Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Protozoan parasites of the genus Cryptosporidium were first identified in the stomach of mice in 1907. The species name Cryptosporidium parvum was proposed in 1912 to describe parasites identified in murine intestines. Although Cryptosporidium was linked to gastrointestinal disease in turkeys in 1955 and to bovine diarrhea in 1971, the first human cases were described only in 1976. Only a handful of cases had been reported before 1982. In the early 1980s, large numbers of cases were noted to be associated with the emerging epidemic of acquired immunodeficiency syndrome (AIDS). Soon studies identified cases among animal handlers and children. Shortly thereafter, Cryptosporidium was associated with waterborne outbreaks of diarrhea, including an outbreak in Milwaukee, Wisconsin in 1993 that affected an estimated 403,000 persons. Studies have now demonstrated that Cryptosporidium is an important cause of diarrhea in normal hosts worldwide and one of the main causes of childhood diarrhea in resource-poor countries, including causing prolonged diarrhea and malnutrition, and of chronic diarrhea in immunocompromised hosts, including patients with AIDS. Cryptosporidiosis is now recognized as a major cause of childhood diarrhea morbidity and mortality.
The genus Cryptosporidium consists of a group of protozoan parasites within the phylum Apicomplexa, which also includes Plasmodium species. Cryptosporidium has been reclassified from the subclass Coccidiasina (coccidia, along with Toxoplasma, Cyclospora, and Cystoisospora ) into the class Gregarinomorphea, subclass Cryptogregaria. The genomes of a number of Cryptosporidium species have been sequenced, including Cryptosporidium hominis , C. parvum , an anthroponotic strain of C. parvum subtype IIc, and others. Compared with other apicomplexan parasites, the genomes are relatively compact (approximately 9.1 Mb), with the loss of approximately 1400 genes, compared with the Plasmodium parasites. Many of these gene deletions may be due to loss of the mitochondria and apicoplast, organelles found in most other apicomplexans but not found in Cryptosporidium spp. Cryptosporidium spp. also lack the genes for variable surface proteins contained in the Plasmodium falciparum genome (e.g., var , rif , and stevor genes). The metabolic pathways are also simplified (e.g., no Krebs cycle), but a number of transporters are present to scavenge molecules from the host. Important metabolic pathways do exist, however, such as fatty acyl-coenzyme A synthetase, and their inhibition in a mouse model reduced parasite oocyst production.
Species were initially named based on the host species. In the late 20th century, human isolates were thought to belong to a single species, C. parvum. Molecular studies subsequently demonstrated that parasites previously termed C. parvum include a number of genotypes and occult species. As of 2018, there were at least 35 named Cryptosporidium spp. thought to be valid, based on host specificity, morphology, and molecular biology studies, and numerous other genotypes that may emerge as separate species. Among the isolates speciated as C. parvum, however, there is also a subtype, IIc, that mainly infects people and shares sequence homology in some regions with C. hominis instead of other C. parvum isolates. Molecular biology studies have demonstrated that humans can also be infected with Cryptosporidium meleagridis, Cryptosporidium cuniculus, Cryptosporidium ubiquitum, Cryptosporidium viatorum, Cryptosporidium canis, Cryptosporidium felis, Cryptosporidium muris, and others. No specific clinical characteristics of rare species have been reported, but volunteer studies have demonstrated mild diarrhea with C. meleagridis and C. muris. C. meleagridis, formerly thought to mainly infect birds, has been identified in most large series and appears to cause approximately 1% of human cryptosporidiosis overall and more in some series from Asia. Other species are either rarely noted to cause human infection or have been noted to infect only reptiles, fish, birds, or nonhuman mammals.
