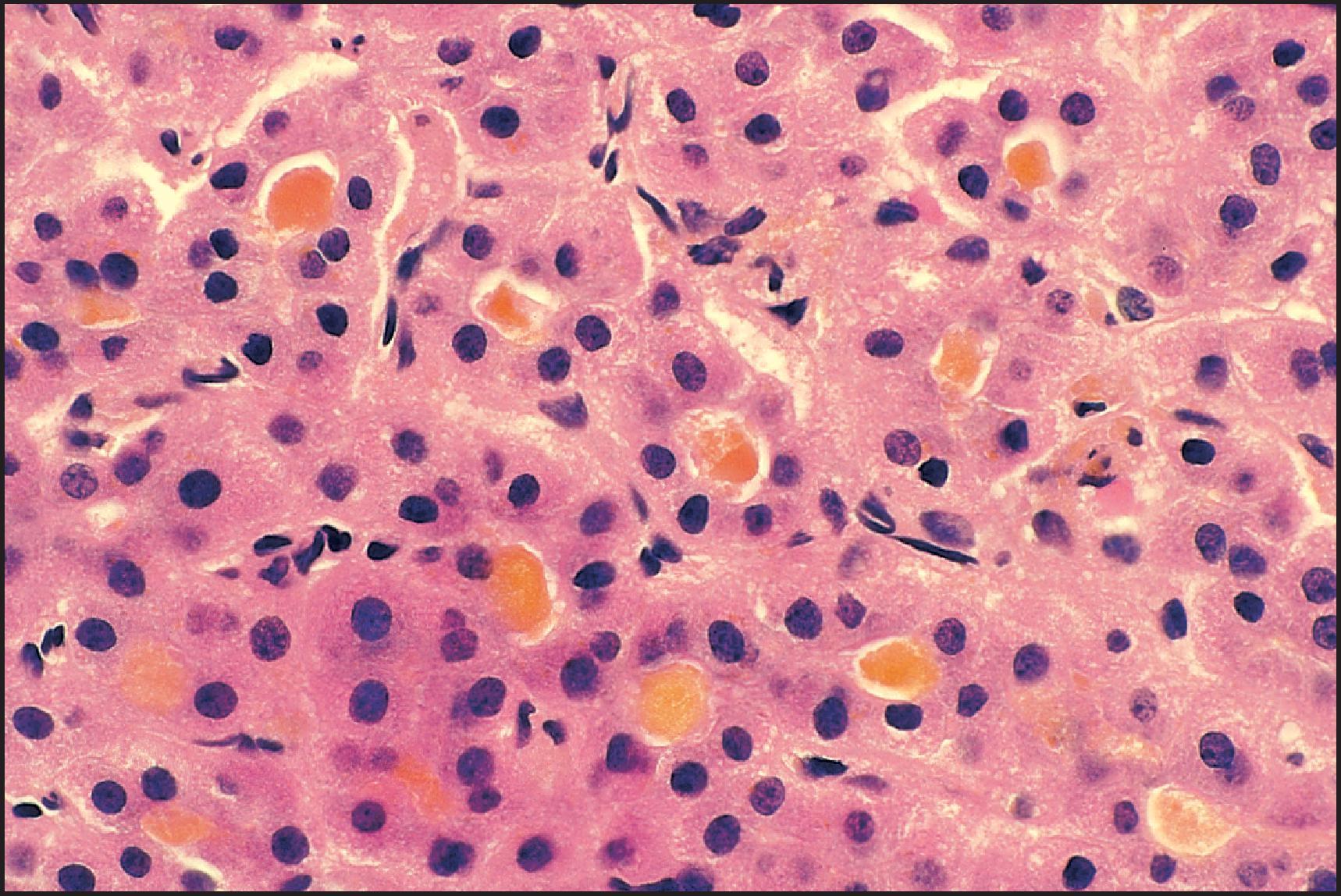
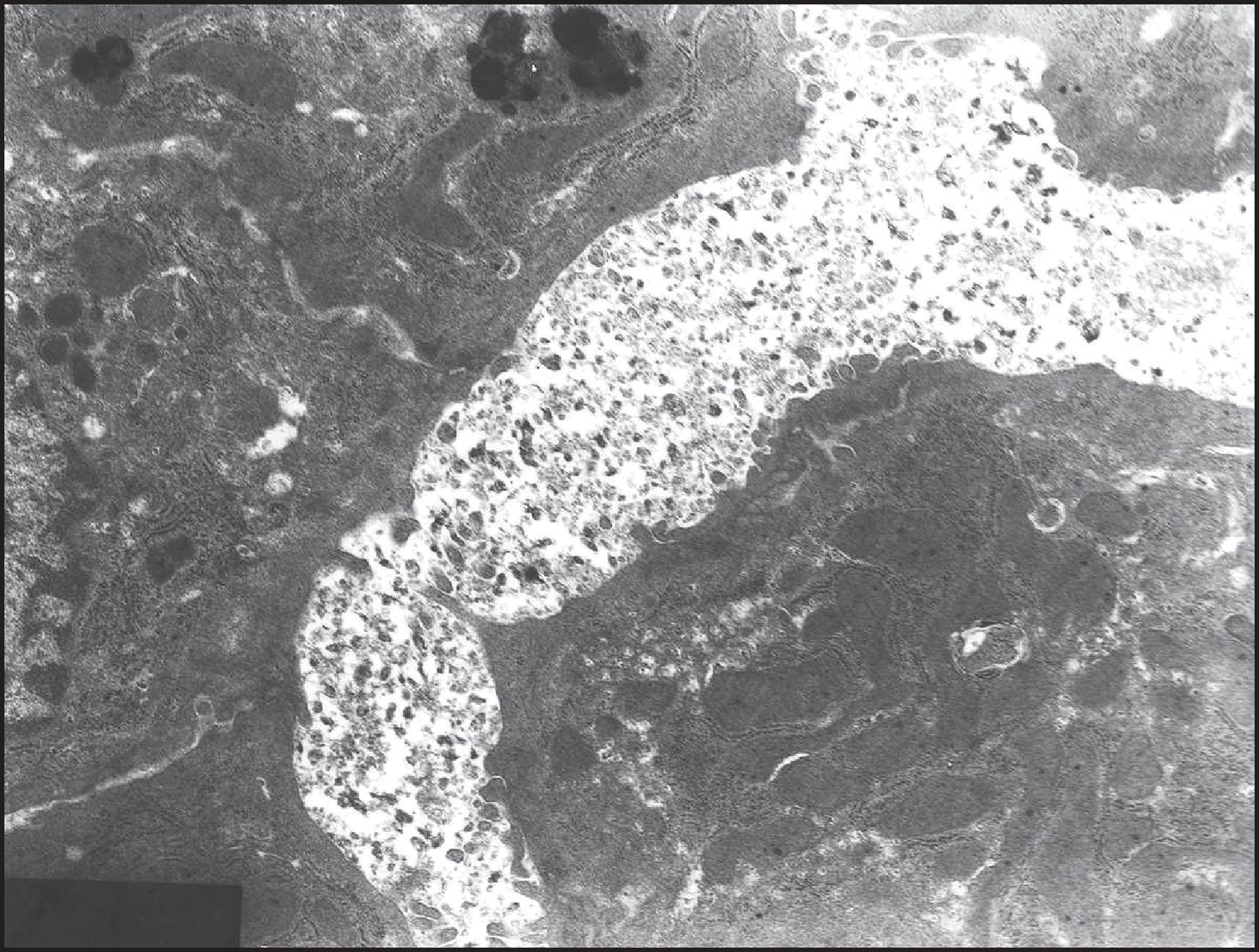
The function of the FIC1 protein, a P-type ATPase, has been unclear. , It may play a role in maintaining the functional integrity of the bile canalicular membrane. Groen et al. have proposed a model by which the concerted action of FIC1 and MDR3, encoded by ABCB4 , is critical in maintaining the asymmetrical lipid composition of the hepatocyte plasma membrane outer leaflet (facing the canalicular lumen) and the inner leaflet (facing the cytoplasm). FIC1 is a flippase , and MDR3 is a floppase. FIC1, along with an accessory protein CDC50A, flips phosphatidylserine from the outer to inner leaflet to complement the action of MDR3, which ‘flops’ phosphatidylcholine from the inner to the outer leaflet so that it can be associated with bile salts to form micelles. This asymmetry would make the membrane outer leaflet resistant to the detergent effect of bile salts, which are at high concentration in the bile duct lumen. The precise mechanism by which a defect in this flippase function leads to cholestasis and extrahepatic manifestations is still not clear. , The reduction in ectoenzymes seen in the canalicular membrane of patients probably reflects the bile acid-dependent extraction seen in mice. This finding has been proposed as an indirect feature of FIC1 deficiency, although in the absence thus far of a specific antibody available for formalin-fixed paraffin-embedded (FFPE) tissue. In patients with FIC1 deficiency, the expression of GGT and neutral endopeptidase (CD10) is lost on the canalicular membrane but not on the cholangiocyte apical surface. In contrast, canalicular expression of integral membrane proteins encoded by ABCB11 and ABCB4 (BSEP and MDR3, respectively) is preserved.
Other rare cholestatic disorders have been identified as FIC1 deficiency. A form of cholestatic syndrome was reported as being specific to the inhabitants of Greenland. Jaundice, bleeding, pruritus, malnutrition, steatorrhoea, osteodystrophy and dwarfism were typical clinical features. Eight of the children died from bleeding or infections between ages 6 weeks and 3 years. Hyperbilirubinaemia, profound hypoprothrombinaemia, thrombocytosis and elevated ALP values were evident. ‘Greenland familial cholestasis’ has now been shown to be FIC1 deficiency, with all affected individuals carrying two copies of the same missense mutation. Ornvold et al. studied liver biopsies from 16 children and characterized the changes as early, intermediate and late. Early changes (up to 5 months of age) were restricted to the perivenular zone and consisted of cholestasis with rosette formation. Intermediate-stage changes (5–14 months) included perivenular and then periportal fibrosis in addition to persistent cholestasis. Changes in the late stage (17–60 months) included progressive cholestasis and portoportal and portocentral fibrosis in seven patients, and cirrhosis in two of the patients. Inflammation and paucity of bile ducts were not seen. Although poorly documented and now largely disappeared, it is likely that the ‘Welsh cholestasis’ was also a form of FIC1 deficiency.
Also known as ‘progressive familial intrahepatic cholestasis type 2’ (PFIC2), BSEP deficiency is caused by mutations in ABCB11 , which encodes the human BSEP (known as BSEP or ABCB11). , The severe form presents in infancy, with a severe hepatitis, obvious giant cells ( Figs 3.48 and 3.49 ), periportal and perivenular sinusoidal fibrosis and eventually cirrhosis. There is usually marked intracellular retention of biliary pigment and considerable hepatocyte disarray. Byler bile is not seen; instead the bile is amorphous or finely filamentous, and microvilli are lost. Mallory–Denk bodies may be present. , , Individual mutations do not correlate with specific histological patterns. Milder forms of BSEP deficiency do not seem to constitute as large a proportion of later-onset disease as seen with FIC1 deficiency; however, there is also clearly a spectrum of disease, which may be considerably underestimated due to difficulties in clinical diagnosis. In the largest multicentre retrospective study to date on 264 patients, genotypic severity was graded into three categories (BSEP1, 2 and 3), the highest associated with the lowest predicted residual BSEP function. This classification predicted long-term native liver survival, risk of developing of HCC and the effect of surgical biliary diversion. A missense mutation in a sibling pair manifested in childhood with a BRIC phenotype but later progressed to advanced fibrosis. BSEP function was impaired, although its canalicular expression was normal, as demonstrated with immunofluorescence. Two cases of HCC in the cirrhotic liver of patients with PFIC , were reported before the characterization of the various subtypes; however, BSEP deficiency appears to be the main risk factor. Mutations predicted to cause a complete loss of this protein seem to be particularly associated with malignancy. HCC complicating BSEP deficiency may reflect the effect of multiple gene mutations. , Cholangiocarcinoma has been observed in two patients with BSEP deficiency.
