Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Cough is an important defense mechanism of the lungs and is a common symptom, particularly during winter months. In most patients, it is self-limited. However, cough can be ominous, indicating serious underlying disease, because of accompanying problems (hemoptysis) or because of serious consequences of the cough itself (e.g., syncope and hemorrhage), or cough may present as an uncommon disorder that mimics a common disease.
The cough reflex serves to prevent the entry of harmful substances into the tracheobronchial tree and to expel excess secretions and retained material from the tracheobronchial tree. Cough begins with stimulation of cough receptors, located in the upper and lower airways, and in many other sites such as the ear canal, tympanic membrane, sinuses, nose, pericardium, pleura, and diaphragm. Receptors send impulses via vagal, phrenic, glossopharyngeal, or trigeminal nerves to the “cough center,” which is in the medulla. Because cough is not only an involuntary reflex activity but also one that can be initiated or suppressed voluntarily, “higher centers” must also be involved in the afferent limb of the responsible pathway. The neural impulses go from the medulla to the appropriate efferent pathways to the larynx, tracheobronchial tree, and expiratory muscles.
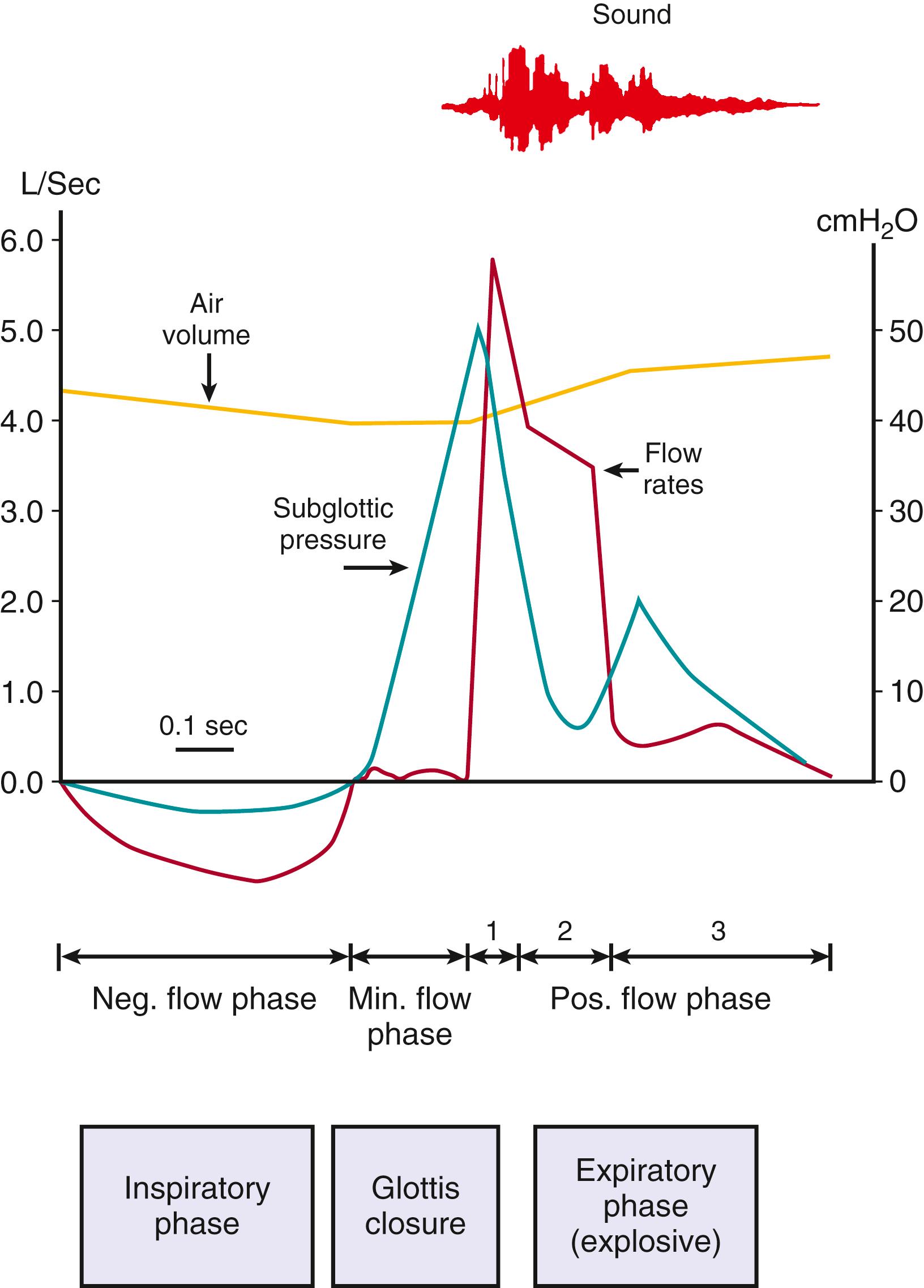
The act of coughing ( Fig. 3.1 ) begins with an inspiration, followed by expiration against a closed glottis (compressive phase), resulting in the buildup of intrathoracic pressures (50–300 cm H 2 O). These pressures may be transmitted to vascular, cerebrospinal, and intraocular spaces. Finally, the glottis opens, allowing for explosive expiratory airflow (300 m/sec) and expulsion of mucus, particularly from the larger, central airways. The inability to seal the upper airway (e.g., endotracheal tube or tracheostomy) impairs the effectiveness of cough. Respiratory muscle weakness (e.g., muscular dystrophy) impairs both the inspiratory and the compressive phase.
The history often provides the most important body of information about a child’s cough. A diagnosis can often be discerned with relative certainty from the environmental and exposure history, family history, timeframe, and characterization of the cough.
