Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Deep venous insufficiency and the techniques used to repair the valve damage or valve incompetence is technically challenging when compared with the treatment of superficial, perforator, and iliofemoral deep venous occlusive disease, and therefore has been relegated to a “last consideration” in the treatment of patients with advanced clinical stage disease. Whether or not this should be the case can be debated but, in practicality, this is the current state and is mainly based on consensus risk/benefit determinations. If we only consider the patient with a venous ulcer as a potential candidate for deep venous valve reconstruction, approximately 2% of the 6 million US citizens with at least clinical stage 4 venous disease (C4) will progress to a venous ulcer (120,000). Medical treatment may heal 70% to 80% of venous ulcers but the recurrence rate is high and dependent on compliance. My best estimate is that 50% will be recurrence free over a 3-year period, leaving about 60,000 with a venous ulcer requiring more advanced treatment. The majority of patients with venous ulcers have reflux in multiple systems and treating all superficial and perforator vein insufficiency (with 89% also afflicted with deep venous insufficiency) will result in healing 80% but with a recurrence rate of 20% at 3 years. This would suggest that 12,000 did not heal and that another 9600 recurred for 21,600 patients still afflicted with a chronic disabling ulcer. If we assume that 55% of these patients have associated deep venous occlusive disease amenable to iliofemoral venous stent treatment and that 60% have long-term healing, we are left with nearly 5000 patients with deep venous insufficiency as the only uncorrected venous pathology to address. The approach to this cohort of patients must be prominent in