Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The placenta is the first organ to form in mammals and is required for establishment of a maternal–fetal vascular interface capable of supplying the bioenergetic needs of the developing conceptus.
Multiple placental cell types engage in highly varied functions, from attachment, invasion, and vascular remodeling to cell fusion, hormone production, and nutrient transport.
Multiple mechanisms allow transport of waste and nutrients across the placenta, including diffusion, transporter protein-mediated (facilitated diffusion and active transporters), and receptor-mediated mechanisms.
The placenta is not an inert transport interface. It consumes 40% to 60% of the oxygen and glucose delivered to the uterus at term. Thus conditions that alter placental metabolism can indirectly affect nutrient transport to the fetus.
In the United States, iatrogenic delivery is responsible for almost half the births that occur between 28 and 37 weeks of gestation, primarily caused by placental pathologies such as preeclampsia or intrauterine growth restriction.
Efforts to standardize placental examination after delivery are in progress so that connections between specific placental problems and poor outcomes can be better defined. In parallel, new advanced imaging techniques and biomarkers for placental function are being developed.
The placenta is a remarkable organ. Its brief existence enables the mammalian fetus to survive and thrive within the otherwise inhospitable confines of the intrauterine environment. To accomplish this, the placenta plays a range of roles, from anchoring the conceptus and preventing its rejection by the maternal immune system to enabling the transport of nutrients and wastes between the mother and the embryo/fetus. As with all organs, multiple specialized cell types derived from lineage-committed precursors are responsible for these functions. Genetic, epigenetic, and physiologic cues direct placental development across gestational stages. Impairments in these processes due to intrinsic or extrinsic insults can lead to placental dysfunction and result in long-lasting increases in disease susceptibility, a process known as fetal programming. Both preterm and term infants are at risk from poor placental function, particularly those that have extremely low birth weight (<1 kg). Preterm infants in particular may suffer from placental dysfunction in utero followed by early loss of placental support, including nutrition, hormones, and immune protection. Preterm delivery rates continue to rise while the survival of preterm infants has increased due to numerous advances in medical management and technology. This convergence has generated an expanded population of patients admitted to and graduating from intensive care nurseries. Not only are these infants more likely to develop complications ssuch as bronchopulmonary dysplasia, failure to thrive, pulmonary hypertension, cerebral palsy, and blindness, but they are also more likely to develop chronic adult ailments such as diabetes and heart disease. Although further improvements in neonatal care are critical for diminishing the long-term consequences of prematurity, prevention or delay of preterm delivery will have the greatest healthcare impact for this at-risk population. A better understanding of the most common placental pathologies is therefore critically important for advancing maternal, fetal, and adult medicine.
The placenta is the first organ to form in mammals. This is because it is required for establishment of a functional maternal–fetal vascular interface capable of supplying the bioenergetic needs of the developing conceptus. The fertilized embryo undergoes a series of cell divisions before implantation to produce up to eight seemingly identical cells called blastomeres. Three further sets of divisions generate the blastocyst, consisting of two distinct cell populations. Surrounding the blastocyst is the trophectoderm (TE), which gives rise to the placenta. The inner cell mass (ICM), located inside the blastocyst, gives rise to the embryo and visceral endoderm. In mice, each blastomere is able to generate either ICM or TE derivatives and is thus totipotent. This also occurs in humans. Once TE or ICM commitment occurs, however, it is considered largely irreversible. Importantly, however, individual blastomeres are found to harbor intrinsic biases regarding which lineage they adopt as early as the four-cell stage and which appear to depend on positional cues.
Multiple factors govern lineage allocation. One major determinant includes differences in polarity and adhesion between inner and outer cells of the blastocyst that are associated with differential activation of the Hippo signaling cascade. Hippo helps restrict expression of key lineage regulatory genes such as Cdx2 that are stochastically expressed as early as the eight-cell stage in mice but restricted thereafter to the TE. Notch signaling also acts in parallel with Hippo to promote Cdx2 expression in this process. Positional cues governed by E-cadherin expression help regulate Hippo signaling. Cell–cell contact within inside cells of the ICM activates Hippo and suppresses nuclear YAP activity. In mice, CDX2 helps repress genes critical for ICM identity, such as Oct4 and Nanog . Its absence in mouse embryos results in the lack of TE differentiation, and all cells of the blastocyst stage embryo express the typically ICM-restricted OCT4 protein. Amazingly, in mice its expression is sufficient to convert embryonic stem cells (ESCs) into trophoblast stem cells (TSCs) that can contribute to all lineages found within the placenta. Cdx2 expression is further maintained in mice via a positive feedback loop driven by the combinatorial activities of the transcription factors Eomesodermin (EOMES), ETS-related transcription factor 5 (ELF5), ETS proto-oncogene 2 (ETS2), and transcription factor AP-2, gamma (TCFAP2c) that help maintain the TE lineage. Interestingly, ELF5, CDX2, and EOMES can collaborate to regulate hundreds of TSC genes by binding to enhancer elements that harbor endogenous retrovirus-derived sequences, indicating that these serve as trophoblast-specific enhancer elements in mice. This opens the exciting possibility that differences in the incorporation of these foreign viral elements across different placental mammals may have contributed to the diversity of placental structures seen across the animal kingdom.
