Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Children with congenital heart disease (CHD) have structural defects of the heart and/or great vessels that are present before birth. Defects range from relatively simple lesions, which neither induce symptoms nor need therapy, to complex life-threatening lesions, which require surgery in the neonatal period.
CHD is the most common birth defect. Recent prevalence estimates for CHD range from 6 to 10 per 1000 live births. Approximately 40,000 infants are born with a congenital heart defect each year in the United States.
One of every four infants with CHD has critical CHD (i.e., a defect that requires either a surgical or a transcatheter procedure within the first year of life for survival). Ductal-dependent heart defects, which require the ductus arteriosus to remain patent after birth to ensure survival, are examples of critical lesions.
Before birth, the fetus is dependent on the utero-placental unit for survival. The relatively oxygen-rich blood from the placenta enters the inferior vena cava via the umbilical vein and ductus venosus. Preferential streaming of blood occurs in the right atrium between the blood returning via the superior vena cava (relatively oxygen poor) and that returning from the inferior vena cava ( ). The more highly saturated blood from the inferior vena cava crosses the foramen ovale to the left side of the heart, facilitating delivery of blood with relatively high oxygen content to the fetal myocardium and brain ( ). Deoxygenated fetal blood returning from the superior vena cava travels through the right ventricle, across the ductus arteriosus, and down the descending aorta to the placenta, where oxygen and carbon dioxide transfer occurs via simple diffusion. Both the fetal right and left ventricles are responsible for blood flow to the systemic circuit and placenta. Because the resistance in the fetal pulmonary vasculature is high, less than 15% of right ventricular output is delivered to the lungs ( ). Thus the parallel fetal circulatory system promotes efficient oxygen delivery in a relatively hypoxic environment ( Fig. 16.1 A).
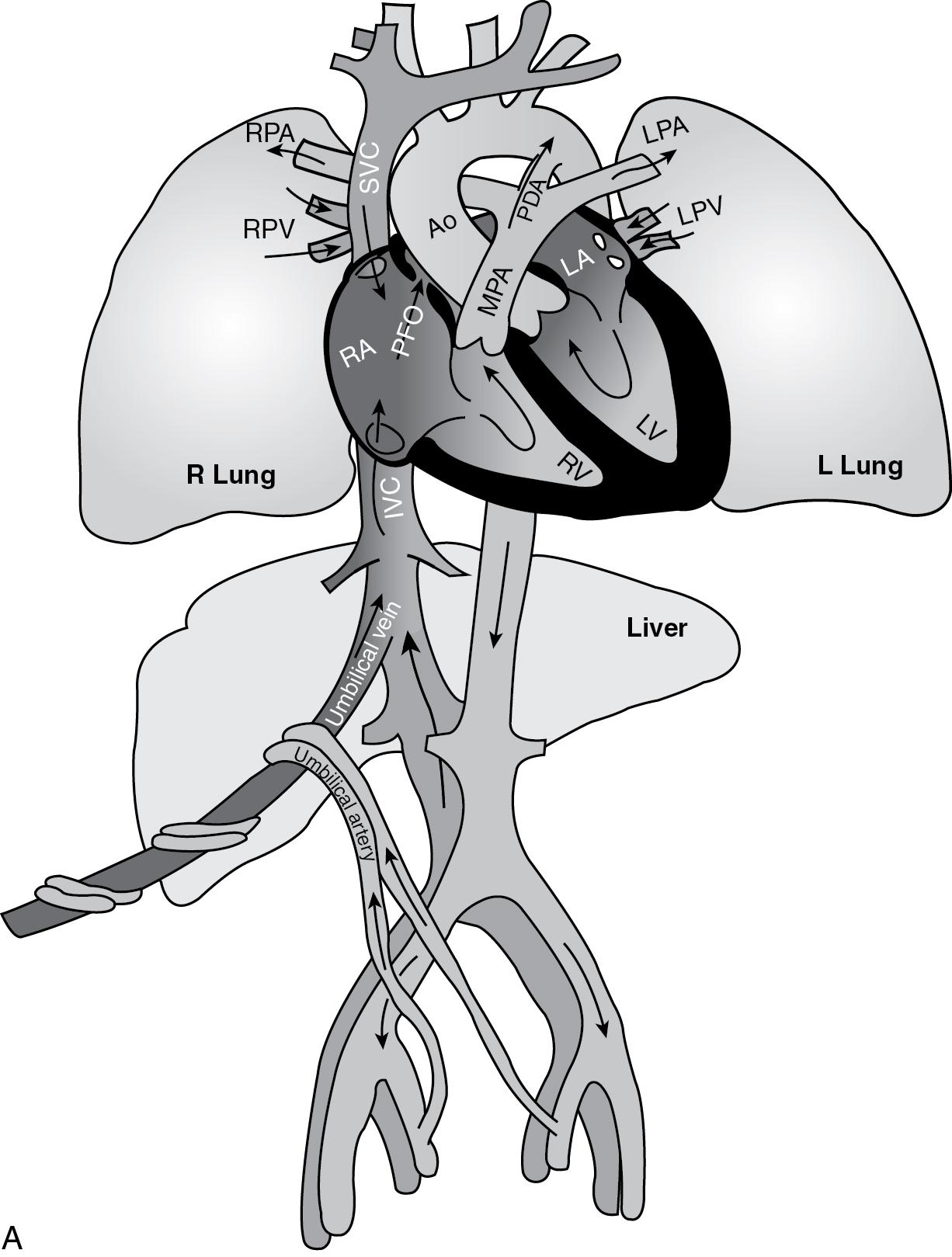
The fetal circulation is forgiving to neonates with even the most severe forms of CHD. Intra- and extracardiac shunts allow fetal circulatory adaptations to abnormal heart anatomy. For example, in neonates with severe obstruction to either ventricular outflow tract, diversion of flow into the other ventricle and great vessel occurs across the foramen ovale and the ductus arteriosus.