the minds of those who care for such patients, or these patients will be deprived of a critical treatment opportunity because of sheer physician ignorance. These estimates are intentionally conservative, so it is certainly important to keep venous valve repair in our treatment toolbox. At least 20 surgeons adept at deep venous reconstruction would be required to treat these patients if each performed 250 operations yearly. If we add those with advanced C4b tissue changes and incapacitating swelling because of venous reflux to those with actual ulceration, the numbers increase significantly.
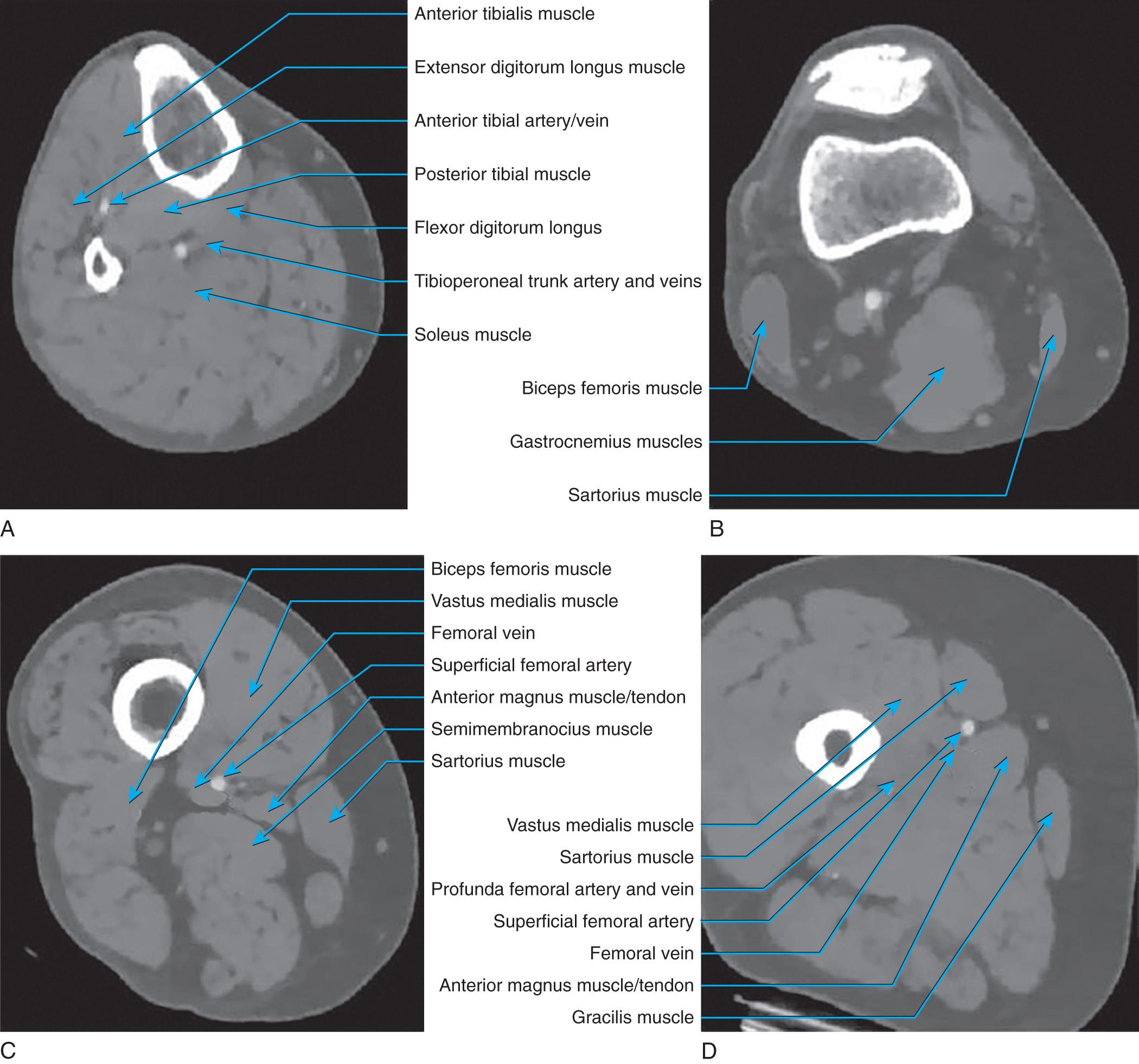
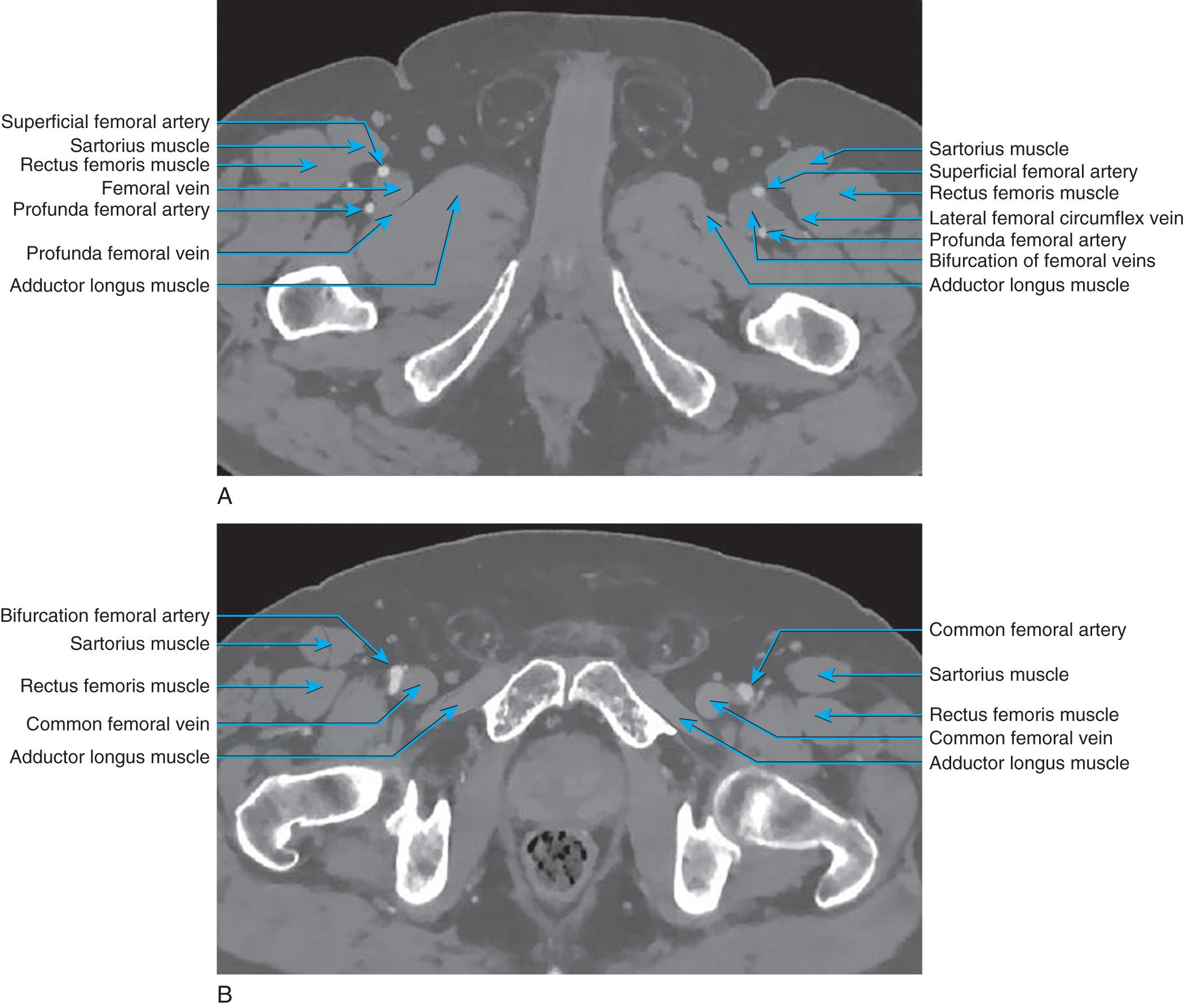
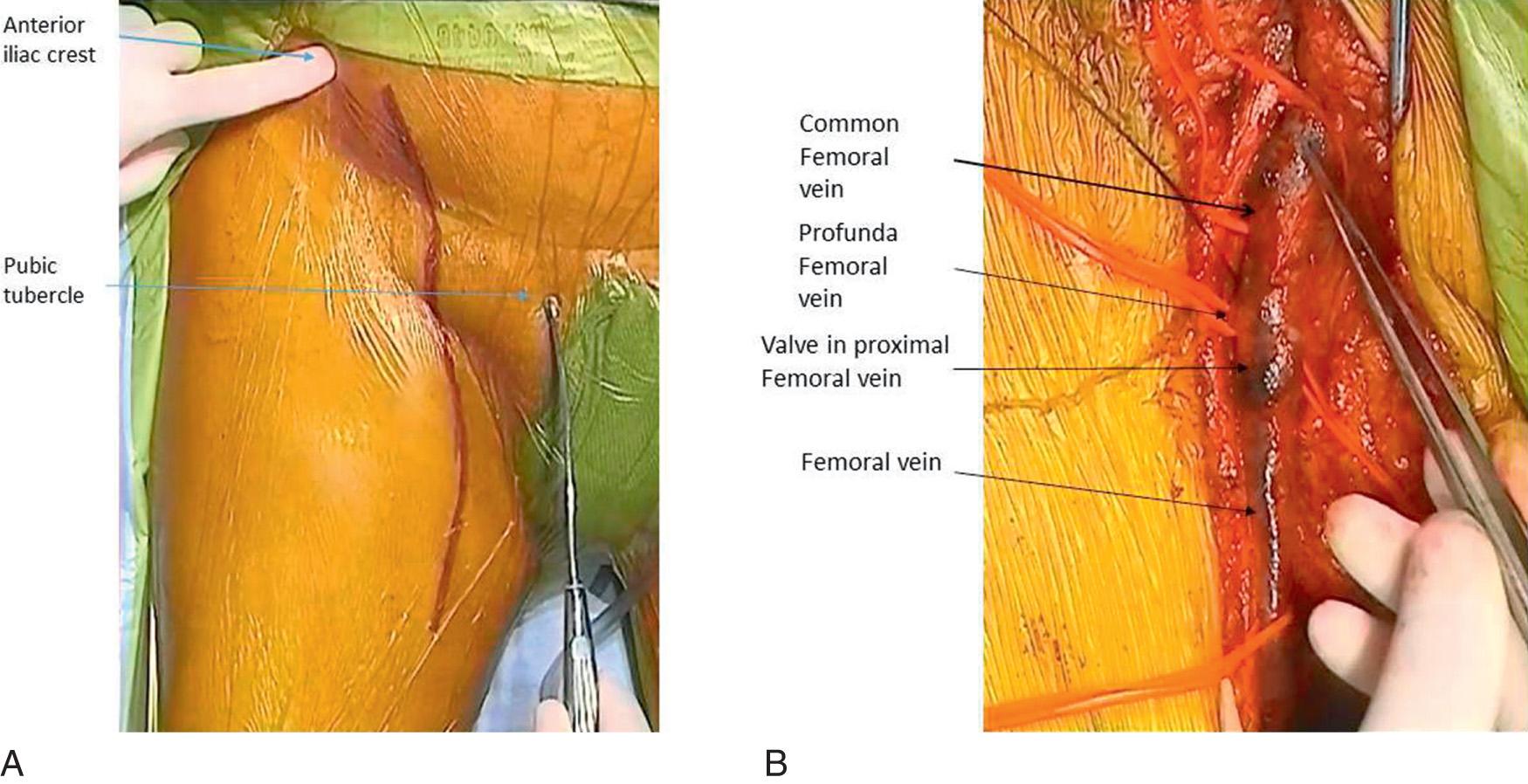
The lower extremity deep venous system with consistent valve presence begins in the foot and ceases at the inguinal ligament. The deep veins lie within the investing fascia of the muscles of the leg and thigh. The anterior tibial, posterior tibial, and peroneal veins are most often dual interconnected veins and follow the like-named arteries. The anterior tibial veins are found in the anterior compartment whereas the posterior tibial and peroneal veins lie within the deep posterior compartment ( Fig. 19.1 ). The anterior tibial veins and closely associated deep peroneal nerve lie between the tibialis anterior and extensor digitorum longus muscles in the proximal leg. The posterior tibial and peroneal veins lie within a rather-thin fascial layer between the superficial and deep posterior muscle groups and, in the upper extent, lie on the tibialis posterior muscle in close association with the tibial nerve. The posterior tibial and peroneal veins can join shortly before joining with the anterior tibial veins as it penetrates the tough interosseous membrane and becomes the popliteal vein(s) (PVs), which can be dual and reside near