Uterine evolution paralleled placental evolution in eutherian mammals. This was recently found to involve large-scale and rapid changes in endometrial gene regulatory networks mediated by ancient transposable elements. These modulate responses to pregnancy hormones as well as other pathways to ensure pregnancy success. Thus, a set of genetic tricks coupled with host–virus interactions enabled the rapid evolution of the placenta–uterus axis in mammals and helped contribute to the diversity of mammalian life forms and modes of procreation observed today.
The placenta is comprised of multiple different cell types that engage in highly varied functions, ranging from attachment, invasion, and vascular remodeling to cell fusion, hormone production, and nutrient transport. Thus trophoblast-specific progenitors need to enact a complex set of lineage restriction decisions to help form a functioning placenta.
In the mouse, the extraembryonic ectoderm differentiates into cells that comprise the chorion and labyrinth, which perform the transport functions of the placenta; whereas the ectoplacental cone, located nearer to the uterine implantation site, differentiates into the spongiotrophoblast layer as well as glycogen trophoblasts and trophoblast giant cells (TGCs) ( Fig. 3.1 ). To function as a transport organ, the placenta must establish an extensive vascular interface between the maternal and fetal circulatory systems. Humans and rodents have a hemochorial placenta, which means that the maternal vascular space comes in direct contact with differentiated trophoblasts, not endothelial cells. In humans, trophoblasts lining maternal arteriolar spaces are relatively well characterized, but very little is known about the cells associated with the draining vascular bed, for example. In mice, there appear to be at least five distinct populations of TGCs that lie at various positions within these maternal vascular spaces and are defined by their location and lineage-specific gene expression. These arise from various sources ( Fig. 3.1 ). Their “giant” size is, in part, a reflection of their DNA content, which continuously replicates without engaging in cell division via a process called endoreplication. Endocycles are thought to be used by cell types that need to be very large or that are highly metabolically active. Consistent with this, TGCs in the mouse placenta are responsible for the bulk of placental hormone production. In humans, placental hormones are produced by the syncytiotrophoblast (SynT) layer, potentially accounting for the reduced ploidy of invasive trophoblast subtypes in this species compared with rodents, although they still appear to exhibit a significant amount of aneuploidy.
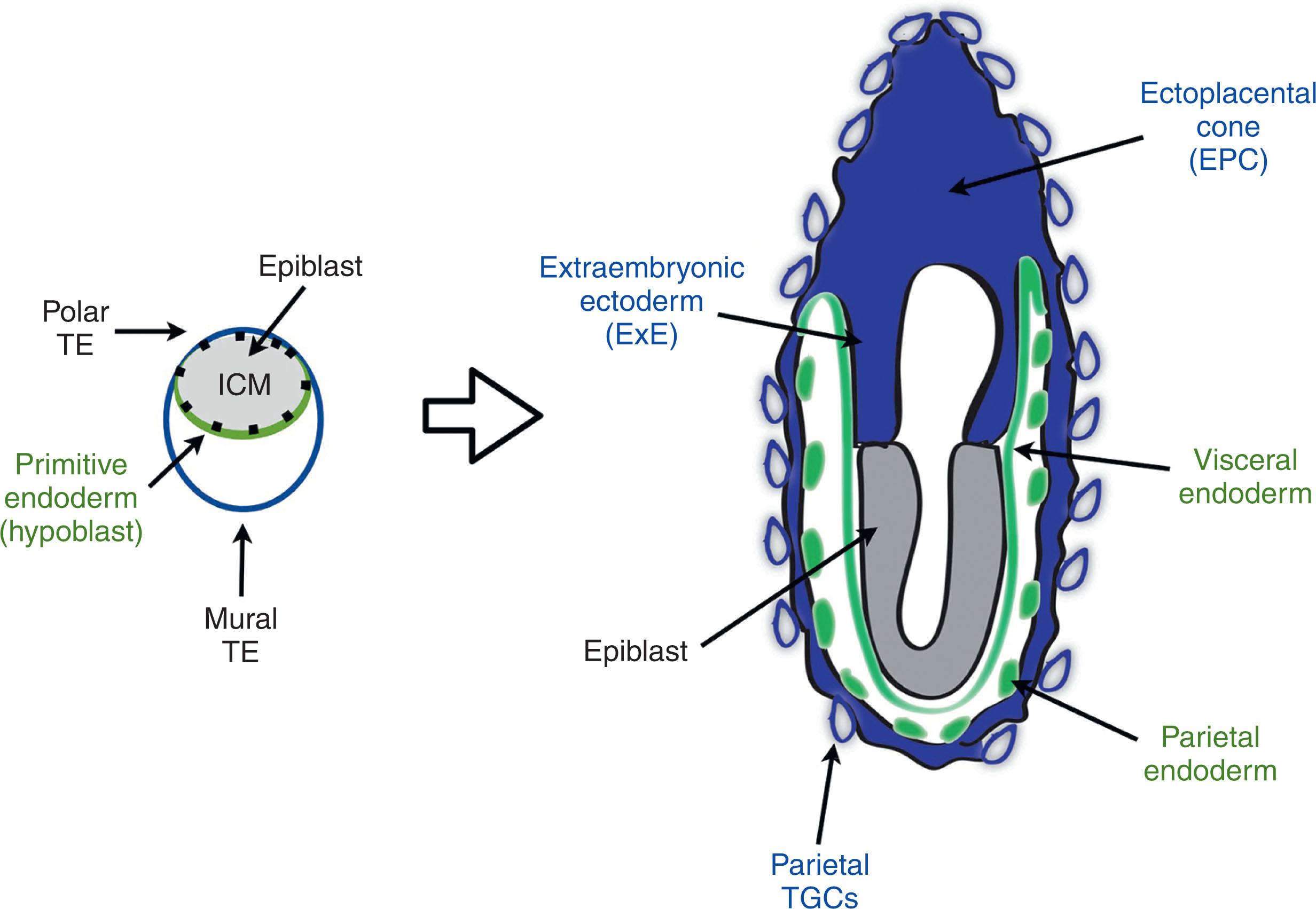
