Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
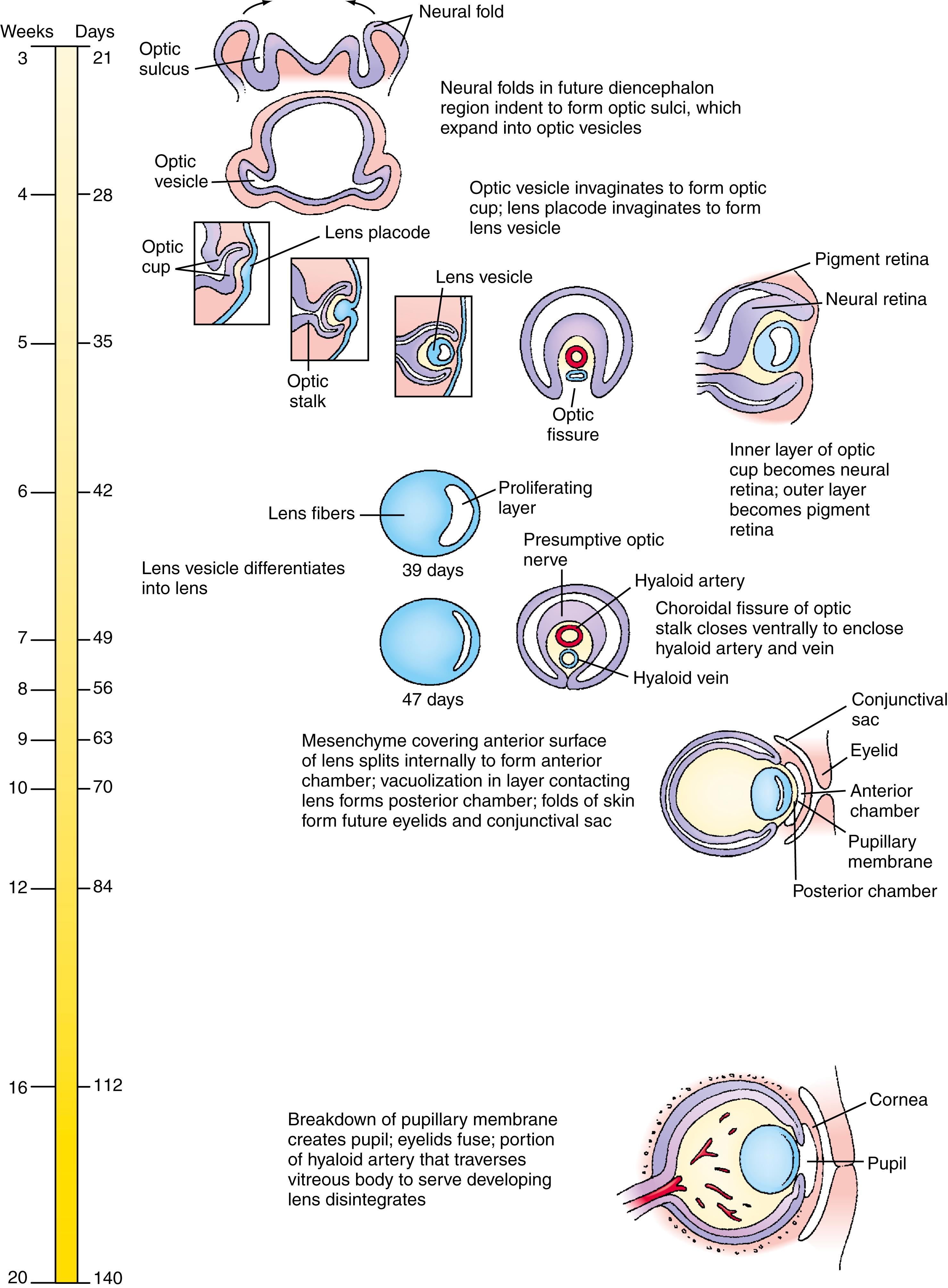
The eyes first appear early in the fourth week in the form of a pair of lateral grooves, the optic sulci , which evaginate from the forebrain neural groove to form the optic vesicles . As soon as the distal tip of the optic vesicle reaches the surface ectoderm, it invaginates, transforming the optic vesicle into a goblet-shaped optic cup that is attached to the forebrain by a narrower, hollow optic stalk . The adjacent surface ectoderm simultaneously thickens to form a lens placode , which invaginates and pinches off to become a hollow lens vesicle . Posterior cells of the lens vesicle form long, slender, anteroposteriorly oriented primary lens fibers . Anterior cells develop into a simple epithelium covering the face of the lens and give rise to the secondary lens fibers , which make up most of the mature lens.