the popliteal artery (see Fig. 19.1 ). The popliteal vein(s) lie(s) behind the knee, can interconnect around the artery, and are noted anterior to the oblique popliteal ligament and popliteus muscle and is surrounded by a small fat pad. The gastrocnemius muscles are positioned laterally and medially, with the hamstrings more cephalad. The tibial nerve runs within this space whereas the common peroneal nerve lies laterally and cephalad, spiraling around the biceps insertion to reach the lateral aspect of the leg. The small saphenous vein may join the PV within this space. The popliteal vein(s) become the femoral vein(s) (FV) as the vein(s) pass(es) through the adductor magnus where it/they split(s) to form the adductor hiatus (see Fig. 19.1 ). The distal connection with the deep femoral vein (DFV) is a constant finding near this location. Two layers of tough fascia separate the quadriceps of the thigh from the adductors medially and hamstrings posteriorly. Superficially, there is an additional piece of fascia separating vastus medialis from adductors and above that the sartorius muscle. The vessels run within this fascial sling. The FV(s) rest in the deep fascia, deep to the sartorius muscle while flanked by the vastus medialis and adductor longus muscles ( Fig. 19.2 ). The inguinal ligament marks the transition from common femoral vein (CFV) to external iliac veins, and the vessels lie beneath the midpoint of the inguinal ligament. In the groin, the femoral vessels lie in the femoral sheath and are flanked laterally by the sartorius muscle and medially by the adductor longus muscle (see Fig. 19.2 ). The fascia lata forms an anterior roof over the femoral triangle and an opening in this fascia allows the lymphatics and great saphenous vein (GSV) to enter. The DFV joins the FV to form the CFV about 1 cm below the inguinal ligament.