Cryptosporidium spp. can complete their entire life cycle within a single host, including both asexual (merogony) and sexual (sporogony) reproductive cycles ( Fig. 282.1 ). In the stomach and upper intestines, the oocysts are activated, producing serine and cysteine proteases and aminopeptidases, which allow the organisms to excyst, releasing four infective sporozoites. Contact with the sialylated carbohydrate surface of the epithelial cells may be an important trigger for excystation. Other factors in excystation may include temperature, stomach acid, bicarbonate pancreatic enzymes, and bile. Each sporozoite contains an apical complex with specialized organelles involved in invasion, including rhoptries, micronemes, and dense granules. The motile sporozoites bind to receptors on the surface of the intestinal epithelial cells. Several parasite ligands (including p30, the galactose- N -acetylgalactosamine lectin; p23, the 1300-kDa circumsporozoite-like antigen; gp900, the thrombospondin-related adhesive protein of Cryptosporidium -1, Cpgp40/15, and CP47) have been implicated in parasite attachment to the intestinal epithelium. The parasites then induce actin polymerization and protrusion of the intestinal epithelial cell membrane, which is mediated by tyrosine kinase growth factor receptor (TKGFR), phosphatidylinositol 3 (PI3) kinase, and CDC42. The membrane surrounds the sporozoite and fuses to form the parasitophorous vacuole, which remains in the microvillus layer on the surface of the epithelium. A band of dense cytoskeletal elements separates the parasite from the host cytoplasm. This band prevents free flow of materials between parasite and the host cell cytoplasm. It also contains an adenosine triphosphate–binding cassette, which likely functions as an efflux pump, contributing to the resistance of the organisms to chemotherapy. Inside the parasitophorous vacuole, the parasites undergo asexual reproduction (merogony). They enlarge into trophozoite forms and divide to form type I meronts, which mature and rupture to release the motile merozoites. The merozoites bind to receptors on the epithelial cells and are engulfed by the cells. They then either repeat the process of merogony or undergo sexual differentiation. In that case, the merozoites differentiate into the microgamonts and macrogamonts. The microgamont releases the microgametes, which penetrate the cells infected with a macrogamont. The macrogamont and microgametocytes fuse to form the zygote form, which then undergoes meiosis to form the oocyst, containing four sporozoites. Two morphologic forms of the oocyst have been described. Thin-walled oocysts are thought to excyst within the same host in a process of self-infection. The thick-walled oocysts are shed into the environment.
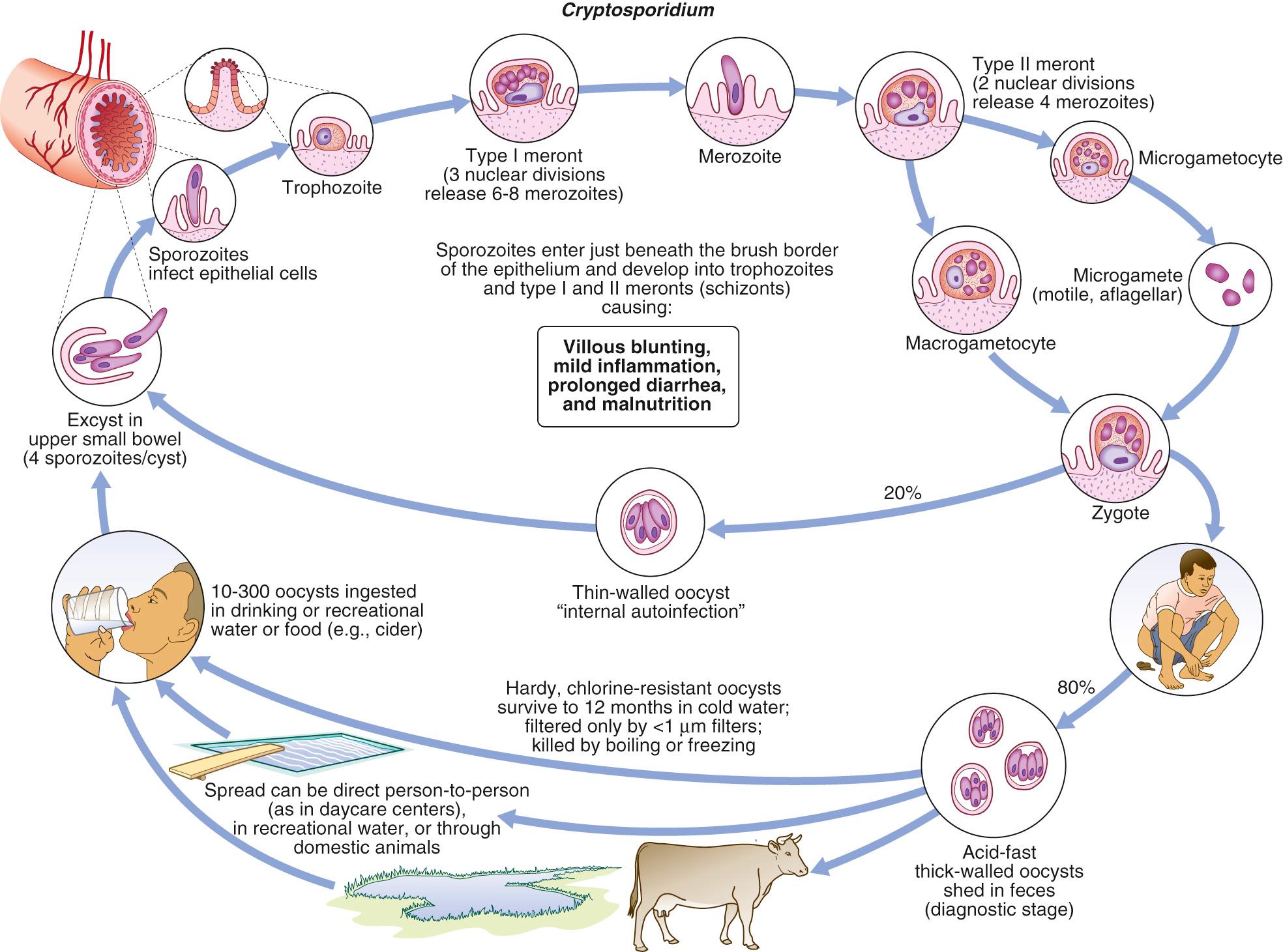
Several factors define the epidemiology of cryptosporidiosis. First, the oocysts are infectious when shed. Thus, parasites are readily transmitted directly from person to person. Second, although Cryptosporidium does not multiply outside of the host, the infectious dose is low, facilitating transmission from sources with low-grade contamination, such as recreational water. Third, the oocyst stage can survive for prolonged periods in the environment and resists disinfection, including chlorination. Its small size and resistance to chlorination facilitate waterborne transmission. Fourth, the host immune response limits the duration and severity of infection such that disease is more commonly recognized in children (preimmune) or in compromised hosts, especially patients with AIDS. Although some genotypes have important animal reservoirs, molecular studies demonstrate that most human infection is caused by species and subtypes that mainly affect humans. Still, transmission of some species (e.g., C. parvum ) is associated with animal contact, rural areas, and exposure to surface water. In contrast to zoonotic species, C. hominis is more common in urban settings and is associated with higher population density (especially the number of children). In some studies, household income is not protective against cryptosporidiosis and may pose an increased risk of C. hominis infection.