The inner wall of the optic cup gives rise to the neural retina , whereas the outer wall gives rise to the thin, melanin-containing pigmented epithelium . Differentiation of the neural retina takes place between the sixth week and the eighth month. Six types of neuronal cells and one glial (Müller) cell are produced in the neural retina, which is proliferative, forming three layers in the mature retina: the ganglion cell layer ; an inner nuclear layer containing the amacrine, horizontal, Müller, and bipolar cells; and an outer nuclear layer containing the rods and cone photoreceptors. Axons from the neural retina grow through the optic stalk to the brain, converting the optic stalk into the optic nerve .
Blood is supplied to the developing lens and retina by a terminal branch of the ophthalmic artery, the hyaloid artery , which enters the optic vesicle via a groove called the optic fissure . The portion of the artery that traverses the vitreous body to reach the lens degenerates during fetal life as the lens matures; the remainder of the artery becomes the central artery of the retina .
As the optic vesicle forms, it is enveloped by a sheath of mesenchyme that is derived from neural crest cells and head mesoderm. This sheath differentiates to form the two coverings of the optic cup: the thin inner vascular choroid and the fibrous outer sclera . The mesenchyme overlying the developing lens splits into two layers to enclose a new space called the anterior chamber . The inner wall of the anterior chamber, overlying the lens, is called the pupillary membrane , which is a transient vascular structure. Deep layers of this wall undergo vacuolization to create a new space, the posterior chamber , between the lens and the thin remaining pupillary membrane. Early in fetal life, the pupillary membrane breaks down completely to form the pupil . The rim of the optic cup differentiates to form the iris and ciliary body. The canal of Schlemm and the trabecular meshwork develop from mesoderm and neural crest cells. Mesoderm adjacent to the optic cup also differentiates in the fifth and sixth weeks to form the extrinsic ocular muscles . The connective tissue components of the extrinsic ocular muscles are derived from neural crest cells. The eyelids arise as folds of surface ectoderm and are fused from the eighth week to about the fifth month.
A boy born with bilateral anophthalmia (missing eyes) is seen at 10 months of age by an endocrinologist. He was referred by his regular doctor because of underdevelopment of his penis (micropenis), undescended testicles (cryptorchidism), and poor linear growth. Although his weight had tracked along the 50th percentile, his height had lagged from the 50th percentile in the past to the 10th percentile now. These findings, and the association of anophthalmia with pituitary abnormalities, prompted the referral. A family history questionnaire does not uncover any relatives with birth defects, and both parents are in good health.
Although the boy had brain imaging in the newborn period that demonstrated a normal pituitary gland, testing reveals deficiency of several pituitary hormones ( hypopituitarism ). Hypogonadotropic hypogonadism due to reduced release of LH and FSH explains the small genitalia, and his parents are informed that testosterone replacement treatment will be required at the age of 12 to initiate puberty. His short stature is explained by reduced release of growth hormone, which can be managed in the future with growth hormone replacement. The rest of the endocrine workup is normal, but the family is warned that other hormone deficiencies can develop, and regular follow-up visits with an endocrinologist are planned.
The family then asks a question that they have asked other doctors, but they have not yet received a conclusive answer, “Will this happen again in our next pregnancy?” To help the family define its recurrence risk and to address the need for other clinical evaluations, genetic testing is discussed.
The clinical picture supports screening of the SOX2 gene, a high mobility group (HMG) box transcription factor (SRY-related) important for development of the hypothalamo-pituitary axis, as well as the eye. Human SOX2 mutations are associated with bilateral anophthalmia or severe microphthalmia in association with pituitary endocrine deficits. Additional variable abnormalities include developmental delay, learning difficulties, esophageal atresia, sensorineural hearing loss, and genital abnormalities.
This patient is found to have a de novo SOX2 nonsense mutation that is predicted to result in a truncated protein with an incomplete transactivation domain and impaired transactivator activity. De novo SOX2 mutations are the most commonly identified cause of syndromic bilateral anophthalmia/microphthalmia and have low risk of recurrence in future pregnancies.
![]()
Animations are available online at StudentConsult .
The eye develops from several embryonic tissue layers. The ectoderm gives rise to the lens and part of the cornea. The neuroectoderm forms the pigmented epithelium and the neural retina, the non-neural ciliary body, and iris structures, including the smooth muscles. Neural crest cells contribute to the stroma of the cornea and iris, the ciliary muscles, and the vascular choroid layer together with the fibrous sclera. The mesoderm contributes to the cornea and forms the angioblasts of the choroid layer and retina.