The axillary vein lies below the lateral margin of the first rib and above the lateral edge of the teres major muscle. It lies on the subscapularis muscle with the lowest segment resting on the teres major and latissimus dorsi insertions. It resides in a cleft formed by muscles originating in the scapula with medial wall being the serratus anterior muscle. The coracoid process lies over the axillary neurovascular bundle and some of the muscle which overlay the neurovascular bundle attach to it. The pectoralis minor muscle, which lies directly over the bundle separates the vein and artery into three parts. The pectoralis major is the most superficial covering whereas the vein parallels the coracobrachialis and short head of the biceps brachii. The brachial vein lies below and within a fascial sheath, which when entered allows displacement of the biceps brachii and triceps muscles exposing the vessels because the vessels run in a parallel fashion with these muscles. Same named arteries and associated nerves lie in close proximity. This anatomy is included because it becomes important when considering a venous valve transplantation operation.
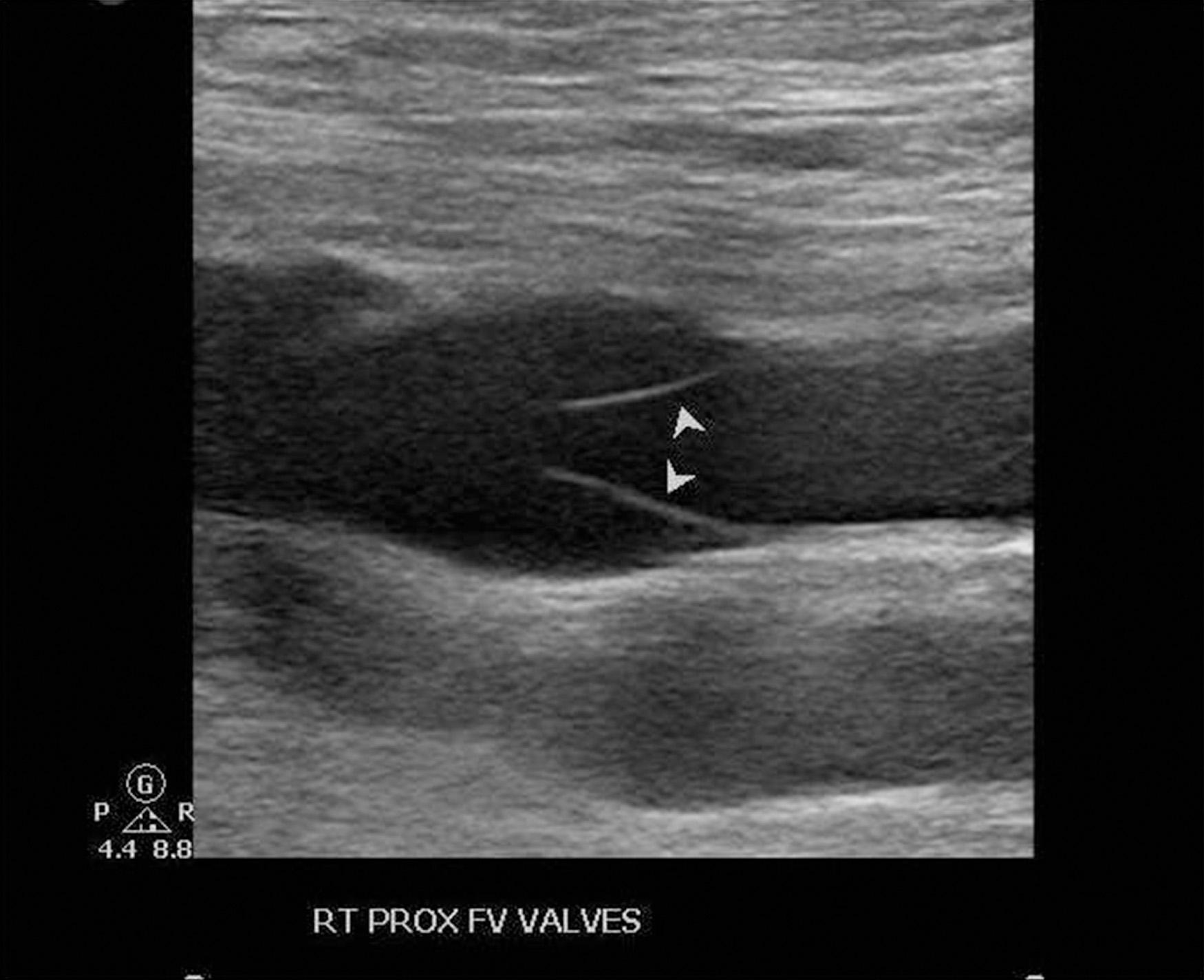