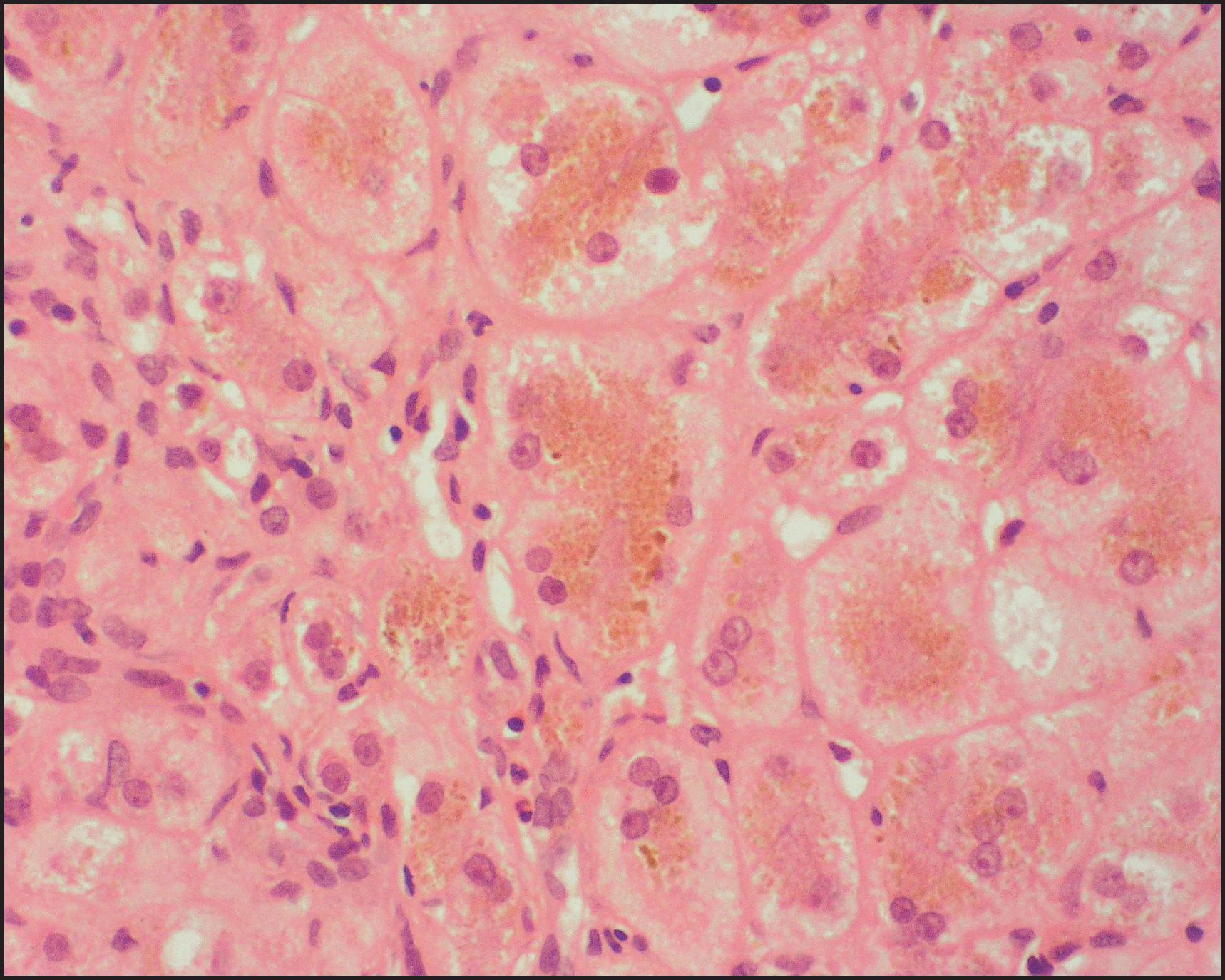
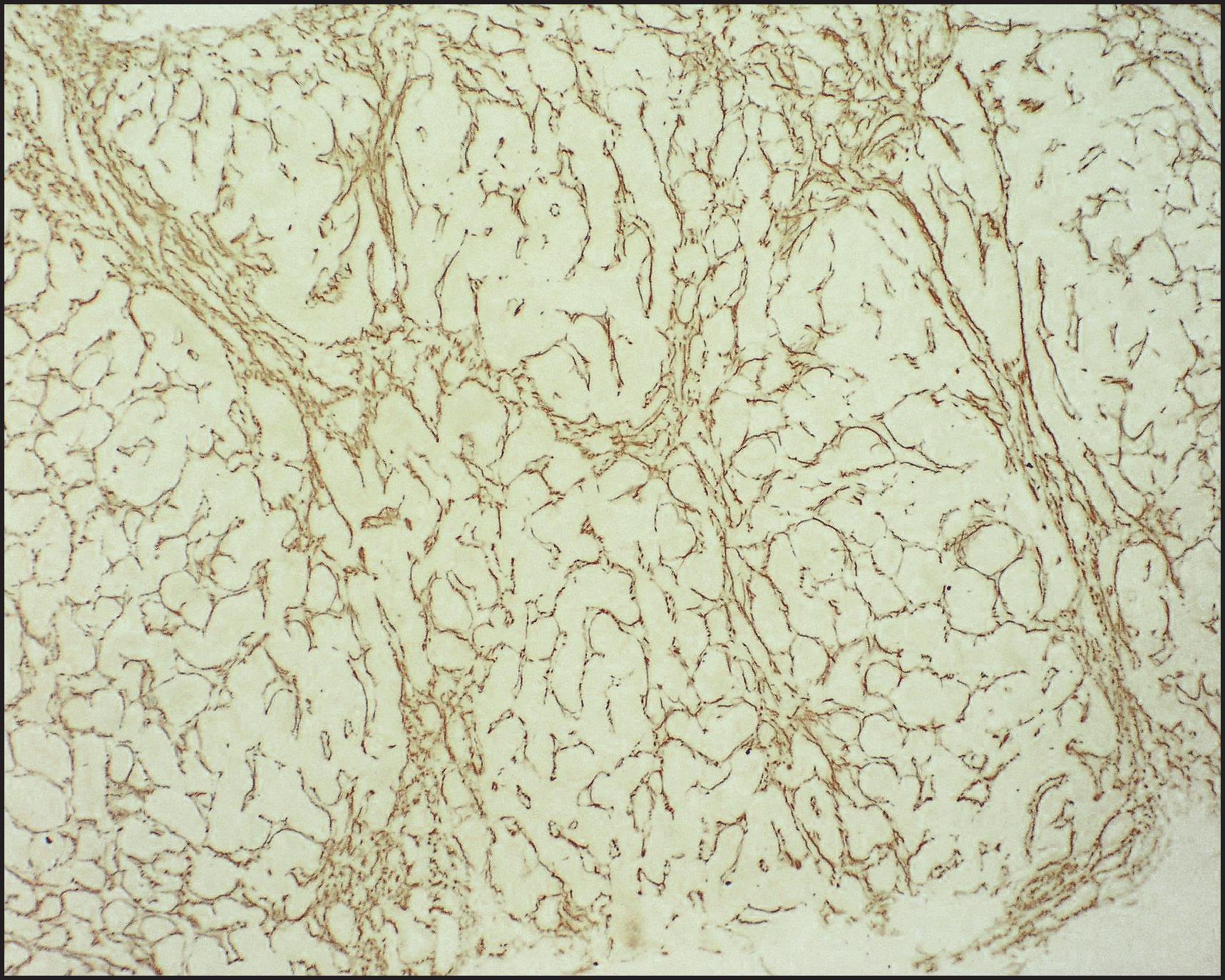
The histological features of BSEP deficiency are not specific. In an attempt to improve this, IHC methodology, using a number of anti-BSEP antibodies, has been developed ( Fig. 3.50 ). IHC may reveal no canalicular expression or focal, patchy and faint canalicular staining. Redistribution of BSEP expression from cytoplasmic to canalicular or de novo expression (retargeting) has been described in children treated with UDCA and biliary diversion.
![Figure 3.50, Bile salt export pump (BSEP) deficiency. The same case as shown in Figs 3.48 and 3.49 . Immunohistochemistry for BSEP shows totally absent marking at canalicular margins, whereas the control stains adequately (inset). (Peroxidase–antiperoxidase [PAP] technique.) Figure 3.50, Bile salt export pump (BSEP) deficiency. The same case as shown in Figs 3.48 and 3.49 . Immunohistochemistry for BSEP shows totally absent marking at canalicular margins, whereas the control stains adequately (inset). (Peroxidase–antiperoxidase [PAP] technique.)](https://storage.googleapis.com/dl.dentistrykey.com/clinical/DevelopmentalandInheritedLiverDisease/49_3s20B978070208228300003X.jpg)
Genetic liver diseases in which the gene is only expressed in the liver have generally been thought to be ideal candidates for LT. BSEP deficiency has recently highlighted a problem, which may have wider significance. Patients have been noted to occasionally have ‘recurrent disease’. It is only recently that the mechanism has been determined to be caused by the presence of antibodies against the ‘novel’ BSEP protein of the liver graft. It appears that the antibodies are capable of inhibiting the function of the protein. Various strategies have been employed to overcome the problem, but several patients have been retransplanted before the mechanism of apparently recurrent disease was understood.
Impaired expression of BSEP may occur as a consequence of other genetic abnormalities. In cholestasis associated with mutations in MYO5B , BSEP may be relatively underexpressed. With mutations in NR1H4 encoding the farnesoid X receptor (FXR), BSEP is undetectable. These disorders must be differentiated from BSEP deficiency due to ABCB11 abnormality.
This entity, previously termed ‘type 3 PFIC’, has been shown to be caused by a deficiency in MDR3, a floppase, caused by mutations in the encoding gene ABCB4 . , This protein is essential for the entry of the main phospholipid, phosphatidylcholine, into bile. In the absence of phospholipids, bile acids cannot form mixed micelles, and the bile is extremely hydrophobic. Much or all of the phenotype is probably due to this toxic bile, in particular the damage it causes to the biliary epithelium resulting in a cholangiopathy. Lipid clefts may be identified in the lumen of bile ducts. In severe cases the features seen include portal inflammation, bile ductular reaction and subsequent fibrosis ( Fig. 3.51A ). In the neonatal setting, conjugated hyperbilirubinaemia in the presence of elevated serum GGT values differs dramatically from the normal GGT levels usually observed in patients with FIC1 and BSEP disease. The histological differential diagnosis on liver biopsy specimens is with BA or other disorders manifesting in a biliary pattern. Copper and copper-binding protein accumulate considerably, and in excess of the levels usually observed with other cholangiopathies, potentially simulating WD. In the somewhat older child, the cardinal diagnostic finding is that cholangiography discloses normal biliary architecture despite the appearance of bile duct obstruction.