Cryptosporidium parasites have been found in every region of the world except Antarctica. Infection is generally more common in warm or moist months. For example, cases in the United States peak in late summer and early autumn. Studies from England found that C. parvum peaks in the spring, whereas C. hominis peaks in autumn. The peak that occurs in spring is thought to be associated with runoff from pastures, and the autumn peak follows the season for swimming in recreational water. Most studies on the prevalence of infection have relied on detection of oocysts in fecal specimens submitted for parasitologic examination. Fewer than 3800 cases per year were reported in the United States from 1995 through 2002, but the number of reported cases increased to more than 7600 per year after then, without other evidence suggesting more illness. This increase coincided with the marketing of nitazoxanide and was thought to be the result of increased awareness and improved diagnosis. Inconsistencies exist in these data, perhaps driven by changes in diagnostic criteria. By contrast, estimates based on antibody prevalence suggest that more than 750,000 persons in the United States are affected each year. The main reason for this difference stems from both insensitivity and underuse of diagnostic tests for Cryptosporidium . With improving diagnostic techniques, more cases are also being identified in high-income countries. Similarly, studies from England and the Netherlands showed that use of polymerase chain reaction (PCR) assays led to a doubling of the number of Cryptosporidium cases identified.
The prevalence of cryptosporidiosis in low- and middle-income countries has been characterized in three large multicenter studies of diarrhea in resource-poor countries; these studies used sensitive techniques to study all of the major pathogens. The Global Enteric Multicenter Study (GEMS) was a case-control study of moderate-to-severe diarrhea conducted at seven centers in sub-Saharan Africa and South Asia. Cryptosporidium was second to rotavirus as a cause of diarrhea in children younger than 2 and was associated with an increased fatality rate in infants and toddlers. Based on these data and a subsequent study of milder diarrhea, the authors estimated that 7.6 million children in South Asia and sub-Saharan Africa develop diarrhea due to cryptosporidiosis annually and that those infections are associated with over 200,000 deaths. The Malnutrition and Enteric Disease Study prospectively studied birth cohorts from India, Bangladesh, Nepal, Tanzania, South Africa, Brazil, and Peru. Cryptosporidium was among the top four causes of diarrheal disease. Among 1486 individuals for whom full data through 2 years were available, 962 (65%) developed a Cryptosporidium infection. The World Health Organization (WHO) Global Rotavirus Surveillance Network collects stool specimens from children hospitalized with acute watery diarrhea from 178 sentinel surveillance sites in 60 countries. A recent study tested 878 stool samples from children with watery diarrhea in sub-Saharan Africa, India, Myanmar, Philippines, and Brazil for enteric pathogens by PCR card. The fraction of diarrheal episodes attributable to Cryptosporidium was 5.8%, with slightly higher rates in children younger than 2. Rates were less than for rotavirus but similar to those of norovirus. A recent report on global burden of diarrheal disease estimated that there are 48,000 child deaths each year from cryptosporidiosis and more than 4.2 million disability-adjusted life-years lost.