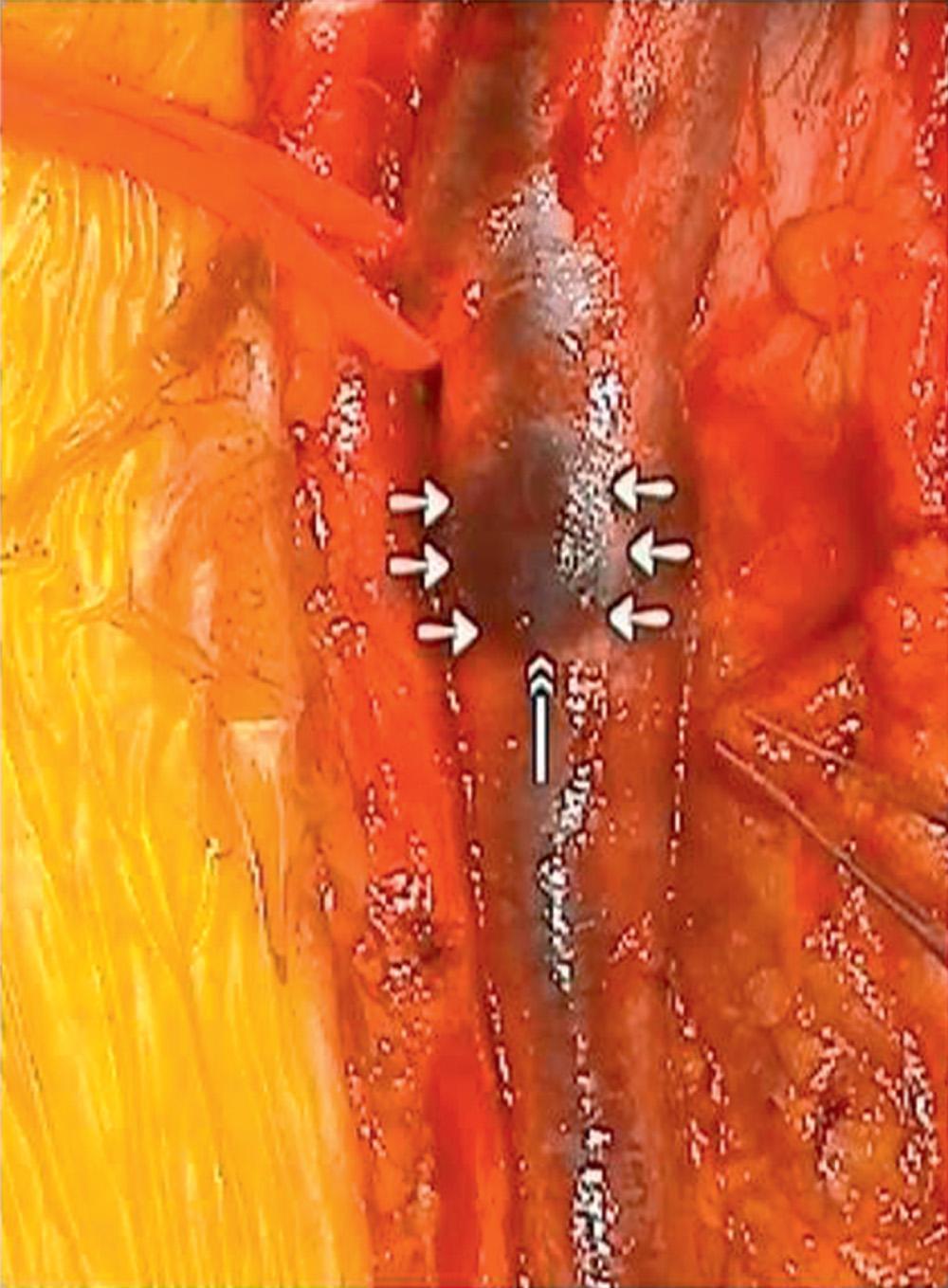
Venous valves are thin but very strong connective tissue structures with minimal muscular media covered by endothelium and are generally bicuspid in nature ( Fig. 19.3 ). The tibial and peroneal veins typically have 3 to 12 valves present in each. The majority of popliteal veins have one to three valves, with a tendency to be located more caudal within the vein. The FV has one to five valves, and in approximately 90% of patients, the most constant valve is located proximally within 1 to 2 cm of joining the DFV. About 90% of DFVs will have venous valves and some have up to four present. Only 30% to 50% of CFVs have a valve, but in those that do, there may be one or even two valves within a few centimeters of the inguinal ligament. Rarely does the external iliac vein have a valve present (~25%), and the common iliac vein lacks a venous valve in most cases. The GSV usually contains more than six valves (4–25), and at least one of these is within a few centimeters of the saphenofemoral junction. Axillary veins have at least one valve, with about 70% having a second, and 30% or less having one-third more distally located valve.
