Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Autoimmune rheumatic diseases include a wide variety of illnesses, such as rheumatoid arthritis (RA) and systemic lupus erythematosus (SLE), in which changes to both the innate and adaptive immune system lead to tissue damage. The etiologies of these diseases are believed to be multifactorial. Genetic susceptibility is believed to play an important role, but is not sufficient for the development of disease. Environmental agents, infections, or drugs are possible inciting factors. For example, tobacco exposure has been identified as a strong risk factor for RA, and ultraviolet light exposure has been associated with the development of SLE. Infection may also play a role through molecular mimicry: studies have shown that Epstein-Barr virus may precede the development of SLE autoantibodies, which are amplified through epitope spreading. Certain major histocompatibility complex haplotypes have been found to be associated with increased risk of particular rheumatological diseases. Classic examples include the link between human leukocyte antigen HLA-B27 in spondyloarthropathy and HLA DRB1*044 in RA.
Autoimmune rheumatic diseases commonly affect the cardiovascular system. The endocardium, myocardium, pericardium, and conducting system may all be injured through different mechanisms by any rheumatological disease. Tissue damage may be due to direct immunologic injury (via direct antibody attack, immune complex formation, or cell-mediated destruction) to the myocardium, endocardium, or pericardium, or to the blood vessels supplying these tissues. Direct inflammatory infiltration or fibrosis frequently causes conduction system damage and various electrophysiological abnormalities. In utero conduction damage may be due to specific autoantibodies, including anti-Ro/SSA and anti-La/SSB passively transferred from maternal circulation through placental blood flow. In addition to the effects of the underlying disease process on the heart, many of the rheumatological diseases confer a higher risk of coronary artery disease (CAD), which is believed to be related to high levels of systemic inflammation.
SLE is a multisystem autoimmune disorder characterized by the production of autoantibodies, with a striking female predominance in the reproductive years (10 : 1 female-to-male) ( Fig. 67.1 ). The diagnosis of SLE requires both characteristic clinical features (e.g., malar rash, alopecia, arthritis, oral ulcers, or nephritis) in addition to supportive serologies. Diagnosis may be difficult due to the heterogeneity of the disease. Classification criteria have been developed by both the Systemic Lupus International Collaborating Clinics and the American College of Rheumatology (ACR) for research purposes, but may be useful as a reference when evaluating a patient with possible SLE. Cardiovascular diseases are one of the most important causes of morbidity and mortality in SLE patients. Autoantibodies and the development of immune complexes with complement activation are believed to be the major mechanisms in cardiovascular injury in SLE. The cardiac manifestations of lupus may affect the pericardium, myocardium, valves, conduction system, and coronary arteries.