Prospective studies of birth cohorts in South Asia have noted that virtually all children are infected by age 2. Summary data from published and unpublished sources in India suggest that that country may see 3.9 to 7.1 million diarrheal episodes, 66,400 to 249,000 hospitalizations, and 5800 to 14,600 deaths each year in children younger than 2 years. Older studies from sub-Saharan Africa have identified Cryptosporidium in 7.5% to 22.2% of cases with use of microscopy. However, studies using PCR or antigen-detection methods have documented Cryptosporidium in up to 30% of acute diarrhea cases. Studies using PCR assays on stool samples from AIDS patients with diarrhea have identified Cryptosporidium DNA in 18% to 77% of cases, significantly higher than with staining alone. Thus, the prevalence is likely higher than suggested by earlier stool studies.
In a systematic review of cryptosporidiosis in low- and middle-income countries, overcrowding, diarrhea in the household, and animal contact were the major risk factors for infection. Breastfeeding was protective. Surprisingly, there was no increased risk associated with water source.
A series of human challenge experiments was performed to determine the infectious dose of Cryptosporidium spp . The initial studies were performed in seronegative volunteers with different strains of C. parvum parasites (maintained in calves). The studies discovered a low infectious dose but considerable variability among isolates, with the dose infecting half of subjects ranging from approximately 1000 oocysts ( C. parvum UCP strain), to approximately 100 oocysts ( C. parvum Iowa strain), to approximately 10 oocysts ( C. parvum TAMU strain, C. hominis TU502 strain). Based on these data, even a single oocyst should result in infection in a portion of those exposed. When the volunteers were rechallenged, they had a higher infectious dose. They also developed less severe manifestations in that they were less likely to shed organisms but frequently developed symptomatic illness, with oocyst shedding detectable only with flow cytometry. When volunteers who were seropositive were challenged, the infectious dose was 20- to 50-fold higher. Because the infectious dose is low, transmission can readily occur from exposure to low doses, such as might be found in waterborne outbreaks or in person-to-person spread.
Oocysts of Cryptosporidium are relatively resistant to environmental conditions. Oocysts can remain infectious for at least 6 months if kept moist, but viability decreases rapidly with desiccation. Oocyst viability does not decrease with storage at temperatures between 0°C and 20°C. Viability decreases over a few hours with freezing (−20°C or lower). The oocysts can also be killed by heat, including pasteurization and microwave heating. Oocysts are highly resistant to chlorination. For example, 80 ppm chlorine inactivated only 90% of oocysts after 90 minutes of incubation, and the concentrations in tap water (e.g., 5 ppm) had no effect. The sensitivity of oocysts to chlorine is further decreased in the presence of fecal contamination. Even incubation of oocysts for up to 2 hours in household bleach failed to decrease infectivity. By contrast, oocysts are sensitive to hydrogen peroxide, ozone, and ultraviolet radiation. Sunlight decreased oocyst viability up to 90%, but the effects vary with water turbidity, radiation intensity, the presence of biofilms, and time.
Surveys have demonstrated that many sources of drinking water were contaminated with oocysts before treatment. Oocysts are more frequent and at higher densities in water contaminated with agricultural runoff, sewage, urban runoff, or recreational use. However, up to 39% of apparently pristine sources were contaminated. Organisms found in the water included both C. hominis and C. parvum genotypes, in addition to animal species. Even groundwater can be contaminated. Low-grade contamination has also been documented in samples of treated water, but this has been decreasing with improvements in water standards.
Numerous outbreaks of cryptosporidiosis have been linked to contaminated drinking water. The largest documented waterborne outbreak of diarrhea occurred in Milwaukee in 1993. One of two city water treatment plants was contaminated. More than 600 cases of Cryptosporidium infection were confirmed by parasitologic examination. Based on telephone surveys, diarrhea episodes were more widespread, with an estimated 403,000 people developing a diarrheal illness. Of interest, water quality never failed to meet the standards current at the time for turbidity and fecal coliform counts. Many of the waterborne outbreaks, including the outbreak in Milwaukee, have been caused by C. hominis. Thus, the outbreaks are thought to result from fecal contamination of drinking water. Other outbreaks are associated with C. parvum. Most outbreaks of C. parvum can be tied to contamination of the watershed with agricultural runoff. Over the past decades, there has been a marked reduction in the number of outbreaks and cases associated with drinking water in the United States, but Cryptosporidium remains one of the more common causes of waterborne outbreaks of disease. Similarly, there has been a marked decrease in cryptosporidiosis in England and Wales associated with improved water standards.