The first morphologic evidence of the eye is the formation of the optic sulcus in the future diencephalic region of the prosencephalic neural groove (forebrain) at 22 days ( Fig. 19.1A,B ). By the time that the cranial neuropore closes on day 24, the optic stalk is evident (see Fig. 19.1C–E ), and the optic primordia have developed into lateral evaginations of the neural tube called optic vesicles (see Fig. 19.1D,E ). The walls of the optic vesicles are continuous with the neuroepithelium of the future brain, and the cavity or ventricle within the optic vesicle is continuous with the neural canal. As the optic vesicle forms, it becomes surrounded by a layer of mesenchyme derived from neural crest cells and head mesoderm. Fate mapping studies in birds and mice have revealed that this mesenchyme gives rise to many ocular tissues, such as the sclera, ocular muscles, connective tissue, and cartilage, together with vascular endothelial cells. The extraocular mesenchyme begins to form on day 22 and will almost completely envelop the optic vesicle by day 26. By day 24, the distal part of the optic vesicle contacts the overlying surface ectoderm. At about this time, the optic cup becomes patterned along its planar axes (see below and the “In the Research Lab” of Chapter 9 entitled “Positional Information Patterns Neural Plate and Neural Tube”).
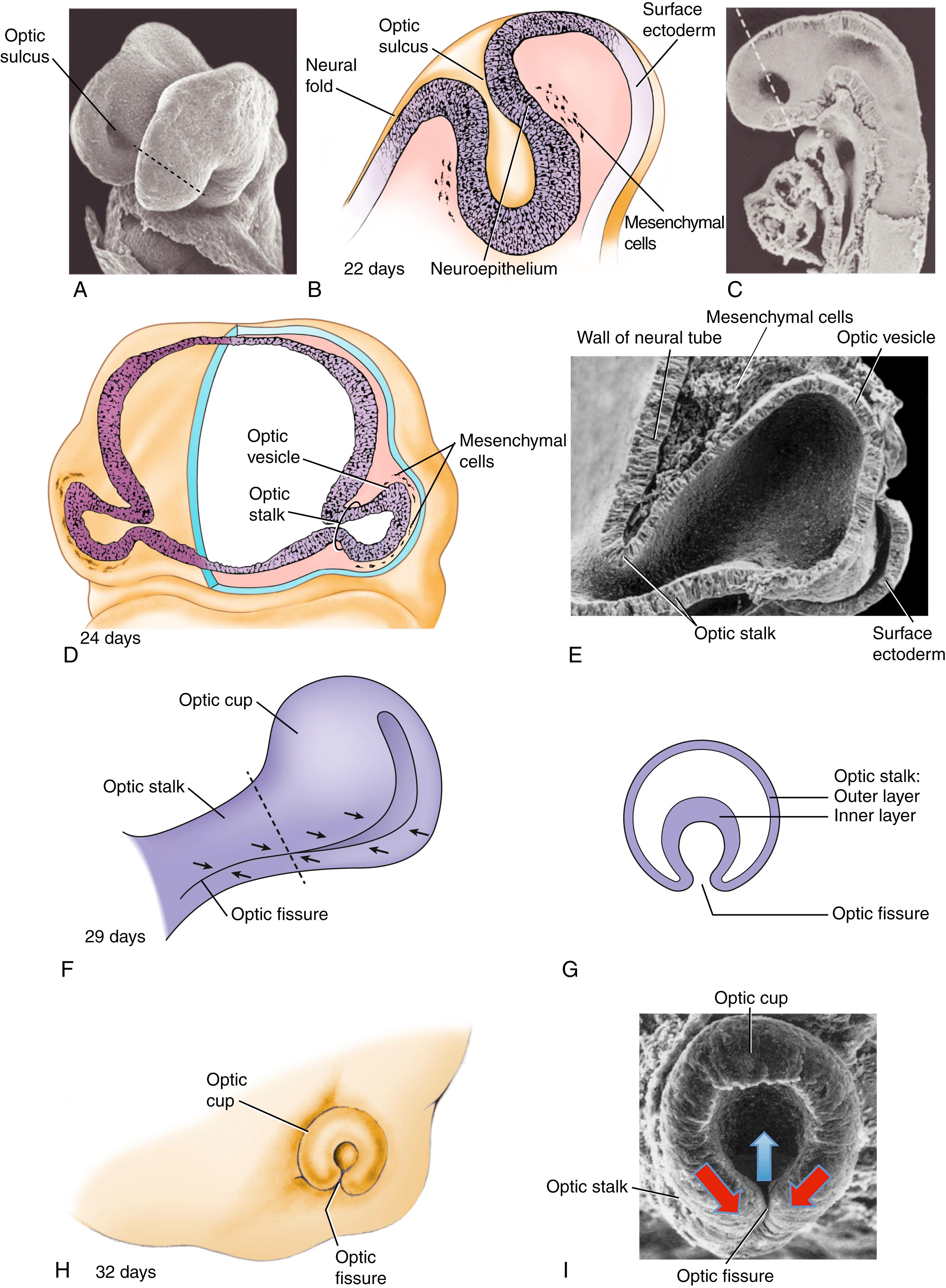
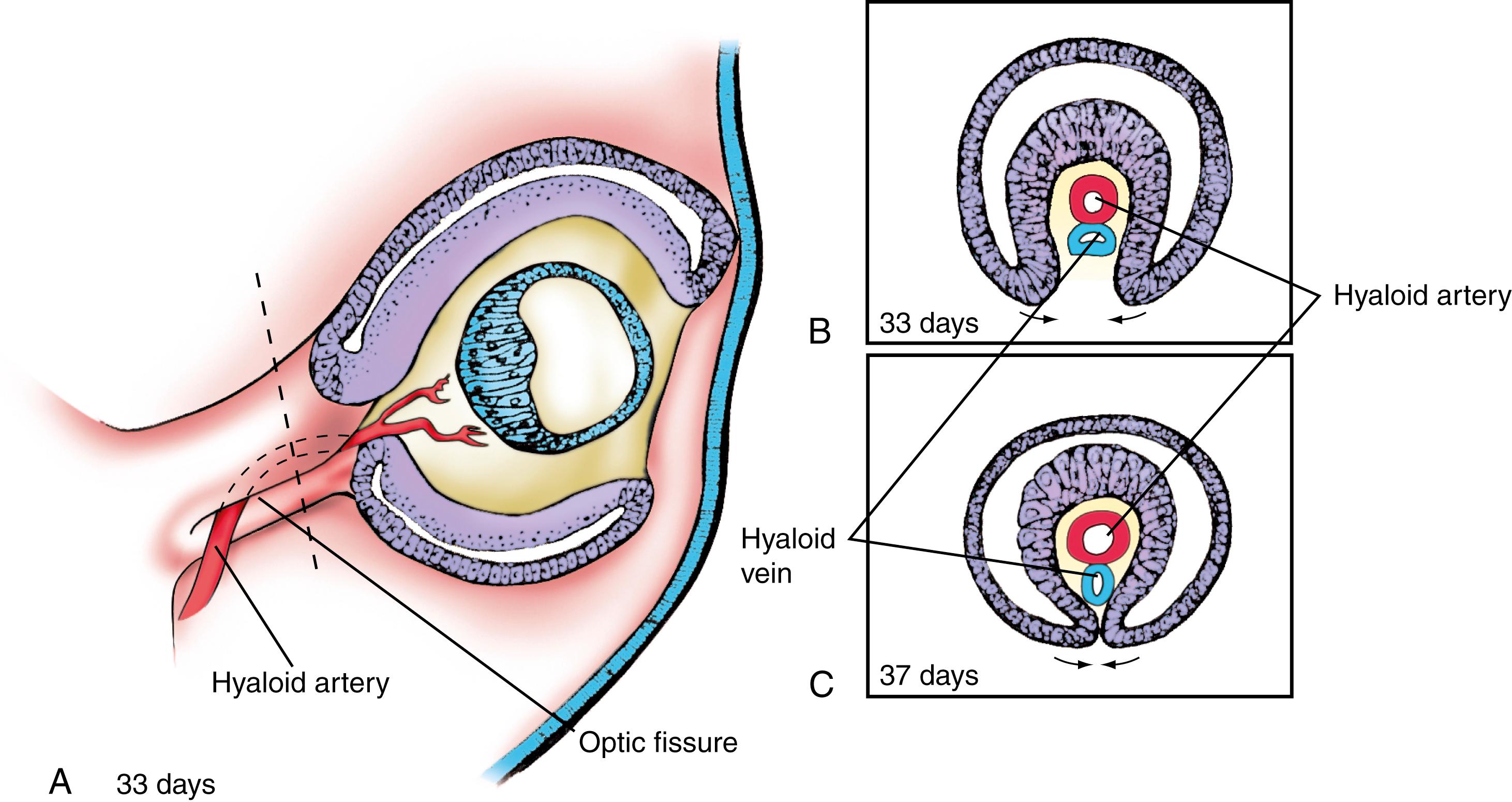
On about day 28, the distal end of the optic vesicle invaginates, converting the optic vesicle into a goblet-shaped optic cup (see Fig. 19.1F–I ). Simultaneously, the ventral part of the optic stalk invaginates, and the dorsal optic cup folds around the invagination to form the optic ( choroidal ) fissure . Blood vessels later enter the optic cup through the optic
