Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Endoscopic cryospray ablation or cryotherapy is a unique method of ablation that offers physicians a versatile choice in the treatment of Barrett’s and early esophageal cancer. The concept of endoscopic cryospray ablation began in the late 1990s with the development of two delivery platforms using liquid nitrogen (LN) and carbon dioxide (CO 2 ). Both methods share some unique characteristics compared to other ablation techniques using radiofrequency energy and bipolar or monopolar electrocautery. The spray application allows the physician to paint the cryogen over the target tissue making it ideal for both flat and uneven surfaces. Similar to a professional painter choosing a spray gun for delivery of their wares, physicians can choose spray cryoablation when the topography of the target lesion is uneven or nodular. Technical challenges in performing cryospray ablation have limited its use to centers specializing in the treatment of Barrett’s esophagus (BE) and esophageal cancer. Cryotherapy is currently indicated in a variety of neoplastic stages including well-established low-grade dysplasia (LGD), high-grade dysplasia (HGD), intramucosal carcinoma (IMC), and palliation of more advanced forms of carcinoma in high-risk patients or for residual disease after definitive traditional oncologic therapy with radiation or chemotherapy. Attempts to use cryospray ablation as a primary palliative ablation therapy for malignant dysphagia have been successful in some patients. Prior to spray cryotherapy, alternative use of cryotherapy with a contact probe has been associated with transmural injury with the risk of perforation in the esophagus .
All types of cryotherapy rely on the polarization of water molecules to disrupt cellular membranes leading to cell death when the tissue freezes. Endoscopic spray cryoablation is performed with a catheter delivering liquid nitrogen (−196°C) under low pressure (2–4 psi), carbon dioxide (−70°C) cooling tissue with the Joules–Thompson principle, or a newer balloon delivery device using nitrous oxide. The familiarity of cryotherapy with patients is due to the widespread application of LN in dermatology, which makes the treatment appealing to patients. Similar stages of tissue destruction are noted in the dermis and esophagus: initial edema followed by submucosal hemorrhage and blistering and then slough of the epithelium. In an acid suppressed environment, slough of the epithelium prompts healing of the ulcerated lumen with normal squamous or more specifically a “neosquamous” epithelium.
Proper informed consent is required before initiation of any ablation therapy. Important details for consideration include number of treatment sessions, adverse events, as well as success and failure rates. Cryospray ablation is similar to other ablation methods in terms of the number of sessions required to obtain a complete response. On average, three to five sessions are performed 2–3 months apart to allow healing and avoid therapy during periods of ulceration. For more advanced lesions, there may be urgency to induce a remission and procedures can be performed as frequently as every 2–3 weeks.
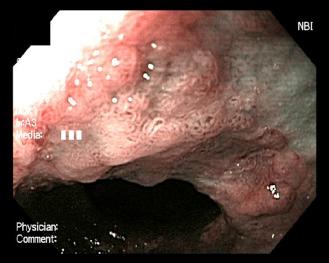
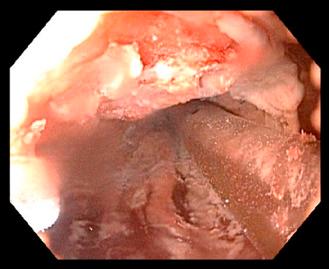
Preparation for endoscopic cryospray ablation includes a prolonged fast for patients with delayed gastric emptying. Deep sedation is generally recommended by most physicians performing cryospray ablation. Very little data is known about the use of cryospray ablation in patients taking anticoagulation medications, however, it is our practice to not discontinue any anticoagulation medications before the endoscopic ablation session due to the risk of thrombosis. High dose acid suppression with twice daily dosing of proton pump inhibitors is required to promote neosquamous epithelialization after ablation of the unwanted columnar lining ( Figs. 12.1 and 12.2 ).
The first use of LN cryogen provided proof of concept and early dosimetry data in the swine model using small Yorkshire pigs (20 kg) . The current commercial device is the second generation with significant improvements over the prototype and first commercial release (CSA Medical, Lutherville, MD). The initial treatment changes observed immediately after LN cryospray were nil to minimal while targeting normal squamous mucosal of swine esophagus. The treated swine mucosa would slough after a blistering stage identical to the dermatologic applications. Later, immediate treatment effects from cryospray were recognized in BE mucosa with intense reddening and slight hemorrhage from the mucosa, which help guide therapy. In the initial animal model, transmural inflammation was found only with higher doses of 30–60 seconds spray duration.
Initial human use was limited to a relatively small number of referral centers where the technology was refined . Initial complications related to gaseous distention responded to an improved dual chamber decompression tube with both active suction and passive venting. In an early retrospective cohort of 98 patients from 9 academic and community centers, complete eradication of dysplasia was noted in 87% of patients completing therapy . An early complete response rate of 84% was found in 16 of 19 patients with T1a mucosal cancer receiving only cryospray ablation and 60% in 6 of 10 patients with T1b submucosal cancers (75% overall success in T1 group) . Cryospray ablation demonstrated remarkable ability to provide endoluminal control in several patients with advanced esophageal cancer (T2–T4), which served as the basis for using LN for salvage treatment after traditional oncologic therapy with chemotherapy and radiation. LN cryospray has demonstrated effectiveness in treating residual BE with dysplasia after primary chemoradiation therapy.
The long-term results of cryospray ablation in BE have been reported by both single center and multicenter groups . In the single center, the authors achieved a complete eradication of HGD in 97% with 31 of 32 subjects with a follow-up range of 24–57 months.
The energy applied to the neoplastic tissue is measured in terms of duration of freeze time multiplied by the number of freeze cycles. In general, the longer the cryogen is applied, the deeper the penetration or depth of ablation. The duration of the freeze time is measured in seconds and begins at the moment the entire targeted area is frozen. The usual target area is approximately 2 cm×2 cm but smaller areas are commonly chosen when there are worrisome lesions or areas of the targeted neoplasia that could be difficult to achieve a complete ablation. Overlap of the treatment areas is extremely important to reduce the risk of incomplete response resulting in persistent neoplasia. The resultant tissue changes immediately after cryospray ablation are the appearance of a uniform cherry red color with slight hemorrhage. Identification of these immediate changes is extremely important to be assured that the neoplastic tissue was properly treated. Nodular areas can be given extra cycles to ensure adequate tissue response.
The typical dosimetry for BE with HGD is 20 second cryospray × 2 cycles based initially on expert opinion then confirmed with a small but important human study in patients undergoing esophagectomy. Cryospray ablation demonstrated consistent mucosal destruction and treatment effect well into the submucosa . In this clinical trial of depth of injury, seven male esophageal cancer patients were treated with LN cryospray ablation applied to segments with less advanced pathology to avoid confounding information on the pathologic review of the most advanced lesion. Two dosimetry treatments were compared using either 4 cycles of 10 second duration cryospray or 2 cycles of 20 second cryospray. Deeper injury was noted in the 2 cycles × 20 second cryospray group with 5.4 mm depth compared to 4.0 mm for the 4 cycles × 10 second group. The two dosimetry protocols were found to be similar in terms of maximum depth of necrosis into the submucosal layer. The 20 second × 2 cycles protocol induced a deeper level of inflammation into the adventitia layer more often.
Efforts to reduce the overall treatment session time with single sessions of longer freeze time (30 seconds) did not provide a reproducible treatment effect (unpublished data) confirming the importance of the freeze–thaw–freeze cycle. Higher doses of LN cryospray ablation with 30 second spray repeated with two to three cycles should be considered for unusually thick or nodular lesions and persistent BE after multiple prior ablation sessions.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here