Outbreaks of cryptosporidiosis associated with contaminated recreational water are common. The number of outbreaks increased throughout the 1990s. In 2007 approximately 5700 people in Utah developed cryptosporidiosis associated with contamination of 450 swimming pools with an epidemic strain of C. hominis. Cryptosporidium is now the most common organism associated with waterborne-disease outbreaks associated with recreational water in the United States. In fact, small outbreaks appear to be an important source of endemic cryptosporidiosis. Swimming is an important risk factor for cryptosporidiosis, and public swimming pools are frequently contaminated with Cryptosporidium. Outbreaks have also involved lakes, rivers, beaches, and fountains. The concentration of chlorine in pool water and limited filtration are often insufficient to disinfect the water. Not surprisingly, most outbreaks associated with fecal accidents are attributable to C. hominis.
Foodborne infection occurs less frequently. Well-documented outbreaks have been tied to contaminated apple cider, unpasteurized milk (cow and goat), salads, raw produce, and shellfish. In resource-poor countries, oocysts are commonly found on vegetables. Oocysts have been frequently identified in shellfish and in flies, but their role in transmission to humans is not clear.
Oocysts of Cryptosporidium are immediately infectious when shed. Thus, Cryptosporidium is associated with direct person-to-person spread. Person-to-person transmission was initially recognized in outbreaks associated with contact with daycare centers. There are also documented cases of nosocomial transmission. The risk of transmission from adult patients is small with standard precautions. However, contact with a person ill with diarrhea or with children in diapers remains a major risk factor for cryptosporidiosis.
Secondary transmission within households is also common. For example, in a study of household contacts of children with cryptosporidiosis in Brazil, Newman and coworkers noted secondary cases in 18 of 31 households (58%) and involving 19% of household members. In daycare-associated outbreaks, secondary transmission is common. By contrast, few cases in adults or school-aged children were associated with secondary transmission.
Cryptosporidiosis in high-income countries is also associated with travel to resource-poor countries. This was first recognized in Finnish travelers to Russia and was thought to reflect contamination of drinking water. Cryptosporidium was thought to rarely cause traveler's diarrhea. However, in studies using molecular detection methods, the actual rates were up to 6% of cases. Most travel-associated cases are caused by C. hominis. However, a number of cases of C. viatorum have been reported in travelers returning to England from India.
Sexual transmission has been postulated to occur. Among human immunodeficiency virus (HIV) patients, men who have sex with men are more likely to develop cryptosporidiosis. Transmission is associated with anal-genital sex and the number of sex partners. Cryptosporidium transmission among men who have sex with men shares risk factors and may occur concurrently with other fecal-oral pathogens, such as Shigella.
C. parvum was thought to infect primarily domestic animals, with zoonotic transmission to humans. However, molecular studies have demonstrated that many of the human C. parvum infections are caused by subtype IIc, which typically only infects humans. Animal contact is also associated with acquisition of C parvum in sporadic cryptosporidiosis and occasional outbreaks. Cryptosporidiosis is common among veterinary students. In addition to cattle, sheep, goats, pigs, and pets have also been implicated in zoonotic infection.
The host immune response plays a key role in susceptibility. In studies from resource-poor countries where there is heavy exposure, most cases of cryptosporidiosis develop in young children, presumably because of rapid development of immunity. Human challenge studies document resistance to infection associated with previous challenge or prechallenge immunity, as documented by anti- Cryptosporidium antibodies. There is also strong evidence of an increased frequency of infection in patients with altered cellular immunity. Among AIDS patients with diarrhea, Cryptosporidium was found in up to three-fourths of patients with chronic diarrhea from resource-poor countries. In a waterborne outbreak affecting a drug treatment center, only 190 of 1392 (13.6%) HIV-negative patients developed cryptosporidiosis, compared with 104 of 339 (30.7%) infected with HIV. Among those with HIV, the infection rate varied with the CD4 cell count, ranging from 23% of those with CD4 cell counts greater than 1000 cells/µL to 46% of those with CD4 cell counts less than 100 cells/µL. However, infection was not more frequent in HIV patients during the Milwaukee outbreak. Cryptosporidiosis has also been noted in other immunodeficient hosts, including patients with primary immunodeficiencies, organ transplants, cancer, and diabetes.
Cryptosporidium organisms are found within parasitophorous vacuoles in the microvillus layer of the epithelial cells ( Fig. 282.2 ). In immunocompetent individuals, the organisms are localized primarily to the distal small intestines (e.g., terminal ileum) and proximal colon. However, studies from Uganda documented frequent infection of the respiratory tract in apparently immunocompetent children and adults. In immunodeficient hosts, the parasites have been identified throughout the gut, in the biliary tract, and even in the respiratory tract. Children with persistent cryptosporidiosis may have villous atrophy and a mild increase in lamina propria lymphocytes. The distribution of parasites is limited and often spares the proximal small intestines. Heavier infection is associated with villous atrophy, crypt hyperplasia, and marked infiltration with lymphocytes, plasma cells, and even neutrophils, and is also associated with extraintestinal involvement.