A 3.2 kg infant is born via cesarean section (due to nonprogression of labor) at 39 weeks’ gestation to a primigravida woman with an unremarkable medical and obstetric history. The infant is breathing comfortably and appears pink. There are no risk factors for infection. At 5 minutes of life, the preductal saturation value in room air is 90% and the postductal value is 82%.
What is the best course of action for this infant?
Continued observation
Consultation with pediatric cardiology
Stat echocardiogram
Four extremity blood pressures
Arterial blood gas determination from the left radial artery.
A
Most of the circulatory changes that happen in the transition from intra- to extrauterine life occur in the first few moments after birth, with additional circulatory adjustments occurring over a period of several weeks. The primary events that trigger the alteration in blood flow patterns are removal of the low resistance placental circuit and the establishment of alveolar ventilation ( ). With establishment of alveolar gas volume, there is a substantial decline in pulmonary vascular resistance and a several-fold increase in pulmonary blood flow. A rise in left atrial pressure results from an increase in pulmonary venous return and allows closure of the foramen ovale, abolishing the atrial level shunt. The higher oxygen tension in the blood initiates postnatal closure of the ductus arteriosus, establishes complete separation of pulmonary and systemic blood flows, and leads to a circulation in series ( Fig. 16.1 B). In full-term infants, functional closure of the ductus arteriosus is initiated within the first hours and days following birth, and anatomic closure follows.
The switch from a parallel fetal circulation to a transitional circulation in series results in a slow rise in hemoglobin oxygen saturation in the first few minutes after birth. The median preductal oxygen saturation in healthy term newborn babies is around 90% at 5 minutes of life and increases to 98% by 15 minutes. Postductal oxygen saturation is significantly lower than the preductal oxygen saturation in the first 15 minutes of life, with oxygen saturation gradient narrowing over time. Babies born by cesarean section have lower pre- and postductal oxygen saturations in the first 15 minutes of life compared with those born vaginally. The neonate described in Case Study 1 is exhibiting a normal postnatal transition and therefore warrants only continued observation.
Postnatal closure of fetal shunts can be life threatening in babies with ductal-dependent CHD. Closure of the ductus arteriosus can lead to hypoxemia and cyanosis when there is severe anatomic obstruction to pulmonary blood flow and decreased perfusion to vital organs when there is severe anatomic obstruction to systemic blood flow. The ductus arteriosus with rare exceptions is patent at birth and hence these signs will rarely be noted in the delivery room during transition.
The transitional period can be life threatening in babies with d-transposition of the great arteries with an intact ventricular septum (d-TGA/IVS). Postnatal closure of the foramen ovale can cause severe hypoxemia in babies with this condition. In this lesion, the aorta arises from the right ventricle and the pulmonary artery arises from the left ventricle. After birth, the circulation remains in parallel and severe hypoxemia may result if the shunt at the atrial level is abolished. Only adequate mixing at the atrial level will permit a stable transition. Transition to extrauterine life may also be difficult in babies with hypoplastic left heart syndrome (HLHS) with mitral atresia and an intact or restrictive atrial septum. Because of restricted egress of pulmonary venous return, pulmonary edema quickly ensues, and hence these babies are severely hypoxemic from birth with signs of inadequate cardiac output. However, with the few exceptions described earlier, most babies with CHD should transition normally.
