Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
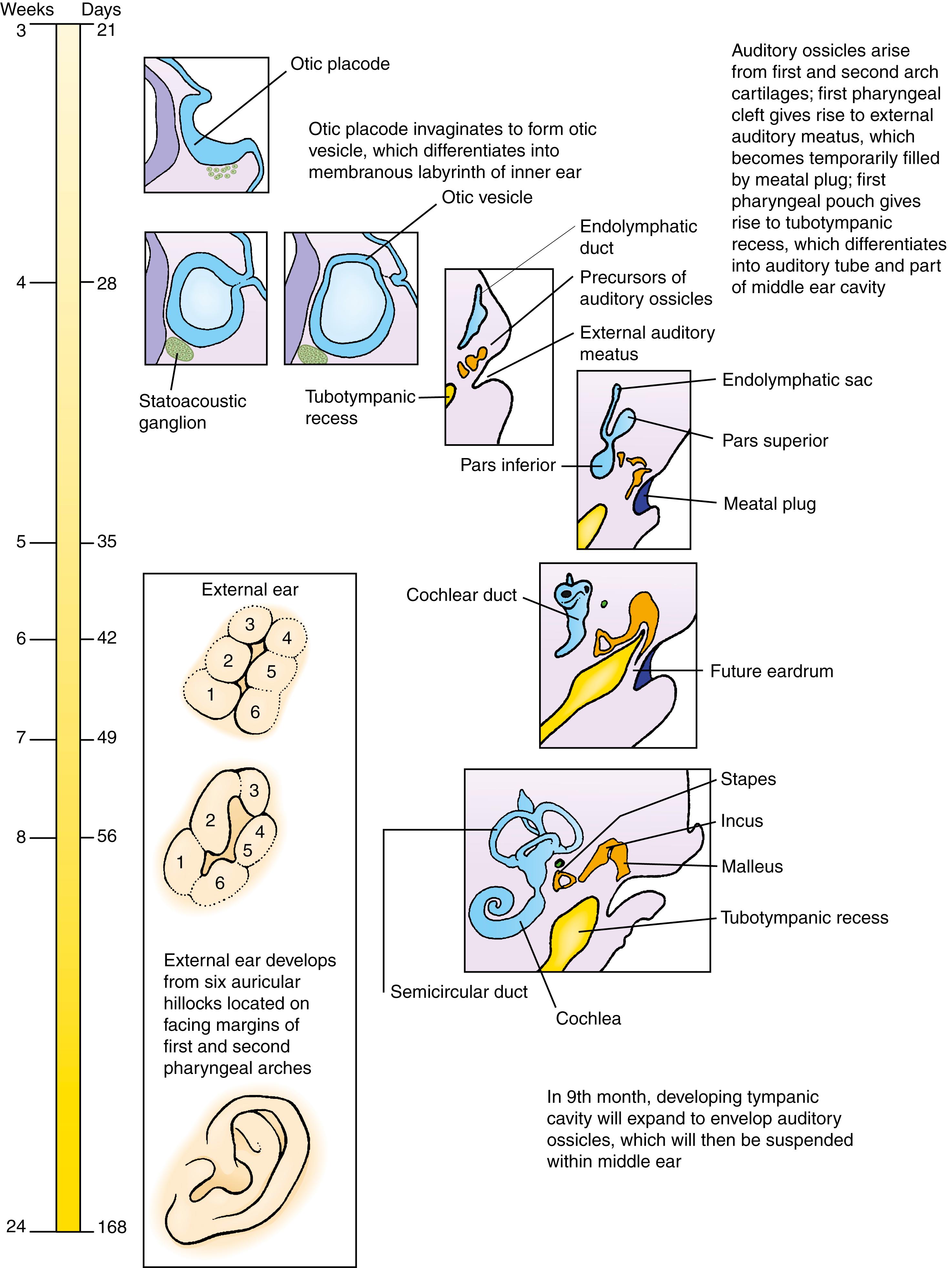
The ear is a composite structure with multiple embryonic origins. The external and middle ears arise from the first and second pharyngeal arches and the intervening pharyngeal cleft, membrane, and pouch. The inner ear, in contrast, develops from an ectodermal otic placode that forms on either side of the neural tube at the level of the future caudal hindbrain. At the end of the third week, this otic placode invaginates and then pinches off to form an otic vesicle (otocyst) within the head mesenchyme. The otic vesicle rapidly differentiates into three subdivisions: a slender endolymphatic duct, the expanded pars superior, and a tapered pars inferior . From the fourth to seventh weeks, the pars superior differentiates to form the three semicircular canals and the utricle. The pars inferior elongates and coils to form the cochlear duct distally and the saccule proximally. All of these otic vesicle derivatives collectively constitute the membranous labyrinth. The otic placode also gives rise to the sensory ganglia of the vestibulocochlear (statoacoustic) nerve (cranial nerve VIII). In addition, neural crest cells contribute to the vestibulocochlear nerve and its glial cells, as well as to melanocytes, which invade the cochlear duct. From weeks 9 to 23, the mesenchymal condensation that surrounds the membranous labyrinth, called the otic capsule, first chondrifies and then ossifies to form a bony labyrinth within the petrous part of the temporal bone.
