Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Cystic diseases of the kidney are a heterogeneous group of disorders that can be inherited, developmental, or acquired ( Box 43.1 ).
Autosomal dominant polycystic kidney disease (ADPKD)
Autosomal recessive polycystic kidney disease
Autosomal dominant or X-linked diseases in the differential diagnosis of ADPKD
Tuberous sclerosis complex
von Hippel-Lindau syndrome
Hepatocyte nuclear factor-1 associated nephropathy
Familial renal hamartomas associated with hyperparathyroidism–jaw tumor syndrome
Orofaciodigital syndrome
Autosomal dominant medullary cystic kidney disease
(autosomal dominant tubulointerstitial kidney disease)
Hereditary recessive ciliopathies with interstitial nephritis and/or cysts
Nephronophthisis
Joubert syndrome
Meckel-Gruber syndrome
Bardet-Biedl syndrome
Alström syndrome
Nephronophthisis variants associated with skeletal defects
Renal cystic dysplasias
Multicystic kidney dysplasia
Other cystic kidney disorders
Simple cysts
Localized or unilateral renal cystic disease
Medullary sponge kidney
Acquired cystic kidney disease
Renal cystic neoplasms
Cystic renal cell carcinoma
Multilocular cystic nephroma
Cystic partially differentiated nephroblastoma
Mixed epithelial and stromal tumor
Cysts not of tubular origin
Cystic disease of the renal sinus
Perirenal lymphangiomas
Subcapsular and perirenal urinomas
Pyelocalyceal cysts
Kidney cysts are fluid-filled cavities lined by epithelial cells that derive primarily from the tubules or collecting duct, losing its connection with their origin tubule once developed. Various imaging techniques can identify kidney cysts, which, depending on their characteristics and distribution and together with clinical and genetic information on the patient, can aid in the differential diagnosis ( Fig. 43.1 ). In some the kidney cysts are the prominent abnormality, whereas in others the cysts are part of a more complex phenotype.
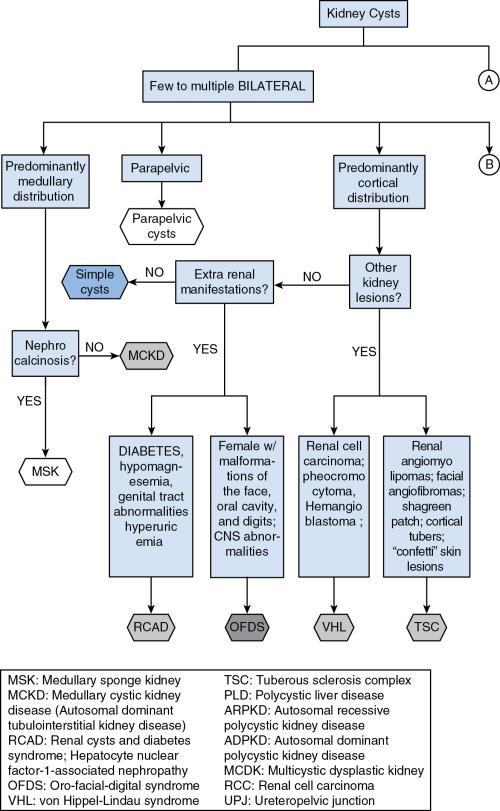
Acquired simple kidney cysts are the most common abnormality and can be single or multiple. They are rare in children and individuals less than 30 years old, but the frequency increases with age. Among the inherited cystic diseases, autosomal dominant polycystic kidney disease (ADPKD) is the most frequent one followed by autosomal recessive polycystic kidney disease (ARPKD).