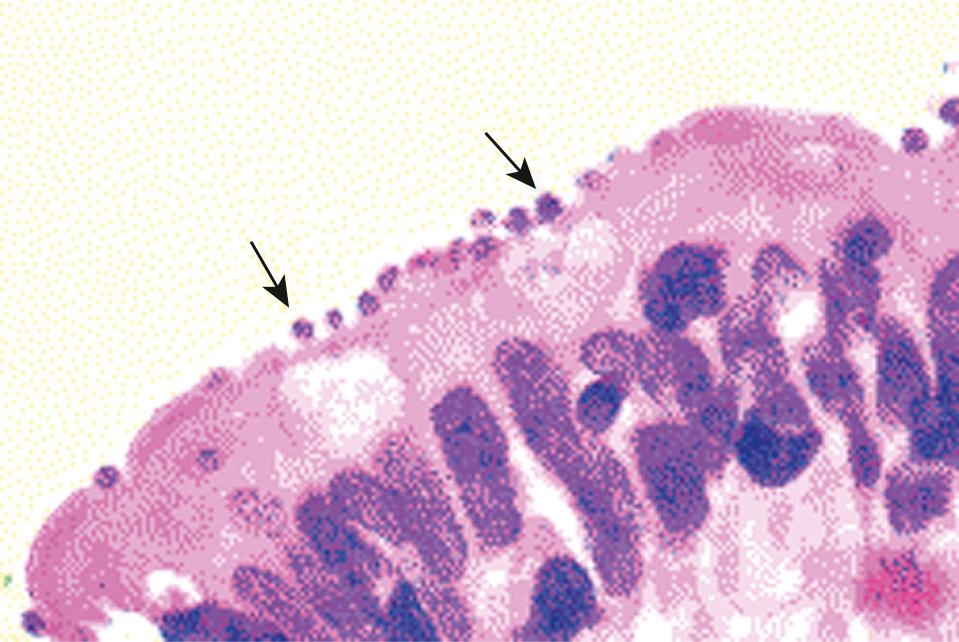
Villous atrophy and crypt hyperplasia are thought to reflect epithelial cell turnover. Although infection of epithelial cells stimulates antiapoptotic mechanisms in infected cells, there is increased apoptosis in adjacent cells that is likely mediated by the interaction of Fas and Fas ligand. Increased epithelial cell apoptosis has been demonstrated in biopsy specimens from infected intestines. In vitro infection models show that infection initially stimulates antiapoptotic signals, but after 24 hours of infection, proapoptotic molecules dominate. Furthermore, as the organisms complete their cycle, they cause necrotic death of the infected cells. The resultant loss of villous surface was, in turn, associated with decreased expression of glucose-stimulated sodium pumps. Similarly, loss of villous surface area has been demonstrated in human infection as d -xylose malabsorption. Studies of AIDS patients with severe cryptosporidiosis have also demonstrated malabsorption of bile acids, vitamin B 12 , and fatty acids. Metabolic studies of AIDS patients with chronic cryptosporidiosis demonstrate fat wasting with a decreased metabolic rate, consistent with decreased absorption.
Cryptosporidiosis is characterized clinically by watery diarrhea and malabsorption. The physiologic processes that are thought to account for these symptoms include sodium malabsorption, electrogenic chloride secretion, and increased intestinal permeability. Increased permeability may result in decreased absorption of fluids and electrolytes and solute fluxes into the gut. Studies of AIDS patients with cryptosporidiosis have demonstrated a direct correlation between the severity of disease and altered intestinal permeability, as measured by ratios of excretion in the urine of lactulose and mannitol. Similar defects have been noted in children with cryptosporidiosis. Cryptosporidium -induced defects in intestinal epithelial cell barrier function can be reversed by antiinflammatory cytokines, such as transforming growth factor-β.
The voluminous, watery diarrhea resembles that of toxin-mediated illnesses, but no secretory activity was detected in formal studies. Prostaglandins, induced by tumor necrosis factor-α (TNF-α), mediate decreased sodium absorption and cause diarrhea in porcine and bovine cryptosporidiosis. However, studies in volunteers and AIDS patients with chronic cryptosporidiosis did not demonstrate any correlation between expression or level of proinflammatory cytokines and symptoms. Furthermore, prostaglandin inhibitors have not proven to be effective symptomatic therapy in human cryptosporidiosis.
Robinson and colleagues demonstrated a correlation between expression of the neuropeptide substance P and the presence and severity of diarrhea in volunteers challenged with C. parvum and AIDS patients with chronic cryptosporidiosis. In monkey models, Cryptosporidium infection is associated with increased expression of substance P and its receptor, and substance P inhibitors blocked Cryptosporidium -induced intestinal permeability, glucose malabsorption, and chloride secretion. Similarly, mice were protected against C. parvum –induced intestinal inflammation and illness by a substance P receptor antagonist.