The first pharyngeal pouch (endoderm) lengthens to form the tubotympanic recess, which differentiates into the auditory (Eustachian) tube and contributes to the tympanic cavity of the middle ear; by contrast, the most superior part of the tympanic cavity is derived from neural crest cells (ectoderm). Three auditory ossicles, the malleus, incus, and stapes, develop in the mesenchyme adjacent to the tympanic cavity. The malleus and incus are formed from neural crest cells in the first pharyngeal arch mesenchyme, whereas the stapes is a second arch derivative, formed from neural crest cells as well, with the exception of a small portion that will form the foot plate of the stapes, which is derived from mesoderm. In the last month of gestation, the mesenchyme surrounding the ossicles regresses and the tympanic cavity expands to enclose the ossicles.
The auricle (pinna) of the external ear develops from six auricular hillocks, which appear during the sixth week on the lateral edges of the first and second pharyngeal arches. The first pharyngeal cleft lengthens to form the primordium of the external auditory canal. The ectoderm lining the canal subsequently proliferates to form a meatal plug that completely fills the inner portion of the canal. The definitive canal is formed by recanalization of this plug during the 26th week. The tympanic membrane is derived from the pharyngeal membrane that separates the first pharyngeal pouch and cleft. It develops as a three-layered structure, consisting of an external layer of ectoderm, a middle layer of ectoderm derived from neural crest cells, and an inner layer of endoderm. The definitive tympanic membrane is formed during recanalization of the external auditory meatus.
A 2-year-old boy with profound hearing loss is admitted to the pediatric service for fever and vomiting. Urinalysis showed leukocytes and bacteria. He is diagnosed with pyelonephritis (urinary tract infection with kidney involvement) and started on intravenous antibiotics.
The boy’s hearing loss was detected by a local Department of Health newborn hearing screening program and verified with a sedated brainstem auditory evoked response (BAER). His hearing loss was determined to be both conductive (caused by abnormalities of the external or middle ear) and sensorineural (caused by defects of the cochlea or cranial nerve VIII). He had been using hearing aids since he was 4 months old.
A renal ultrasound done on admission showed small, dysplastic kidneys and hydronephrosis (dilation of the ureter and renal pelvis) on the right side. Later that night, while researching the differential diagnosis of hearing loss and kidney abnormalities, the medical student on call finds the description of branchio-oto-renal (BOR) syndrome. Intrigued by this possibility, the student returns to the patient’s bedside and finds that the boy has cup-shaped ears, preauricular pits, and small cysts over the sternocleidomastoid muscle ( Fig. 18.1A ). These cysts are later determined to be pharyngeal (branchial) cysts (persisting rudiments of the pharyngeal apparatus, as covered in Chapter 17 ). During his hospital stay, the patient has a thin-cut computed tomography (CT) of the temporal bone that shows malformations of the middle ear bones and hypoplastic cochlea.

As suspected by the medical student, the combination of pharyngeal (branchial) arch, otic (ear), and renal (kidney) abnormalities seen in the patient suggests the diagnosis of BOR. Also known as Melnick-Fraser syndrome, BOR is most often caused by mutations in the EYES ABSENT HOMOLOG 1 (EYA1) gene. As the name suggests, mutations in the Drosophila homolog of this gene (Eya) affect the eyes (see Fig. 18.1B ). Humans with EYA1 mutations rarely have abnormalities of the eyes, likely because of functional redundancy of multiple EYA genes (four EYA homologs are present in humans) acting during eye development.
![]() .
.
Animations are available online at StudentConsult.