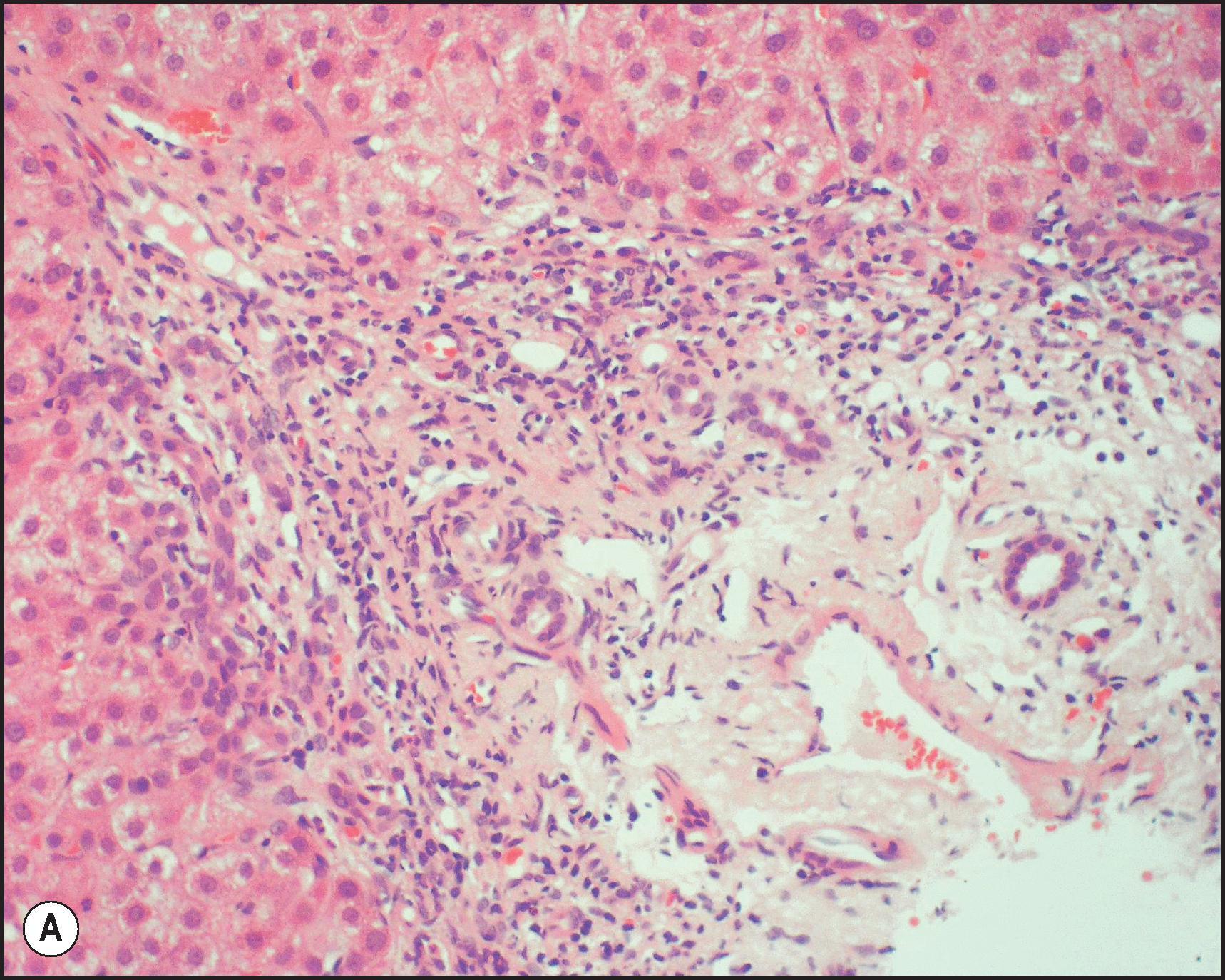
The result of partial loss of function of MDR3 may have more widespread clinical importance than the neonatal disease. Degiorgio et al. have shown that a substantial proportion of adults with cholestatic liver disease, including primary sclerosing cholangitis, have ABCB4 mutations. Progressive cholestatic liver failure in the adult members of a Transylvanian family was characterized histologically by a small duct cholangiopathy with ductopenia and associated with an ABCB4 , c.2362C>T (p.Arg788Trp). Heterozygosity for ABCB4 mutations has been observed in children with cholestatic liver disease. Furthermore, a reduction in biliary phospholipid not only results in parenchymal damage but also reduces the amount of cholesterol that may be maintained in solution. As with BSEP, anti-MDR3 antibodies have been raised. Although this may be a useful method of establishing a complete loss of protein ( Fig. 3.51B ), it is less clear-cut in milder cases, as also evident in cell lines. Partial loss of MDR3 function may lead to a more slowly progressive disease ( Figs 3.52 – 3.54 ), but may still require LT.

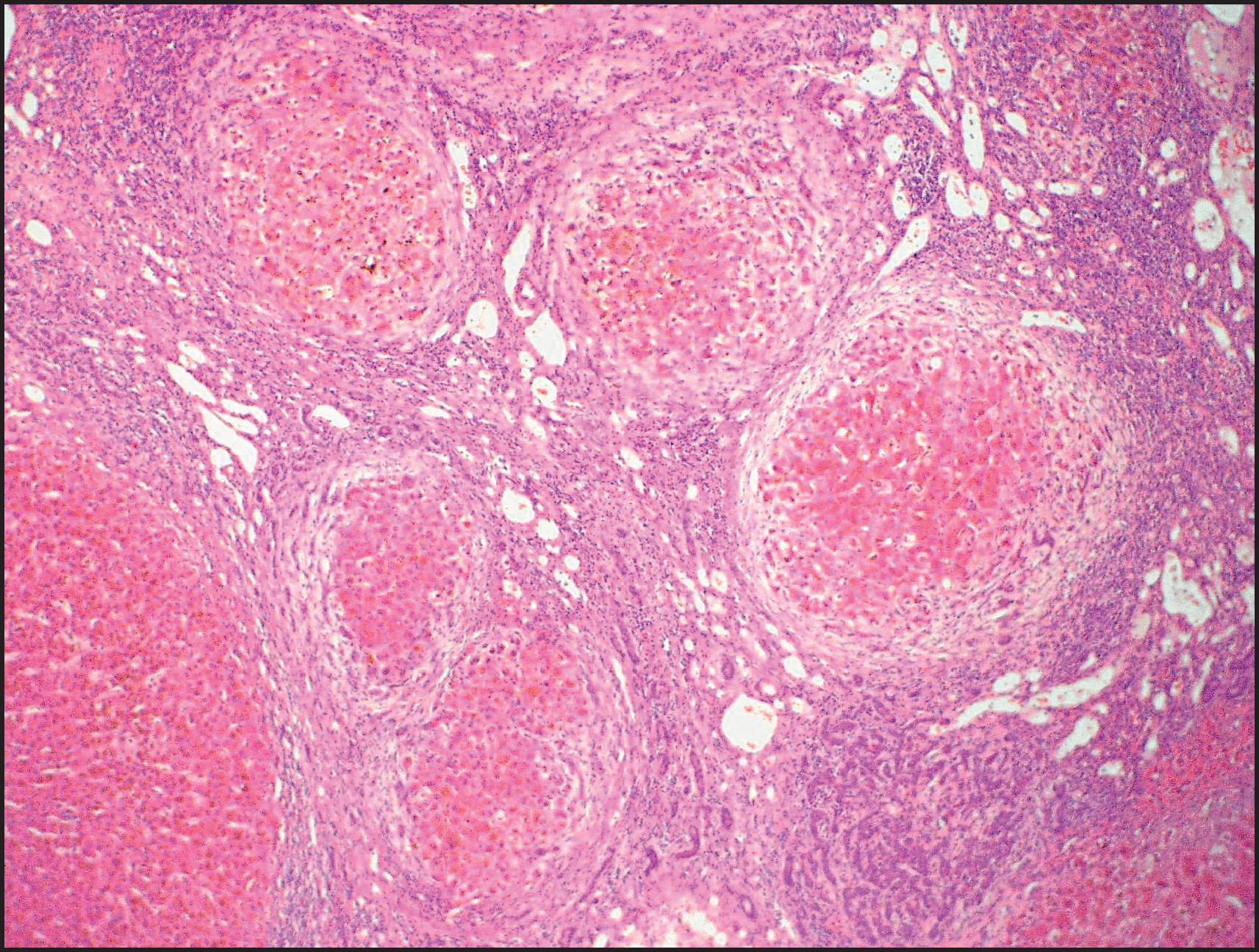
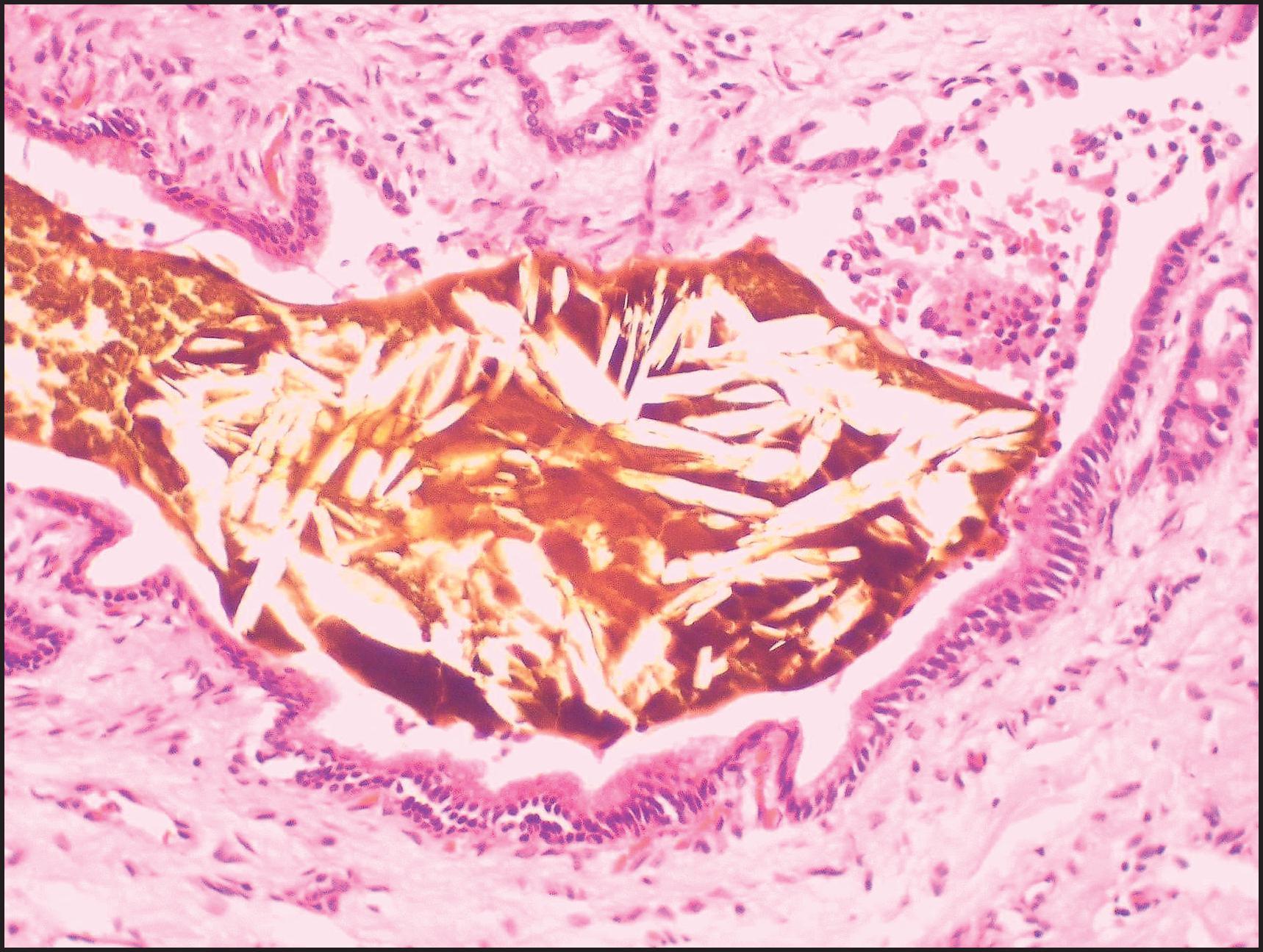
Cholestasis during pregnancy has also been reported in individuals heterozygous for mutations in ABCB4 (MDR3 deficiency). , Histopathological findings include bile ductular reaction with inflammation, resembling the changes in the mouse model. One diagnostic test is the detection of low bile phospholipid levels. When compared to the control bile samples of cholestatic children with sclerosing cholangitis (mean phospholipid, bile acid and cholesterol concentration of 29.1 mmol/L, 116.5 mmol/L and 3.4 mmol/L, respectively), the bile of patients with a PFIC3 phenotype had much lower phospholipid concentration (mean phospholipid, bile acid and cholesterol concentration of 1.4 mmol/L, 25.7 mmol/L and 0.74 mmol/L, respectively). In patients with MDR3 deficiency, the biliary bile salt/phospholipid and cholesterol/phospholipid ratios are approximately five times higher than control bile. Biliary phospholipids in MDR3 deficiency patients are 1–15% of total biliary lipids (normal range, 19–24%). The residual percentage relates to the severity of the mutation (<2% associated with nonsense frameshift mutations). Mutations in ABCB4 may also manifest as low-phospholipid-associated cholelithiasis syndrome, characterized by biliary symptoms before 40 years of age, intrahepatic microlithiasis and recurrence of biliary symptoms after cholecystectomy.
