Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Bare-metal stents (BMS) overcome many of the drawbacks of balloon angioplasty but are limited by restenosis, which develops in 20% to 40% of cases.
Drug-eluting stents (DES)—which consist of a metallic stent coated with a drug carrier vehicle (usually a polymer) that controls the dose and timing of the elution of an antiproliferative agent—have been shown to significantly reduce in-stent late loss, resulting in reduced rates of angiographic and clinical restenosis.
First-generation DES, including sirolimus-eluting and paclitaxel-eluting stents, have resulted in similar rates of mortality and myocardial infarction (MI) as BMS in a broad spectrum of patients and lesions; however, concern over an increased rate of late stent thrombosis led to reliance on extended dual antiplatelet therapy (DAPT), and studies have demonstrated an increase in stent thrombosis and MI beyond 1 year with these devices.
Second-generation DES have been developed to overcome the drawbacks of their predecessors, principally by using improved stent platforms with thinner struts and enhanced deliverability, and more biocompatible or bioabsorbable polymers compared with first-generation devices.
Second-generation DES have been shown to be safer and more effective than not only first- generation DES, but also may be safer than BMS. The enhanced safety of second-generation DES is especially apparent in specific high-risk and complex situations, including acute MI, bifurcation lesions, chronic total occlusions (CTO), diabetic patients, and saphenous vein grafts. The utility of DES compared with bypass surgery has also become accepted in some but not all cases of left main and multivessel disease.
The improved safety profile of second-generation DES has challenged the notion of prolonged DAPT after DES implantation. Evidence has emerged that in low-risk patients, 3- or 6-month DAPT after second-generation DES implantation may be as effective as and safer than DAPT continued for 1 year. However, in patients with a low risk of bleeding and high risk of atherothrombotic events, prolonged DAPT for several years or longer after stent implantation may reduce the ongoing risk of very late stent thrombosis and prevent MI arising from untreated atherosclerosis.
Even with the improvements with current-generation DES, adverse ischemic events arising from the stent site continue to accrue at late follow-up due to neoatherosclerosis, strut fractures, compliance mismatch, and altered vascular physiology. In this regard, fully bioresorbable vascular scaffolds (BVS) may further improve chronic outcomes, but the increased rates of stent thrombosis observed with some of these devices has raised concern over their safety.
The history of percutaneous coronary intervention (PCI) may be described as a series of transformative steps, beginning with the introduction of coronary balloon angioplasty. Development of the implantable metallic stent enabled PCI to become a durable approach for the vast majority of patients with coronary artery disease, and one that most operators can apply safely. From balloons to atherectomy to lasers to bare-metal stents (BMS) and now drug-eluting stents (DES), the evolution of interventional cardiology has progressively and uncompromisingly advanced through the insights and inventions of thousands of physicians and scientists, coupled with the recognition of the importance of optimal adjunctive pharmacotherapy, on the foundation of thousands of randomized trials and registries performed around the world which provide an unparalleled evidence base for daily clinical decision making. The present chapter will trace the evolution and development of the coronary stent from its initial applications to treat balloon angioplasty failures to its widespread global adoption for the treatment of patients with ischemic coronary heart disease.
The performance of the first successful balloon angioplasty by Andreas Gruentzig on September 16, 1977, in Zurich, Switzerland, was a landmark event heralding the inception of percutaneous management of obstructive coronary artery disease. Although this initial procedure set the stage for the millions of PCI procedures that have since taken place, stand-alone balloon angioplasty was a highly unpredictable experience. While the majority of vessels tolerate the focal plaque dissections caused by balloon dilatation and heal sufficiently to result in an adequate lumen, the injury to the vessel wall may unpredictably result in severe dissections, which along with acute recoil and chronic constrictive remodeling, result in balloon angioplasty’s two major limitations: abrupt closure (occurring acutely, or within the first several days after angioplasty) and restenosis (occurring later, within months, and, rarely, years after the procedure). The coronary stent was thus devised as an endoluminal scaffold to create a larger initial lumen, to seal dissections, and to resist recoil and late vascular remodeling, thereby improving upon the early and late results of balloon angioplasty.