The ear can be divided into three parts, the external, middle, and inner ear, each of which has distinct tissue origins. The external ear consists of the pinna (or auricle ) and external auditory canal (ear canal). The middle ear contains the auditory ossicles—the malleus, incus , and stapes —arranged in a chain in the tympanic cavity . The external and middle ear capture and carry the sound waves to the inner ear. The inner ear consists of the cochlea and vestibular apparatus. The three semicircular canals, the utricle, and the saccule comprise the vestibular apparatus. The cochlea perceives sound waves, whereas the vestibular apparatus perceives orientation, movement, and acceleration due to gravity, and it is necessary for maintaining one’s balance. The derivatives of the inner ear are collectively known as the membranous labyrinth. Innervated by the vestibulocochlear nerve (cranial nerve VIII), the inner ear receives contributions from neural crest cells in the form of melanocytes and Schwann cells.
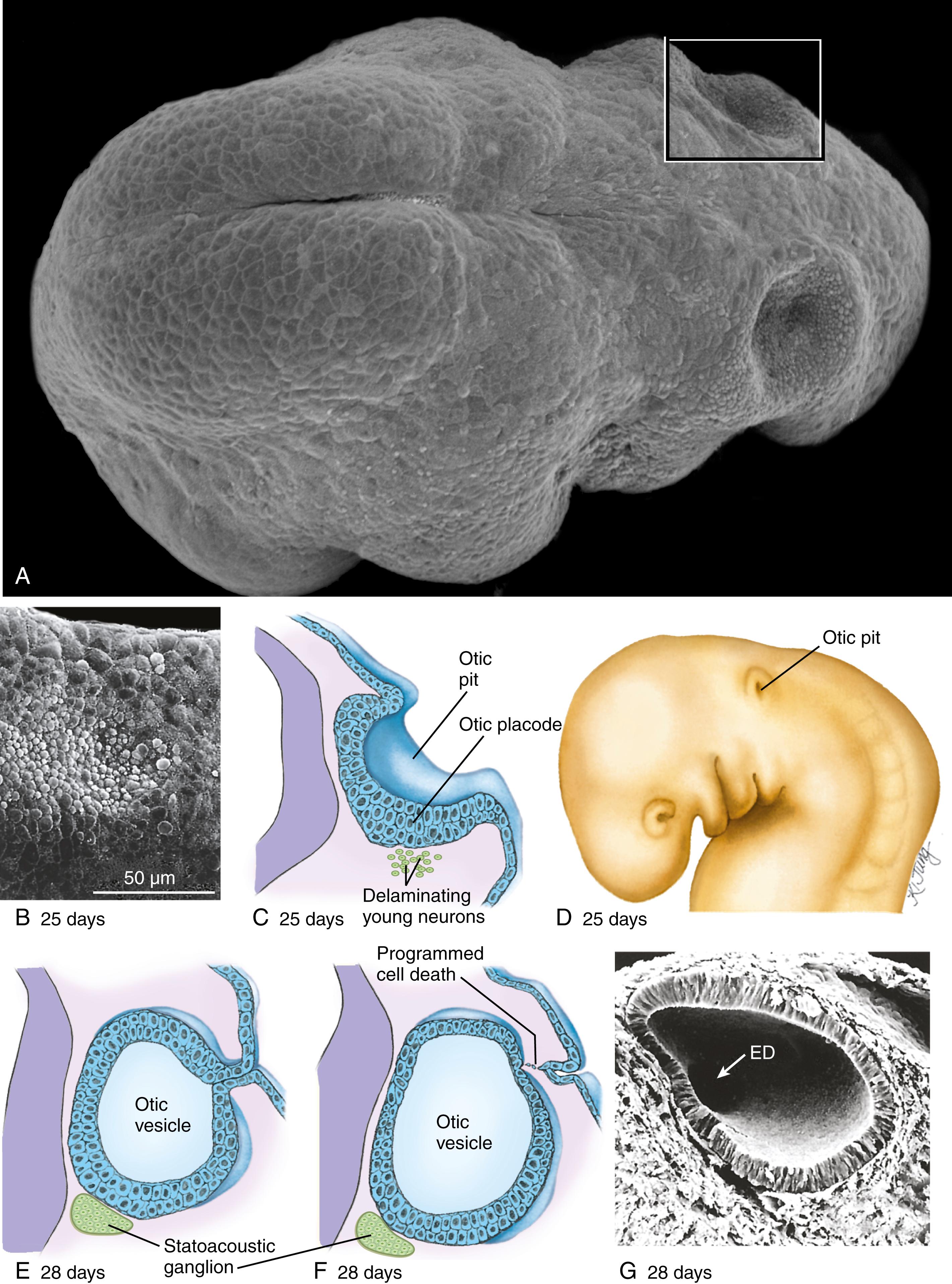
All of the inner ear derivatives arise from ectoderm. Late in the third week, a thickening of the surface ectoderm called the otic placode or otic disc forms next to the hindbrain ( Fig. 18.2A,B ). During the third and fourth weeks, the otic placode gradually invaginates to first form an otic pit , and then a closed, hollow otic vesicle or otocyst (see Fig. 18.2C–G ), which is connected briefly to the surface by a stem of ectoderm (see Fig. 18.2E,F ). Young neurons delaminate from the ventral otocyst to form the statoacoustic (vestibulocochlear) ganglion (see Fig. 18.2C ).