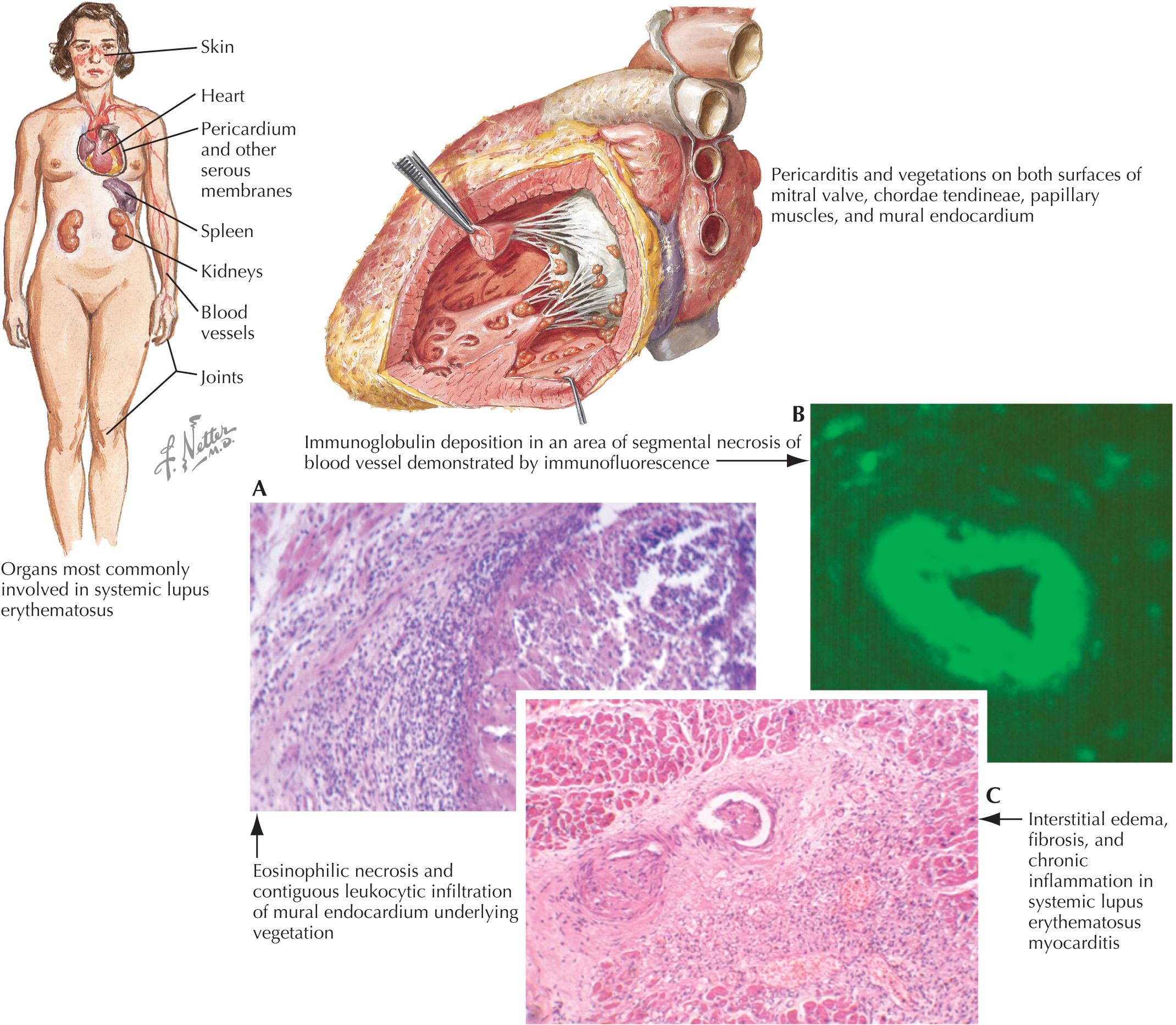
Serositis in SLE is often associated with disease flares. Pericarditis is the most common cardiac manifestation of lupus, with approximately 25% of all patients with SLE developing symptomatic pericarditis during the course of their disease. The prevalence by echocardiography and in autopsy studies is as high as 60% in individuals with SLE. Cardiac tamponade is rare and occurs in 1% to 2% of patients; however, some retrospective studies found 13% to 22% of patients with symptomatic pericarditis had tamponade. Constrictive pericarditis is even less common. Analysis of pericardial fluid has revealed decreased pH and neutrophil predominant inflammatory exudate. Pericardial biopsy has revealed mononuclear cells, fibrinous material, and immune complex deposition; however, biopsy is not necessary for diagnosis.
Myocarditis is clinically evident in <10% of patients but can cause severe systolic dysfunction. Cardiac MRI shows delayed gadolinium enhancement in lupus myocarditis, although this may be seen in myocarditis from other causes. Biopsy has a low yield and is not required for diagnosis; however, it may be of benefit in some patients. Myocarditis often develops with other organ involvement and may occur early in the course of disease. Treatment with steroids or cytotoxic agents can be lifesaving. Cardiomyopathy may be directly caused by SLE, but the most common cause is coexisting hypertension or CAD. Hydroxychloroquine, which is frequently prescribed for patients with SLE, is a rare cause of cardiomyopathy in SLE patients.
Asymptomatic valvular involvement, which is usually mitral and/or aortic, has been found in up to 70% of patients by transesophageal echocardiography. In a meta-analysis from 20 case–control studies that included 1117 SLE patients and 901 healthy control subjects from January 1, 1992 to July 31, 2013, patients with SLE who were evaluated with echocardiography had 11 times the increased risk of combined valvular alterations compared with healthy control subjects. There was a tenfold increase in mitral valve thickening, aortic valve thickening, mitral valve regurgitation, and mitral valve vegetation. Libman and Sacks first described sterile endocarditis-like lesions in SLE in 1924. These lesions most commonly involve the mitral valve, but may occur in any valve. These lesions consist of thrombotic-fibrinous clusters with proliferating endothelial cells, edema, and areas of necrosis, and have only rarely been reported to embolize. Antiphospholipid syndrome, which may accompany SLE, may be associated with valvular pathology. Acute valvular insufficiency in SLE can lead to hemodynamic instability and require surgical correction.