The first implantation of stents in human coronary arteries occurred in 1986 when Ulrich Sigwart and Jacques Puel and colleagues placed the Wallstent-sheathed self-expanding metallic mesh scaffold (Medinvent, Lausanne, Switzerland) in the peripheral and coronary arteries of eight patients. Further experience with this device demonstrated high rates of thrombotic occlusion and late mortality, although patients without thrombosis had a 6-month angiographic restenosis rate of only 14%, suggesting for the first time that stenting could improve late patency in addition to stabilizing the acute results obtained after conventional balloon angioplasty. Contemporaneously, Cesare Gianturco and Gary Roubin developed a balloon-expandable coil stent consisting of a wrapped stainless steel wire resembling a clamshell. Completion of a phase II study using the Gianturco-Roubin stent to reverse postangioplasty acute or threatened vessel closure led to United States Food and Drug Administration (FDA) approval for this indication in June 1993. However, high rates of restenosis with this device relegated its use mainly for bailout of occlusive dissections and recoil after balloon angioplasty.
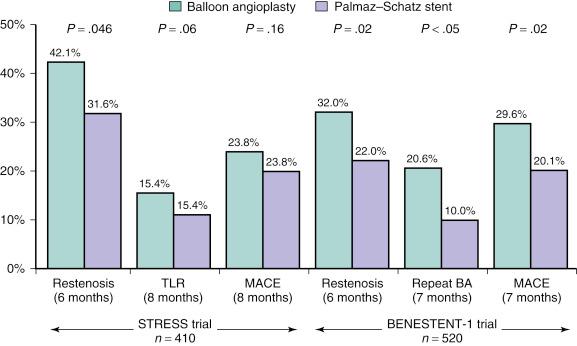
While these stents were being developed and tested, in 1984 Julio Palmaz designed a balloon-expandable stainless steel “slotted tube stent.” In this stent design, rectangular slots were cut into a 15-mm-long, thin-walled stainless steel tube, such that balloon inflation within the stent deformed these rectangular slots into diamond-shaped windows or cells. While this design allowed for relatively straightforward deployment (and greater resistance to recoil than the clamshell design), the rigidity of this stent made it difficult to deliver in the coronary vasculature. In 1989, a design modification was suggested by Richard Schatz, consisting of the placement of a 1-mm central articulating bridge connecting two rigid 7-mm slotted segments, creating the 15-mm Palmaz–Schatz stent (Johnson and Johnson Interventional Systems, Warren, NJ) ( Fig. 15.1 ). The first coronary Palmaz–Schatz stent was placed in a patient by Eduardo Sousa in São Paulo, Brazil, in 1987. The Palmaz–Schatz stent was subsequently investigated in two landmark randomized trials comparing balloon angioplasty to elective stenting. In the STent REStenosis Study (STRESS) and Belgian Netherlands Stent study (BENESTENT)-1 studies, routine use of the Palmaz–Schatz stent was associated with a 20% to 30% reduction in clinical and angiographic restenosis within 6 to 12 months compared with conventional balloon angioplasty ( Fig. 15.2 ). The Palmaz–Schatz stent also resulted in markedly improved initial angiographic results, with a larger postprocedural minimal luminal diameter and fewer residual dissections, which translated into a lower rate of subacute vessel closure. These results led to approval of the Palmaz–Schatz Stent by the FDA in 1994. Long-term follow-up up to 15 years has subsequently demonstrated a low (but not zero) rate of clinical angiographic recurrences from years 1 to 5 after coronary stent implantation, with slight and progressive decrements in luminal size thereafter extending beyond 10 years. The mechanisms of this late progression of disease are not entirely known, but, as discussed later, is usually described as atherosclerotic transformation of neointimal hyperplasia, known as neoatherosclerosis.