The patient’s age ( Table 3.1 ) helps to focus the diagnostic possibilities. Congenital anatomic abnormalities may be symptomatic from birth, whereas toddlers, who may have incomplete neurologic control over swallowing and often put small objects in their mouths, are at risk for foreign body aspiration; adolescents may experiment with smoking traditional cigarettes, e-cigarettes, or vaping. Socioeconomic factors must be considered; a family that cannot afford central heating may use a smoky wood-burning stove; spending time at a daycare center may expose an infant to respiratory viruses; and several adult smokers in a small home expose children to a high concentration of respiratory irritants.
| Age Group | Acute | Recurrent | Chronic (>4 wk) |
|---|---|---|---|
| Infants | Infection 1 ∗ Aspiration 2 Foreign body 3 |
Asthma 1 CF 1 GER 1 Aspiration 2 Anatomic abnormality 3 † Passive smoking 3 |
Asthma 1 CF 1 GER 1 Aspiration 2 Pertussis 2 Anatomic abnormality 3 † Passive smoking 3 |
| Toddlers | Infection 1 Foreign body 2 Aspiration 3 |
Asthma 1 CF 1 GER 1 Aspiration 2 Anatomic abnormality 3 Passive smoking 3 Protracted bacterial bronchitis 1 |
Asthma 1 CF 1 GER 1 Aspiration 2 Pertussis 2 Anatomic abnormality 3 Passive smoking 3 Protracted bacterial bronchitis 1 |
| Children | Infection 1 Foreign body 3 |
Asthma 1 CF 1 GER 1 Passive smoking 3 Protracted bacterial bronchitis 1 |
Asthma 1 CF 1 GER 2 Pertussis 2 Mycoplasma 3 Habit 3 Anatomic abnormality 3 Passive smoking 3 Protracted bacterial bronchitis 1 |
| Adolescents | Infection 1 Vaping |
Asthma 1 CF 1 GER 1 Aspiration 2 Anatomic abnormality 3 Protracted bacterial bronchitis 1 |
Asthma 1 CF 1 GER 2 Smoking 2 Tuberculosis 3 Habit 2 Pertussis 3 Aspiration 3 Anatomic abnormality 3 Tumor 3 Protracted bacterial bronchitis 1 |
∗ Infections include upper (pharyngitis, sinusitis, tracheitis, rhinitis, otitis) and lower (pneumonia, abscess, empyema) respiratory tract disease.
† Anatomic abnormality includes tracheobronchomalacia, tracheoesophageal fistula, vascular ring, abnormal position, or take-off of large bronchi.
The various cough characteristics can help determine the cause of cough. The causes of acute, recurrent, and chronic coughs may be quite different from each other ( Fig. 3.2 ; see also Table 3.1 ). A cough can be paroxysmal, brassy, productive, weak, volitional, and “throat-clearing,” and it may occur at different times of the day ( Tables 3.2 and 3.3 ). The previous response or lack of response to some therapies for recurrent and chronic cough can provide important information (see Table 3.3 ). Furthermore, some coughs may be caused or worsened by medications ( Table 3.4 ).
| Characteristic | Think of |
|---|---|
| Staccato, paroxysmal | Pertussis, cystic fibrosis, foreign body, Chlamydia species, Mycoplasma species, parapertussis |
| Followed by “whoop” | Pertussis |
| All day, rarely during sleep, honking | Habit |
| Barking, brassy | Croup, habit, tracheomalacia, tracheitis, epiglottitis |
| Staccato | Chlamydia |
| Hoarseness | Laryngeal involvement (croup, recurrent laryngeal nerve involvement) |
| Abrupt onset | Foreign body, pulmonary embolism |
| Follows exercise | Asthma |
| Accompanies eating, drinking | Aspiration, gastroesophageal reflux, tracheoesophageal fistula |
| Throat clearing | Postnasal drip |
| Productive (sputum) | Infection |
| Sputum casts | Plastic bronchitis |
| Night cough | Sinusitis, asthma |
| Seasonal | Allergic rhinitis, asthma |
| Immunosuppressed patient | Bacterial pneumonia, Pneumocystis jirovecii , Mycobacterium tuberculosis , Mycobacterium avium–intracellulare , cytomegalovirus |
| Dyspnea | Hypoxia, hypercarbia |
| Animal exposure | Chlamydia psittaci (birds), Yersinia pestis (rodents), Francisella tularensis (rabbits), Q fever (sheep, cattle), hantavirus (rodents), histoplasmosis (pigeons) |
| Geographic | Histoplasmosis (Mississippi, Missouri, Ohio River Valley), coccidioidomycosis (Southwest), blastomycosis (North and Midwest) |
| Workdays with clearing on days off | Occupational exposure |
| Cause | Abrupt Onset | Only When Awake | Yellow Sputum | Responds to Inhaled Bronchodilator (by History) | Responds to Antibiotics (by History) | Responds to Steroids (by History) | Failure to Thrive | Wheeze | Digital Clubbing |
|---|---|---|---|---|---|---|---|---|---|
| Asthma | + | ++ | ++ | +++ | + | +++ | + | +++ | – |
| Cystic fibrosis | + | ++ | ++ | + | +++ | + | ++ | ++ | +++ |
| Infection | + | + | ++ | – | ++ | – | + | + | – |
| Aspiration | + | + | + | + | + | + | ++ | ++ | + |
| Gastroesophageal reflux | + | ++ | – | – | – | + | ++ | ++ | – |
| Foreign body | +++ | + | ++ | + | ++ | + | + | ++ | + |
| Habit | – | +++ | – | – | – | – | – | – | – |
| Drug | Mechanism |
|---|---|
| Tobacco, marijuana | Direct irritants |
| β-Adrenergic blockers | Potentiate asthma |
| ACE inhibitors | ↑ Bradykinin (protussive mediator) |
| Bethanechol | Potentiates asthma |
| Nitrofurantoin |
|
| Antineoplastic agents | Various (including pneumonitis/fibrosis, hypersensitivity, noncardiogenic pulmonary edema) |
| Sulfasalazine |
|
| Penicillamine |
|
| Diphenylhydantoin | Hypersensitivity pneumonitis |
| Aspirin, NSAIDs | Potentiate asthma |
| Nebulized antibiotics |
|
| Inhaled/nebulized bronchodilators | Increases tracheal/bronchial wall instability in airway malacia; or via reaction to vehicle |
| Theophylline, caffeine | Indirect, via worsened gastroesophageal reflux (relaxation of lower esophageal sphincter) |
| Metabisulfite | Induces allergic asthma |
| Cholinesterase inhibitors | Induce mucus production (bronchorrhea) |
A history of accompanying signs or symptoms, whether localized to the respiratory tract (e.g., wheeze, stridor, transient tachypnea of the newborn) or elsewhere (e.g., failure to thrive, frequent malodorous stools) can give important clues ( Table 3.5 ; see also Tables 3.2 and 3.3 ). It is essential to remember that the daily language of the physician is full of jargon that may be adopted by parents but with a different meaning from that understood by physicians. If a parent says that a child “wheezes” or “croups” or is “short of breath,” it is important to find out what the parent means by that term and ask them to mimic the sound or action.
|
Because many disorders of childhood have genetic or environmental familial components, the family history can provide helpful information:
Are there older siblings with cystic fibrosis (CF) or asthma?