By day 28, the dorsomedial region of the otic vesicle begins to elongate, forming an endolymphatic appendage ( Fig. 18.3A ; see Fig. 18.2G ). Shortly thereafter, the rest of the otic vesicle differentiates into an expanded pars superior and an initially tapered pars inferior (see Fig. 18.3B,C ). The endolymphatic appendage elongates over the following week, and its distal portion expands to form an endolymphatic sac , which is connected to the pars superior by a slender endolymphatic duct (see Fig. 18.3C ).
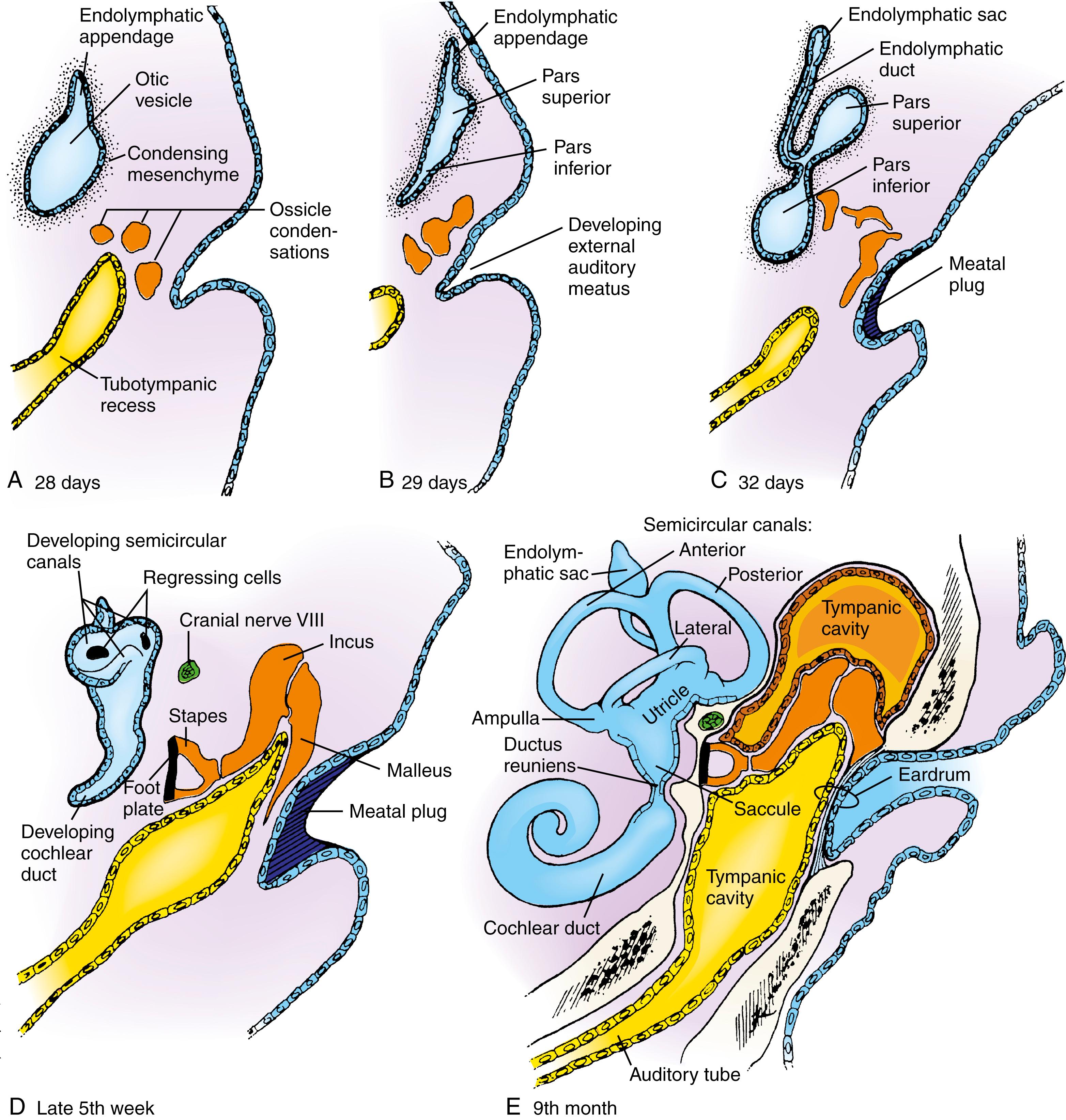
During the fifth week, the ventral tip of the pars inferior begins to elongate and coil, forming the cochlear duct , which is the primordium of the cochlea (see Fig. 18.3D,E ). The pars inferior also gives rise to the saccule, which is connected to the cochlea by a narrow channel called the ductus reuniens . During the seventh week, cells of the cochlear duct differentiate to form the spiral organ of Corti (the structure that contains the sensory hair cells responsible for transducing sound vibrations into electrical impulses; see Fig. 18.5B ). The sensory hair cells in the different regions of the cochlea are activated by different frequencies of sound waves.
Beginning late in the fifth week, flattened bilayered discs grow dorsally and laterally from the pars superior (see Fig. 18.3D ). In the center of the discs, the epithelial walls meet, and in these regions the epithelium regresses, leaving the rudiments of the semicircular canals. The semicircular canals are oriented perpendicularly to each other and consist of anterior, posterior, and lateral semicircular canals (see Fig. 18.3D,E ). A small expansion called the ampulla, which houses the sensory cells, forms at one end of each semicircular canal (see Fig. 18.3E ; see also Fig. 18.5A,B ).