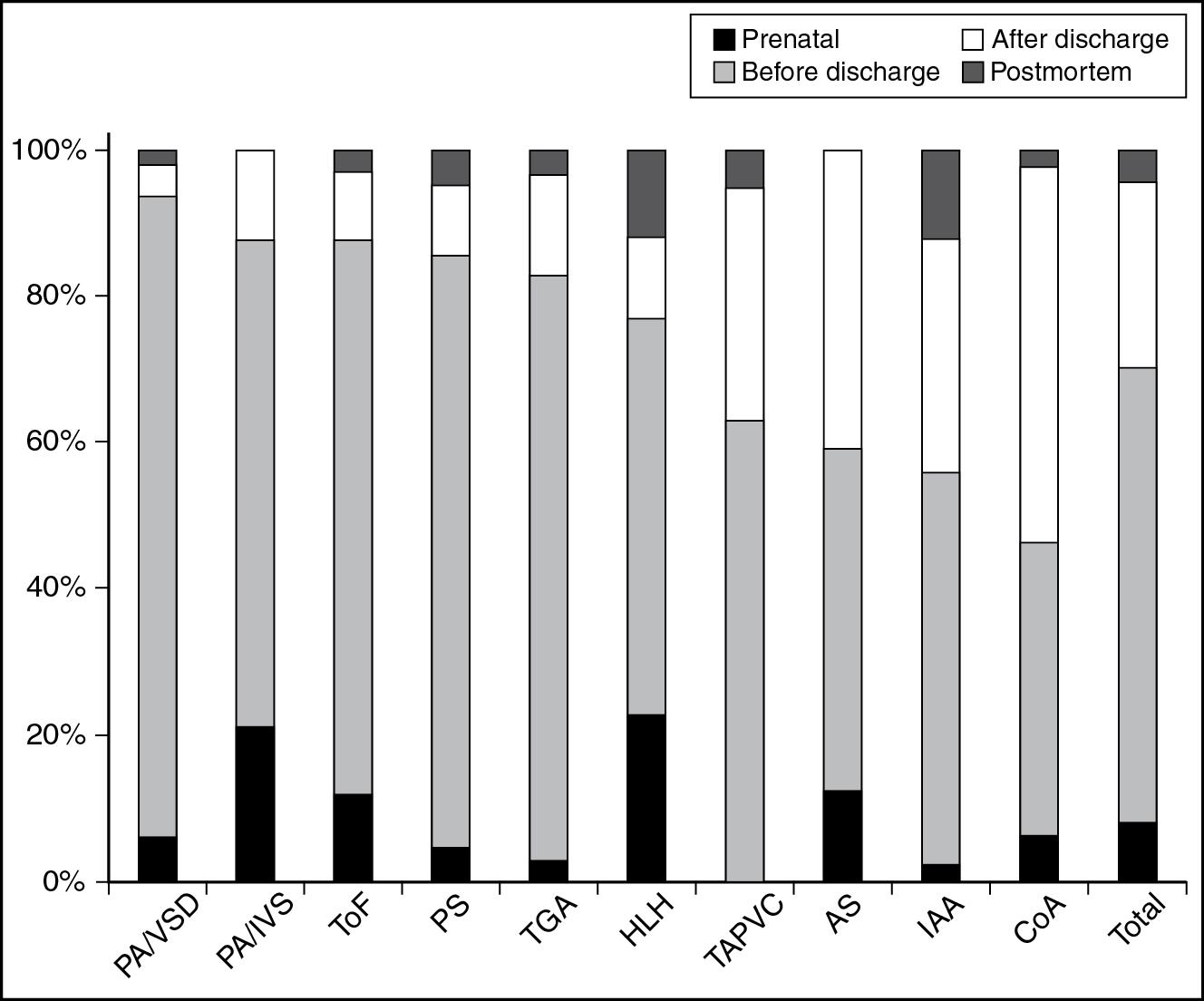
Although antenatal diagnosis of CHD is increasing, a significant proportion of babies with CHD are not diagnosed before birth. Postnatal diagnosis of CHD in the delivery room or the newborn nursery is possible only if signs of CHD manifest during the hospital stay or if there is a universal screening protocol utilizing pulse oximetry. In a review of 20-year trends in the diagnosis of life-threatening cardiovascular malformations, 62% of babies with such lesions presented before discharge from the hospital, 25% of babies with critical CHD were diagnosed after discharge from the nursery, and 5% were diagnosed at autopsy ( Fig. 16.2 ) ( ). Babies with left sided obstructive lesions such as coarctation of the aorta were more likely to be diagnosed after discharge from the nursery ( Fig. 16.2 ), whereas those who had cyanotic CHD were more likely to be identified while still in the nursery.
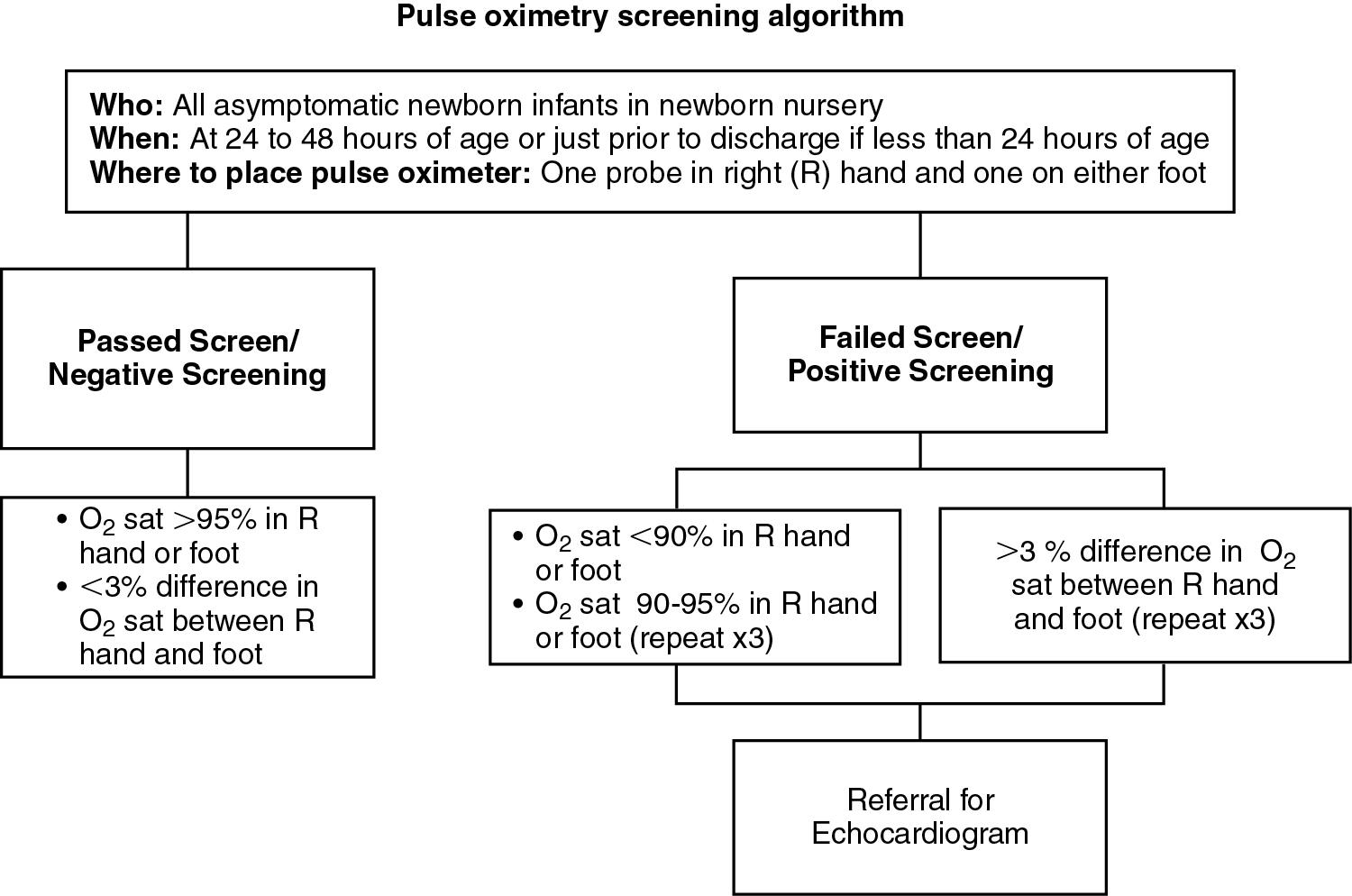
Critical CHD is difficult to uncover in asymptomatic newborn infants because several congenital heart defects do not produce visible central cyanosis despite severe hypoxemia. Screening by pulse oximetry provides an opportunity to detect clinically silent hypoxemia in critical CHD. In September 2011, the U.S. Department of Health and Human Services recommended that all newborns be screened for critical CHD before discharge from the newborn nursery using pulse oximetry. This recommendation has been endorsed by several national organizations, including the American Academy of Pediatrics. Pulse oximetry has now been included in the Recommended Uniform Screening Panel for newborns across several states. The proposed newborn screening strategy is described in Fig. 16.3 .
Screening by pulse oximetry is highly specific for detection of critical CHD (99.9%) and has moderate sensitivity (70%). The false positive rate is very low (0.035%) when screening occurs after 24 hours of age ( , ). It is important to note that although pulse oximetry is generally a reliable screening tool, it can miss lesions, particularly those that involve obstruction to systemic blood flow.
Neonates with critical CHD may escape detection at all three stages of screening (i.e., antenatal ultrasound, routine neonatal examination in the nursery, and pulse oximetry). A high index of suspicion, along with prompt and timely recognition of babies with critical CHD, improves prognosis.
Most babies with critical CHD follow an ordinary transition to extrauterine life, and perinatal history is often unremarkable. The absence of important clinical information that would foster the consideration of an alternative diagnosis is a notable feature of CHD. For example, babies with severely obstructed totally anomalous pulmonary venous connection commonly present with respiratory distress and cyanosis within the first 24 hours of life. However, respiratory distress is a common symptom in neonates and has several