BRIC was also described in 1965. Although the name and the original description suggest a distinct condition, it is now understood that BRIC is really a form of bile canalicular transporter disorders. When associated with ATP8B1 mutations, it is called BRIC1, and when associated with ABCB11 mutations, BRIC2; its association with as yet undetermined transporter defects is still possible. The clinical definition of BRIC involves specific criteria: at least two episodes of jaundice separated by a symptom-free interval lasting several months to years; laboratory findings consistent with intrahepatic cholestasis, notably elevated serum GGT and total serum bile acids; and severe pruritus and normal intra- and extrahepatic bile ducts confirmed by cholangiography. The clinical presentation is usually jaundice with pruritus, but there is much interindividual variation in terms of severity, frequency and duration of episodes. Symptoms can be triggered by factors such as pregnancy, oral contraceptives, drugs or infection. There is an association with gallstones. Severe cholestasis in a young adult with polymorphism in ABCB11 and ATP8B1 preceded the diagnosis of Hodgkin lymphoma by 7 months. More recently variants in ABCB11 were suspected in instances of cholestasis in young men taking bodybuilding supplements. In one series, early onset of the first attack was associated with a more severe course, in terms of frequency and duration of attacks; some patients developed kidney stones, pancreatitis and diabetes. The authors proposed dropping the adjective ‘benign’ because of the profoundly detrimental effect that the disease has on the long-term quality of life of affected patients. The suggestion that BRIC is in fact not benign is supported by the finding that some cases do progress, if slowly. , There is a spectrum of severity, with some degree of genotype to phenotype correlation.
Histopathological observations of biopsy specimens from 22 patients at the milder end of the BRIC spectrum were detailed by Brenard et al. During attacks of jaundice, cholestasis (hepatocellular and canalicular) occurred in all patients, and infiltration of portal areas with mononuclear cells in seven patients. Other findings included focal mononuclear cell infiltration with or without focal necrosis, infiltration of portal areas with many eosinophils, ductular reaction (one patient) and periportal fibrosis (one patient).
The primary medical therapies used in the three main bile canalicular transporter disorders include UDCA and treatments for pruritus; response to medical therapy occurs but is variable. Some patients with FIC1 deficiency respond poorly to UDCA treatment. It may be of particular benefit in MDR3 deficiency, where bile salt hydrophobicity is thought to be particularly important in the disease pathogenesis; findings in the mouse model for MDR3 deficiency support this concept. Partial bile diversion by a cholecystojejunal cutaneous conduit and/or ileal diversion produces complete resolution of the liver disease, but the results are variable from one institution to another. Recent observations indicate that some BSEP genotypes show a more favourable response to partial external biliary diversion than others. The surgery must be performed before significant fibrosis has developed.
New treatment modalities are in development. The possibility of chemical chaperones for certain mutations is being explored. Specifically, 4-phenylbutyrate has been proposed as treatment for BSEP deficiency. Clinical trials are currently ongoing to investigate the efficacy of inhibitors of the IBAT (also referred to as ASBT, apical sodium-bile acid transporter, encoded by SLC10A2 ).
A wealth of data, mainly unpublished, regarding LT in bile canalicular transporter disorders exists. It appears to show generally good results in both BSEP deficiency and MDR3 deficiency. The results in FIC1 deficiency are less positive: in keeping with the widespread expression of the gene, extrahepatic manifestations, graft dysfunction and nutritional status can be problematic. In one series, hepatic transplantation was performed in 14 children with ‘Byler disease’. One patient died postoperatively from arterial thrombosis. In the remaining 13 patients, graft function, growth and quality of life were good after an average follow-up of 17 months, without evidence of disease recurrence. Steatosis and steatohepatitis may affect the liver allograft of patients transplanted for FIC1 deficiency. ,
NAIC is one of several disparate disorders identified as ribosomopathies, of which Blackfan–Diamond syndrome is perhaps the best known. Weber et al. studied 14 children, all from the Cree and Ojibwa–Cree tribes, with familial occurrence of neonatal hepatitis syndrome. Conjugated hyperbilirubinaemia occurred neonatally in nine children but disappeared before the end of the first year. Subsequent reports clarified that this was a high-GGT cholestatic disorder. On light microscopy, there was giant cell transformation and bilirubinostasis. Progressive liver damage was documented by persistently high levels of serum ALP, moderate elevation of aminotransferases, severe pruritus and morphologically by fibrosis and cirrhosis. Early portal hypertension and variceal bleeding necessitated portosystemic shunts in seven children. In some patients, LT has been required. Initial ultrastructural and immunohistochemical studies suggested that this group of children might represent a human model of microfilament dysfunction-induced cholestasis.
Investigation of the molecular genetics of NAIC has clarified its pathogenesis. The disease locus was mapped to chromosome 16q22. A single mutation was identified in all affected individuals in a gene labelled CIRH1A . The encoded protein, cirhin, is a nucleolar protein. Cirhin, also known as human Utp4, is prominent in ribosomal biogenesis. It interacts with the nucleolar protein NOL11, and the complex has a role in ribosomal 18S rRNA maturation. The common mutation found in patients with NAIC disrupts that interaction. A zebrafish model suggests a role in biliary development mediated via a p53-dependent process. Pathogenetic mechanisms involving p53 are implicated in some other ribosomopathies. Cirhin may have other actions in hepatocytes leading to cell damage.
In familial hypercholanaemia, clinical jaundice is classically uncommon in affected infants, who usually present clinically with nutritional sequelae of chronic cholestasis, such as failure to thrive, fat-soluble vitamin deficiency, rickets and bleeding tendency. Pruritus may be severe. Serum GGT is normal or low, but serum bile acids are elevated.
The genetic defect in familial hypercholanaemia is complex. Most cases exhibit oligogenic inheritance. The genes involved are the tight junction protein 2 gene ( TJP2 , previously known as ZO2 ) and BAAT. Some patients are homozygous for a disease-causing mutation in TJP2 and have normal BAAT ; others have mutated TJP2 plus they are heterozygous for a mutation in BAAT ; still others are homozygous for a mutation in BAAT and have normal TJP2 . Some children are homozygous for the TJP2 mutation, with normal BAAT , and they are entirely asymptomatic. TJP2 encodes a tight junction protein (TJP2, formerly ZO-2) and BAAT encodes the protein bile acid CoA:amino acid N -acyltransferase, which plays an important role in amidation, the process by which bile acids are conjugated to taurine or glycine (see earlier). The postulated mechanism is that bile acids do not enter the bile because of BAAT mutation or leak back into plasma because of faulty tight junctions caused by TJP2 mutation. Other genes have not been excluded. Mutations in the microsomal epoxide hydrolase gene ( EPHX1 ) have also been reported.
Truncating mutations in TJP2 can produce a much more severe clinical phenotype. Affected children present in the first 3 months of life with severe cholestatic liver disease and low serum GGT (without mutations in ATP8B1 and ABCB11 ). They were found to have homozygous mutations on TJP2 in the absence of BAAT mutations. In these patients, claudin 1 is normally expressed in the cytoplasm but cannot co-localize at tight junctions without TJP2. These children have unfavourable outcomes: the majority have required LT.
Recent studies have shown that pathogenetic variants of USP53 encoding ubiquitin carboxyl-terminal hydrolase 53, a protein which interacts with TJP2, may be associated with a low-GGT cholestatic disorder. In one report, affected individuals presented with jaundice before the age of 7 months; treatment included UDCA and cholestyramine; jaundice resolved by age 2 years. Other observations indicate the potential for chronic liver disease. Some children had deafness. Hearing loss was reported in another affected kindred.
Likewise, BAAT mutations may be associated with more severe liver disease, classified as an amidation disorder of bile acid synthesis (see earlier).
This disorder, which is caused by an abnormal mitochondrial protein ( citrin, a mitochondrial aspartate-glutamate carrier; see urea cycle disorders later), encoded by the gene SLC25A13 on chromosome 7q21, has different clinical presentations dependent on age. Infants with citrullinaemia type II typically present with infantile conjugated hyperbilirubinaemia. The features are distinctive enough to be called ‘neonatal intrahepatic cholestasis with citrin deficiency’ (NICCD). This name is misleading, however, in that the degree of hepatic inflammation can be very severe. Moreover, the infant can have various metabolic defects affecting glucose metabolism (resulting in hypoglycaemia), urea synthesis and fatty acid synthesis. The infant may appear to have galactosaemia due to decreased uridine diphosphate (UDP)-galactose epimerase activity, but in citrullinaemia type II, measurement of serum amino acids shows elevated plasma levels of citrulline and methionine. , Liver biopsy may display steatosis in addition to inflammatory changes.
Although primary sclerosing cholangitis can present clinically in infancy, it is extremely rare for it to present in the first 6 months of life. By contrast, some infants with neonatal conjugated hyperbilirubinaemia clear the jaundice and experience a period of apparent recovery. Later, cholestasis recurs, and imaging of the biliary tree reveals an appearance similar to that of primary sclerosing cholangitis. These infants are said to have NSC. It was first described in 1987, with subsequent reports. , Serum GGT is elevated. The disease mechanism is likely multiple, and it has not been determined for every case. Affected infants should not undergo a Kasai portoenterostomy; medical management is that of childhood chronic cholestatic liver disease; with the development of end-stage biliary cirrhosis, LT is the required intervention.