Is there a coughing sibling whose kindergarten class has been closed because of pertussis or COVID-19?
Is there an adolescent or adult with chronic cough (bronchitis) who may have pertussis or tuberculosis?
Was the child premature, and if so, did they spend a month on the ventilator, and do they now have chronic lung disease (bronchopulmonary dysplasia)?
Did the toddler choke on a carrot or other food a few months ago?
Did the child have respiratory syncytial virus (RSV), bronchiolitis, or rhinovirus infection as an infant?
Did the child receive a bone marrow transplant?
Is the child fully immunized?
Did the infant have a tracheoesophageal fistula repaired in the neonatal period?
Initial inspection often reveals the seriousness of an illness:
Is the child struggling to breathe (dyspnea)?
Does the child have an anxious look?
Is the child in respiratory distress?
Can the child be calmed or engaged in play?
Are there audible respiratory sounds?
Is the child’s skin blue (representing cyanosis) or ashen?
Does the child appear wasted, with poor growth that may indicate a chronic illness?
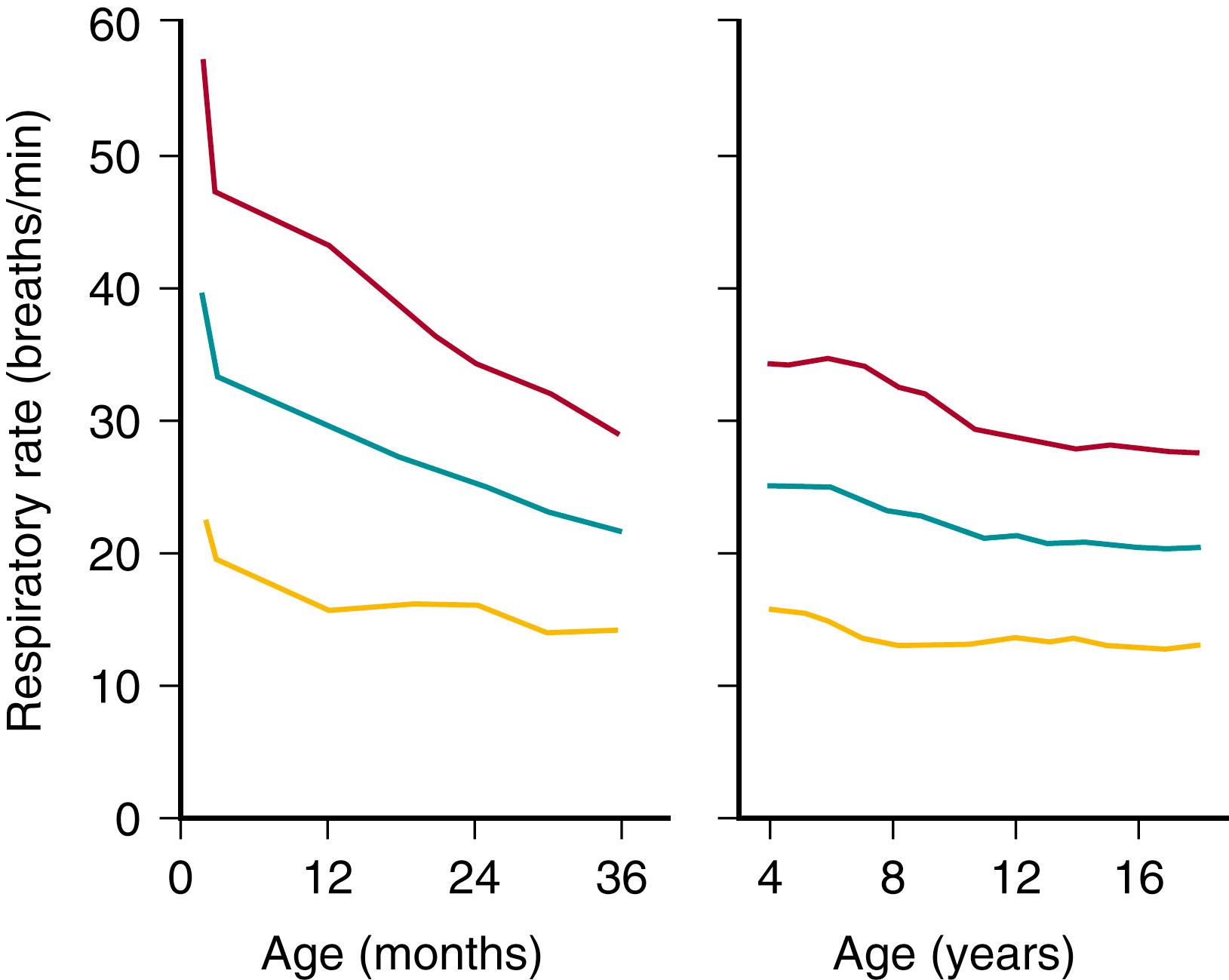
The respiratory rate is often elevated with parenchymal lung disease or extrathoracic airway obstruction. Respiratory rates vary with the age of the child ( Fig. 3.3 ) and with pulmonary infection, airway obstruction, activity, wakefulness and sleep, fever, metabolic acidosis, and anxiety.
Odors may also give helpful clues. Does the examining room or the clothing smell of stale cigarette smoke? Is there a foul odor from a diaper with a fatty stool, which may suggest pancreatic insufficiency and CF? Is the child’s breath malodorous, as can be noticed in sinusitis, nasal foreign body, lung abscess, or bronchiectasis?