Dynamic duplex imaging has demonstrated that the large intramuscular veins (femoral[s]/popliteal), are supported on all sides by connective tissue, and therefore, when subjected to changes in volume, expand or collapse in direct response to those changes and in a circular manner. There compliance curves mimic those of an artery in that pressure changes reflect volume changes, they act as conduits rather than compliance vessels. The vein valves are not crushed together when the vein empties as might be expected in a completely compliant vessel. The valves within the vein function on a four-phase cycle consisting of opening, equilibrium, closing, and closed. After opening, the equilibrium phase demonstrates separation occurring at the valve edge on duplex imaging and the flow splits into two streams, with one directed into the valve sinus possibly to prevent stasis (clot prevention). When maximally open, the two cusps create a 35% narrowing of the outflow tract, which might aid in overall flow. Most of the time, the valve is in the open position. Valve closure normally occurs rapidly in response to the loss of the forward pressure/flow gradient and less so to retrograde blood flow. In fact, in normally functioning valves, closure is likely instantaneous without reverse blood flow noted. In clinical practice when evaluated by duplex imaging, the precise location of the valve is often not known and so reverse flow as an estimate of normal valve closure time is less than 500 ms for the deep femoral and tibial veins, although slightly longer for the FV and popliteal vein at 1 second. The hemodynamic result of a normal functioning venous system, both as conduit for blood to return to the heart and with valve function to prevents reflux, is decreased venous blood pooling and prevention of sustained/consistent high venous pressures in the lower leg when in the erect position.
The rare patient may congenitally lack vein valves. In primary venous insufficiency, the valve may be structurally normal but floppy or redundant; the vein diameter may be enlarged preventing cusp apposition; or asymmetric insertion or development may result in a malfunctioning valve. There is no clear inciting event for the changes noted and except for developmentally defective valves, tightening of the valve leaflets can reestablish function.
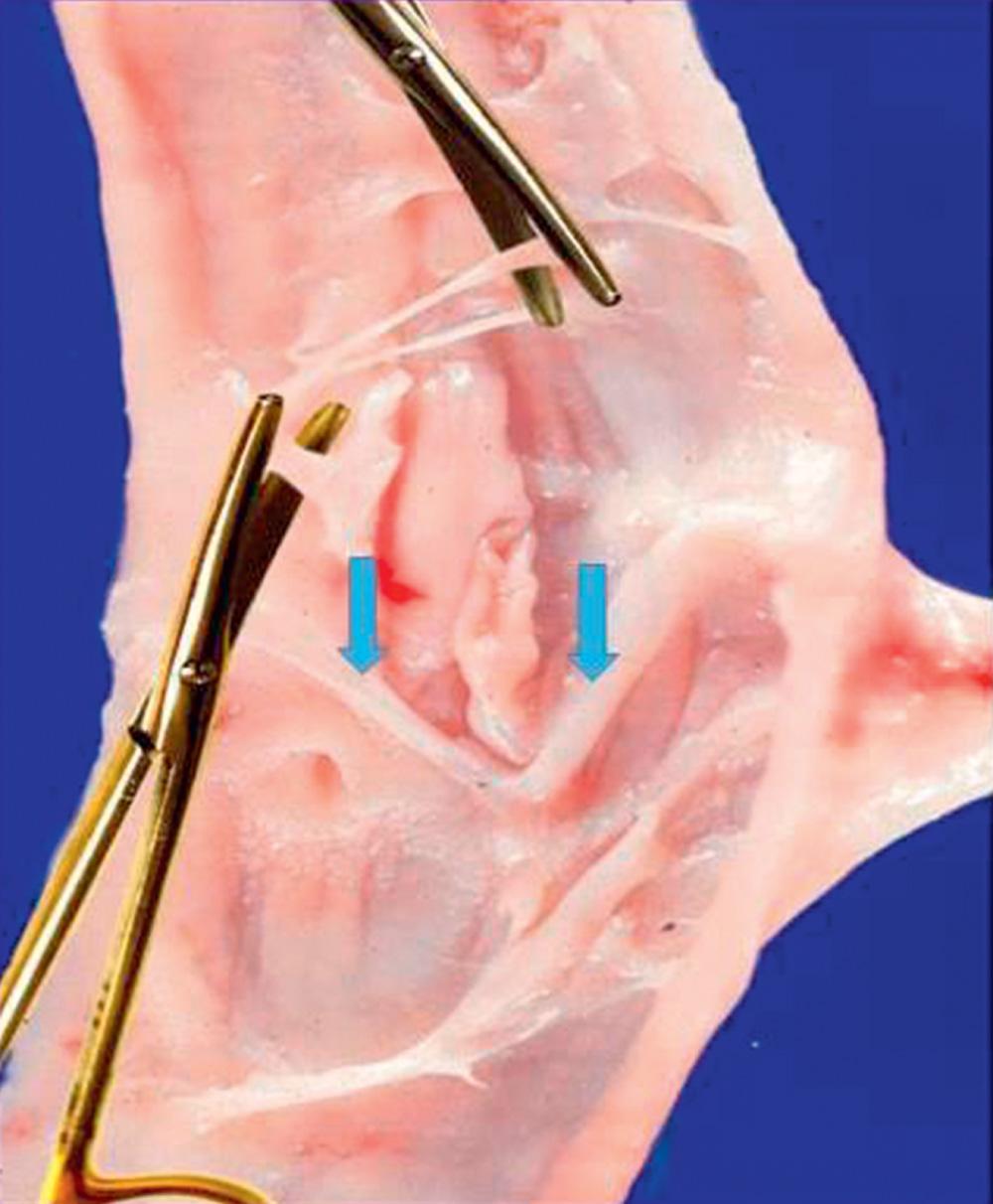
In contradistinction to primary venous insufficiency described earlier, secondary venous insufficiency has an inciting event, which is often acute venous thrombosis. The associated inflammation can result in scarring following recanalization demonstrated as valve fibrosis, foreshortening, perforation, and wall adhesion in a potential environment of luminal narrowing ( Fig. 19.4 ). The valve leaflets are not repairable and other options to reestablish a lower extremity deep venous system free of reflux is required. Because acute thrombosis and recanalization is variable in extent and resolution, there are conditions in which a valve is preserved either within a normal vein segment or within a damaged shrunken vein, potentially the result of isolated wall scarring.