The genetic basis of NSC is increasingly being characterized. In neonatal ichthyosis-sclerosing cholangitis (NISCH) affected infants have prominent cutaneous features (scanty scalp hair, scarring alopecia, ichthyosis) as well as hepatic findings of NSC. It involves mutations in the gene CLDN1 (found on chromosome 3q27-q28), which encodes claudin-1, a tight junction protein. Biliary damage may be caused by disturbed regulation of hepatic paracellular permeability. The severity of the neonatal liver disorder may resemble biliary atresia BA. Serum GGT level is increased. Liver biopsy shows features of duct obstruction. Seven of 24 NSC patients were found to harbour truncating variants in the doublecortin domain containing 2 gene ( DCDC2 ), encoding a protein expressed in cholangiocyte cilia. Lack of primary cilia and expression of DCDC2 and the ciliary protein acetylated α-tubulin (ACALT) were shown by EM and IHC. More recently, likely deleterious variants in KIF12 , an orphan member of the kinesin family of molecular motors, were found in three families with a clinical picture of NSC. Of note, KIF12 function, together with PKHD1 , is regulated by HNF1β.
The full-blown arthrogryposis, renal dysfunction and cholestasis (ARC) syndrome involves skeletal changes, renal tubular dysfunction and cholestasis, and some infants also have severe ichthyosis, CNS deformity (lissencephaly) and prominent growth failure. Approximately 10% have cardiac defects. However, ARC syndrome is frequently incomplete, lacking the skeletal changes. Infants with incomplete ARC may present with conjugated hyperbilirubinaemia plus renal tubular dysfunction. This appears to be a disorder characterized by normal serum GGT. The genetic abnormality is twofold. Some patients have germline mutations in the gene VPS33B on chromosome 15q26, encoding a protein that regulates vesicular membrane fusion by interacting with SNARE proteins. Others have mutations in VIPAS39, which encodes the VIPAR protein. Mutations in VPS33B are approximately twice as frequent as those in VIPAS39 .
The histological pattern in the liver includes giant cell transformation of hepatocytes, lobular cholestasis and paucity of bile ducts. , However, liver biopsy can be hazardous in ARC syndrome because of intrinsic platelet dysfunction severe enough to lead to haemorrhage after liver biopsy ; consequently, mutation analysis is the diagnostic test of choice. The prognosis for patients with ARC syndrome is generally poor, with limited survival beyond infancy. Cirrhosis may develop. Milder ARC syndromes with longer survival have been described.
Three of four adult siblings in a family studied for three generations had clinical and/or laboratory evidence of slowly progressive intrahepatic cholestasis. Slight hyperpigmentation, facial hypertrichosis and hypothyroidism were seen in affected individuals, who also had prolonged increases in serum aminotransferases, GGT and ALP levels. A biopsy of one patient who was jaundiced showed cholestasis and other changes indistinguishable from those of extrahepatic obstruction. Asymptomatic intervals were characterized by abnormal BSP retention, reduced N -demethylation capacity, elevated fasting total bile acid levels and normal light microscopic findings. A high serum α-lipoprotein level was found in affected individuals. The inheritance is believed to be autosomal recessive. A defect in prekeratin–keratin metabolism is postulated to account for the lesions in liver and other tissues.
This syndrome is characterized by café-au-lait spots, polyostotic fibrous dysplasia and sexual precocity. Silva et al. reported two patients with McCune–Albright syndrome (MAS) who presented with neonatal conjugated hyperbilirubinaemia. Despite the severity of presentation, which can resemble BA on a nondraining hepatobiliary scan, both patients cleared their jaundice in 6 months but continued to have mild LFT abnormalities. The patients have been shown to carry an activating mutation in the gene encoding the α-subunit of the G protein. , The protein stimulates adenylcyclase in liver tissue, suggesting that this metabolic defect could be responsible for their cholestatic syndrome. Serum GGT is elevated in MAS. A variety of hepatobiliary and pancreatic lesions have been observed in MAS patients, including inflammatory hepatocellular adenomas, atypical focal nodular hyperplasia and hepatoblastoma.
Also known as lymphoedema cholestasis syndrome type 1 (LCS1), Aegenaes syndrome is a rare disorder with limb oedema and cholestasis, originally reported in Norwegian kindreds. , , Infants present clinically with conjugated hyperbilirubinaemia and elevated serum GGT along with a lymphatic disorder, typically lower-limb oedema, although a generalized oedema or lymphangiomas may occur. The intrahepatic cholestasis may be severe, with fat-soluble vitamin deficiencies and pruritus. The neonatal hepatitis syndrome may predate the lymphatic disorder and resolve before the lymphatic component is evident. The typical course is relapsing with intermittent bouts of cholestasis. Progression to end-stage liver disease is uncommon; however, a few young children have required LT. HCC was reported in a teenager who was thought to have LCS1. In adulthood the lymphatic disorder predominates. The disease mechanism is unknown; the gene has been localized to chromosome 15q, but there may be genetic heterogeneity.
Some patients with microvillus inclusion disease (MVID), associated with mutations in the MYO5B gene, develop intrahepatic cholestasis with jaundice, severe pruritus, normal serum GGT and increased serum bile acid concentrations. Importantly, MYO5B disorder can be associated with cholestasis without MVID being present. , The cholestatic disorder shows low/normal γGT. Trafficking of ABC transporters such as BSEP and MDR3 from trans-Golgi to the canalicular membrane via endosome depends on the interaction between MY05B and RAB978-0-7020-8228-3. MY05B deficiency thus impairs canalicular bile secretion. , In patients with MVID, the degree of cholestasis may worsen after intestinal transplantation.
Very rare patients with defects in NR1H4 encoding for farnesoid X receptor (FXR) have presented neonatally with a clinical and histological picture similar to BSEP (ABCB11) deficiency. , Bile acids are the natural ligand for FXR, and in turn FXR regulates ABCB11 . The disorder shows low γGT and undetectable BSEP; severe coagulopathy may reflect the role of FXR in regulating coagulation. Implicated mutations in NR1H4 result in loss of FXR function.
A recent study has shown two other potential candidate genes, WDR83OS and lipolysis-stimulated lipoprotein receptor ( LSR ). The patient with a novel variant of the LSR gene also had low GGT. Mutations in SCYL1 were found in children with episodes of low-GGT cholestasis triggered by infection (characterized as ‘ALF’) and progressive neurodegeneration: hepatic involvement was mainly found after the neonatal period. Histological changes included advanced fibrosis, microvesicular steatosis and depletion of SCYL1 by IHC. TEM showed enlarged Golgi cisternae. The extensive diversity within the genetic basis for inherited cholestatic disorders is thus becoming apparent.
Patients present with asymptomatic jaundice from elevated serum unconjugated bilirubin (Crigler–Najjar syndrome I and II, Gilbert syndrome) or conjugated bilirubin (Dubin–Johnson syndrome, Rotor syndrome). Bilirubin metabolism is discussed in Chapter 9 ; see also reviews by Bosma and Erlinger et al.
Crigler–Najjar syndrome is characterized by severe nonhaemolytic unconjugated hyperbilirubinaemia beginning in the newborn period. It was named after the physicians who provided the first description in 1952. Patients with Crigler–Najjar syndrome type I can develop kernicterus at any age. The diagnosis should be entertained in the first 3 days of life. Levels of unconjugated bilirubin are usually greater than 20 mg/dL and may be as high as 50 mg/dL before therapy. Routine LFTs are in the normal range.
Crigler–Najjar syndrome type I is rare, with an estimated incidence of 1 per 1 million general population. Inheritance is autosomal recessive. Patients lack the enzyme UDP-glucuronosyltransferase completely and consequently are unable to conjugate bilirubin. Mutations in exons 2–5 of the UGT1A gene affect all isoforms influencing glucuronidation of bilirubin and other substrates (type Ia). Mutations of exon 1 affect bilirubin glucuronidation only (type Ib).
Therapy is difficult and limited. Patients do not respond to phenobarbital. Phototherapy has been used long-term with plasmapheresis for acute bilirubin elevations and, if initiated promptly, such therapy can reverse early bilirubin encephalopathy. , Therapeutic effectiveness decreases during adolescence. , Currently, light-generating blankets or mattresses have proved effective. LT results in a cure. , , This can also be achieved by an orthotopic auxiliary transplant. Experimental hepatocyte transplantation has decreased the hours needed for phototherapy. Gene therapy is under investigation. The Gunn rat has been a valuable animal model.
The histopathology of the liver in Crigler–Najjar syndrome type I is characterized by intrahepatic cholestasis ( Fig. 3.55 ), , but the liver may be normal. Of 15 UGT1A1 c.222C>A homozygotes (median age, 14.6 years; range, 4.4–23.6 years), 9 had mild to severe fibrosis during long-term follow-up.
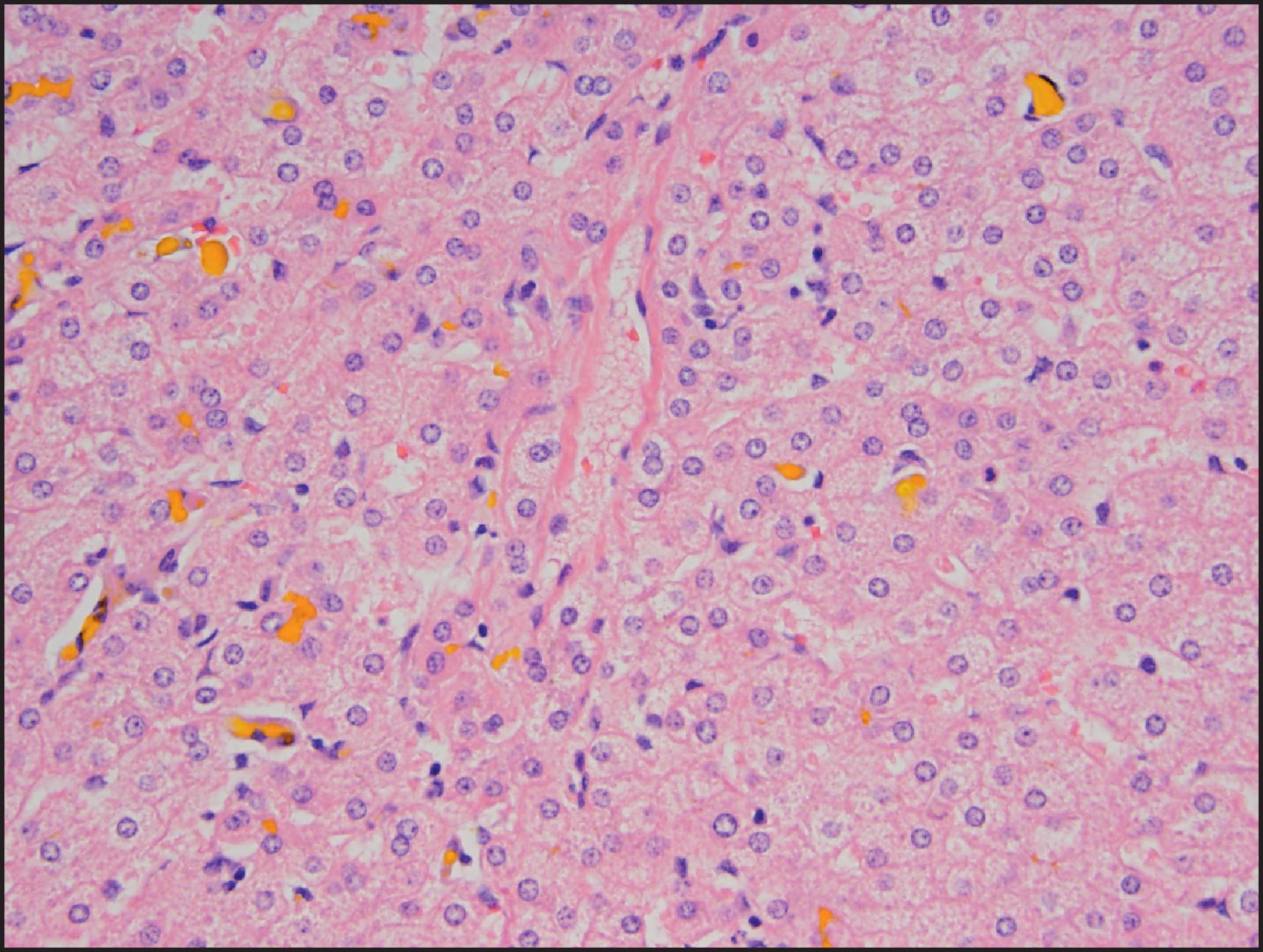
Crigler–Najjar syndrome type II appears to be a milder variant of type I. It is also characterized by unconjugated hyperbilirubinaemia. The deficiency of bilirubin UDP-glucuronosyltransferase is less severe, but the mechanism of this difference is not entirely clear.