ADPKD is a genetically heterogeneous disease, for which two different genes have been identified. PKD1 , located on the short arm of chromosome 16, is responsible for approximately 85% of the cases in which a mutation has been identified, whereas PKD2, located on chromosome 4, accounts for nearly 15% of remaining cases with an identified mutation. PKD1 and PKD2 encode for polycystin-1 and polycystin-2, respectively. These membrane-bound glycoproteins are located in the plasma membrane of the primary cilia and regulate calcium homeostasis. PKD1 is associated with more severe disease compared with PDK2. This is due to the fact that patients with PKD1 develop more cysts at an early age rather than faster cyst growth. There have been hundreds of different pathogenic mutations identified in PKD1 and PKD2 . It is thought that kidney cysts may develop from loss of functional polycystin with somatic inactivation of the normal allele consistent with a “two-hit” mechanism, but other genetic mechanisms may play a role. Between 7% and 10% of ADPKD cases remain genetically unresolved after genetic screening. Data suggest that mutations in GANAB , a coding gene that encodes for a glucosidase-II alpha subunit, may account for approximately 3% of the genetically unresolved ADPKD cases. However, due to the mild phenotype presentation of patients with mutations in GANAB, it is possible that this account for a larger proportion of the missing genetic causes of ADPKD and may be underdiagnosed.
In patients older than 18 years with a family history of ADPKD, the diagnosis is primarily established by imaging studies. Patient counseling should always be done before performing imaging studies. Ultrasound is commonly used because it is noninvasive and inexpensive. Diagnostic criteria for ADPKD by ultrasound are summarized in Table 43.1 . There are no preestablished diagnostic criteria for computed tomography (CT) or magnetic resonance imaging (MRI), but ultrasound criteria could reasonably be applied to CT or MRI if restricted to cysts measuring ≥1 cm in diameter. Contrast-enhanced CT and MRI provide better anatomic definition than ultrasonography and are more helpful to ascertain the severity and prognosis of the disease. In addition, both techniques are used to better characterize cystic lesions and complications. MRI is the preferred imaging modality for following disease progression in these patients. It provides great cystic-parenchyma definition without requiring intravenous (IV) contrast. CT with contrast is also highly sensitive (detects cysts as small as 3 mm), and both techniques may provide useful information regarding extrarenal manifestations of the disease (liver, pancreas, spleen, and other organs). CT with contrast should be avoided if there is evidence of significant risk of contrast nephropathy (see Chapter 13 ). CT (with and without contrast) is also helpful in assessing and identifying complications, such as nephrolithiasis, complex cysts, or cyst wall calcifications from old cyst hemorrhage. Kidneys, ureter, and bladder scan and tomograms may be helpful to differentiate uric acid stones from calcium stones, whereas dual energy computed tomography may help discriminate uric acid stones from other kidney stones. Indium 111 ( 111 In) scans may be useful to identify infected kidney or liver cysts. Likewise, positron emission tomography (PET) scans may identify infected liver cysts.
| CRITERIA | PKD1 | PKD2 | UNKNOWN ADPKD GENE TYPE | |
|---|---|---|---|---|
| Revised Unified Diagnostic Criteria for ADPKD | ||||
| 15–29 | ≥3 cysts, unilateral or bilateral | SEN = 94.3% | SEN = 69.5% | SEN = 81.7% |
| 30–39 | ≥3 cysts, unilateral or bilateral | SEN = 96.6% | SEN = 94.9% | SEN = 95.5% |
| 40–59 | ≥2 cysts in each kidney | SEN = 92.6% | SEN = 88.8% | SEN = 90% |
| >60 | ≥4 cysts, in each kidney | SEN = 100% | SEN = 100% | SEN = 100% |
| Revised Ultrasound Criteria for Exclusion of ADPKD | ||||
| 15–29 | ≥1 cyst | SPEC = 97.6% | SPEC = 96.6% | SPEC = 97.1% |
| 30–39 | ≥1 cyst | SPEC = 96.0% | SPEC = 93.8% | SPEC = 94.8% |
| 40–59 | ≥2 cyst | SPEC = 98.4% | SPEC = 97.8% | SPEC = 98.2% |
Genetic testing (linkage analysis and direct DNA sequencing) is available as a clinical test and can detect PKD1 and PKD2 mutations in approximately 90% of confirmed cases. The ADPKD Mutation Database ( http://pkdb.mayo.edu ) lists known mutations of the PKD1 and PKD2 genes. Counseling should be done before genetic testing. Evaluation of family history may also provide important clues about the genetic variant, which has prognostic implications. For example, the presence of at least one affected family member who reached end-stage kidney disease (ESKD) at or before 55 years old is highly suggestive of a PKD1 mutation. Contrarily, the presence of at least one affected family member with either preserved kidney function or ESKD developed after 70 years old is highly indicative of a PKD2 mutation. Lastly, in patients with multiple bilateral cysts (10 or more per kidney) but negative family history, a presumptive diagnosis of ADPKD may be considered.