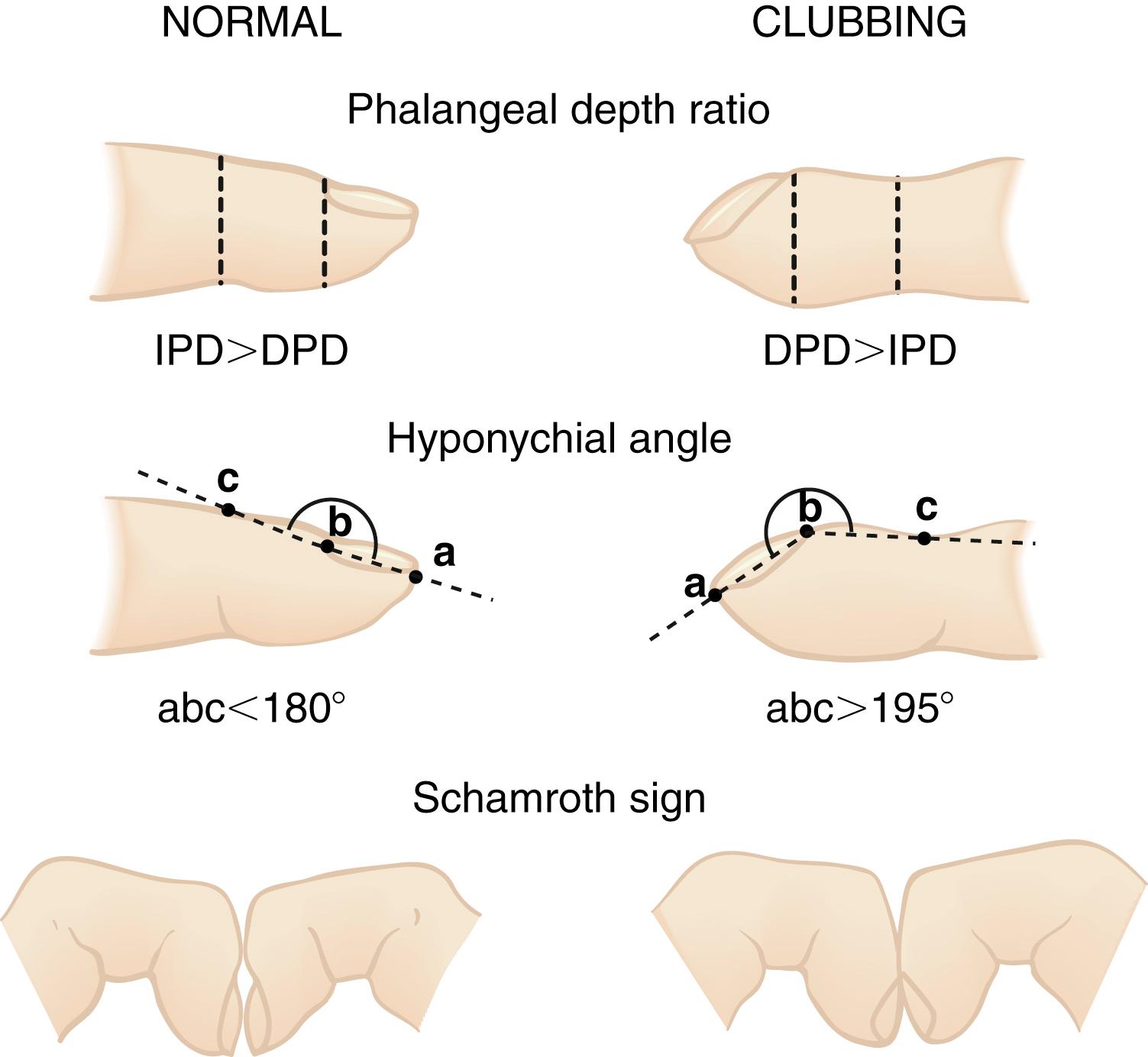
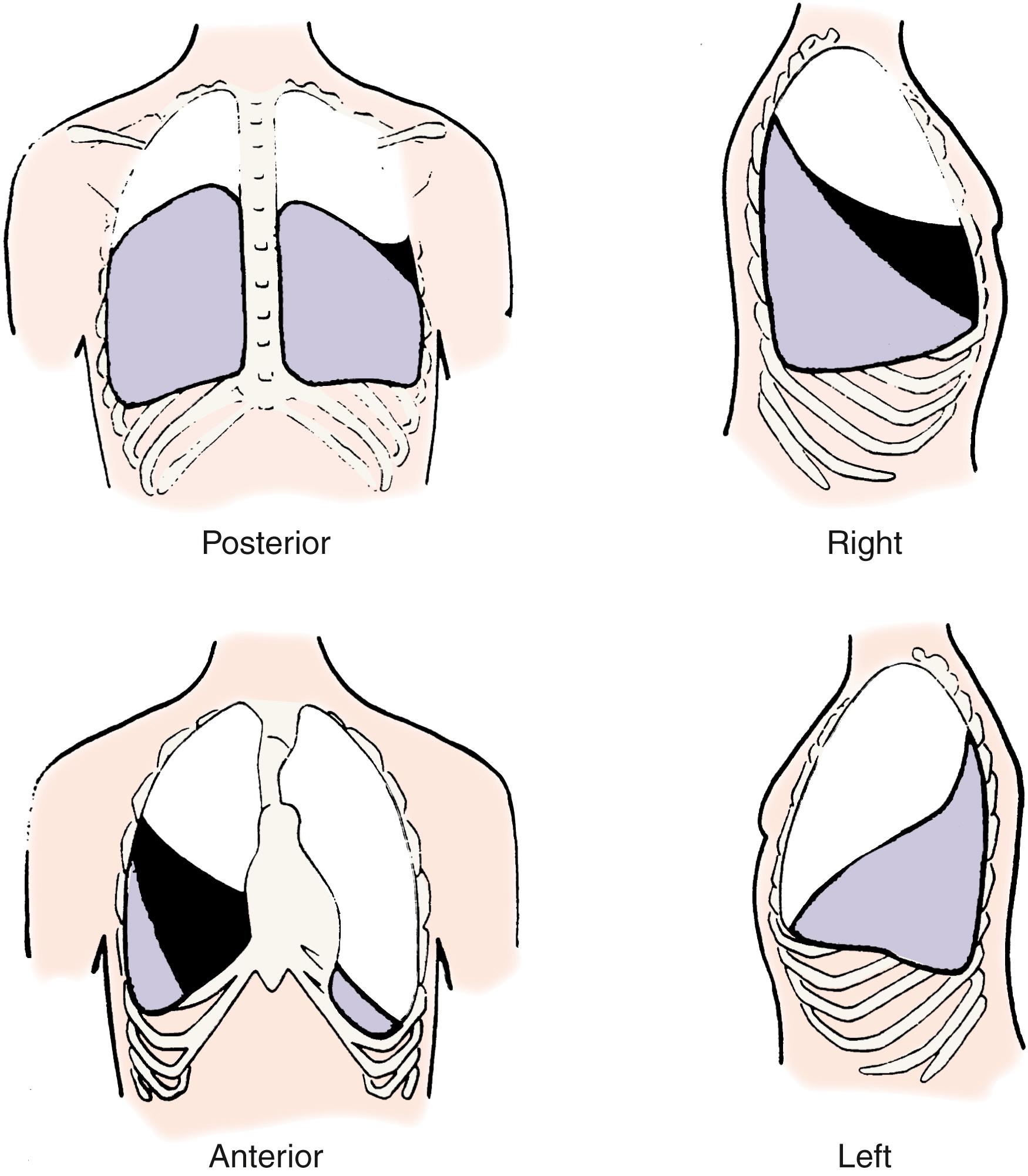
Cyanotic nail beds suggest hypoxemia, poor peripheral circulation, or both. The examiner looks for the presence of digital clubbing ( Fig. 3.4 ), which makes asthma or acute pneumonia extremely unlikely. The absence of digital clubbing but a history of severe chronic cough in an older child makes CF unlikely.