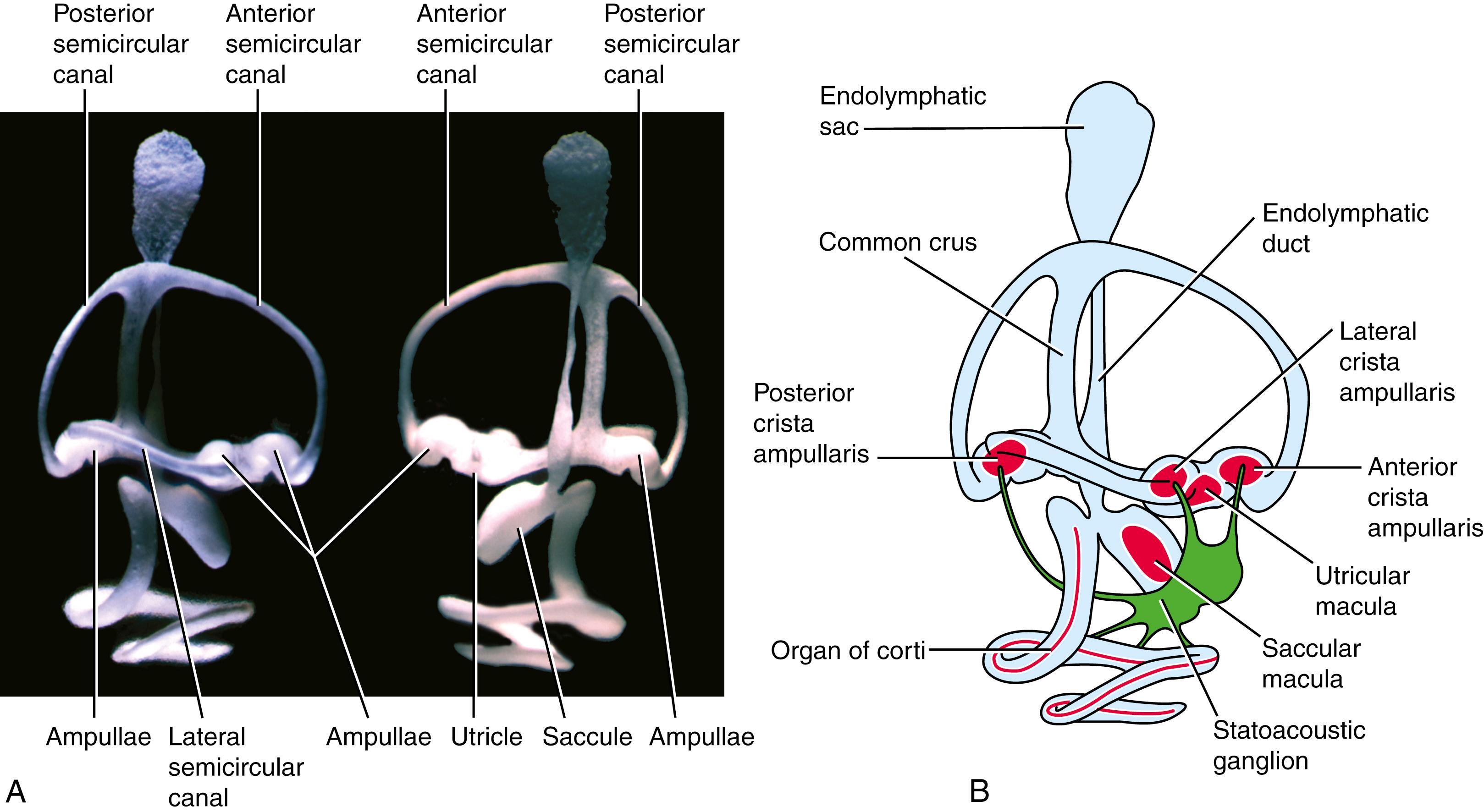
The morphogenesis of the mouse inner ear closely resembles that of the human inner ear. Fig. 18.4 shows the morphogenesis of the mouse inner ear over a 7-day period of embryogenesis, using an injection procedure in which the cavity of the otocyst is filled with an opaque paint and the head of the embryo is cleared. This approach provides a more three-dimensional view of the developing inner ear. Because the embryo can be turned and photographed in various orientations, the relationships of the three semicircular canals can be readily understood ( Fig. 18.5A ).
The placodes (otic, epibranchial, trigeminal, olfactory, adenohypophyseal, and lens; see Fig. 4.21 in Chapter 4 ) arise from a horseshoe-shaped domain surrounding the anterior neural plate, called the preplacodal region (see Fig. 3.9D ). The preplacodal region is initially multipotent, is competent to form all of the placodal derivatives, and is characterized by the expression of the Six and Eya families of transcription factors (specifically, Six1, Six4, Eya1, and Eya2) throughout the preplacodal domain. This domain, together with neural crest progenitors, is established by Fgf signaling and intermediate levels of Bmp signaling (low levels of Bmps specify the neural plate, and high levels the ectoderm; see Chapter 4 for further coverage). The preplacodal region progressively becomes regionalized to form the precursors for the distinct placodes. For example, following formation of the preplacodal region, the presumptive olfactory and lens placodes are