etiologies. Fortunately, in most of these other disorders, the etiology is apparent from the history. Respiratory distress caused by surfactant deficiency is seen in preterm or late-preterm infants and is rarely encountered in infants born at term. Respiratory distress caused by an invasive bacterial infection is likely if there is a maternal history of prolonged rupture of membranes, a history of chorioamnionitis or maternal vaginal carriage of group B Streptococcus . In the presence of such historical data, a diagnosis of the usual etiologies of respiratory distress (e.g., sepsis or surfactant deficiency) is easily made. It is the absence of such relevant historical information that makes a diagnosis of CHD more likely.
Babies with cyanotic CHD usually present with cyanosis or cyanotic “spells.” Lesions that cause obstruction to pulmonary blood flow or poor mixing often present during the initial hospital stay in the nursery. In these patients, cyanosis may not be noticed at birth because the ductus arteriosus is still patent. Parents or medical caretakers may initially note transient cyanosis during crying or feeding. As the ductus arteriosus begins to close, cyanosis becomes more apparent and persistent. Most importantly, despite the cyanosis, a history of respiratory distress with dyspnea is usually not elicited.
Parents of babies with left sided obstructive lesions may report an increase in the rate of breathing, irritability, and progressive difficulty in feeding. These symptoms emerge as systemic blood flow becomes compromised with closure of the ductus arteriosus. Infants usually present after nursery discharge, typically within the first 2 weeks of life. As circulatory failure ensues, patients may present to the emergency room in extremis.
Babies with large left-to-right shunts manifest symptoms of heart failure (e.g., rapid respirations, diaphoresis, feeding difficulties). These symptoms are subtle at first, usually appear by 4 to 6 weeks of age, and worsen over time.
Anthropometric measurements: Weight, height, and head circumference should be measured. A small head circumference is noted in some babies with CHD, such as hypoplastic left heart syndrome. In babies with congestive cardiac failure who are several weeks old, comparison of current weight to birth weight may uncover inadequate interim growth.
Vital signs: Tachycardia (normal heart rate 120–160 beats/minute) in babies with CHD may be reflective of depressed ventricular function. An increased respiratory rate (normal 40–60 breaths/minute) may be caused by pulmonary edema, excessive pulmonary blood flow, or metabolic acidosis. Blood pressure should be measured in all four extremities. Normally, the measured blood pressure in the lower extremities is a little higher than that measured in the upper extremities. A blood pressure gradient of greater than 10 to 20 mm Hg between the right arm and lower extremities may indicate juxta-ductal coarctation of the aorta or interruption of the aortic arch. Systemic hemoglobin oxygen saturation should be measured using pulse oximetry. Ideally, both preductal (right hand) and postductal (any foot) hemoglobin oxygen saturation values should be recorded simultaneously. Normally, both preductal and postductal saturations are above 95% with minimal difference in measurements. Low (<95%) preductal or postductal saturation may be suggestive of cyanotic CHD especially in the absence of respiratory symptoms.