Eye development starts with the formation of a single eye field in the cranial neural plate (i.e., the presumptive forebrain during gastrulation and neurulation). The transcription factor Otx2 is necessary for forebrain development. At the neural plate stage, the morphogen sonic hedgehog (Shh) is secreted by the underlying prechordal plate and is essential for separating the initially single eye field into two individual optic primordia ; failure of Shh signaling results in the persistence of a single eye field, and ultimately, cyclopia (a single, midline eye). In addition, failure of Shh signaling by the prechordal plate results in holoprosencephaly (a single forebrain vesicle rather than paired vesicles; covered in Chapter 17 in the “In the Clinic” entitled “Holoprosencephaly”). Several transcription factors, which regulate normal eye development, are specifically expressed in the eye field and are necessary for eye field specification. Eye field transcription factors include Pax6, Six3, Six6 (also known as Optx2), Rx/Rax, and Lhx2. Their loss results in failure of eye development. For example, the homeobox gene Rx/Rax is expressed in the eye field in both mice and humans. When deleted in mice, it leads to arrest of eye development at the neural plate stage. This results in anophthalmia (absence of the eye) or microphthalmia (small eye). Moreover, ectopic expression of individual eye field transcription factors such as Pax6 or Six3, which are expressed in the developing eyes of model organisms as diverse as Drosophila and mouse, results in the formation of ectopic eyes. These and other findings support the idea that progressive patterning of the neural plate, and subsequently of the eye field, is regulated by a feedback network of eye field transcription factors.