Unrelieved ambulatory venous hypertension in the lower leg (especially in the gaiter area) is a major factor causing the signs and symptoms of chronic venous disease (CVD). Correcting free reflux into the lower leg by even one competent valve has demonstrated clinical improvement for prolonged periods as long as that valve remained competent. This has been demonstrated by the results of valve repair preformed for this condition and will be reviewed in detail later in this chapter. The anatomic fact that the popliteal vein is directly connected to both the FV and DFV establishes it in a unique position as “gatekeeper” for reflux into the lower leg. This is clearly demonstrated by the fact that an isolated FV valve repair may clinically fail if the deep femoral system is also incompetent (free reflux into the popliteal and then the lower leg), although isolated femoral repair will be successful when the deep femoral valve(s) is/are competent. FV occlusion may result in significant DFV dilation, such that correction of reflux in the DFV will dramatically improve venous hemodynamics. Alternatively, preventing reflux through the DFV (gatekeeper) can correct significant axial insufficiency into the lower leg and has been associated with delayed healing when not addressed. Repairing multiple tibial vein valves can have a similar effect.
A history and physical examination with handheld Doppler examination of lower leg veins (level of investigation 1) can provide an idea of the etiology of the patient's condition and rule out other causes of the patient's symptoms. Patients considered for venous valve surgery should have symptomatic advanced disease: C3 (unrelenting edema), C4 (skin damage), C5 (healed venous ulcer), or C6 (active venous ulcer).
When considering a deep venous valve repair, a more in-depth evaluation is essential for clarifying your patient's precise anatomic situation and planning the appropriate operation. A complete noninvasive venous duplex evaluation that typically includes plethysmography (level of investigation 2) is essential in determining the location of reflux, which specific veins are involved, and provides some indication of overall lower extremity hemodynamic effects attributable to the insufficiency present. Assuming that other venous systems in the lower extremity are functionally normal or have been corrected by intervention before considering a valve repair, this investigation specifically defines the deep venous system. Prolonged reflux time (valve closure time), after a provocative test (compression distal to the femoral junction) conducted in the standing position and throughout the deep venous system, is standard protocol for determining reflux. Although some studies have demonstrated that 1 second or more should be the cut-off abnormal value for the FV and popliteal vein, current consensus suggests that a valve closure time 0.5 second or more is best considered abnormal for all vein segments. Venous duplex imaging is variably capable of valve visualization to determine cusp presence and its function or lack thereof (see Fig. 19.3 ). Venous plethysmography is not a routine study in standard clinical practice but adds some insight into differentiation between hemodynamic reflux and obstruction when considering a deep venous valve repair. It also provides the potential to demonstrate quantitative improvement after repair. It may help to establish the effect an abnormal calf pump function may have on the patient's condition. The ejection volume and residual volume fraction are good indicators of calf muscle pump function and are abnormal when the ejection volume is less than 60% and the residual volume fraction is greater than 35%. The residual volume fraction reflects the ambulatory venous pressure quite well.