General examination: CHD often has an underlying genetic etiology with recognizable patterns indicative of a chromosomal abnormality or syndrome. Underlying hypoxemia with central cyanosis is best recognized in the buccal mucosa, lips, and tongue. Cool extremities, feeble pulses, mottled skin, and prolonged capillary refill time indicate poor cardiac output and systemic perfusion. Peripheral pulses are globally diminished when ventricular function is depressed. Disparity in both pulses and blood pressure between the upper and lower extremities suggests juxtaductal coarctation of the aorta or interruption of the aortic arch distal to the origin of the left subclavian artery.
Cardiovascular examination: The site of precordial activity should be noted. Dextrocardia should be suspected if the precordial impulse or activity is noted over the right hemithorax rather than the left. Parasternal impulse rather than an apical impulse is normal in neonates and signifies right ventricular dominance. A prominent parasternal impulse is noted with right ventricular pressure overload (right ventricular outflow tract obstructive lesions, pulmonary hypertension, and d-TGA). A diminished parasternal impulse is noted in right ventricular inflow obstruction (e.g., tricuspid atresia or tricuspid stenosis with hypoplasia of the right ventricle). Left ventricular volume overload in infants with left-to-right shunts (large ventricular septal defect, large patent ductus arteriosus) causes a prominent and hyperdynamic apical impulse.
Abnormalities of the first heart sound are rarely appreciated in newborns. Split S 1 may be seen in infants with Ebstein’s anomaly. Soon after birth, when the pulmonary vascular resistance is still elevated, closure of both aortic and pulmonary valves occurs almost simultaneously. Hence, a single S 2 is commonly heard. As the pulmonary vascular resistance falls, the pulmonary valve closes after the aortic valve and a split S 2 becomes apparent. A rapid heart rate makes it difficult to appreciate physiologic splitting of the second heart sound in newborn infants. Fixed splitting of the second heart sound in newborns is heard when pulmonary blood flow is excessive, as in unobstructed total or partial anomalous pulmonary venous connection. Wide, fixed splitting of the second heart sound occurs in atrial septal defects but is not typically heard in the newborn period. A split second heart sound is also appreciated when there is right ventricular obstruction or conduction delay. A single second heart sound is appreciated when there is only one semilunar valve, as in pulmonary or aortic atresia or truncus arteriosus. A loud pulmonic component is heard in pulmonary hypertension, whereas a soft P 2 may suggest pulmonary stenosis. Stenosis of semilunar valves, a bicuspid aortic valve, or a dysplastic truncal valve (truncus arteriosus) may produce additional sounds or ejection clicks. A midsystolic click is sometimes appreciated in Ebstein’s anomaly or with mitral valve prolapse.
Murmurs are often associated with structural abnormalities of the heart. Quite often, the murmurs are innocent and bear little clinical significance. It may be difficult for the inexperienced practitioner to distinguish innocent from pathologic murmurs. A systematic approach to evaluation may assist in identifying an underlying anatomic malformation causing the cardiac murmur. The intensity, quality, location, radiation, duration, and timing of the murmur should be assessed. Murmurs can occur during systole, diastole, or continuously during the entire cardiac cycle. Timing and duration of murmurs during the different phases of systole or diastole should be noted. A harsh murmur of at least grade 3 intensity—best heard in the left lower sternal border and occupying the whole duration of systole—is likely to be secondary to a ventricular septal defect. A harsh 3- to 4-grade intensity murmur with a crescendo–decrescendo configuration, best heard in the upper right sternal border and radiating to the carotids, may suggest stenosis of the aortic valve. A murmur heard continuously across systole and diastole and best appreciated in the left upper sternal border is probably due to a widely patent ductus arteriosus. Innocent murmurs are common in the newborn period. Innocent murmurs are softer, occur in systole, and do not have accompanying symptoms. It is extremely important to note that the absence of murmur does not rule out CHD.
Pulmonary examination: Respiratory rate, effort, quality of breath sounds, and the presence of adventitious sounds should be assessed. Babies with significant left-to-right shunts and increased pulmonary blood flow are tachypneic and show an increased respiratory effort. Most babies with cyanotic CHD exhibit normal respiratory activity despite low oxygen saturation. A normal respiratory examination in the presence of cyanosis strongly suggests CHD.
Abdominal examination: Location and size of the liver should be assessed. A left sided liver is present in situs inversus and a midline liver is often noted in heterotaxy syndromes. Hepatomegaly suggests hepatic congestion and right ventricular dysfunction or volume overload. Neonates with hepatic arteriovenous malformation and high output cardiac failure may have a bruit over the liver.
Chest radiograph: Characteristic chest radiographs may be useful in the diagnosis of some CHD. However, in most cases of CHD, chest radiographs are rarely diagnostic.
Electrocardiogram: A 12-lead electrocardiogram (ECG) often reveals typical ECG findings in some types of CHD. However, a normal ECG should not rule out the presence of a serious underlying CHD.
Hyperoxia test: Hyperoxia test is helpful in differentiating hypoxemia caused by structural heart disease from that caused by lung disease. Arterial blood gas is obtained at baseline and after exposure to 100% oxygen under an oxyhood for at least 15 minutes. Babies with structural heart disease do not show a significant increase in Pa o 2 (remains less than 150 mm Hg) after exposure to 100% oxygen.