Primary TGCs differentiate from the mural TE, and this represents the first terminally differentiated cell type in mice to aid in implantation. In humans, the initial wave of invasion following implantation is thought to occur via formation of a primitive syncytium, through which invasive CTBs push following approximately day 13 or 14 of gestation. This early wave of syncytialization does not occur in rodents. In mice, the remaining secondary TGCs differentiate either from trophoblast-specific protein alpha (Tpbpa)/4311+ outer ectoplacental cone cells or from Tpbpa/4311−chorionic progenitors. Secondary TGCs come in various forms that have differing locations as well as characteristics. As the name implies, spiral artery (SpA) TGCs invade the spiral arteries and displace the smooth muscle and endothelial cells to remodel them, canal TGCs line the large vascular spaces delivering maternal blood to the base of the labyrinth, sinusoidal TGCs sit within the small vascular spaces of the labyrinth, and parietal TGCs surround large pools of deoxygenated blood that ultimately drain into the maternal uterine veins.
Trophoblasts that invade and line blood vessels appear to do so via two different mechanisms: (1) vascular invasion with endothelial mimicry and (2) vasculogenic mimicry. In the former, trophoblasts invade and displace maternal endothelial cells from within maternal arterioles and include SpA-TGCs in mice or endovascular trophoblasts (EVTs, also known as extravillous trophoblasts) in humans. During vasculogenic mimicry, however, trophoblasts undergo morphogenesis to create vascular tubes de novo. Sinusoidal TGCs perform this function in mice. Whether this also occurs in human placentation is not clear. Transplanting trophoblasts subcutaneously in mice or culturing them as 3-dimensional trophospheres in vitro allows one to visualize them from de novo vascular spaces, where they generate tumors harboring large blood sinuses surrounded by trophoblasts, as opposed to host endothelium. Many pathways known to regulate endothelial development also drive trophoblast differentiation and formation of the maternal–fetal vascular interface. Furthermore, the endothelium and trophoblast are primary regulators of hemostasis in the adult and fetal circulation. Trophoblasts can regulate the coagulation cascade like endothelial cells and produce such molecules as thrombomodulin, tissue factor, tissue factor pathway inhibitor, annexin V, and endothelial protein C receptor. These factors are critical for preventing thrombotic or hemorrhagic events from occurring in the developing placenta. Thus, mammalian placentas have solved the problem of hemochorial placentation by having trophoblasts take over functions typically performed by endothelial cells.