Advancement to the third level of investigation includes invasive venography and is recommended before valve repair. Descending venography provides detail of valve anatomy and the degree of reflux within the deep system. Kistner used a four-grade system with grade 4 (the most severe) involving reflux down the entire lower leg venous system, whereas grade 3 reflux went through the popliteal vein during descending venographic evaluation. Both are considered for valve repair. Visualization and interrogation of the DFV is an important component of this investigation as a potential alternate source of axial reflux and an indication for valve repair within the DFV itself or placement of the valve in the popliteal vein rather than the upper FV. The success of FV valve repair depends upon elimination of axial reflux by all axial routes, including femoral-popliteal, deep femoral-popliteal, and other random anatomic pathways. Descending venography is not perfect, but when paired with duplex imaging and ascending venography is the best available preoperative diagnostic combination to define repairable, deep venous reflux. Ascending venography provides some anatomic detail of segmental obstructions and the opportunity to measure ambulatory venous pressure as an estimate of venous insufficiency. Computed tomographic imaging and magnetic resonance imaging are performed in the supine position, which renders the study unreliable for valve anatomy and function, although such can be useful to rule out other cause of venous pathology.
These anatomic and functional investigations lead to a precise determination of your patient's current venous pathologic state to be compared with a complete clinical application of CEAP (clinical, etiologic, anatomic, pathophysiologic) including full determination of clinical presentation (with or without symptoms), etiology, anatomy involved, and pathophysiology by anatomic segment. Included in the classification is the level of investigation (potentially 3) and the date of investigation. Integral to demonstrating success after an intervention, it is helpful to have a baseline physician determined disease severity score (Venous Clinical Severity Score) and a patient-estimated impact on life and functional status score (Quality of Life survey[s]). Measurement of clinical and pathologic status using current assessment tools (for planned venous interventions) can be given a grade 1 recommendation.
General anesthesia is generally required for these open procedures, which can be technically challenging and therefore lengthy. Confirmation of respiratory and cardiac stability for a safe operative intervention and the elimination of arterial occlusive disease as a confounding issue, especially in a patient with an ulcer, are highly recommended. A patient with diabetes, renal failure, or collagen vascular disorders requires optimal medical treatment before operative intervention. Perioperative antibiotics are frequently used (first-generation cephalosporin) and heparin is recommended for open vein procedures, so allergies must be considered.
The exposure of the lower extremity veins mimics that used for exposure of the associated artery. If reconstruction of the proximal FV or DFV is planned, a generous groin incision ( Fig. 19.5A ) is made in the direction of the vessels to expose the first and second femoral valves. Extending the dissection distally through the fascia will allow lateral displacement of the sartorius muscle and exposure of a longer length of either vein as needed ( Fig. 19.5B ). Ligate any apparent lymphatic chains to prevent postoperative lymphocele or lymph leak. To determine if valve cusps are present and to allow later valve repair, dissection of the vein's adventitial layer is critical ( Fig. 19.6 ). It will allow confirmation of a valve (which may have been missed on primary imaging) or determine the lack of a valve in cases where one was thought present. A lack of clear valve attachment lines signifies high likelihood of significant postthrombotic valve damage and suggests that techniques other than in situ repair will be required for success. This incision also provides exposure of the lower CFV, proximal FV, and DFV as needed for valve transposition, transplantation, or other less-conventional valve reconstructions. The GSV insertion into the FV is also within this field and medial dissection in the subcutaneous tissues will quickly expose its proximal extent and associated branches.
Distal femoral, proximal popliteal, or even tibial veins can be exposed through medial incisions as one would use to expose the associated artery. Detailed descriptions of these exposures are available for review. The best exposure to the popliteal vein is a posterior S-shaped incision with the transverse incision made in the posterior knee crease to decrease the chance of scar contraction. Bear in mind the associated nerves (as noted in the anatomy section), which are prone to iatrogenic injury in this confined area of dissection.
Following the generous dissection of the target vein with valve within, the “strip test” is used to determine valve incompetence. The strip test is performed by occluding antegrade venous blood flow (finger compression or instrument) distal to the valve and pushing the trapped blood into the vein above the valve. If flow into the empty vein below the valve is observed upon applying pressure on the vein above the valve, valve incompetence is confirmed. If the valve is totally competent, the segment above the valve balloons out and the segment below the valve remains collapsed.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here