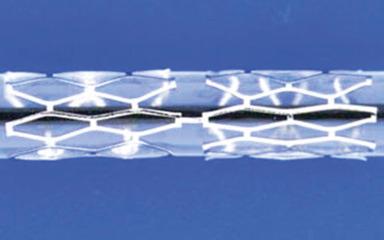
Despite the success of the Palmaz–Schatz stent in improving the early and late results of conventional balloon angioplasty, widespread adoption of stent technology was initially hindered by high rates of subacute stent thrombosis necessitating an intense antithrombotic and antiplatelet regimen (consisting of aspirin, dextran, dipyridamole, heparin, and warfarin). Further refinements in the stent procedure and periprocedural pharmacotherapy regimens were thus required. Antonio Colombo and colleagues demonstrated reduced rates of stent thrombosis with more aggressive intravascular ultrasound (IVUS)-guided deployment techniques including routine high-pressure adjunctive dilatation (>14 atmospheres), along with the use of aspirin and a second antiplatelet agent (thienopyridine ticlopidine) rather than prolonged warfarin therapy. These modifications significantly reduced the incidence of stent thrombosis to ∼1% to 2%, along with a marked reduction in bleeding and femoral arterial complications. The confirmation of these initial findings in several randomized clinical trials definitively established the superiority of dual antiplatelet therapy (DAPT) (with aspirin and ticlopidine) over anticoagulation with warfarin for prevention of stent thrombosis, and facilitated widespread adoption of coronary stenting by the late 1990s.
Coronary stents may be classified based on their composition (e.g., metallic or polymeric), configuration (e.g., slotted tube vs. coiled wire), bioabsorption (either inert [biostable or durable] or degradable [bioabsorbable]), coatings (either none, passive [such as heparin or polytetrafluoroethylene {PTFE}], or bioactive [such as those eluting rapamycin or paclitaxel]), and mode of implantation (e.g., self-expanding or balloon-expandable). In theory, the ideal coronary stent would be made of a nonthrombogenic material and have sufficient flexibility in its unexpanded state to allow passage through small guiding catheters and tortuous vessels. It would also have an expanded configuration that provides uniform scaffolding of the vessel wall with low recoil and maximal radial strength, while at the same time being conformable on bends. In addition, the stent should be sufficiently radiopaque to allow fluoroscopic visualization to guide accurate placement and management if restenosis occurs, but not so opaque as to angiographically obscure important vascular details.
In the early stent experience, the most widely used stent material was 316 L stainless steel. More recently, cobalt chromium and platinum chromium alloys have been employed to allow lower-profile thin stent struts (60 to 82 μm vs. 100 to 150 μm in most stainless steel stents) that maintain strength and visibility. Most self-expanding stents utilize nitinol, a nickel-titanium alloy, which, after being baked at a high temperature, maintains shape memory for a predetermined size and configuration.
Other than gold (which has been shown to increase restenosis), there is little evidence that thrombosis or restenosis rates vary with the specific stent metal, though the final stages of surface finishing, smoothing, and purification or passivation may affect early thrombotic and late restenotic processes. While a meta-analysis including nine randomized trials and 11,313 patients reported a better safety profile with DESs that use cobalt chromium platforms compared to stainless steel platforms, due to a significant reduction in the 30-day rates of myocardial infarction (MI), comparisons such as these may also take into account different polymers used in specific DES, thereby making stent-material-specific comparisons difficult to interpret. There is a growing interest in polymeric and metallic fully biodegradable stents (bioresorbable scaffolds), which theoretically offer the advantages of increased longitudinal flexibility, compatibility with noninvasive imaging, and complete bioresorption over a period of months to a year or longer, with the potential to restore underlying vascular reactivity (see Chapter 16 ).
Stents can be assigned to one of three distinct subcategories, based on construction/design elements: wire coils, slotted tubes/multicellular, and modular designs. The vast majority of stents in current use are either slotted tube/multicellular or modular in design. Within these subcategories, stents are often classified as having open cells or closed cells, based upon the design of connecting links between adjacent stent struts. Open-cell designs tend to have varying cell sizes and shapes, which provide increased flexibility, deliverability, and side branch access, with staggered cross-linking elements to provide radial strength. Closed-cell designs typically incorporate a repeating, unicellular element that provides more uniform wall coverage with less tendency for plaque prolapse, but at the expense of flexibility and side branch access.