Arias et al. described Crigler–Najjar syndrome type II in 1969. Patients present with neonatal jaundice, but the jaundice is less severe than in patients with type I. Serum bilirubin levels fluctuate between 7 and 20 mg/dL. These patients generally do not develop the severe intellectual compromise of type I patients, although during an intercurrent illness, serum bilirubin levels may rise to 40 mg/dL and cause kernicterus.
Crigler–Najjar syndrome type II is inherited as an autosomal recessive disease, although occasional patients with autosomal dominant inheritance have been reported. Type II patients also have significantly reduced levels of bilirubin UDP-glucuronosyltransferase 1. Analysis of bile before administration of phenobarbital demonstrates the presence of significant quantities of bilirubin monoglucuronide, which are not seen in type I disease. Patients with Crigler–Najjar II and significant bilirubin elevations can be distinguished from type I patients by the response to phenobarbital: in type II patients, the drug lowers the serum bilirubin by 30%.
Gilbert syndrome is a mild, and usually intermittent, form of unconjugated hyperbilirubinaemia. Patients often present at puberty, when jaundice is first noticed. Bilirubin levels increase following starvation or other stress. In newborns it can exacerbate neonatal jaundice in the first 2 days and can contribute to higher bilirubin levels in patients with concomitant glucose-6-phosphate dehydrogenase (G6PD) deficiency. Mild haemolysis and hepatocyte bilirubin uptake defects have been found. , Liver enzymes are in the normal range.
Gilbert syndrome is the most common genetic bilirubin defect in White populations. , The incidence is 3–16% of the population; inheritance is autosomal recessive. Hepatic bilirubin UDP-glucuronosyltransferase-1 activity may be 25% of normal. Mutations have been found in both the promoter and coding regions of the UDP-glucuronosyltransferase-1 gene in patients with Gilbert syndrome, resulting in reduced enzyme expression.
As with Crigler–Najjar syndrome type II, analysis of bile before administration of phenobarbital demonstrates the presence of significant quantities of bilirubin monoglucuronide. Diagnosis of Gilbert syndrome is clinical. Liver biopsy, even if direct measurement of bilirubin UDP-glucuronosyltranserfase can be performed, is not justified.
No therapy is necessary for Gilbert syndrome. Prolonged fasting should be avoided. A primate animal model has been reported. Increased lipofuscin pigment accumulation in liver cells has been described in Gilbert syndrome. The pigment granules do not exhibit the coarse granularity of those of the Dubin–Johnson syndrome. Ultrastructurally, hepatocytes reveal hypertrophy of the SER.
Dubin–Johnson syndrome was first described in 1954 by Dubin and Johnson and Sprinz and Nelson. Dubin subsequently reviewed in detail 50 cases reported up to 1958. Patients typically present with asymptomatic conjugated hyperbilirubinaemia, but they have normal liver enzymes and no other evidence of hepatic dysfunction. Some patients have hepatomegaly and abdominal pain. Cholecystitis associated with minute bilirubinate stones may develop.
Elevations of predominantly conjugated bilirubin range from 2 to 5 mg/dL. Sulphobromophthalein is retained in these patients, with levels increased at 60–90 minutes over those at 45 minutes after intravenous (IV) administration. In urine the total coproporphyrin level is normal, but isomer I is >80%, which is characteristic of the syndrome.
Age of onset may be as late as the sixth decade. Disease onset may occur during pregnancy or in relation to taking oral contraceptives. Dubin–Johnson syndrome is rare in infancy, , except in an inbred population of Iranian Jews, where the incidence is as high as 1 in 1300. Inheritance is autosomal recessive, with reduced penetrance in females. The underlying defect is complete absence of the cMOAT. The ABCC2 gene is localized to chromosome 10q24. It was cloned in humans in 1996. Human multidrug-resistance protein 2 ( MRP2 ), is the same gene with 32 exons. Multiple gene mutations have been documented and can be detected in fibroblasts. , , On liver biopsy, IHC for cMOAT (MRP2 protein, also called cMOAT for multidrug organic anion transporter or ABCC2 for ATP-binding cassette subfamily C member 2) reveals a complete absence of staining ( Fig. 3.56C ). Animal models include the Corriedale sheep and the transport-deficient (TR) rat. Pigment accumulation resembling that of the Dubin–Johnson syndrome has been described in the howler monkey.
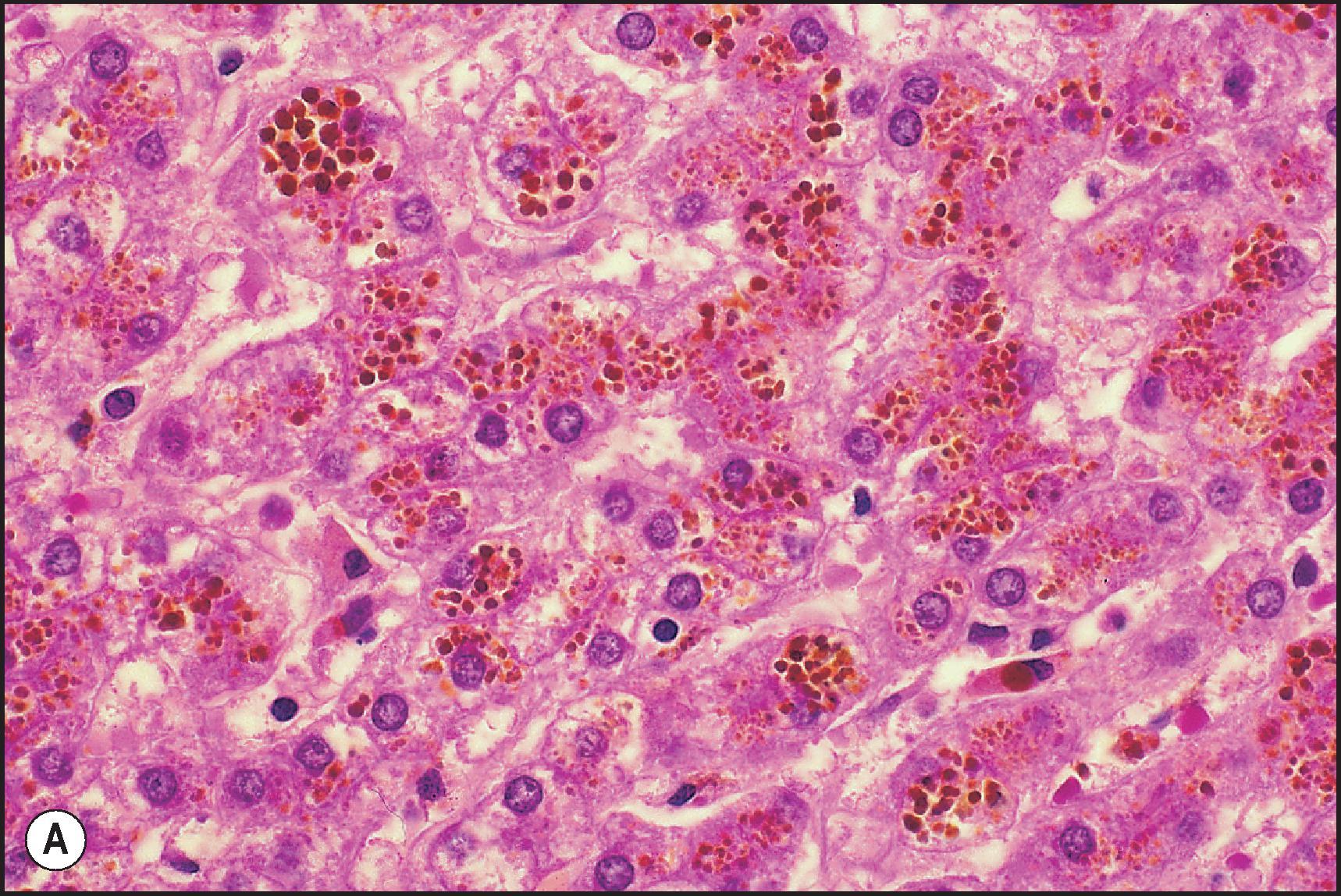
Accumulation of a coarsely granular brown pigment, called lipomelanin , in liver cells is characteristic of the Dubin–Johnson syndrome , ( Fig. 3.56A ). Lobular distribution is diffuse, although more heavily concentrated in the perivenular zone. The accumulation of the pigment in hepatocytes gives the liver a grey to black colour grossly, although this feature may be less prominent in younger patients. Thus the gross (black) colour of the liver biopsy core may be diagnostic. The pigment shares some of its physicochemical properties with lipofuscin and melanin in that it is Oil Red O-positive (in frozen sections), stains black with the Fontana stain ( Fig. 3.56B ), is variably PAS-positive and is autofluorescent when examined by ultraviolet microscopy. Ultrastructurally, the Dubin–Johnson pigment is lysosomal and thought to differ from lipofuscin in being pleomorphic and having a more variegated appearance and less lipid. , An electron spin resonance spectroscopy study suggested that the pigment is not melanin but rather is a stable free radical with unusual properties. Phillips et al. consider the lysosomes distinctive ultrastructurally in having very dense areas as well as moderately electron-dense, finely stippled areas.
A form of conjugated hyperbilirubinaemia, Rotor syndrome was reported in 1948. Although clinically similar to the Dubin–Johnson syndrome, there is no abnormal liver pigmentation. Total coproporphyrin excretion in the urine is increased, and isomer I is elevated but is less than 80% of the total. Inheritance is autosomal recessive and related to homozygous variants in both the SLCO1B1 and SLCO1B3 genes on chromosome 12, encoding for organic anion transporting polypeptide 1B1 and 1B3 ( OATP1B1 , OATP1B3 ). These proteins mediate reuptake across the sinusoidal hepatocellular membrane of several compounds including bilirubin glucuronide. , Hepatobiliary scanning reveals either no or minimal visualization of the liver, a clue to the diagnosis. There is no pigment storage or other morphologic change in Rotor syndrome. A number of ultrastructural changes were described by Phillips et al., including distinctive ‘two-tone’ lysosomes.