fissure ( Fig. 19.2 ), following which the two lips of the fissure fuse together (see Fig. 19.2C ). Upon closing of the fissure, the primitive ciliary epithelium secretes aqueous fluid, establishing intraocular pressure.
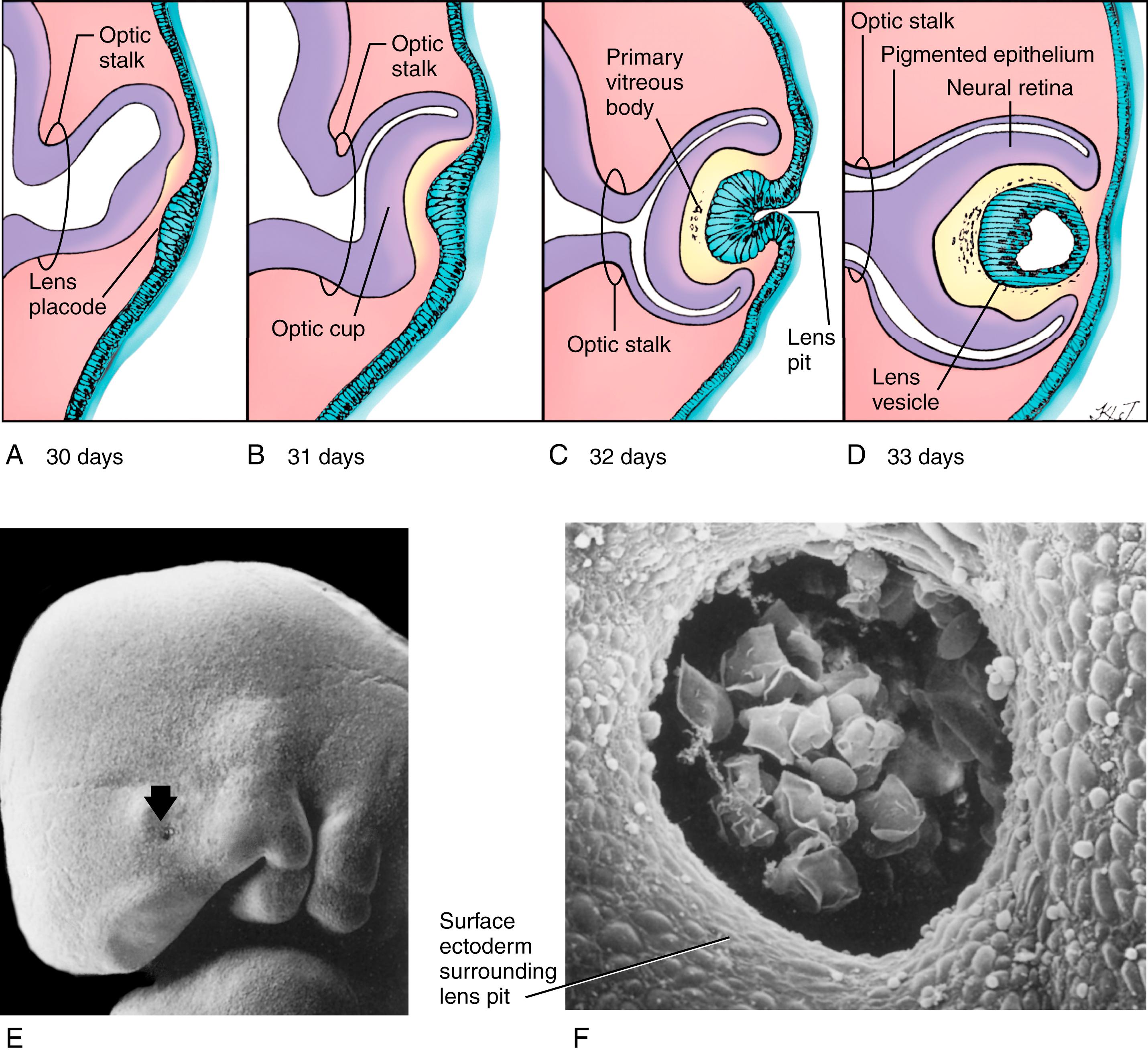
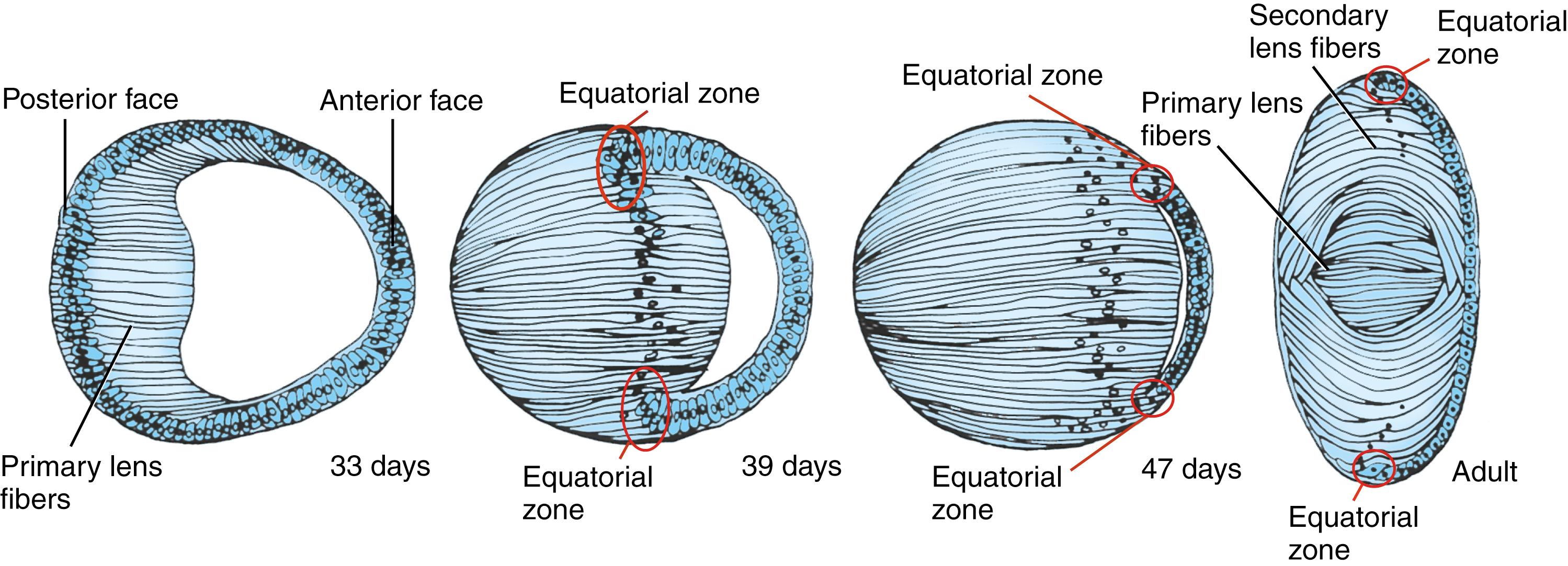
As soon as the optic vesicle contacts the surface ectoderm, the ectoderm apposed to it thickens to form a lens placode ( Fig. 19.3A,B ). Shortly thereafter, the lens placode invaginates to form a lens pit (see Fig. 19.3C,E,F ). By day 33, the placode separates from the surface ectoderm, becoming a hollow lens vesicle (see Fig. 19.3D ), surrounded by a basal lamina (lens capsule). Mesodermally derived mesenchymal cells migrate into the lentiretinal space between the lens vesicle and the inner wall of the expanding optic cup and secrete a gelatinous matrix called the primary vitreous body (see Fig. 19.3C,D ). Beginning on day 33, the cells of the posterior (deep) wall of the lens vesicle differentiate to form long, anteroposteriorly oriented primary lens fibers that express crystallins (α, β, and γ) necessary for the transparency of the lens ( Fig. 19.4 ). Elongation of these cells transforms the lens vesicle into a rounded lens body , obliterating the cavity of the lens vesicle by the seventh week. Anterior lens epithelial cells closest to the cornea remain proliferative throughout life. They migrate peripherally to the lens equatorial zone , giving rise to future secondary fetal and adult cortical lens fibers (lens bow). Secondary lens fibers start to be formed by the third month.