Stent design may significantly impact acute and late vascular responses. Stents that possess better conformability, less rigidity, and greater circularity experimentally produce less vascular injury, thrombosis, and neointimal hyperplasia. Clinical studies have suggested that thin stent struts may be associated with reduced neointimal hyperplasia and lower rates of restenosis. In addition, an experimental study using an ex vivo modified Chandler loop generating pulsatile flow found that thick strutted stents (162 μm) are significantly more thrombogenic than otherwise identical thin-strutted stents (81 μm). Stent design may also significantly impact longitudinal integrity of the stent, which principally depends on the number of connectors between hoops. Longitudinal distortion may manifest as length change, strut overlap, or strut separation which may obstruct the lumen, predisposing to stent thrombosis or restenosis.
A variety of coatings have been used to attempt to reduce the thrombogenicity or restenosis of metallic stents ( Table 15.1 ). Experimental studies have demonstrated that coating stents with inert polymers may reduce surface reactivity and thrombosis, though until recently, most polymers used were found to provoke intense inflammatory reactions. With the advent of DES came a renewed interest in the study of stent coatings, primarily to act as drug-carrier vehicles. However, concerns regarding the long-term safety of DES and the requirement for extended- duration DAPT have led to the development of new stent coatings, which are either more biocompatible or bioabsorbable. Specifically, fluorinated copolymers have been shown in vitro to have thromboresistant properties, due to preferential absorption and retention of albumin, which passivates the stent surface. Finally, covered stents (metallic stents covered by a distensible microporous PTFE membrane) are of unquestioned clinical utility in treating life-threatening perforations. They are also used for excluding giant aneurysms, pseudoaneurysms, and clinically significant fistulae (see Chapter 29 ). More recent is the application of polyethylene terephthalate (PET) mesh-covered stents (MGuard, Inspire MD, Tel Aviv, Israel) to minimize the risk of distal embolization in patients with ST-segment elevation myocardial infarction (STEMI) (see Chapter 20 ).
|
Balloon-expandable stents are mounted onto a delivery balloon and delivered into the coronary artery in their collapsed state. Once the stent is in the desired location, inflation of the delivery balloon expands the stent against the arterial wall, following which the stent delivery system is removed. Almost all stents implanted in human coronary arteries are balloon-expandable. Self-expanding stents incorporate either specific geometric designs or nitinol shape-retaining metal to achieve a preset diameter, and are released from a resisting sheath once placed in position. While self-expanding stents are more flexible than their balloon-expandable counterparts, greater rates of restenosis have been observed with this design, presumably from chronic ongoing vascular injury, limiting their use in coronary arteries. Recently, a renewed interest in self-expanding stents with reduced outward expansion force for the treatment of patients with acute coronary syndromes or vulnerable plaque has surfaced.
Stenting after failed balloon angioplasty. Stents may be used either on a routine (planned) basis or after failed balloon angioplasty for acute or threatened vessel closure (“bail-out” stenting). One of the major benefits of stenting (indeed, the initial reason for the genesis of stents) is the ability to reverse abrupt closure due to dissection and recoil, thus eliminating the need for high-risk emergency bypass surgery. These data, coupled with the fact that routine stent implantation compared to balloon angioplasty provides superior acute results and greater event-free survival in almost every patient and lesion subtype studied to date, has for the most part relegated balloon dilation to the rare lesion that is too small (<2.0 mm) for stenting, or to which a stent cannot be delivered because of excessive vessel tortuosity or calcification, or in patients in whom thienopyridines cannot be taken or are contraindicated.
The utility of routine stent implantation as a modality to reduce acute vessel closure and late restenosis was first demonstrated in the STRESS and BENESTENT-1 trials, which enrolled patients undergoing PCI of discrete, focal lesions. As a result, the types of lesions treated in these trials (discrete de novo lesions coverable by one stent, with reference vessel diameter [RVD] 3.0 to 4.0 mm) became known as “Stress/Benestent” lesions, to differentiate them from more complex stenoses. Despite initial concerns regarding diminished safety and efficacy of coronary stents (which were also more costly than balloon angioplasty alone) with more generalized use of these devices, numerous randomized trials and observational studies comparing stenting to balloon angioplasty demonstrated an advantage to coronary stenting over conventional balloon angioplasty across a wide range of patient and lesion subsets.