Echocardiogram: An immediate cardiology consultation should be requested when CHD is suspected. Echocardiogram is often the only definitive procedure required to confirm a diagnosis of structural heart disease.
Blood tests: Baseline blood work includes complete blood count to help rule out infection, serum chemistry to assess for electrolyte and renal function abnormalities, and an arterial blood gas with lactate level to assess gas exchange and the presence or absence of lactic acidosis.
Airway, breathing, and circulation must be assessed in patients with signs consistent with pulmonary or cardiac disease. In patients presenting with severe hypoxia and increased respiratory effort, intubation and mechanical ventilation may assist in improving gas exchange. Circulation cannot be reestablished without a patent ductus arteriosus in ductal-dependent lesions. Prostaglandin E1 (PGE-1) infusion can reopen a closing ductus arteriosus and must be initiated as soon as ductal-dependent CHD is suspected. It is not necessary to wait for an echocardiogram for confirmatory evidence before initiating a PGE-1 infusion except in cases of totally anomalous pulmonary venous connection where an infusion of PGE-1 has the potential to increase pulmonary edema, thereby worsening oxygenation. Reopening the ductus arteriosus will improve oxygen saturation in patients with ductal-dependent pulmonary circulation, and systemic perfusion will improve in patients with ductal-dependent systemic circulation after initiation of a PGE-1 infusion. Correction of hypovolemia and initiation of cardiotonic drugs to enhance inotropy may be required in patients presenting in cardiogenic shock. Metabolic derangements including hypoglycemia and hypocalcemia should be corrected. Early transfer to a cardiac center is important.
The three major presenting features of CHD in the newborn period are central cyanosis, decreased perfusion to the body, and tachypnea. The predominant clinical manifestation depends on the type of CHD. The following case studies provide examples of typical presentations of CHD.
A 32-year old gravida 2, para 1 woman delivers a male infant at 39 weeks by elective cesarean section. Routine prenatal laboratory tests are unremarkable. Normal fetal anatomy was noted on an 18-week screening ultrasound.
A term male infant with a vigorous cry is handed to you. Apgar scores of 8 and 8 at 1 and 5 minutes respectively are assigned. You provide free flow oxygen at 10 minutes of life for central cyanosis. There is minimal improvement in skin color. At 20 minutes of life, you note that the baby’s face, oral mucosa, and tongue continue to have a bluish hue but the abdomen and both legs appear pinker. The baby is breathing comfortably (respiratory rate is 50 breaths/minute) with neither grunting nor subcostal retractions, that is, without dyspnea. The lungs are clear to auscultation with equal air entry. The precordium is quiet, the heart rate and rhythm are normal, and there are no murmurs heard. The second heart sound is loud. Peripheral pulses are normal. There is no hepatomegaly. A chest radiograph shows well-aerated lung fields with no focal pathology, a normal heart size, and a left aortic arch.