The shape of the chest gives information. Is the anteroposterior (AP) diameter increased, which indicates hyperinflation of the lungs from obstruction of small airways (e.g., asthma, bronchiolitis, CF)? Is this diameter small, as can be seen with some restrictive lung diseases with small lung volumes (e.g., muscular dystrophy, spinal muscular atrophy)? The normal infant has a “round” chest configuration, with the AP diameter of the chest about 84% of the transverse (lateral) diameter. With growth, the chest becomes more flattened in the AP dimension, and the AP-to-transverse ratio is between 70% and 75%. Although obstetric calipers can be used to give an objective assessment of the AP diameter of the chest, most clinicians rely on their subjective assessment of whether the diameter is increased: does the patient look “barrel-chested”?
Intercostal, subcostal, suprasternal, and supraclavicular retractions (inspiratory sinking in of the soft tissues) indicate increased effort of breathing and reflect both the contraction of the accessory muscles of respiration and the resulting difference between intrapleural and extrathoracic pressure. Retractions occur most commonly with obstructed airways (upper or lower), but they may occur with any condition leading to the use of the accessory muscles. Any retractions other than the mild normal depressions seen between an infant’s lower ribs indicate a greater-than-normal work of breathing. Audible end-expiratory “grunting” is suggestive of alveolar fluid from pulmonary, cardiac, or inflammation; it is often associated with severe disease.
Less easy to notice than intercostal retractions is their bulging out with expiration in a child with expiratory obstruction (asthma). Contraction of the abdominal muscles with expiration is easier to notice and is another indication that a child is working harder than normal to push air out through obstructed airways.
Inspection of the spine may reveal kyphosis or scoliosis. There is a risk of restrictive lung disease if the curvature is severe.
Palpating the trachea, particularly in infants, may reveal a shift to one side, which suggests loss of volume of the lung on that side or extrapulmonary gas (pneumothorax) on the other side. Placing one hand on each side of the chest while the patient breathes may enable the examiner to detect asymmetry of chest wall movement, either in timing or in degree of expansion. The former indicates a partial bronchial obstruction, and the latter suggests a smaller lung volume, voluntary guarding, or diminished muscle function on one side. Palpating the abdomen gently during expiration may allow the examiner to feel the contraction of the abdominal muscles in cases of expiratory obstruction. Hyperinflation may push the liver down, making it palpable below the costal margin.
Palpation for tactile fremitus, the transmitted vibrations of the spoken word (“ninety-nine” is the word often used to accentuate these vibrations), helps determine areas of increased parenchymal density and hence increased fremitus (as in pneumonic consolidation) or decreased fremitus (as in pneumothorax or pleural effusion).
The percussion determined by the examiner’s tapping of one middle finger on the middle finger of the other hand, which is firmly placed over the patient’s thorax, may be dull over an area of consolidation or effusion and hyperresonant with air trapping. Percussion can also be used to determine diaphragmatic excursion. The lowest level of resonance at inspiration and expiration determines diaphragmatic motion.
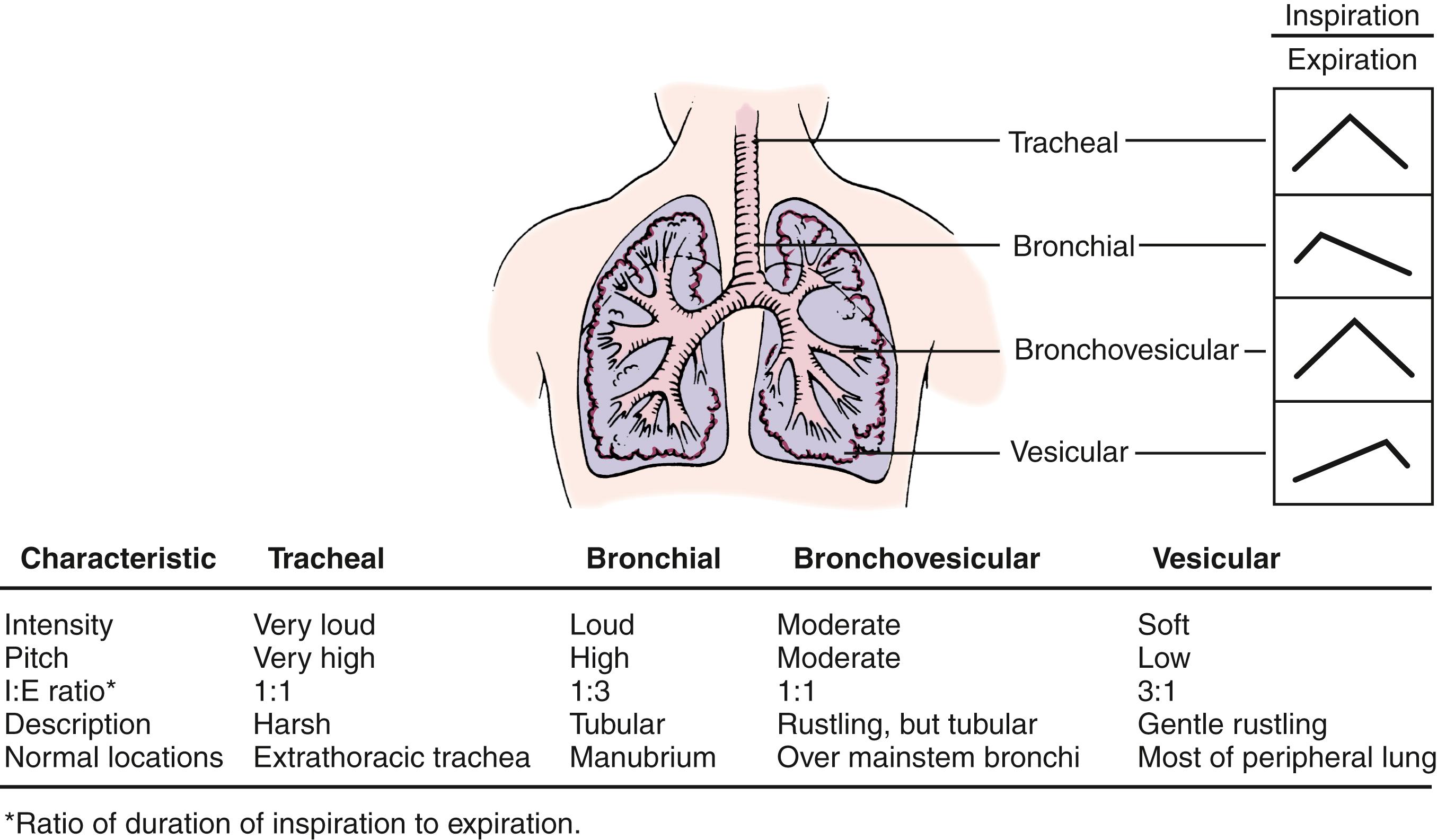
Because lung sounds tend to be higher pitched than heart sounds, the diaphragm of the stethoscope is better suited to pulmonary auscultation than is the bell, whose target is primarily the lower-pitched heart sounds ( Table 3.6 ). The adult-sized stethoscope generally is superior to the smaller pediatric or neonatal diaphragms, even for listening to small chests, because its acoustics are better ( Figs. 3.5 and 3.6 ).