By the late 1990s, stent implantation became the predominant treatment for most patients with coronary artery disease as a result of more predictable acute and late angiographic results compared with conventional balloon angioplasty, atherectomy, and laser therapy. Improvements in procedural technique (including IVUS and optical coherence tomography [OCT] guidance), more effective antiplatelet regimens, and the introduction of increasingly lower profile, more flexible and deliverable devices additionally contributed to the ascendancy of stenting. With improvements in stent deliverability and reductions in rates of subacute stent thrombosis to less than 1%, restenosis emerged as the major persistent limitation of coronary stenting. While coronary stents increase acute luminal diameters to a greater extent than balloon angioplasty (greater acute gain), the greater vascular injury caused by stent implantation compared to balloon angioplasty elicits an exaggerated degree of neointimal hyperplasia, resulting in greater decreases in luminal diameter (late loss). Importantly, however, comparing stents to balloon angioplasty, the mean incremental gain in luminal dimensions with stenting is statistically greater than the mean incremental increase in late loss, resulting in a larger net gain in minimal luminal dimensions. This observation led Kuntz and Baim to formulate the “bigger is better” concept: the greater the acute gain, the greater the late gain and the lower the ultimate rate of restenosis. Nonetheless, even with optimal stent implantation, restenosis after BMS implantation still occurred in approximately 20% to 40% of patients within 6 to 12 months, in part due to stenting more complex patient and lesion subsets than in the balloon angioplasty era. As such, coronary restenosis became known as the “Achilles’ heel” of coronary stenting, with significant resources devoted to its prevention and treatment (see Chapter 34 ).
DES, which maintain the mechanical advantages of BMS while delivering an antirestenotic pharmacologic therapy locally to the arterial wall, have been shown to effectively and safely reduce the amount of in-stent tissue that accumulates after stent implantation, resulting in significantly reduced rates of clinical and angiographic restenosis. These devices were designed specifically to prevent the neointimal hyperplasia resulting after conventional BMS placement, and have been highly successful in this regard. In numerous randomized trials, the reduction in neointimal hyperplasia that occurs with DES compared to BMS has been shown to result in a 50% to 75% reduction in binary angiographic restenosis and target-lesion revascularization (TLR). The initial results of the pivotal randomized trials that led to device approval have been replicated and validated in numerous subsequent trials and real-world registries across the spectrum of disease and lesion subtypes.
The three critical components of a DES that must be optimized to ensure its safety and efficacy are (1) the stent itself (including its delivery system); (2) the pharmacologic agent being delivered; and (3) the drug carrier vehicle, which controls the drug dose and pharmacokinetic release rate ( Fig. 15.3 ).
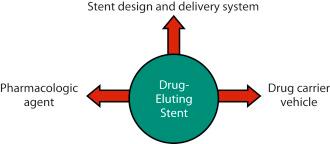
The stent component of early DES platforms was typically that of the predicate BMS without design modifications (i.e., relatively thick strut designs composed of stainless steel). Indeed, first-generation DES designs often appropriated approved and “off-the shelf” stent designs in order to expedite device development and regulatory approval. Subsequent DES have incorporated newer materials, thinner struts, and more flexible designs, with resultant improvements in device delivery and performance. Additionally, newer dedicated DES designs have included modifications aimed at either optimizing local drug delivery while reducing total drug dose (e.g., drug delivery limited to the abluminal direction), or modifying the stent surface to facilitate direct drug delivery and/or arterial healing following implantation (without a drug carrier vehicle per se). The latest evolution has been the introduction of drug-eluting fully bioresorbable scaffolds (BRS), wherein the stent frame is composed of a bioabsorbable polymer or metal that provides a scaffolding function for 6 to 12 months and then completely resorbs over the next 1 to 3 years, recapitulating the underlying vessel anatomy and physiology.