Indicate whether the following statements are true (T) or false (F):
Peripheral cyanosis signals underlying arterial hypoxemia.
Clinical recognition of central cyanosis is easier in neonates with polycythemia than in those with a normal hemoglobin concentration.
This infant’s cyanosis is most likely related to lung disease.
What is the differential diagnosis of central cyanosis in a newborn infant?
A: F; B: T; C: F
See Fig. 16.4 .
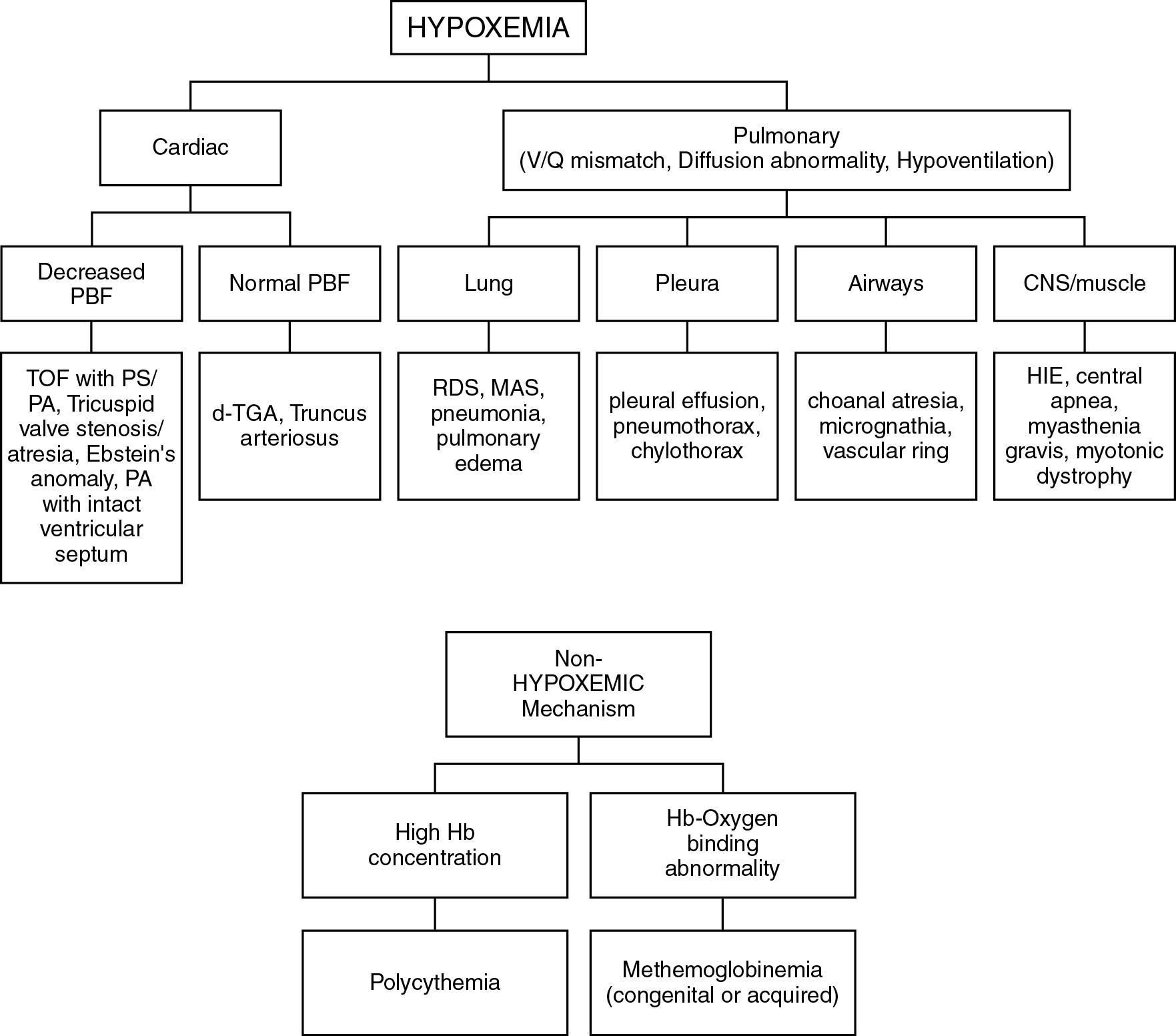
Because the normal systemic arterial oxygen saturation in fetal life is around 60% to 65%, generalized cyanosis at birth is a normal finding but is transient. In most babies who are born at term, skin color improves rapidly as alveolar ventilation is established. Persistent cyanosis is abnormal. Cyanosis signals the presence of elevated levels of deoxyhemoglobin in the underlying capillaries. At least 3 to 5 g/dL of deoxyhemoglobin should be present in the microcirculation for cyanosis to be apparent. Cyanosis may not be recognized if deoxyhemoglobin levels are less than this critical amount. For example, let us assume that this infant has a total hemoglobin concentration of 18 g/dL and an oxygen saturation of 83%. The calculated oxyhemoglobin concentration for this infant would be 15 g/dL (0.83 × 18 g/dL) and the calculated deoxyhemoglobin level would be 3 g/dL. At this absolute concentration of deoxyhemoglobin, cyanosis is likely to be apparent. In the same example, if the absolute concentration of deoxyhemoglobin were 2 g/dL, this infant would not appear cyanotic despite an abnormal hemoglobin oxygen saturation of 89% (oxyhemoglobin concentration = 16/18 g/dL or 89%).
It is important to note that hemoglobin concentration determines the saturation level at which cyanosis is visible and detected ( Fig. 16.5 ). Cyanosis may not be appreciated in newborn infants with normal hemoglobin levels unless oxygen saturation falls below 85%. Cyanosis is identified at a higher level of hemoglobin saturation in newborns with polycythemia. For example, if the hemoglobin concentration were 22 grams/dL, cyanosis would be detected at a saturation of 86% (22 − 3 grams/dL = 19 grams/dL, 19/22 = 86%). Cyanosis is more difficult to detect in patients with anemia. In anemic infants, the hemoglobin saturation has to fall profoundly before cyanosis is visible. For example, with a hemoglobin concentration of 6 grams/dL, cyanosis would be noticeable only if the hemoglobin saturation fell below 50% (6 − 3 grams/dL = 3 grams/dL, 3/6 = 50%).
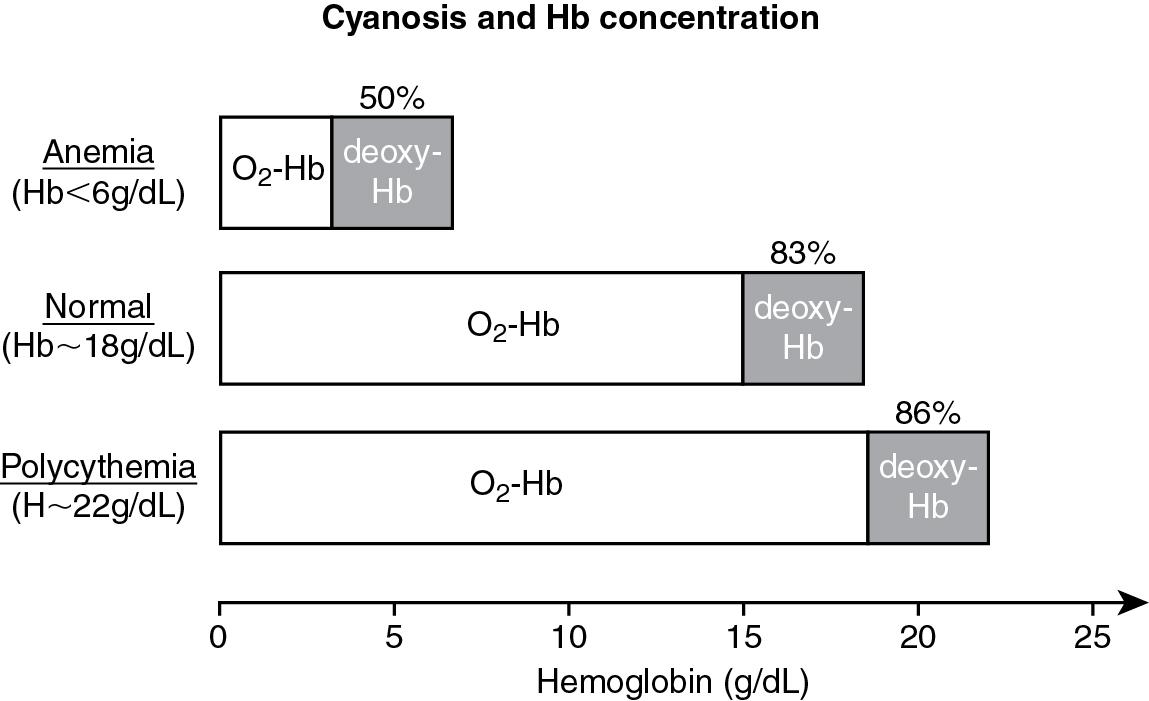
Other factors that affect detection of cyanosis include skin pigmentation, fetal hemoglobin concentration, and conditions that influence the position of the hemoglobin-oxygen dissociation curve.