Infection of intestinal epithelial cells leads to activation of nuclear factor kappa B (NF-κB). Upstream signals may include Toll-like receptor 2 (TLR2) and TLR4 signaling via MyD88 (myeloid differentiation primary response 88). NF-κB then activates several hundred target genes, including genes for antiapoptotic molecules such as osteoprotegerin. These antiapoptotic molecules allow the parasite to form and release merozoites before cell death. However, NF-κB activation also leads to upregulation of a proinflammatory cascade, including expression of chemokines, chemokine receptors, and cytokines. Many of these effects are mediated by upregulation or downregulation of micro-RNAs, including let-7, miR-27b, and miR-98. These mediate secretion of exosomes, containing defensins (LL-37 and β-defensin 2), which may limit infection. The parasite also secretes noncoding RNAs into the host cytoplasm, which are transported to the nucleus by host HSP70. This in turn leads to activation of host histone methylation, increased expression of proinflammatory molecules (e.g., interleukin [IL]-8, IL-6, and CXCL2), and decreased expression of other host molecules, including the alarmin IL-33. Both biopsy specimens and stool studies from human infection also demonstrate increased expression of proinflammatory cytokines and markers of inflammation, including TNF-α, IL-1β, IL-8, and lactoferrin. Chemokines, including IL-8 and CXCL10, are produced by the infected epithelial cells. Among the chemokines, CCL20 actually is microbicidal to extracellular forms of Cryptosporidium. It is downregulated by the parasite via upregulation of MiR21. Studies of infected human tissues noted that Cryptosporidium infection leads to upregulation of the TNF receptor family decoy receptor osteoprotegerin. It was also detected in intestinal tissues after experimental infection. Its ligand, TRAIL (TNF-related apoptosis-inducing ligand), was able to eliminate infected cells in vitro, but this effect was reversed by osteoprotegerin, suggesting that TRAIL may be a key mediator of parasite clearance.
Both innate and adaptive immune responses are critical for control of cryptosporidiosis. CD4 + T cells play a key role in the adaptive immune response to cryptosporidiosis. In patients with HIV infection, cryptosporidiosis is usually self-limited in individuals with CD4 cell counts higher than 150/µL, is often chronic in patients with CD4 cell depletion to less than 100/µL, and can be fulminant in some of those with counts less than 50/µL. Similarly, infection is chronic in mice without functional CD4 cells (e.g., nude mice, SCID mice, or RAG knockout mice). These defects can be reversed by infusion of CD4 + cells, particularly CD4 + intraepithelial lymphocytes. Kirkpatrick and colleagues noted an association between DQ alleles, which present antigen to CD4 + T cells, and susceptibility to infection. Furthermore, resolution of cryptosporidiosis among AIDS patients in response to effective antiretroviral therapy (ART) is associated with an influx of CD4 cells into the intestines. The role of other cell populations has been less clear. Although major histocompatibility complex (MHC) class I deficiency and CD8 depletion have little effect on murine cryptosporidiosis, there are associations of MHC class I types and human infection. CD8 cells produce interferon-γ (IFN-γ) in response to Cryptosporidium antigens, and sensitized human CD8 T cells can clear parasites from infected cells in vitro.
The role of antibody in the immune response to cryptosporidiosis is controversial. Early studies noted cases of chronic cryptosporidiosis in patients with low antibody levels, but these studies did not exclude coexisting T-cell dysfunction. In animal models, inactivation of B cells by inactivation of the muMT gene did not affect clearance of cryptosporidiosis. Treatment with high concentrations of anti- Cryptosporidium antibody did facilitate clearance in mice. Anecdotes suggested that hyperimmune bovine colostrums might improve cryptosporidiosis in AIDS, but a large randomized, controlled trial demonstrated no clinical benefit and decreases in oocyst shedding only at very high doses. High levels of serum and fecal antibodies to C. parvum have been found in AIDS patients with chronic cryptosporidiosis. Studies of the fecal antibody response in volunteers challenged with C. parvum demonstrated specific fecal antibody in most volunteers. However, the presence and timing of antibody correlated with oocyst shedding rather than clearance or resistance to infection. Similarly, cytokines such as TGF-β that stimulate immunoglobulin A (IgA) production often develop only after resolution of illness. Studies have noted higher antibody responses to specific antigens in patients with acute versus persistent diarrhea, but this may have been a marker for prior exposure. Also, specific antibody in breast milk was associated with decreased risk of infection in infants. Thus the importance of antibody in cryptosporidiosis remains unclear.