Following lens induction (see the “In the Research Lab” of Chapter 18 entitled “Development of Placodes”), the optic cup influences growth, differentiation, and maintenance of the developing lens. If the portion of the optic cup in contact with the ectoderm is removed, the lens eventually degenerates. Studies using null mutations in mice have demonstrated that several genes are required for induction and maintenance of the lens placode, including Pax6, Bmp4, and Bmp7. Using conditional mutagenesis, Pax6 has been specifically knocked out in the lens ectoderm, resulting in absence of all lens structures.
Several different growth factor families and transcription factors regulate differentiation of lens fibers. These include, respectively, Fgfs, Maf, and Prox-1. For example, once the lens vesicle has formed, Fgf in the aqueous humor (produced by the retina) induces cells in the posterior region of the lens to differentiate. Lower levels of Fgf signaling from the vitreous humor, and notch signaling, maintain proliferation in the anterior lens epithelium. The homeobox gene FoxE3 also maintains proliferation, whereas Prox1, which is expressed at the equatorial zone of the lens, induces cell differentiation and the expression of crystallin genes.
Malformations of the anterior segment of the eye can include failure of the lens to undergo separation from the surface ectoderm, resulting in a persistent lens stalk and leading to an arrest of lens development. Several genes regulating lens vesicle separation have been identified in mouse (e.g., FoxE3, Pitx3, Ap-2α). Lens defects are also associated with corneal and iris abnormalities, showing that signals from the lens are important for initiating differentiation of overlying ectoderm and mesenchyme. For example, Tgfβ signaling from the lens induces expression of Pitx2 and Foxc1 in the anterior mesenchyme, which is necessary for differentiation of the corneal epithelium and cornea (see “In the Clinic” entitled, “Abnormalities of Eye”). Conversely, corneal abnormalities can lead to secondary lens defects.
The two walls of the optic cup give rise to the two layers of the retina: the thick pseudostratified inner wall of the cup develops into the neural retina , which contains the light-receptive rods and cones plus associated neural processes, and the thin outer wall of the cup becomes the cuboidal melanin-containing pigmented epithelium ( Fig. 19.5A ; see also Fig. 19.3D ). These two walls are initially separated by a narrow intraretinal space . The intraretinal space between the neural retina and pigmented epithelium disappears by the seventh week. However, the two layers of the retina never fuse firmly, and various types of trauma—even a simple blow to the head—can cause retinal detachment (i.e., mechanical separation of these two layers).
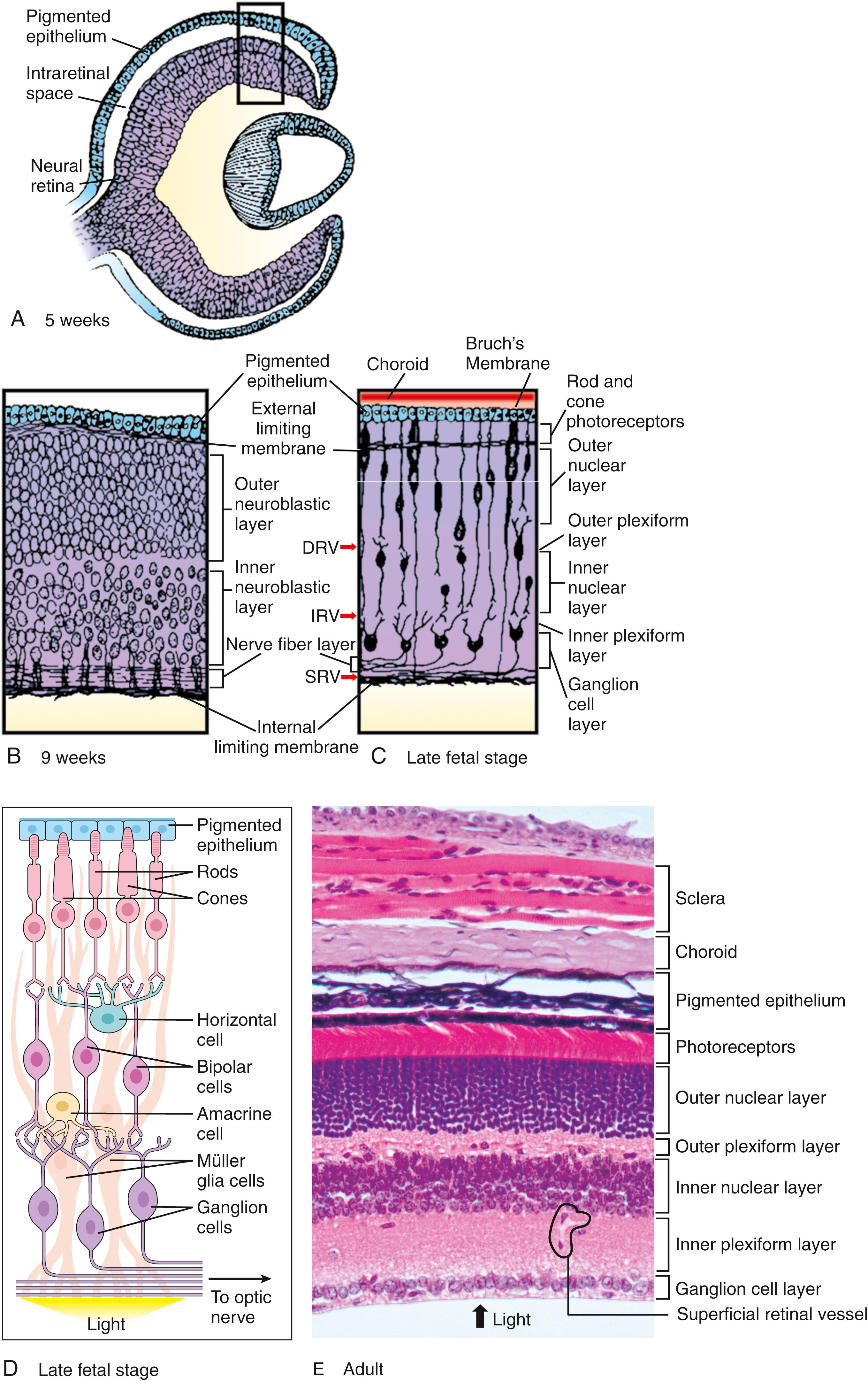