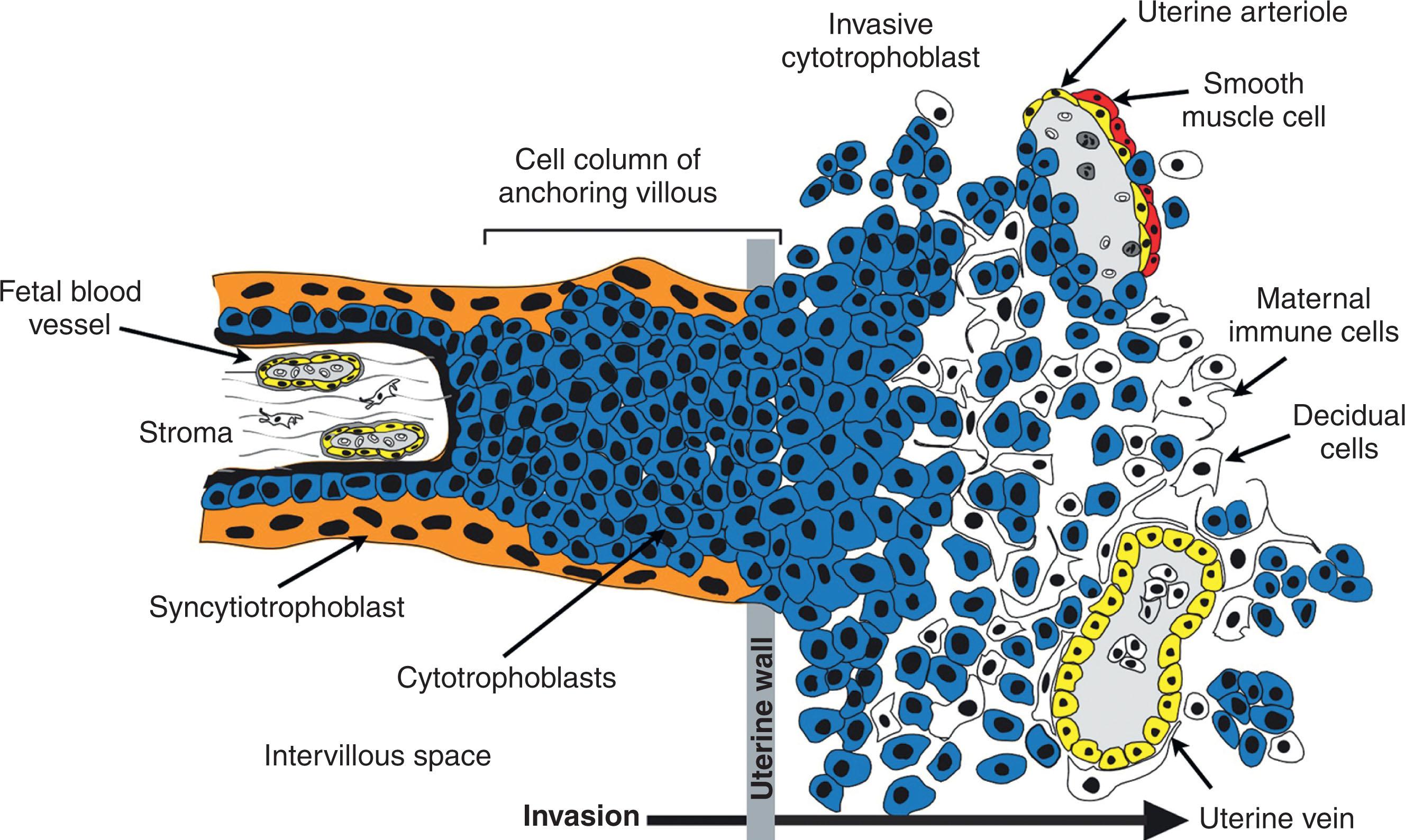
Remodeling of uterine vasculature is critical for successful pregnancy in humans and mice. The equivalents of TGCs in humans, invasive EVTs, are derived from column CTB progenitors located at the tips of anchoring villi ( Fig. 3.2 ). They migrate through the uterine parenchyma via interstitial invasion, in search of maternal spiral arterioles and veins. This invasion peaks early in pregnancy, around 9 to 12 weeks of gestation. EVTs then breach the spiral arterioles via a process termed endovascular invasion and replace resident endothelial and smooth muscle cells. This results in these high-resistance vessels being remodeled into low-resistance/high-capacitance conduits necessary for proper fetal perfusion as well as modulation of maternal hemodynamics. While EVT interactions with veins are largely confined to the inner surface of the uterus, they migrate along much of the intrauterine course of maternal arterioles. Although endovascular invasion begins quite early and typically begins within the center of the placental bed, uterine arterial blood only begins to flow into the intervillous space by the end of the first trimester. Before this point, EVTs paradoxically plug these vessels, preventing blood flow to the placenta. As a result, all of first trimester placental development occurs in a highly hypoxic environment with the bulk of placental nutrients being provided by endometrial secretions (i.e., histiotrophic nutrition). Only about one-third of the uterine SpAs are actually invaded by 18 weeks’ gestational age, indicating that the more lateral arteries are only invaded throughout the second and third trimesters in a progressive manner because most are completely remodeled when examined following delivery at term.
