Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Iodinated contrast agents can be classified based on osmolarity (high, low, or iso) and ionitiy (ionic or nonionic).
A variety of iodinated contrast agents have been developed and are distributed by different manufacturers. The agents all consist of iodinated benzene ring derivatives, and ionic agents are typically formulated as sodium and/or meglumine salts. Two generic classes of agents include:
High-osmolar contrast agents (HOCAs, “ionics”)
Low-osmolar contrast agents (LOCAs, “nonionics”); includes iso-osmolar agents
A previously used low-osmolar ionic contrast agent called Hexabrix (ioxaglate) was discontinued in 2015.
| Bayer Healthcare | Urovist | Ultravist |
| Angiovist | ||
| Mallinckrodt | Conray | Optiray |
| Vascoray | ||
| Squibb (Bracco) | Renovue | Isovue |
| Renografin | ||
| Renovist | ||
| GE Healthcare | Hypaque | Omnipaque |
| Visipaque | ||
| Guerbet | Oxilan |
For nonionic agents, the I content is easy to determine because it is written on the label. For example, Omnipaque 300 contains 300 mg I/mL of solution. For ionic agents, the iodine content has to be calculated because contrast concentrations are expressed on a salt weight basis. For example, Conray 60 contains 60% meglumine iothalamate = 600 mg salt/mL = 282 mg I/mL. Because meglumine and sodium have different molecular weights, a 60% (weight/volume) solution of contrast contains different amounts of iodine, depending on the accompanying cation:
60% meglumine diatrizoate: 282 mg I/mL
60% sodium diatrizoate: 358 mg I/mL
There are two major ionic agents on the market, differing in the R-group:
Diatrizoate (i.e., Hypaque)
Iothalamate (i.e., Conray)

The osmolarity of ionic agents depends on the concentration, which typically ranges from 30% (550 milliosmole [mOsm]/kg H 2 O) to 76% (~2000 mOsm/kg H 2 O). The ionic agents have been in clinical use since the 1950s.
Nonionic LOCAs have a lower incidence of adverse reactions when used intravenously and are equally effective as imaging agents. Intravascular contrast agents in current use are thus exclusively nonionic LOCAs. The main nonionic agents on the market are:
Iodixanol (Visipaque)
Iopamidol (Isovue)
Ioversol (Optiray)
Iopromide (Ultravist)
Nonionic agents do not require an accompanying cation and therefore have lower osmolarity. The osmolarity depends on the concentration, which typically ranges from 300 mg I/mL (= 670 mOsm/kg H 2 O) to 370 mg I/mL (= 800 mOsm/kg H 2 O).
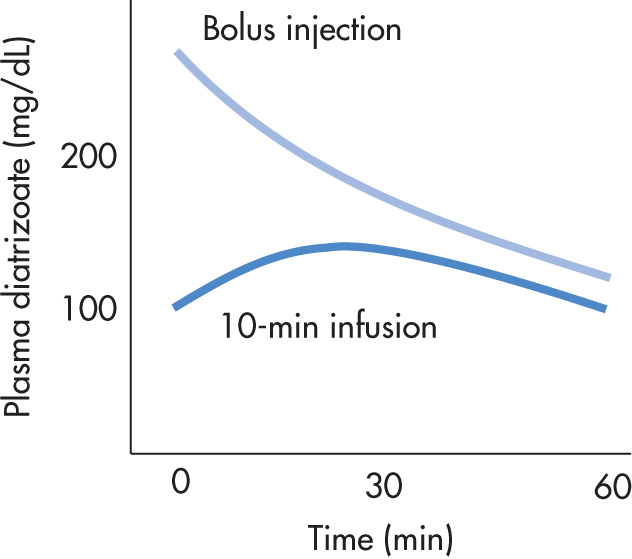
Plasma levels of iodinated agents depend on:
Rate of administration (intravenous [IV] bolus, IV drip)
Blood half-life
Distribution
Rapid exchange between plasma and extracellular space
Exclusion from intracellular space
Agents do not cross intact blood-brain barrier.
Excretion
Glomerular filtration with no resorption in
the tubules.
Hepatic excretion (vicarious excretion)
increases in renal failure.
Frequency of reactions has decreased considerably with the use of modern nonionic LOCAs, with an overall incidence of 0.2%–0.7%.
Acute reactions can be categorized as either allergic like or physiologic and subclassified as mild, moderate, or severe.
A history of allergies or asthma increases the risk of an acute reaction.
No evidence to support a relationship between shellfish/seafood allergy and contrast reaction
Allergic like: typically mediated by type 1 hypersensitivity mechanism, small minority related to immunoglobulin E (IgE) mechanism
Physiologic: results from disruption of homeostasis related to physical/chemical characteristics of the agent such as osmolality and viscosity or from chemotoxicity
Most reactions occur within 1 hour, many within 5 minutes.
Delayed reactions can occur between 1 hour and 1 week after exposure and are typically cutaneous and self-limiting.
In patients with a previous contrast reaction, subsequent reactions are most likely to be of a similar severity, though could be either more or less severe.
Premedication may help reduce mild or moderate reactions, though is not appropriate for patients with severe reactions as there is no proven benefit.
Elective premedication: prednisone 50 mg orally (PO) at 13, 7, and 1 hour before contrast injection, as well as diphenhydramine (Benadryl) 50 mg PO 1 hour before injection
Emergent premedication: methylprednisolone 40 mg or hydrocortisone 200 mg IV every 4 hours until contrast injection, plus diphenhydramine 50 mg IV 1 hour before the injection
| Allergic Like a | Physiologic a | |
|---|---|---|
| Mild |
|
|
| Moderate |
|
|
| Severe |
|
|
a Classification of acute contrast reactions (adapted from ).
Decrease in renal function following recent intravascular administration of iodinated contrast
Usually occurs within 24–48 hours of injection; generally self-limited
Incidence rate is controversial due to contamination of published studies by bias.
Primary risk factor is preexisting renal insufficiency; evidence suggests increased risk of CIN if glomerular filtration rate (GFR) is <30 mL/min.
Can consider prehydration with GFR of 30–45 mL/min; 100 mL/hour for 6–12 hours before and 4–12 hours after contrast injection if inpatient, more rapid volume expansion if outpatient
No proven benefit for pretreatment with N -acetylcysteine or sodium bicarbonate
Patients with anuric end-stage renal disease (ESRD) can receive iodinated contrast with no further potential for damage as kidneys are already nonfunctional.
Iodinated contrast is toxic to soft tissues and skin.
Serious complications such as compartment syndrome or skin ulceration/necrosis are uncommon but can occur.
Patient should always be evaluated by the radiologist.
Management generally includes elevating the site of injection and applying warm or cold compresses.
Obtain surgical consultation if:
Progressive swelling or pain
Skin blistering or ulceration
Altered tissue perfusion/skin discoloration
Change in sensation distal to site of extravasation
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here