| Disease Process | Mediastinal Deviation | Chest Motion | Fremitus | Percussion | Breath Sounds | Adventitious Sounds | Voice Signs |
|---|---|---|---|---|---|---|---|
| Consolidation (pneumonia) | No | Reduced over area, splinting | Increased | Dull | Bronchial or reduced | Crackles | Egophony, ∗ whispering pectoriloquy increased † |
| Bronchospasm | No | Hyperexpansion with limited motion | Normal or decreased | Hyperresonant | Normal to decreased | Wheezes, crackles | Normal to decreased |
| Atelectasis | Shift toward lesion | Reduced over area | Decreased | Dull | Reduced or absent | None or crackles | None |
| Pneumothorax | Tension deviates trachea and PMI to opposite side | Reduced over area | None | Resonant, tympanitic | None | None | None |
| Pleural effusion | Deviation to opposite side | Reduced over area | None | Dull | None | Friction rub; splash, if hemopneumothorax | None |
∗ Egophony is present when e sounds like a .
† Whispering pectoriloquy produces clearer-sounding whispered words (e.g., “ninety-nine”).
Adventitious sounds come in a few varieties, namely, stridor, crackles, rhonchi, and wheezes. Other sounds should be described in clear, everyday language.
Stridor is a continuous musical sound usually heard on inspiration and is caused by narrowing in the extrathoracic airway, as with croup or laryngomalacia.
Crackles are discontinuous, representing the popping open of air-fluid menisci as the airways dilate with inspiration. Fluid in larger airways causes crackles early in inspiration (congestive heart failure). Crackles that tend to be a bit lower in pitch (“coarse” crackles) than the early, higher-pitched (“fine”) crackles are associated with fluid in small airways (pneumonia). Although crackles usually signal the presence of excess airway fluid (pneumonia, pulmonary edema), they may also be produced by the popping open of noninfected fibrotic or atelectatic airways. Fine crackles are not audible at the mouth, whereas coarse crackles may be. Crackles is the preferred term, rather than the previously popular “rales.”
Rhonchi , or “large airway sounds,” are continuous gurgling or bubbling sounds typically heard during both inhalation and exhalation. These sounds are caused by movement of fluid and secretions in larger airways (in asthma, viral upper respiratory infection [URI]). Rhonchi, unlike other sounds, may clear with coughing.
Wheezes are continuous musical sounds (lasting longer than 200 msec), caused by vibration of narrowed airway walls, as with asthma, and perhaps vibration of material within airway lumens. These sounds are much more commonly heard during expiration than inspiration.
The chest radiograph is often the most useful diagnostic test in the evaluation of the child with cough. Table 3.7 highlights some of the radiographic features of the most common causes of cough in pediatric patients. Radiographic findings are often similar for a number of disorders, and thus these studies may not indicate a definitive diagnosis. Chest radiographs are normal in children with psychogenic (habit) cough and in children with sinusitis or gastroesophageal reflux (GER) as the primary cause of cough. A normal chest radiograph indicates the unlikelihood of pneumonia caused by RSV, influenza, parainfluenza, adenovirus, Chlamydia species, or bacteria. Although children with cough resulting from CF, Mycoplasma species, tuberculosis, aspiration, a bronchial foreign body, or an anatomic abnormality usually have abnormal chest radiographs, a normal radiograph does not exclude these diagnoses. Hyperinflation of the lungs is commonly seen on chest radiographs of infants with RSV bronchiolitis or Chlamydia pneumonia, and a lobar or round (coin lesion) infiltrate is the radiographic hallmark of bacterial pneumonia. Normal sinuses on radiograph or CT scan exclude sinusitis. In some causes of cough, the chest x-ray may be unrevealing; CT angiography is often very helpful in these circumstances, which may include COVID-19, vaping injury, pulmonary embolism, bronchiectasis, granulomatous diseases, and diseases associated with hilar or mediastinal lymphadenopathy.