Conduction abnormalities, including atrioventricular (AV) block, bundle branch block, and dysautonomia, have been found in up to 10% of patients with SLE. However, most of these are not clinically significant. In pregnant patients with SLE, screening for anti-Ro/SSA and anti-La/SSB antibodies is important to identify those at risk for having an offspring with neonatal lupus. In neonatal lupus, maternal autoantibodies cross the placenta, which affects the conduction system of the developing fetus. Complete heart block may occur, which is associated with fetal myocarditis. Two percent of fetuses develop heart block in the presence of anti-Ro/SSa antibodies, which rises to 18% in subsequent pregnancies if the first child is affected. Weekly fetal echocardiography between 17 and 24 weeks of gestation is recommended. Hydroxychloroquine use may reduce the risk of recurrent neonatal lupus in subsequent pregnancies. Although there is increased risk of having a child with neonatal lupus, adults with anti-Ro/SSA and anti-La/SSB are not prone to heart block. However, one study showed statistically significant prolongation of the QTc interval to 445 ms compared with 419 ms in control subjects.
Atherosclerosis remains the most prevalent and significant mortality risk in patients with SLE. The development of premature CAD in SLE patients is believed to be related to systemic inflammation. Some studies have shown a 2- to 10-fold increased risk of myocardial infarction. Long-term steroid use also increases the risk of subclinical cardiovascular disease and independently predicts the risk of cardiovascular events. Hydroxychloroquine modulates cardiovascular risk by improving both the lipid profile and glycemic control. Two prospective lupus cohort studies have shown a 50% to 60% decreased risk for cardiovascular disease with hydroxychloroquine use.
RA is a systemic illness characterized by a symmetric, destructive polyarthritis, and occurs in 1% of most populations. The diagnosis of RA is based upon the presence of a chronic symmetric inflammatory arthritis, elevated inflammatory markers, and supportive serologies (rheumatoid factor and/or anti-CCP antibody). The 2010 ACR/European League Against Rheumatism classification criteria, developed for research purposes, uses a scoring system based on these domains. The presence of extra-articular manifestations in RA is associated with increased severity of disease and may involve the skin, eyes, lungs, hematologic system, nervous system, and the heart ( Fig. 67.2 ). Cardiovascular disease causes almost one-half of all deaths in patients with RA. Manifestations include pericarditis, cardiomyopathy and/or myocarditis, cardiac amyloidosis, coronary vasculitis, arrhythmia, valvular disease, congestive heart failure (CHF), and CAD. The most frequent cardiac manifestations in RA patients are pericarditis and valvular heart disease. Pericarditis is seen in between 30% and 50% of autopsy cases and up to 30% by echocardiography; however, the clinical prevalence is lower. Pericarditis is more common in male, seropositive patients with nodular RA. Most patients develop pericarditis after the onset of arthritis, but rarely, it may be the initial manifestation of RA. Pericardial fluid is exudative, serosanguinous, or hemorrhagic with low glucose, neutrophilic predominance, and low pH. Cholesterol crystals may be seen in persistent effusions. Adhesions and loculations are common, often making pericardiocentesis difficult. A significant proportion of patients with clinical pericarditis have constriction or tamponade with a grave prognosis. These patients, under some circumstances, may benefit from surgical pericardiectomy.