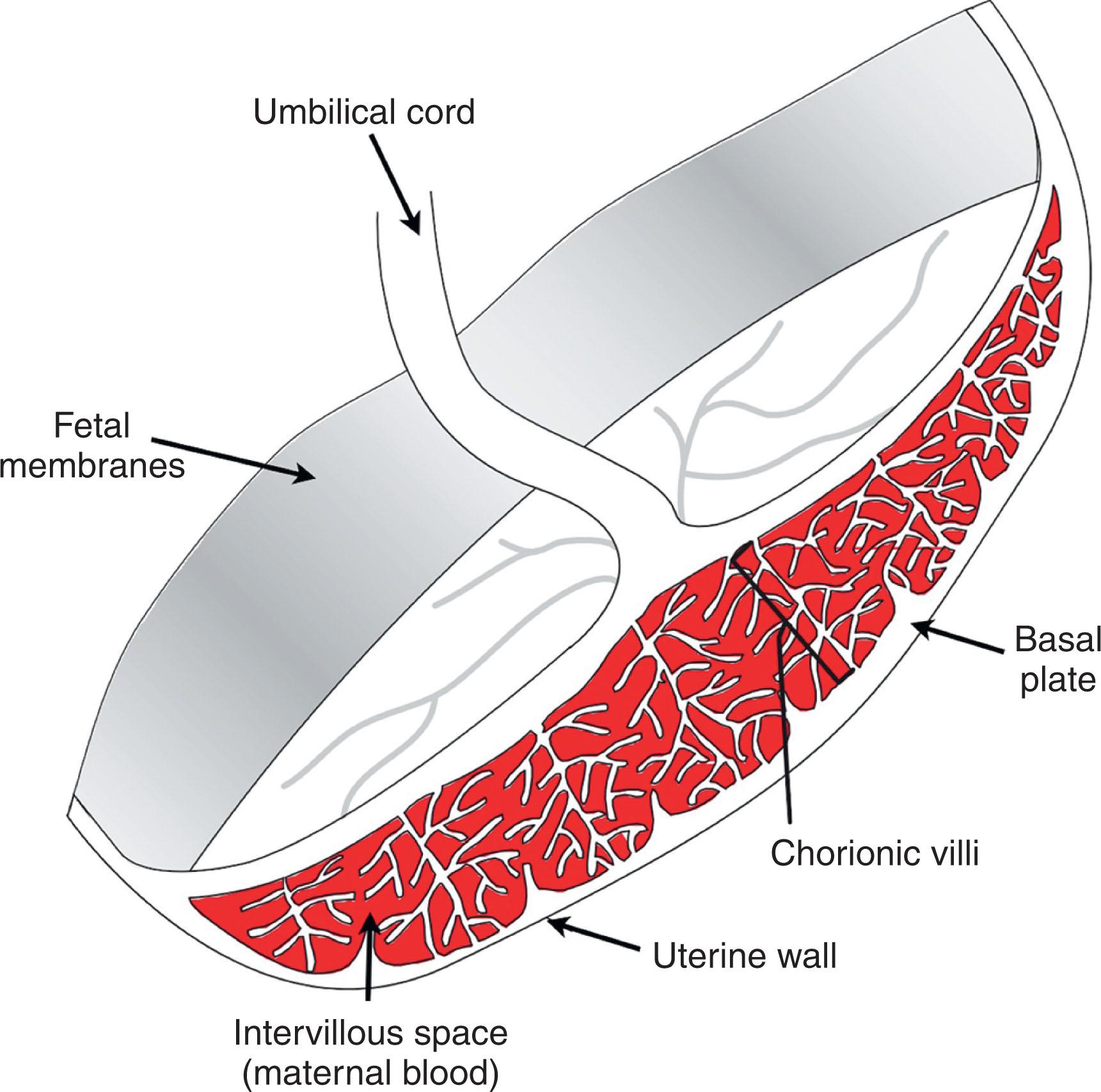
Following unplugging of these vessels, maternal blood begins to bathe floating chorionic villi that are covered by a layer of multinucleated SynTs. SynTs form as a result of the fusion of lineage-committed progenitors. The need for multinucleated syncytium formation is not clear but may have been driven evolutionarily by a response to viral infections that may help minimize pathogen transmission to the fetus. A combination of fusogenic protein expression, particularly syncytins, and dramatic cytoskeletal rearrangement appears to be essential for this trophoblast fusion. Additionally, caspase 8 activity—frequently implicated in apoptosis—aids this process during human SynT formation. These cytoskeletal changes are frequently accompanied by another apoptosis-associated process—externalization of phosphatidylserine to the outer leaflet of the plasma membrane. Typically acting as an “eat me” signal for the clearance of apoptotic cells, phosphatidylserine externalization is associated with SynT fusion. Apoptosis is not completed during SynT formation, however, and the syncytium is maintained in this “preapoptotic” state until being sloughed off into the maternal circulation. As a result of the dramatic cytoskeletal changes required for cell fusion, in addition to changes in the composition of the membrane lipid bilayer, the biophysical properties of the SynTs change to become much more rigid, possibly aiding the infection barrier properties of the placenta.