| Chest Radiograph | Abnormal Sinus Radiograph | Complete Blood Count | IMMUNOGLOBULINS | + NP PCR | Other | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Normal | Hyper | Lobar Infil | Diff Infil | Other | ↑WBC | ↑LY | ↑EOS | ↑PMN | ↑IgG | ↑IgM | ↑IgE | ||||
| Asthma | + | ++ | – | – | – | + | + | + | ++ | – | + | + | ++ | +Bdilator 1 | |
| Cystic fibrosis | + | ++ | + | + | ++ | +++ | ++ | + | + | ++ | ++ | + | + | See Table 3.8 | |
| Other infection | |||||||||||||||
|
++ | + | + | + | ++ 2 | – | – | + | + | – | Paraflu +++ |
||||
|
++ | + | + | + | ++ 3 | – | +++ | + | + | +++ | Direct look | ||||
|
+++ | – | – | – | – | +++ | ++ | – | + | +++ | ++ | + | |||
|
– | +++ | + | ++ | + | – | + | + | + | + | RSV, human metapneumovirus +++ |
||||
| Pneumonia | |||||||||||||||
|
– | ++ | + | ++ | + | – | ++ | – | – | + | +++ | ||||
|
– | + | ++ | + | – | ++ | – | – | + | +++ | |||||
|
- | ++ | + | ++ | + | – | ++ | – | – | + | +++ | ||||
|
– | + | ++ | + | – | ++ | + | + | ++ | +++ | |||||
|
++ | + | – | + | + | – | ++ | +++ | + | + | ++ | + | – | ++ 4 | |
|
– | +++ | + | +++ | + | – | + | + | ++ | + | +++ | +++ | – | +++ | |
|
+ | + | + | + | ++ 5 | + | + | + | + | + | – | ++ | – | ++ | +Cold agglutinin |
|
+ | – | ++ | + | ++ | – | + | + | + | + | +PPD, QuantiFERON | ||||
|
– | + | +++ | + | ++ 5 | – | +++ | + | + | +++ | ++ | + | + | + | +Bld cult 6 |
| Foreign body | – | ++ 7 | ++ | – | ++ 7 | – | ++ | + | + | ++ | Bronch | ||||
| GE reflux | +++ | + | – | – | – | + | – | – | – | – | – | – | + | – | Esoph pH 8 |
| Aspiration | + | + | + | + | ++ 9 | + | + | – | + | + | – | – | + | – | 10 |
| Anatomic | + | + | + | – | ++ 11 | – | + | – | – | + | – | – | – | – | 12 |
| Habit | +++ | – | – | – | – | – | – | – | – | – | – | – | – | – | |
1 Positive response to bronchodilators, either as a home therapeutic trial or in a pulmonary function test in the laboratory.
2 “Steeple” sign: narrowing of upper tracheal air column.
3 “Swollen thumb”: sign of thickened epiglottis.
4 Low yield in paroxysmal stage.
5 Pleural effusion relatively common.
6 Blood culture positive in 10%; needle aspiration of pleural fluid or lung fluid may yield organism; bacterial antigen in urine. In older infants and children, common pathogens include pneumococci and group A streptococci; Staphylococcus aureus is rare and may be associated with pneumatoceles or empyema.
7 Localized hyperinflation is common; localized atelectasis is common; inspiratory-expiratory radiographs may show ball-valve obstruction.
8 Esophageal biopsy specimen shows esophagitis.
9 Multilobular or multisegmental, dependent lobes.
10 (?) Lipid-laden macrophages from bronchoscopy or gastric washings; barium swallow or radionuclide study showing aspiration.
11 Right-sided arch, mass effect on airways, mass identified; MRI.
The white blood cell (WBC) count may help exclude or include certain entities for a differential diagnosis. For example, a WBC count of 35,000 with 85% lymphocytes strongly suggests pertussis, but not every child with pertussis presents such a clear hematologic picture. The presence of a high number or large proportions of immature forms of WBCs suggests an acute process, such as a bacterial infection. Immunoglobulins provide supportive evidence for a few diagnoses, such as chlamydial infection, which rarely occurs without elevated serum concentrations of immunoglobulin G (IgG) and immunoglobulin M (IgM).
Specific bacteriologic or virologic diagnoses can be made in a number of disorders causing cough, including RSV, influenza, parainfluenza, coronaviruses, adenovirus, enteroviruses, metapneumovirus, Mycoplasma, and Chlamydia pneumonia. In most cases, the viruses can be rapidly identified with amplification of the viral genome through polymerase chain reaction (PCR). In bacterial pneumonia, the offending organism can be cultured from the blood in a small proportion (10%) of patients. A positive culture provides definitive diagnosis, but a negative culture specimen is not helpful. Throat cultures are seldom helpful (except in CF) in identifying lower respiratory tract bacterial organisms. Sputum cultures and Gram stains may help guide initial empirical therapy in older children with pneumonia or purulent bronchitis, but their ability to identify specific causative organisms with certainty (with the exception of CF) has not been shown clearly.
Infants and young children usually do not expectorate but rather swallow their sputum. Specimens obtained via bronchoscopy may be contaminated by mouth flora, but heavy growth of a single organism from a bronchoalveolar lavage in the presence of polymorphonuclear neutrophils certainly supports the organism’s role in disease. If pleural fluid or fluid obtained directly from the lung via needle aspiration is cultured, the same rules apply: Positive cultures and nucleic acid amplification tests are definitive, but negative cultures are not.
A number of specific tests can help to establish diagnoses in a child with cough (see Table 3.7 ). These include a positive response to bronchodilators in a child with asthma; visualizing the red, swollen epiglottis in epiglottitis (to be done only under very controlled conditions); the bronchoscopic visualization of the peanut, plastic toy, or other offender in foreign body aspiration; a positive purified protein derivative (PPD) or QuantiFERON assay in tuberculosis; and several studies of the esophagus in GER. Several imaging techniques, such as CT or MRI, can help to delineate various intrathoracic anatomic abnormalities, pulmonary embolism, and bronchiectasis. Multiple tests can be employed to confirm the diagnosis of CF ( Table 3.8 ).
| Usefulness | Test | Sensitivity | Specificity |
|---|---|---|---|
| Definitive | Sweat chloride test | .99+ | .95+ |
| DNA analysis | .85–.90 | .99 | |
| Suggestive | Throat or sputum culture ∗ positive for mucoid Pseudomonas aeruginosa | .70–.80 | .85 |
| Sinus radiographs | |||
| Pansinusitis | .95 | .90 | |
| Positive IRT newborn screen | .98 | .25 | |
| Supportive | Fecal elastase | ||
| Pulmonary function tests: | |||
|
.70+ | ? | |
| Chest radiograph: | |||
|
.70+ | ? | |
| Throat or sputum culture ∗ : | |||
|
.20 | .20 | |
|
.05–.20 | .15 |
Infections are the most common cause of acute cough in all age groups and are responsible for some chronic coughs. The age of the patient has a large impact on the frequency of the type of infection.