Melanin first appears in the cells of the developing pigmented epithelium on day 33. Soon afterward, the basal lamina of the pigmented epithelium, Bruch’s membrane , develops. Differentiation of the neural retina begins at the end of the sixth week, as the layer of retinal progenitor cells adjacent to the intraretinal space (which is homologous to the proliferative neuroepithelium lining the neural tube; covered in Chapter 4, Chapter 9 ) begins to produce waves of cells that migrate inward toward the vitreous body. By the sixth week, two cellular embryonic retinal layers are present: an outer neuroblastic layer and an inner neuroblastic layer . By the ninth week, two additional membranes develop to cover the two surfaces of the neural retina. An external limiting membrane is interposed between the pigmented epithelium and the proliferative zone of the neural retina, and the inner surface of the retina is sealed off by an internal limiting membrane (see Fig. 19.5B,C ).
The definitive cell layers of the mature neural retina arise from multipotent precursors that can give rise to all cell types within the pseudostratified neuroblastic layers (see Fig. 19.5B ). The progenitors divide at the apical side of the neuroepithelium, and the differentiated cells then move into the appropriate layers. Six major cell classes of neurons and one glial cell type are produced in an evolutionarily conserved order (see Fig. 19.5D ): ganglion cells , cone photoreceptors , and horizontal cells are born early; amacrine cells and rod photoreceptors are born next; and bipolar cells and Müller glia are born last. The axons of the ganglion cells form the definitive fiber layer that lines the inner surface of the retina and courses to the developing optic nerve (see Fig. 19.5B,C,D ). By the 16th week, the developing neuropil (i.e., the network of neuronal processes within the wall of the neural retina) becomes organized into inner and outer plexiform layers between the nuclear layers (see Fig. 19.5C ). All cell layers of the definitive retina are apparent by the eighth month, and the morphology of the late fetal retina is essentially equivalent to that of the adult (see Fig. 19.5E ).
Cellular differentiation progresses in a wave from the central to peripheral retina. Macular differentiation occurs around the sixth month, when cone precursor cells and multiple rows of ganglion cells accumulate in the central macular area. At 7 months, the central macular depression or primitive fovea forms. By several months postpartum, the fovea centralis , the region of the eye with the highest visual acuity, contains only a dense population of cone photoreceptors. This region is also totally avascular, reducing light scattering within the eye.
There are two types of photoreceptors: rods and cones. Rods are required for vision in low light; cones function in daylight and are necessary for color vision. There are three types of cones; each expresses distinct pigments and responds to one of the three different color wavelengths. S-cones respond to short wavelengths (blue light), M-cones respond to medium wavelengths (green), and L-cones respond to longer wavelengths (red). Color blindness is due to the absence of one or more types of cones. Protanopes lack L-cones, deuteranopes lack M-cones, and tritanopes lack S-cones. The genes OPN1LW and OPN1MW encode photopigments. Color blindness occurs frequently in males (>2%), as these genes are located on the X chromosome. Achromatopsia (rod monochromatism, resulting in total color blindness) can be caused by mutations in CNGA3, CNGB3, and GNAT2.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here