The transport functions of the placenta are performed by the multinucleated SynTs that sit at the maternal–fetal interface. Multiple mechanisms allow transport of waste and nutrients across the placenta. The simplest is diffusion. The high surface area of the placental transport interface, along with the hemochorial nature of the rodent and human placentas, enables efficient diffusion across the placenta. The rate of diffusion depends on the molecular properties and concentrations of the solute, however, in addition to the composition of the exchange barrier. In the human placenta at term, a single SynT layer separates maternal blood from fetal capillary endothelium, whereas in the mouse, two SynT layers as well as an sinusoidal (S)-TGC layer, surprisingly, separate the vascular spaces. These layers are progressively thinned out to minimize their barrier properties and increase the surface area for exchange. Oxygen is transported across the placenta via passive diffusion, aided by the high affinity of fetal hemoglobin and the concentration differential across the maternal–fetal vascular beds. The orientation of the maternal and fetal vascular blood spaces produces a countercurrent exchange mechanism in mice. This maximizes transport efficiency in rodents, whereas the human placenta has a less efficient multivillous arrangement that necessitates a larger placental size relative to the mouse.
Hydrophilic molecules do not readily cross plasma membranes. Transporter protein-mediated mechanisms are typically required for transporting hydrophilic molecules. Classic transporter proteins include facilitated diffusion transporters (i.e., the glucose transporter [GLUT] family ) as well as active transporters (i.e., transporters associated with calcium transport and the amino acid transporters ). Transport can occur down a concentration gradient, as is the case with GLUT1-mediated glucose transport, or against a concentration gradient, as is the case with calcium and amino acid transport. Interestingly, nearly all amino acids in the fetal circulation are found at higher levels than in the maternal circulation, indicating active uptake and/or synthesis of these nutrients via the placenta or fetus.
The fetal–placental unit is both physically and metabolically interconnected with each other and the maternal circulation. Ultimately, all fetal–placental metabolism is constrained by the nutrients delivered from the maternal circulation. However, the placenta and fetal liver are both capable of producing and metabolizing various nutrients that impact their levels in the fetal–placental circulation largely independent of placental transport mechanisms. This has been well described in ovine species, wherein the fetal liver of sheep in utero actively produces large quantities of serine and glutamate that are consumed by its placenta. In the placenta, serine is converted to glycine via a process that contributes to one-carbon metabolism-dependent DNA methylation pathways that play important roles in cell fate regulation and growth mechanisms. Additionally, nonglucose carbohydrates such as fructose, mannose, inositol, and sorbitol are also either transported by or synthesized in the placenta and play important roles in regulating fetal growth as well as in redox regulation.
Finally, antibody-mediated immunity is transferred from mother to fetus across the placenta via receptor-mediated mechanisms. Immunoglobulin G (IgG) transport across the human placenta, for example, begins at approximately 16 weeks’ gestation, and fetal serum IgG levels reach maternal levels by 26 weeks’ gestational age. This process is highly efficient, enabling fetal concentrations to exceed maternal values at term.