Viral upper respiratory infections (common cold); croup (laryngotracheobronchitis); viral bronchiolitis, particularly with RSV or human metapneumovirus; and viral pneumonia are the most frequently encountered respiratory tract infections and hence the most common causes of cough in infancy. Viral illness may predispose to bacterial superinfection (e.g., croup and Staphylococcus aureus tracheitis or influenza and Haemophilus influenzae or S. aureus pneumonia).
Viral URI symptoms and signs usually include nasal congestion and discharge, sore throat, and sneezing. There may be fever, constitutional signs (irritability, myalgias, and headache), or both. Cough is common and may persist for 5–7 days. The mechanism by which URIs cause cough in children is undetermined. In adults, it is generally thought that “postnasal drip”—that is, nasal or sinus secretions draining into the posterior nasopharynx—causes cough and, in fact, may be one of the most frequent causes of cough. Indeed, sinus CT in older patients with URIs often reveals unexpected involvement of the sinus mucosa. Other authorities believe that cough in a child with a URI indicates involvement (inflammation or bronchospasm) of the lower respiratory tract. Over-the-counter cough and cold medications are commonly used. Evidence of efficacy of these medications for children with URI is lacking. Because of the known risk for unintentional overdose from these medications, their use is not recommended in children under age 4 years.
Common viral pathogens include rhinovirus, RSV, coronaviruses, and parainfluenza viruses. The differential diagnosis includes allergic rhinitis, which often demonstrates clear nasal secretions with eosinophils and pale nasal mucosa, and sinusitis, which presents with mucopurulent nasal secretions containing neutrophils and erythematous mucosa.
Infectious croup (see Chapter 4 ) is most common in the first 2 years of life. Its most dramatic components are the barking (“croupy”) cough and inspiratory stridor, which appear a few days after the onset of a cold. In most cases, the patient has a low-grade fever, and the disease resolves within a day or two. In severe cases, the child can be extremely ill and is at risk for complete laryngeal obstruction. There may be marked intercostal and suprasternal retractions and cyanosis. Stridor at rest signifies significant obstruction . Diminishing stridor in a child who is calm is a good sign, but diminishing stridor in and of itself is not necessarily good: If the child becomes fatigued because of the tremendous work of breathing through an obstructed airway and can no longer breathe effectively, smaller-than-needed tidal volumes make less noise.
It is important to distinguish croup from epiglottitis in the child with harsh, barking cough and inspiratory stridor because the natural histories of the two diseases are quite different (see Table 3.7 ). Epiglottitis is uncommon but occurs in unimmunized patients (see Chapter 4 ).
Treatment of mild croup is usually not needed. For decades, pediatricians have recommended putting a child with croup in a steamy bathroom or driving to the office or emergency department with the car windows rolled down. It is likely that these remedies are effective because of the heat exchange properties of the upper airway; air that is cooler or more humid than the airway mucosa will serve to cool the mucosa, thus causing local vasoconstriction and probably decreasing local edema.
In a child who has stridor at rest, evaluation is indicated. Symptomatic, often dramatic relief through decreased laryngeal edema can usually be achieved with aerosolized racemic epinephrine (2.25% solution, 0.25 to 0.5 mL/dose). It is essential to remember that the effects of the epinephrine are transient, lasting only a few hours, although the course of the illness is often longer. The result is that when the racemic epinephrine’s effect has worn off, the child’s cough and stridor will probably be as bad or even worse than before the aerosol was administered. This is not a “rebound” effect: The symptoms are not worse because of the treatment but, rather, because of the natural progression of the viral illness. Repeating the aerosol will probably again have a beneficial effect. A child who responds favorably to such an aerosol needs to be observed for several hours because further treatment may be needed. A single dose of dexamethasone (0.6 mg/kg orally, intramuscularly, or intravenously, maximum dose 16 mg) reduces the severity and hastens recovery.
Bronchiolitis is a common and potentially serious lower respiratory tract disorder in infants (see Chapter 4 ). It is caused usually by RSV but on occasion by parainfluenza, influenza, human metapneumovirus, adenovirus, enterovirus, and human rhinovirus. It mostly occurs in the winter months, often in epidemics. RSV bronchiolitis is seen uncommonly in children older than 4 years. Typically, “coldlike” symptoms of rhinorrhea precede the harsh cough, increased respiratory rate, and retractions. Respiratory distress and cyanosis can be severe. The child’s temperature is seldom elevated above 38°C.
The chest is hyperinflated, widespread crackles are audible on inspiration, and wheezing marks expiration. The chest radiograph invariably reveals hyperinflation, as depicted by a depressed diaphragm, with an enlarged retrosternal air space in as many as 60% of patients, peribronchial thickening in approximately 50%, and consolidation and/or atelectasis in 10–25%.
The diagnosis is confirmed with demonstration of RSV by PCR of nasopharyngeal secretions. In most cases, no treatment is needed because the disease does not interfere with the infant’s eating or breathing. Apnea is a common complication of RSV bronchiolitis in neonates and may necessitate close monitoring. In severe cases, often those in which there is underlying chronic heart, lung, or immunodeficiency disease, RSV can be life threatening. In severe cases, hospital care with supplemental oxygen and intravenous fluids is indicated. Suctioning of secretions is an essential part of the treatment. Many other treatment modalities have been tried for hospitalized infants with bronchiolitis. Aerosolized bronchodilators and systemic glucocorticoids do not seem to alter clinical outcome and are not recommended in most patients. Nebulized saline may reduce the length of hospitalization. Use of a high-flow nasal cannula may reduce the need for more invasive forms of respiratory support in infants with impending respiratory failure.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here