The porphyrias comprise eight disorders of the biosynthesis of porphyrins and haem that lead to the excessive accumulation and excretion of porphyrins and porphyrin precursors. Caused by a defect in one of the eight enzymes involved in haem synthesis, porphyrias are classified into hepatic or erythropoietic or, more recently, as acute hepatic and non-acute, depending on where overproduction of the haem precursors takes place. Acute hepatic porphyrias are associated with primary liver cancer, in particular HCC. Only two of the porphyrias importantly affect the liver: porphyria cutanea tarda (PCT) and the erythrocytic protoporphyrias. Severe liver injury is increasingly recognized in association with X-linked protoporphyria, the variability of its manifestations probably depending on random X chromosome inactivation. LT is used to treat the liver disease complicating porphyria or to correct the genetic defect causing intractable neurovisceral involvement. Lead poisoning affects three enzymes involved in the synthesis of haem and causes an increase in porphyrin and its precursors. Severe hepatobiliary disorders can cause asymptomatic elevation in porphyrins.
The classification, clinical aspects and therapy of PCT and other porphyrias are covered in several comprehensive reviews. , PCT is classified as sporadic or familial. Most patients with PCT have the sporadic form (type I). Decreased activity of uroporphyrinogen decarboxylase is restricted to the liver, and the family history is negative. Familial (type II) PCT, accounting for approximately 20% of cases of PCT, is inherited as an autosomal dominant trait. The gene ( UROD , encoding enzyme hepatic uroporphyrinogen decarboxylase) is located on chromosome 1p34. The enzyme defect (50% reduction of activity) is present in all tissues. Clinical penetrance is low, with less than 10% of affected persons developing symptoms. Of note, some familial PCT occurs with mutations in gene(s) other than UROD ; this is classified as PCT type III. Familial (type II) PCT has an earlier onset than sporadic (type I) PCT, with some cases presenting in childhood.
Sporadic (type I) PCT occurs typically in male patients between 40 and 50 years of age. Precipitating factors in the sporadic form include toxins (e.g. hexachlorobenzene), oestrogens, alcohol, mutations in the HFE gene and viral infection. , An isolated case of PCT has followed IFN-alfa therapy of hepatic metastases. The interaction of the HFE mutation and susceptibility to sporadic PCT has been discussed in several studies. Mild to moderate iron overload is found in 60–70% of patients with PCT; 10% have increases in the range of hereditary haemochromatosis. In almost all studies, a high prevalence of HFE gene mutations has been detected, with differences between patients of Northern European ancestry and those of Mediterranean origin. In a U.S. study, 19% of the PCT patients tested were homozygous for the C282Y mutation, and 7% were compound heterozygous (0.5% and 1.0%, respectively, in the general population). Viral infections may trigger PCT. Initial reports documented a high incidence (40%, 57% and 70.7% in three separate series) of hepatitis B serological markers in PCT and anecdotal cases of HIV infection. The strong association between hepatitis C virus (HCV) and PCT is most important. , In the series reviewed by Elder, the prevalence of antibodies to HCV in PCT has ranged from 9% to 79%. This is broadly related to the level of endemicity of HCV infection in the general population in various countries. A subsequent meta-analysis allowed Gisbert et al. to conclude that the mean prevalence of HCV infection, detected by PCR in PCT patients, is approximately 50%, much higher than that reported in the general population. Prevalence similarly varied depending on the country and the type of PCT (57% in sporadic, 25% in familial). It must be noted that HCV infection in itself is unlikely to derange the haem synthesis pathway; only a small proportion of HCV-infected patients show an increase in urinary porphyrins, and the prevalence of PCT among HCV-infected patients is 1–5%. , Treating PCT patients with chloroquine apparently does not affect the course of the underlying liver disease.
Histopathological findings in the liver in PCT include the accumulation of uroporphyrin in the cytoplasm of hepatocytes. Needle-shaped cytoplasmic inclusions have been identified in hepatocytes by light microscopy, fluorescent microscopy and EM in biopsy specimens; the crystals are also birefringent under polarizing light. , According to Cortes et al., the inclusions are best seen by light microscopy in unstained paraffin sections. The crystals can be specifically stained in paraffin sections by the ferric ferricyanide reduction test ( Fig. 3.57 ). They are often found close to ferritin-like deposits. Morphometric studies have shown significantly greater amounts of uroporphyrin crystals in familial than in sporadic PCT. The crystals have been induced experimentally in mice by iron overload. Ultrastructurally, they reveal alternating areas of differing electron density. The crystals appear to be a mixture of porphyrinogens and porphyrins surrounding uroporphyrin crystals. Other changes in liver biopsy specimens from patients with PCT have been described in several series. , , They include steatosis, variable haemosiderosis in periportal hepatocytes and fibrosis. Cortes et al. found periductal lymphoid aggregates in 43% of their cases. The incidence of cirrhosis in two series was 33% and 34%. , It should be emphasized that tests for chronic viral hepatitis, particularly chronic HCV infection, were not performed in these series.
HCC has been reported in association with PCT , , and also in patients with acute intermittent porphyria, variegate porphyria and hereditary coproporphyria. The risk in patients with acute hepatic porphyria is high particularly after 50 years of age. Factors related to an increased risk of HCC in PCT are a long symptomatic period before start of therapy, chronic hepatitis (particularly hepatitis C), iron overload and advanced fibrosis or cirrhosis. In one series, there was a direct relationship between increasing age and extent of liver damage, with fibrosis present at a mean of 48 years, cirrhosis at 57 years and HCC at 66 years. A somatic second-hit mutation has been proposed as the critical mechanism for the development of HCC in acute porphyrias.
EPP was first reported in 1926, with further definition of the dermatological manifestations in 1961, and liver disease reported in 1965. Clinical presentation begins with photosensitivity, usually before 6 years of age; patients have an extreme burning sensation with erythema and swelling. The onset of liver disease is usually later; most cases are diagnosed after 30 years of age, although liver disease can become evident in adolescence. Males are affected twice as often as females. Patients with EPP often present with right upper quadrant pain radiating to the back. Cirrhosis develops in only 1–10% of patients. , There is no evidence of biliary obstruction. Death occurs within 3–5 months (range, 1 month to 2 years) following the development of jaundice. A few patients with EPP have exhibited haemolysis and neurological dysfunction.
Ferrochelatase , the defective enzyme, catalyses the insertion of iron into protoporphyrin as the final step in haem synthesis. Functional enzyme levels are usually less than 50%. The diagnosis of EPP is by measurement of elevated plasma, erythrocyte and faecal protoporphyrin. Cord blood analysis is not a reliable prognostic tool. The bone marrow contributes 80% of the protoporphyrins and the liver up to 20%. Protoporphyrin is poorly water soluble and is secreted by hepatocytes only into bile. When this hepatobiliary transport is overwhelmed, insoluble aggregates of protoporphyrin form crystals in hepatocytes, canaliculi and proximal bile ducts, resulting in ‘black liver disease’. Autosomal dominant inheritance is found in most families, but autosomal recessive inheritance has been documented. The incidence of EPP is 1 in 75,000 to 1 in 200,000 live births worldwide.
Approximately 90 different mutations have been identified in the ferrochelatase gene, localized on chromosome 18, including point mutations, nucleotide deletions and a partial chromosome deletion. , The ferrochelatase gene is composed of 11 exons and 10 introns with a size of approximately 4.5 kilobases (kb), and the enzyme is localized to the matrix side of the inner mitochondrial membrane. Patients with EPP who develop liver disease usually have a mutation in one ferrochelatase allele that alters enzyme function, together with a polymorphism in the nonmutant allele that causes low gene expression. This results in a significant increase in the hepatobiliary excretion of protoporphyrin, which can damage the liver through both cholestatic injury and oxidative stress.
Haematin has been the most successful medical therapy. , , , LT is appropriate for patients with liver failure or end-stage cirrhosis. , However, recurrence of protoporphyric liver disease has been reported. Bone marrow transplantation (BMT; stem cell transplant) is needed after LT to prevent disease recurrence in younger patients. A mouse model is available for future evaluation of therapy. The hepatobiliary alterations in this murine model have been reversed by BMT. It has thus been suggested that BMT may be an option for EPP patients at risk of developing hepatic complications. A recent study has confirmed that bone marrow-derived cells play a significant role in restoring and regenerating hepatic tissue in this mouse model.
Macroscopically, the liver affected by EPP is black. The first description of the characteristic, if not pathognomonic, changes was published by Cripps and Scheuer. Sections from percutaneous hepatic biopsy specimens from five patients showed focal accumulations of a dense, dark-brown pigment in canaliculi, interlobular bile ducts, connective tissue and Kupffer cells ( Fig. 3.58 ). The pigment had an intense red autofluorescence in frozen sections examined by fluorescence microscopy with an iodine-tungsten-quartz light source. Two of the patients had portal and periportal fibrosis, and in one case, the portal areas were infiltrated by mononuclear cells. Cholelithiasis occurs in some patients with EPP, and the calculi contain protoporphyrin. Crystals isolated from the liver in one EPP patient had the same fluorescence spectrum as protoporphyrin.