The placenta is not an inert transport interface. It consumes 40% to 60% of the oxygen and glucose delivered to the uterus at term, despite only comprising approximately 10% to 20% of the total mass of the uterus at that time. Changes in this metabolism can regulate placental biology. Mitochondrial fusion, a process that enables greater mitochondrial bioenergetic capacity, is critical for invasive TGC formation in mice, triggering placental failure when compromised. Interestingly, mitochondrial fusion can promote cardiomyocyte differentiation as well, highlighting conserved mechanisms between cellular metabolism and cell fate determination. Thus, alterations of placental metabolic function can impact oxygen and nutrient delivery to the fetus, both by altering placental metabolic demand intrinsically as well as by impacting cell fate regulatory pathways. Primary culture of human CTBs indicates that they exhibit high rates of aerobic glycolysis when compared with other terminally differentiated adult cells, much like rapidly proliferating cancer cells that rely on high glycolytic flux rates to augment biosynthetic precursor production. Aerobic lactate production (i.e., the Warburg effect), also allows the placenta to produce and transfer large amounts of lactate to the fetus, which can readily oxidize it. Interestingly, the placenta also appears able to metabolize lactate during midgestation but loses this ability by term. These studies additionally suggest that glycogen breakdown (glycogenolysis) helps supply the high rates of glucose consumption in proliferating trophoblasts before differentiation into terminally differentiated SynTs. Interestingly, excess glycogen accumulation has been noted within the SynT layer of some human preeclampsia (PE) placentas, consistent with their altered turnover and suggesting a potential link to altered glucose metabolism in the setting of this pregnancy complication. Importantly, epidemiologic studies confirm that derangements in glucose and fatty acid metabolism may drive pregnancy complications. For example, maternal gestational diabetes mellitus (GDM) and obesity are independently and additively associated with elevated rates of PE as well as spontaneous preterm birth. There may be shared mechanisms involving impaired trophoblast invasion contributing to PE and preterm labor (PTL) pathogenesis. For example, up to 30% of patients with spontaneous PTL have placental lesions consistent with impaired SpA remodeling typically observed during PE. Thus, improving our understanding of the links between placental metabolism and placental development may shed light on the growing epidemic of preterm birth.
Metabolic stressors such as hypoxia at high altitude or placental underperfusion associated with intrauterine growth restriction (IUGR) alter placental metabolism in particular ways. During isolated hypoxia induced at high altitude, for example, the human placenta appears to decrease its consumption of oxygen in favor of glycolysis to maintain its bioenergetic needs, which preserves placental growth. While preserving fetal oxygen (O 2 ) delivery, this comes at the expense of glucose, however. Given that fetal hypoglycemia is strongly associated with fetal growth restriction, this limits fetal growth. With maternal undernutrition, however, where uterine O 2 delivery is relatively spared, glucose delivery to the fetus is compromised in a manner associated with restricted placental growth. Given that the placenta is a complex organ with multiple different cell types, it is likely that changes in placental cellular composition due to alterations in cell fate regulatory pathways play important roles in the reallocation of placental metabolic flux patterns. Consistent with this, maternal calorie restriction leads to a loss of glycogen trophoblasts in the mouse. Importantly, isolated hypoxia results in increased hypoxia-inducible factor-1 (HIF-1) levels and target gene expression in the human placenta, and HIF activity regulates trophoblast cell fate decisions, suggesting a potential contribution to these pathologic changes. HIF activity can mediate metabolic adaptation to hypoxia via numerous ways, including repressing mitochondrial O 2 consumption while increasing glycolysis and modulating glucose transport as well as amino acid metabolism.
In addition to performing the essential transport functions of the placenta in humans, SynTs secrete numerous pregnancy-related hormones. Mammalian placenta produces a greater diversity of hormones in greater quantity than any other single endocrine tissue. Near term, steroid hormones (primarily estrogens and progestins) are being made at the rate of 0.5 g/day, and protein hormones (lactogens, growth factors, and other hormones similar to those of the hypothalamic–pituitary–adrenal [HPA] axis) are being made at more than twice this rate. Many of these hormones are species specific, but the categories of hormones (i.e., steroids, pituitary-like, hypothalamic-like etc.) and their endocrine, paracrine, and autocrine functions in pregnancy are frequently conserved ( Table 3.1 ).
| 1.Placental Vascular Processes |
|
| 2.Placental Inflammatory Immune Processes |
|
| 3.Other Placental Processes |
|
The placenta is an “incomplete” steroidogenic organ and does not express a complete set of enzymes for de novo production of estrogens and progestins. Steroid hormone synthesis in the placenta is dependent on precursors from mother and fetus, leading to the concept of an integrated maternal–fetal–placental unit . Fig. 3.3 diagrams the tissues and enzymes that participate in the biosynthesis of progestins and estrogens. The concentration of steroid hormones in the maternal circulation increases dramatically throughout gestation.
Maternal cholesterol, derived from low-density lipoprotein, is transported to the placenta and bound to low-density lipoprotein receptors on SynTs, where it is incorporated by endocytosis and hydrolyzed to free cholesterol in lysosomes. There is no significant 3-hydroxy-3-methylglutaryl coenzyme A activity in human placenta, and thus maternal cholesterol must be used for production of pregnenolone—the first step in steroid synthesis. Cholesterol is converted to pregnenolone in the mitochondria by cytochrome P450 cholesterol side-chain cleavage enzyme. After transfer to the cytosol, progesterone is produced from pregnenolone by type-1 3β-hydroxysteroid dehydrogenase. Before the ovarian–placental shift, the corpus luteum of pregnancy is the primary source of progesterone, but by 35 to 47 days postovulation the placenta produces enough progesterone to maintain pregnancy. The majority (>90%) of progesterone goes to the mother and the rest to the fetus. A limited amount of pregnenolone is also released into the circulation. The fetus has the enzyme activity needed for pregnenolone synthesis but has minimal ability to produce progesterone. High levels of circulating fetal progesterone are of placental origin so that circulating progesterone levels thus reflect placental function, not fetal well-being.