Following promising cell culture and in vitro development, t he antirestenotic properties of a wide range of pharmacologic agents have been tested in humans ( Fig. 15.4 ). Among these, the two most clinically effective classes of agents are the “rapamycin-analog family” of drugs and paclitaxel. The principal mechanism of action of rapamycin (also known as sirolimus), and its analogs (including zotarolimus, everolimus, biolimus A9, and novolimus) is inhibition of the mammalian target of rapamycin (mTOR), which prevents cell cycle progression from the G1 to S phase. mTOR is also localized on platelet membranes where it mediates platelet activation and aggregation. Inhibition of mTOR by sirolimus analogs has been shown to prevent platelet spreading on fibrinogen coating cover slips, can inhibit platelet aggregation, and produce platelet diasaggregation under shear flow conditions. In addition, rapamycin or rapamycin analogs are able to inhibit mTOR-dependent clot retraction. Although somewhat varying in lipophilicity and potency, most rapamycin analogs have been clinically comparable in their safety and efficacy effects. Two other rapamycin analogs that in the past (but not present) have been used on DES platforms—tacrolimus and pimecrolimus—have a different mechanism of action, binding directly to FKBP506, thereby inhibiting the calcineurin receptor with downregulation of cytokines and inhibition of smooth muscle cell activity ; unlike the mTOR inhibitors, these agents have not shown significant antirestenotic potential.
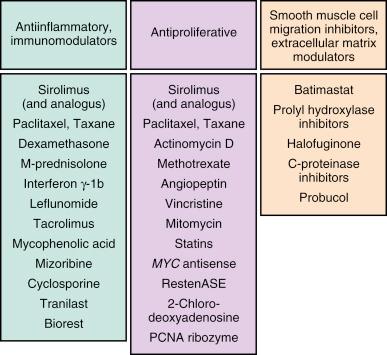
The other agent that has been used effectively not only in DES but also on drug-eluting balloons is paclitaxel. By interfering with microtubule function, paclitaxel has multifunctional antiproliferative and antiinflammatory properties, prevents smooth muscle migration, blocks cytokine and growth factor release and activity, interferes with secretory processes, is antiangiogenic, and impacts signal transduction. At low doses (similar to those in DES applications), paclitaxel affects the G0 to G1 and G1 to S phases (G1 arrest) resulting in cytostasis without cell death.
The inability to predictably deliver a specific dose of active drug over the right time frame to the arterial wall led to the failure of early DES programs. To ensure accurate drug dosing, a drug delivery vehicle became necessary, which for most first-generation stents was a durable (nonerodable) polymer. A wide range of polymer systems have since been developed, and are DES-specific (discussed in subsequent sections of this chapter). While the polymer is instrumental in regulating the pharmacokinetics of drug delivery to the arterial wall (which is necessary for reduced neointimal hyperplasia), the polymer may elicit deleterious vascular responses. Specifically, histopathologic studies have demonstrated hypersensitivity and eosinophilic inflammatory reactions and delayed endothelialization with DES that were not previously seen with BMS. Whether these maladaptive vascular responses are directly related to the polymer and/or to toxic reactions from the drug itself is speculative, but in animal models these effects can be attenuated by modification of the polymer vehicle. It is believed that in selected patients, excessive inflammation and delayed endothelialization play a role in the development of late stent malapposition, aneurysm formation, and stent thrombosis and restenosis. For these reasons, there has been great interest in developing inert and biocompatible polymers, bioabsorbable polymers (BP), and even polymer-free DES. For example, fluorinated copolymers coating some second-generation DES have been shown to possess thromboresistant properties in blood contact applications, and have been shown to reduce platelet adhesion compared to an otherwise identical BMS. On the other hand, optimizing BP performance entails consideration of biocompatibility, composition, and degradation time of the polymer, which can be affected by the use of long polymer chains, decreased polymer hydrophobicity, and greater polymer crystallinity. Polymer degradation can also be associated with significant inflammatory reactions and a persistent immune-mediated response to monomer breakdown product.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here