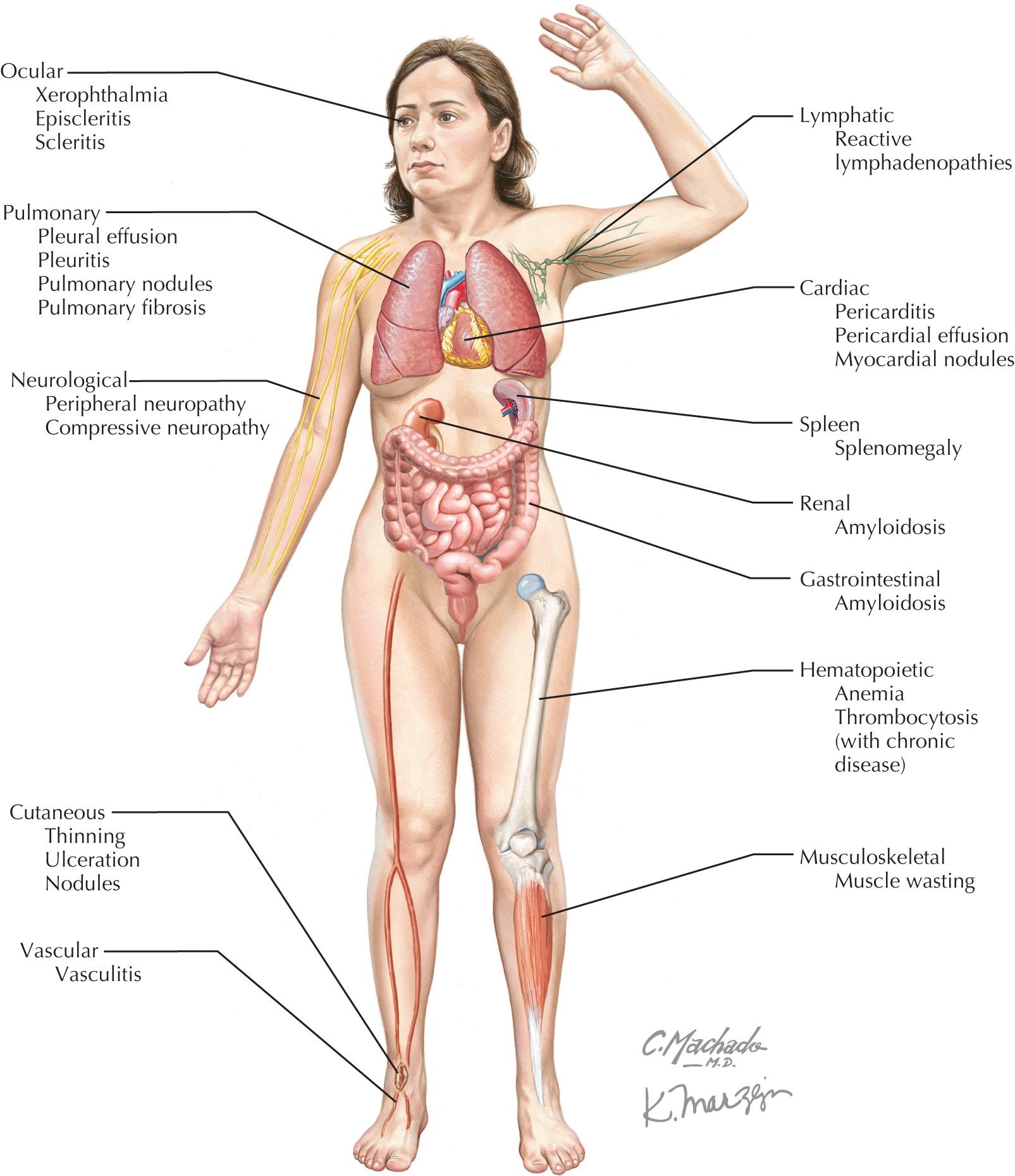
Valvular lesions occur frequently (up to 70%); however, they are rarely symptomatic in RA. Pathologically, endocardial lesions can be caused by fibrosis, nonspecific inflammation, or less frequently, rheumatoid granulomas. Mitral or aortic valve insufficiency are the most common, with 30% to 80% and 9% to 33% frequency in a small case series, respectively. Myocarditis is rarely clinically evident but can be associated with arrhythmias. Cardiomyopathy secondary to RA may be from focal, nonspecific diffuse necrotizing or granulomatous myocarditis. In a small case series of 30 RA patients, cardiomyopathy was found in 37% by echocardiography. Restrictive cardiomyopathy from cardiac amyloidosis may rarely occur in RA. It should be noted that tumor necrosis factor (TNF)-α blockers, which are often used for treatment of RA and other rheumatic diseases should be used with caution in patients with known New York Heart Association functional class III or IV heart failure due to the risk of worsening CHF.
Vasculitis of coronary vessels has been described, although the clinical significance is unknown. It has been seen in up to 20% in postmortem studies. Increased cardiovascular risk occurs in RA independent of traditional risk factors and has been attributed to ongoing inflammation. The prevalence of cardiovascular disease in RA is increased to an extent that is comparable to that of the prevalence in the diabetic population. Arrhythmias may occur due to ischemia, as well as conduction abnormalities due to nodules, amyloidosis, and CHF. It has been hypothesized that increased sympathetic activity plays a role in development of ventricular tachyarrhythmias.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here