Progesterone can be metabolized to 17-hydroxyprogesterone (17-OHP), but relative efficiency of the enzymes favors progesterone production. 17-OHP levels do rise in the third trimester as progesterone levels peak. Additional progesterone metabolites, particularly 5-dihydroprogesterone (5-DHP) and its metabolite allopregnanolone, are also produced in the SynT at increased levels during gestation. These steroids have been hypothesized to play an endocrine role in fetal brain development and provide neuroprotection in the face of hypoxia.
Progesterone or a synthetic form of 17-OHP is used therapeutically in gestation as an adjunct for pregnancy maintenance after in vitro fertilization or in the second half of gestation for prevention of preterm delivery in women with a prior history of preterm birth. Progesterone is required for the maintenance of pregnancy in part by means of its suppressant effect on uterine contractions. Progesterone inhibits genes that promote contractility and has immunosuppressive activity that may promote uterine quiescence. Progesterone also counteracts uterine estrogen effects. Unlike the drop in progesterone levels prior to labor seen in most mammals, there is no progesterone withdrawal per se that occurs before labor in women; however, modulation of progesterone receptor expression in combination with a shift in the progesterone to estrogen balance is presumed to play the same biological role. The relationship of therapeutic response to normal physiologic mechanisms at work in the maternal–fetal–placental unit is not yet understood.
Unlike the requirement for maternal precursors for progesterone production, estrogen production relies on fetal precursors. In pregnancy, estrogens are synthesized from C19 steroids, primarily from dehydroepiandrosterone sulfate (DHEA-S) made in the fetal adrenals. The fetal adrenals rapidly inactivate steroids through sulfatization. Pregnenolone is sulfated and converted to DHEA-S, which then may be hydroxylated in the fetal liver. These biologically inactive androgens are then transferred back to the placenta. Placental sulfatases rapidly cleave the sulfate, and placental 3β-hydroxysteroid dehydrogenase converts DHEA or hydroxylated DHEA to androstenedione or hydroxylated androstenediones, respectively. These androgens are then aromatized to estrone (E1), 16α-OH estrone, or 15α-OH estrone and then converted to estradiol (E2), estriol (E3), or estetrol (E4) respectively by placental 17β-hydroxylation. E3 is the major estrogen of pregnancy with the majority secreted into the maternal compartment; E1 is the only estrogen preferentially secreted into the fetal compartment. Although maternal DHEA-S serves as 40% of the precursor for E2 synthesis, E3 and E4 are formed predominantly from fetal precursors because the maternal liver has limited 5α-hydroxylation or 16α-hydroxylation capabilities. E3 and E4 are thus indicators of fetal function, although neither is a clinically useful marker because of rapid shifts in circulating levels. The primary function of high E3 levels remains unclear, but it does increase uteroplacental blood flow.
Estrogens influence uterine growth, blood flow, contractility, metabolism, and breast development. However, high estrogen levels are not apparently needed for pregnancy. Parturition can proceed in the absence of fetal and placental sulfatase or aromatase, although in the latter case both fetus and mother are virilized. In such pregnancies, there is still circulating estradiol. There are no reports of pregnancy without detectable estrogen levels, suggesting that a basal level of estrogen is likely required. Before parturition, an increase in the estrogen to progesterone ratio occurs within the intrauterine tissues and may increase prostaglandin (PG) and oxytocin (OT) activity. Steroid hormone production is altered by trophic hormones and other factors, including hypothalamic-like releasing or inhibiting hormones. In turn, estrogens affect other endocrine systems (i.e., renin–angiotensin system) and support organ maturation, such as surfactant production in the lung.
In addition to the sex steroids, circulating levels of glucocorticoids and mineralocorticoids are increased in pregnancy. The placenta has the ability to produce cortisol and to convert it to inactive cortisone via 11β-hydroxysteroid dehydrogenase type 2. This enzyme also converts maternal cortisol to cortisone at the placental interface. The primary role of this system appears to be to protect the fetus from elevated cortisol exposure, which may play a role in long-term reprogramming of the fetal HPA axis. Dual oxidative and reductive enzymatic activity regulates the balance between cortisol and cortisone. In the placenta, oxidation of cortisol to cortisone predominates, whereas in the decidua the reverse reaction dominates, potentially providing localized hormone exposure.
A combination of pituitary-like growth hormones is required to support fetal growth while maintaining maternal metabolic homeostasis.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here