No, because the utility of ultrasound to exclude ADPKD is limited in individuals under 30 years old with a family history of the disease, particularly those with PKD2 mutations. In these individuals, MRI, CT, or genetic testing should be considered. Nevertheless, most ADPKD cases can be confirmed or ruled out on imaging testing. Furthermore, a study has shown that fewer than five kidney cysts on MRI are sufficient for excluding ADPKD in at-risk subjects between 16 and 40 years of age.
Besides kidney cysts, patients with ADPKD frequently present extrarenal disease, including hepatic cysts, cysts in other organs, cardiovascular manifestations, diverticular disease, and abdominal hernias.
Polycystic liver disease (PLD) is the most common extrarenal manifestation of ADPKD and is characterized by multiple cysts distributed throughout the liver parenchyma. Hepatic cysts are commonly seen in patients with ADPKD, and their prevalence is as high as 90% on screening MRI. In a minority of patients, it can result in severe PLD requiring surgical intervention. The disease is usually found in association with ADPKD but can occur in isolation, with only a small number of kidney cysts (or even the total absence of cysts). Mutations in protein kinase C substrate 80K-H (PRKCSH) gene on chromosome 19p13 and SEC63 homologue, protein translocation regulator on chromosome 6q, account for more than 30% of isolated cases. Although most patients remain asymptomatic with preserved liver function, abdominal pain, distension, and liver decompensation may occur due to compressive effects of enlarged cysts.
Pancreatic cysts have been found in approximately 10% of patients with ADPKD. These are more prevalent with increasing age. Pancreatic cysts are nearly always asymptomatic, but in very rare occasions, cyst compression of the main pancreatic duct may cause recurrent pancreatitis. In addition, a few cases of combined ADPKD and pancreatic carcinoma have been reported, suggesting genetic interactions between ADPKD and pancreatic carcinogenesis. Asymptomatic arachnoid membrane cysts and spinal meningeal cysts have been reported in a small proportion of patients with ADPKD (8% and 2%, respectively). Arachnoid membrane cysts can increase the risk of subdural hematomas, and spinal meningeal cysts can leak and present with orthostatic headache due to intracranial hypotension.
Ovarian cysts are not associated with ADPKD, whereas cysts of the seminal vesicles occur in 40% to 60% of men with ADPKD but rarely result in infertility.
Vascular manifestations are the most important noncystic complications and include intracranial aneurysms (IAs), dolichoectasias, thoracic aortic and cervicocephalic artery dissections, and coronary artery aneurysms. They are caused by alterations in the vasculature directly linked to mutations in PKD1 or PKD2. Polycystin-1 and polycystin-2 are known to be expressed in vascular smooth muscle cells. IAs represent the most feared vascular manifestation of ADPKD because their rupture carries a 35% to 55% risk of combined severe morbidity and mortality. The rate of IA in ADPKD patients ranges from 6% (negative family history of IA) to 16% (positive family history of IA), approximately five times more common than in the general population (1% to 2%). They are often asymptomatic (Irazabal et al., 2015). However, focal findings such as cranial nerve palsy or seizure may result from compression of local structures.
Cardiac valvular abnormalities are common in patients with ADPKD. Mitral valve prolapse is the most frequent valvular abnormality found in up to 25% of patients on echocardiography, whereas aortic regurgitation and tricuspid prolapse may occur in a small proportion. Nevertheless, they rarely require valve replacement, and screening echocardiography is not indicated unless a cardiac murmur is detected on clinical examination.