Production of IFN-γ is a key mediator of the adaptive immune response to Cryptosporidium. In murine models, IFN-γ knockout mice develop chronic infection. Furthermore, inactivation or depletion of IFN-γ causes further exacerbation of infection even beyond that noted with CD4 depletion. Lymphocytes from persons who have recovered from cryptosporidiosis produced IFN-γ after antigen stimulation in vitro, including HIV patients. Approximately half of volunteers challenged with C. parvum express IFN-γ in the intestinal mucosa. Treatment with IFN-γ can directly activate intestinal epithelial cell lines to prevent C. parvum infection, but this effect was not confirmed with primary cells. Similarly, inactivation of IL-12, the major factor stimulating production of IFN-γ, causes chronic infection.
Surprisingly, IFN-γ expression in normal volunteers was limited to the subset with evidence of previous exposure (either seropositive before challenge or demonstrating resistance to infection). Similarly, IFN-γ production by cells from HIV patients during active cryptosporidiosis and from Haitian children with active cryptosporidiosis was very low despite the fact that they had self-limited disease. Thus, other factors appear to be involved in limiting human infection after initial exposure. In murine models, inactivation of IFN-γ expression resulted in only a mild chronic infection in BALB/c mice but fatal infection in C57BL/6 mice. Mild disease in BALB/c mice was associated with expression of IL-12, IL-4, and TNF-α. In the absence of IFN-γ, IL-12 treatment only worsened cryptosporidiosis. Similarly, IL-12 treatment of AIDS patients with chronic cryptosporidiosis was associated with gastrointestinal side effects. IL-4 synergizes with IFN-γ in preventing infection of epithelial cells, and IL-4 knockout mice displayed prolonged oocyst shedding. IL-4 treatment did not modulate infection in IFN-γ knockout mice and IL-4 expression did not correlate with protection in human volunteers. By contrast, TNF-α limited infection in IFN-γ knockout mice, activated human epithelial cells to limit infection, but did not correlate with resolution in human infection.
Studies have increasingly focused on the innate immune response to cryptosporidiosis. Mononuclear phagocytes likely play a key role in control of cryptosporidiosis. Takeuchi and colleagues demonstrated that mice can be rescued from a fatal infection by type I macrophages. Macrophages can be a source of IL-15 and IL-18, which have been implicated in control of infection. IL-18 stimulates IFN-γ production, natural killer (NK) cell activation, and secretion of defensins. Depletion of dendritic cells (CD11c + ) increased susceptibility to C. parvum infection in a mouse model, and dendritic cells stimulated with sporozoites and cocultured with CD4 + and CD8 + lymphocytes produce higher levels of IFN-γ, and the subsequent control of infection is sustained. Dendritic cells were also important in a neonatal mouse system in which they limited infection in part via stimulation of Th1 responses. NK cells have a role in parasite clearance in some animal models, and human NK cells can clear infection of cell lines in vitro. In seronegative normal volunteers experimentally infected and AIDS patients recovering from cryptosporidiosis in response to ART, control of infection was associated with expression of IL-15. This effect is likely mediated by activation of NK cells. Mannose-binding lectin levels have been shown to be low in AIDS patients and children with cryptosporidiosis, and mice with the gene inactivated are more susceptible to infection. Antimicrobial peptides seem to be a key host defense mechanism against the luminal forms of the parasite. β-Defensins and CCL20 are induced in response to Cryptosporidium infection and can kill the parasites in vitro. More frequent or severe cryptosporidiosis has been noted in a number of primary immunodeficiencies. Some cases have primary T-cell defects. However, recent descriptions primarily involve innate responses. For example, hyper-IgM syndrome, caused by a defect in CD40 ligand (also termed CD154), is associated with increased frequency and severity of Cryptosporidium infection. This syndrome is associated with profound defects in the ability of antigen-presenting cells to produce IL-12 and TNF-α and to stimulate production of IFN-γ. In murine models, recovery required expression of CD40 on donor spleen cells but not recipient epithelial cells. Similarly, treatment of patients with hyper-IgM syndrome with CD40 agonist antibody can lead to resolution of severe forms of cryptosporidiosis in patients with CD40L defects. The prevalence of cryptosporidiosis ranges from 6% to over half of cases. Furthermore, patients often developed biliary disease, primarily sclerosing cholangitis, which is usually due to Cryptosporidium. Studies using PCR assays have demonstrated that most biliary tract infections were caused by Cryptosporidium. An association with sclerosing cholangitis and cryptosporidial infection has also been reported with other primary immunodeficiencies affecting T cells, including dedicator of cytokinesis 8 (DOCK 8) deficiency, and NIK (nuclear factor kappa B–inducing kinase) loss-of-function mutations.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here