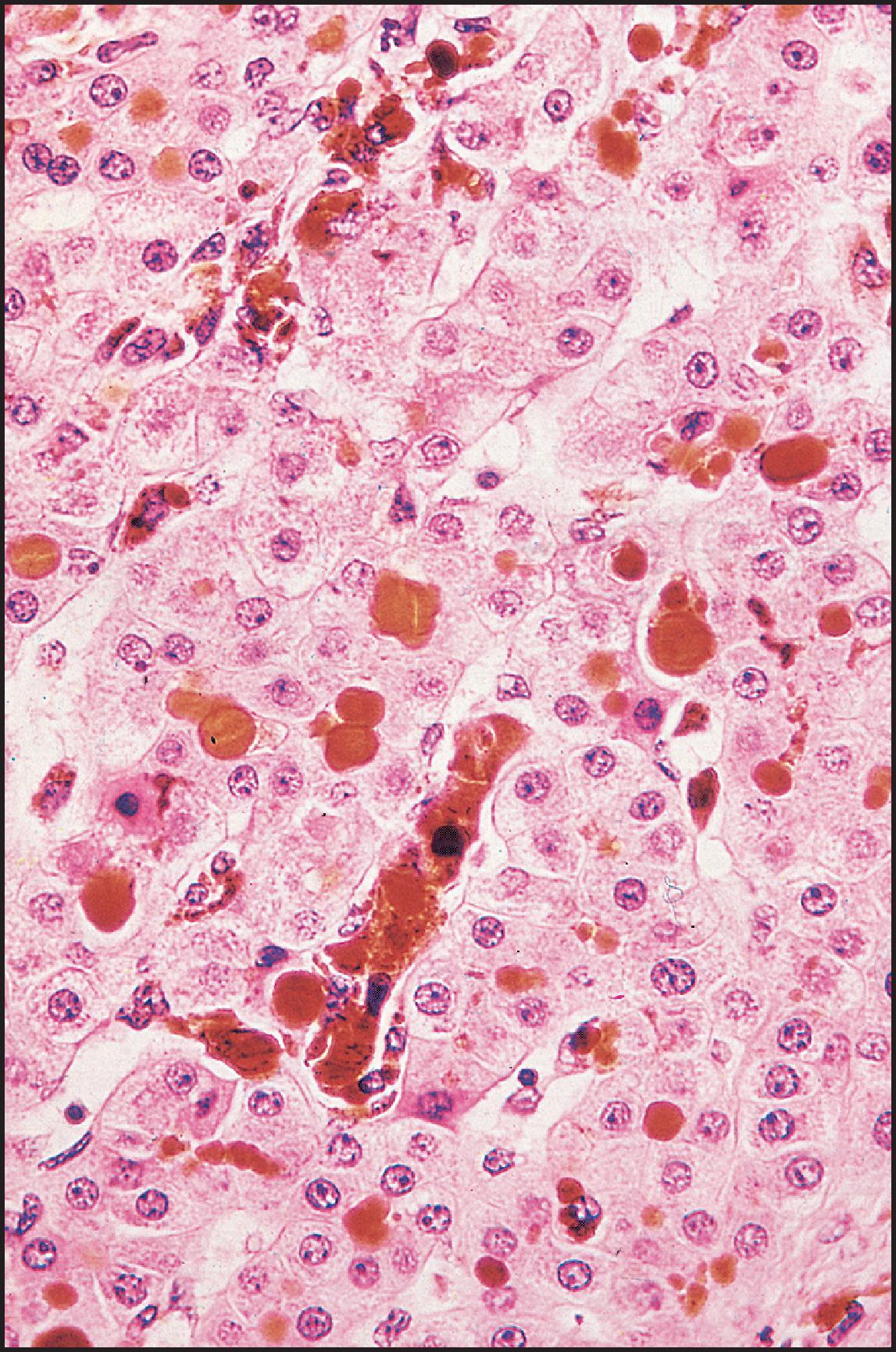
The deposits in canaliculi, bile ducts and Kupffer cells display bright-red birefringence with a centrally located, dark Maltese cross ( Fig. 3.59 ). Some of the larger deposits and most of the smaller ones in the cytoplasm of hepatocytes and Kupffer cells appear as clusters of brilliantly illuminated granules on polarizing microscopy.
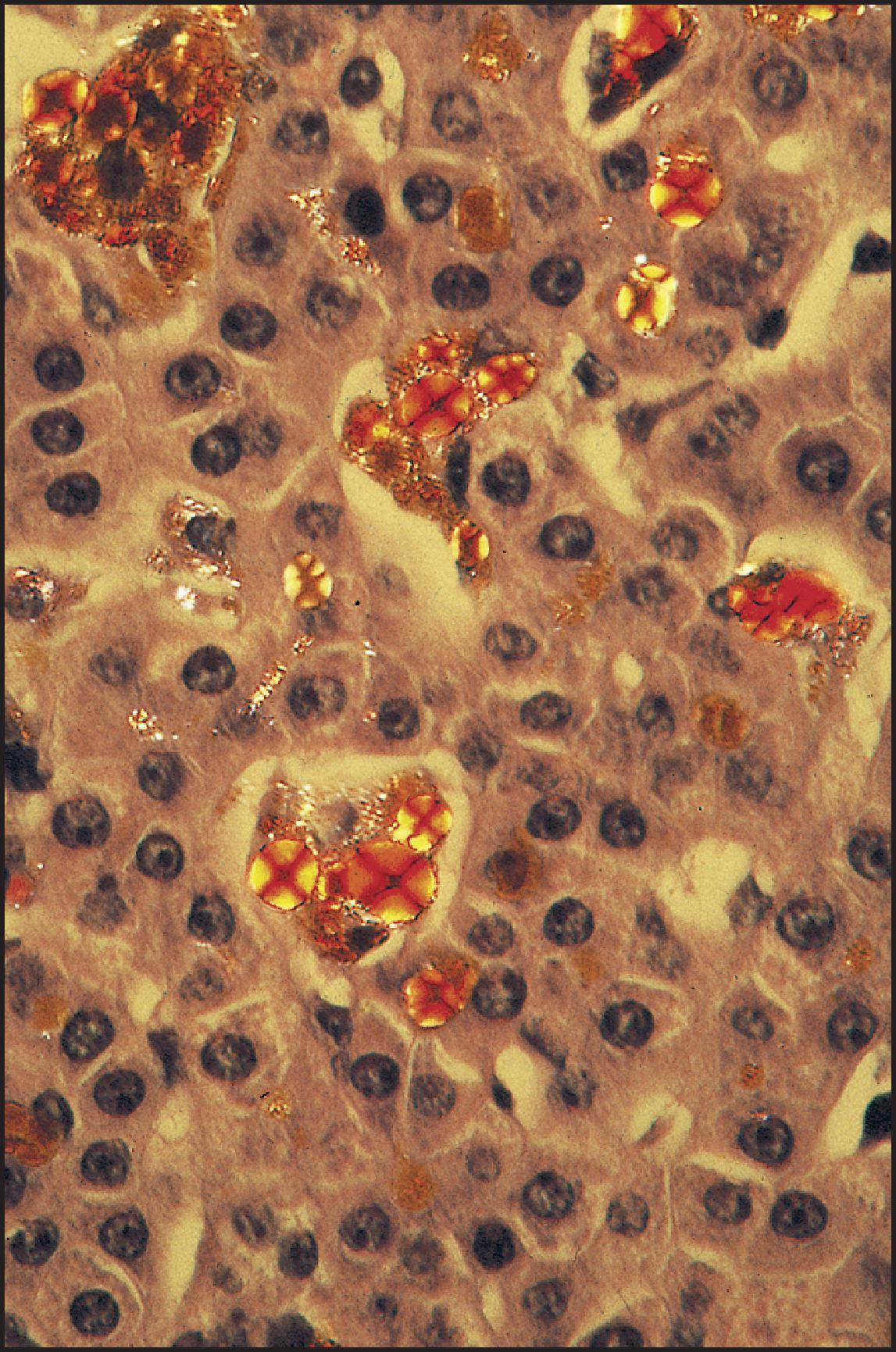
Transmission EM studies have demonstrated that the deposits of protoporphyrin in EPP consist of numerous, slender, electron-dense crystals arranged singly in sheaves or in a ‘star-burst’ pattern , ( Fig. 3.60 ). The crystals are straight or slightly curved and are 43–646 nm in length and 6.1–22.0 nm in width. The crystalline accumulations in the cytoplasm of liver cells are not surrounded by a membrane, but in Kupffer cells they are intralysosomal. Non-membrane-bound crystals have also been demonstrated in the cytoplasm of bile duct cells. The protoporphyrin casts in canaliculi are readily visualized by scanning EM ( Fig. 3.61 ).
![Figure 3.61, Erythropoietic protoporphyria. Rounded protoporphyrin casts in canaliculi show fracture lines. Casts are solid and have a granular structure at higher magnification. Hypertrophied Kupffer cell (upper left) probably contains phagocytosed protoporphyrin. (Scanning electron microscopy [SEM] × 1250.) Figure 3.61, Erythropoietic protoporphyria. Rounded protoporphyrin casts in canaliculi show fracture lines. Casts are solid and have a granular structure at higher magnification. Hypertrophied Kupffer cell (upper left) probably contains phagocytosed protoporphyrin. (Scanning electron microscopy [SEM] × 1250.)](https://storage.googleapis.com/dl.dentistrykey.com/clinical/DevelopmentalandInheritedLiverDisease/60_3s20B978070208228300003X.jpg)
As already noted, the liver disease in EPP may progress to cirrhosis, although the overall incidence of significant hepatic disease is probably small. , Hepatic failure may be precipitated by viral infection (e.g. Epstein–Barr virus [EBV]) or by alcohol. , A direct toxic effect of protoporphyrin in the pathogenesis of liver disease is suggested by the changes observed in protoporphyrin-perfused rat livers. The occurrence of cirrhosis in two sisters with EPP also raises the possibility of a genetic predisposition for hepatic disease. A unique association of two rare diseases, Langerhans cell histiocytosis and EPP, has been reported.
A separate disorder closely resembling erythropoietic protoporphyria is X-linked dominant protoporphyria (XLP). It is caused by gain-of-function mutations in ALAS2 , the 5-aminolaevulinate synthase gene on Xp11.2. The mutation is typically a deletion of two or four bases in exon 11. The increased activity of the mutated enzyme generates high levels of protoporphyrin in erythrocytes, found either as free protoporphyrin or as zinc-chelated. These patients may be at higher risk for hepatic complications. Phenotypic variation is considerable. Prevalence in a North American series was greater than that in European series, accounting for 10% of patients with a disorder appearing to be EPP.
Liver disease is significant with GSD types I, III, IV, VI and IX. Strictly speaking, GSD type IV is a storage disorder of an amylopectin-like molecule. GSD type II and type XI may be associated with hepatomegaly ( Table 3.4 ). The diagnosis is traditionally based on enzyme assays. Some can be performed on skin fibroblasts, lymphocytes or erythrocytes, but others require liver or muscle biopsies. Next-generation sequencing on peripheral blood samples using customized gene panels has now become the method of choice for the diagnosis of GSD. GSDs can also be classified into primary and secondary, depending on whether the genetic defect affects the function of enzyme involved directly in the glycogen/glycolysis pathways (primary) or on the function of ‘regulatory’ proteins having an indirect impact on glycogen metabolism, in relation to the proteasome and autophagy pathways (e.g. Lafora disease, RBCK1 E3 ubiquitin ligase). Glycogen deposits can be of abnormal quantity or quality. The latter, typical of GSD IV, are characterized by the accumulation of polyglucosan, a polysaccharide similar to the amylopectin of plants, with fewer branching points than glycogen, which is insoluble and creates deposits called polyglucosan bodies. ,
| Type | Enzyme | Gene locus | Enzymatic test | Liver abnormalities |
|---|---|---|---|---|
| 0 | Glycogen synthase | 12p12.2 | Liver | Steatosis |
| Ia | Glucose-6-phosphatase | 17q21 | Liver | Steatosis and GHD Adenoma, later HCC |
| Ib (non-a) | Glucose-6-phosphate translocase | 11q23 | Freshly removed liver | Steatosis and GHD Adenoma, later HCC |
| II | Lysosomal-γ1-4 and-γ1-6-glucosidase | 17q25 | Leukocytes, liver, muscle, amniocytes | Cytoplasmic vacuoles Lysosomal monoparticulate glycogen in EM |
| IIIa/b | Amylo-1-6 glycosidase (debrancher) | 1p21 | a. Liver, muscle, heart b. Liver |
GHD and steatosis, fibrosis, rarely cirrhosis |
| IV | Amylo-1-4 glycan-6-glycosyltransferase | 3p12 | Leukocytes, liver, amniocytes | Ground-glass, diastase-resistant inclusions Non-membrane-bound fibrillar material on EM |
| VI | Liver phosphorylase E | 14q21-22 | Liver | GHD, steatosis, fibrosis, rarely cirrhosis |
| IX | Liver phosphorylase kinase | Xp22.1-22.2 16q12-13 16p11.2-12.1 |
Liver, muscle, erythrocytes, leukocytes | Nonuniform GHD, steatosis |
| XI | GLUT2 transporter | 3q26.1-q26.3 | Liver | GHD |
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here