The prevalence of colonic diverticulosis and diverticulitis in patients with ESKD with ADPKD is significantly higher than in individuals with other kidney diseases (83% vs. 32%). However, whether ADPKD patients with preserved kidney function show propensity for diverticular disease remains unknown. The mechanisms implicated in the development of colonic diverticula may include alterations in polycystin function, which can exacerbate aging-induced smooth muscle dysfunction.
Abdominal hernias (inguinal, incisional, and paraumbilical) are frequent in the ADPKD population. Importantly, hernias are associated with complications (intestinal incarceration or strangulation) and may cause problems in patients undergoing peritoneal dialysis.
Hypertension: The majority of patients with ADPKD present with abnormal blood pressure levels. Studies have shown that hypertension is diagnosed 15 years earlier in ADPKD patients compared with those with essential hypertension. Indeed, the prevalence of hypertension reaches 50% in patients aged 20 to 30 years, representing the initial presentation for 30% of ADPKD patients, but increases to 100% of those with ESKD.
The pathogenesis of hypertension in ADPKD is complex, and the most plausible hypotheses include activation of the renin-angiotensin-aldosterone system, cyst expansion, intrarenal ischemia, and decreased expression levels of polycystin-1 and 2, and nitric oxide (NO) availability. Importantly, uncontrolled blood pressure is associated with increased mortality rates from valvular heart disease and aneurysms, increases the risk of proteinuria and hematuria, and increases speed of decline of kidney function. Therefore earlier detection and treatment of hypertension and more rigorous blood pressure control are important on these patients.
Pain: Approximately 60% of patients with ADPKD suffer from pain, the most common symptom reported by adults with ADPKD. Pain is most commonly located in the flank, back, and abdomen and is primarily attributed to cystic compression of the kidney capsule and parenchyma, but it can also be caused by hemorrhage into a cyst, cyst rupture, or infection. Importantly, kidney stones or tumor should be always ruled out. Acute causes of kidney pain include renal hemorrhage, passage of stones, and urinary tract infections (UTIs). Cyst hemorrhages often resolve within a week, but if it is prolonged or if the initial episode occurs after the age of 50 years, screening to exclude neoplasm should be done. Although most patients with hepatic cysts are asymptomatic, cystic enlargement of the liver may also cause mechanical back pain.
Nephrolithiasis: Kidney stones occur in 20% to 36% of patients with ADPKD. The most common types of stones are composed of uric acid or calcium oxalate. Metabolic factors such as low urine citrate and urine pH, and decreased ammonia excretion may predispose to stone formation, yet urinary stasis due to distorted kidney anatomy may also prompt stone formation. Abdominal CT before and following contrast enhancement is important to detect renal calculi. Stones may be missed if only a contrast-enhanced CT is performed.
Urinary infections: In ADPKD, UTIs are more frequent in women than in men, as in the general population. UTIs are usually caused by gram-negative enteric bacteria (Enterobacteriaceae) . Although CT and MRI can detect cyst complications and provide anatomic definition, none of these findings are specific for infection. Alternatively, nuclear imaging with 67 Ga- or 111 In-labeled leukocyte scans may be used, but false negative and false positive may occur. An 18-F-fluorodeoxyglucose PET scan is a promising agent for detection of infected cysts. However, its efficacy for diagnosing UTI is limited. When a kidney cyst infection is suspected, delayed images should be obtained. Finally, in the event that the clinical setting and imaging are suggestive of infection, but blood and urine cultures are negative, cyst aspiration should be considered.
ESKD: ADPKD is the leading inheritable cause of ESKD, with 45% of patients with ADPKD developing ESKD by 60 years of age. Men tend to progress faster than women, which may be partly attributed to a greater prevalence and severity of hypertension. There is a strong relationship between kidney volume growth and glomerular filtration rate (GFR) decline.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here