initially characterized by the expression of Pax6, whereas the presumptive otic and epibranchial placodes express Pax2. Later, Pax6 is specifically expressed in the developing lens, whereas the developing olfactory placode switches off Pax6 expression and is now distinguished by Dlx5 expression. Differential and combinatorial signaling of Shh, Fgfs, Bmps, and Wnts from the surrounding tissues gradually restricts the developmental competence of the preplacodal region. These factors act to promote or inhibit the development of specific placodes. Fgf signaling from the mesoderm, endoderm, and anterior neural ridge (the cranial U-shaped junction between the ectoderm of the neural plate and the non-neural, or surface, ectoderm) is initially required for olfactory, trigeminal, otic, and epibranchial placode development but is inhibitory for lens induction. Signals (Tgfβ) from neural crest cells also inhibit lens development; hence the lens develops only in the ectoderm in contact with the optic cup, where neural crest cells are excluded. Ablation of neural crest cells in chicks and amphibians results in ectopic lens development. Canonical Wnt signaling from the hindbrain is also needed for otic induction, whereas sustained Fgf signaling, together with Bmps from the pharyngeal pouches, is required for formation of the epibranchial placodes. Shh from the prechordal mesoderm (see Chapter 17 for coverage of other roles of Shh signaling in the head mesoderm) is required for development of the adenohypophyseal placode. In the absence of Shh signaling, the adenohypophyseal placode fails to develop and the lens placode expands.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here