In peripheral cyanosis, cyanosis is restricted to the periphery (e.g., nail beds, hands, and feet). Sluggish peripheral circulation associated with hypothermia, vasomotor instability, or polycythemia causes increased oxygen extraction by the tissues and elevated levels of deoxyhemoglobin in the microcirculation. However, in peripheral cyanosis, oxygen tension and saturation of hemoglobin in the systemic circulation are normal. Peripheral cyanosis is a common finding in newborn infants. It is usually innocuous unless it is associated with low cardiac output states.
Central cyanosis is more ominous and never a normal finding. It is caused by elevated levels of deoxyhemoglobin and reduced levels of oxyhemoglobin in the circulation. Unlike peripheral cyanosis, central cyanosis is most often indicative of underlying hypoxemia. Hypoxemia results from two underlying pathophysiologic mechanisms: (1) reduced oxygen tension in pulmonary venous blood (and thereby in the aorta) or (2) extrapulmonary right-to-left shunting of systemic venous blood with low Pa o 2 into the systemic arterial circuit.
Conditions that lead to ventilation/perfusion mismatch and intrapulmonary right-to-left shunting or those that result in impairment of oxygen diffusion across the alveolar epithelium lead to decreased oxygen tension in pulmonary venous blood. Etiologies for low oxygen tension in pulmonary veins include pulmonary (respiratory distress syndrome, meconium aspiration, pneumonia), airway abnormalities (choanal atresia), neurologic, neuromuscular or muscular (myotonic dystrophy), and skeletal anomalies (severe scoliosis, thoracic dystrophies). Extrapulmonary right-to-left shunting occurs in the setting of cyanotic CHD or persistent pulmonary hypertension. Other pathophysiologic mechanisms that may cause central cyanosis but that are not associated with hypoxemia include polycythemia (excessive hemoglobin concentration and high levels of circulating deoxyhemoglobin) and abnormalities of hemoglobin-oxygen binding (congenital or acquired methemoglobinemia). Fig. 16.4 lists conditions that cause central cyanosis in newborn infants.
It is possible to distinguish cyanosis caused by CHD from other conditions that cause hypoxemia and systemic arterial hemoglobin oxygen desaturation. Diseases involving the lung parenchyma (e.g., pneumonia, meconium aspiration) or involving the pleural space (e.g., effusion or pneumothorax) commonly affect gas exchange and oxygenation ( Fig. 16.4 ). In these conditions, other clinical features suggestive of respiratory disease (e.g., nasal flaring, grunting, dyspnea) accompany cyanosis, as does hypercarbia. Infants born with congenital neurologic, muscular, or neuromuscular conditions may present with cyanosis and hypercarbia caused by decreased respiratory effort from hypotonia. In neonates with cyanotic CHD, cyanosis is often the sole clinical feature. The absence of respiratory distress and hypercarbia in a cyanotic infant should raise a strong suspicion of CHD.
Hypoxemia caused by extrapulmonary right-to-left shunting in CHD can be distinguished from that caused by pulmonary venous desaturation by employing the hyperoxia test.
The primary determinant of hemoglobin-oxygen association is the partial pressure of oxygen in the blood ( Fig. 16.6 ). Oxygen binds readily to hemoglobin in the lungs where the partial pressure of oxygen is high and dissociates from hemoglobin in tissues where the partial pressure is much lower. Because of the sigmoidal properties of the hemoglobin-oxygen dissociation curve, increasing partial pressure of oxygen beyond 100 mm Hg does not produce significantly greater binding of oxygen to hemoglobin; hence, there is negligible increase in hemoglobin oxygen saturation and oxygen content when alveolar partial pressure of oxygen is increased beyond 100 mm Hg. The hyperoxia test uses the sigmoidal properties of the hemoglobin-oxygen dissociation curve to differentiate hypoxemia caused by intrinsic lung disease from that caused by cyanotic CHD. The Pa o 2 from an arterial blood gas is measured at baseline and after administering 100% oxygen for at least 10 to 15 minutes. The partial pressure of oxygen in the alveolus, pulmonary vein, and aorta is reduced in babies with parenchymal lung disease. An increase in inspired oxygen concentration to 100% increases alveolar partial pressure of oxygen, which in turn results in a higher pulmonary vein oxygen tension and a higher oxygen tension and saturation in the aorta. Typically, in babies with intrinsic lung disease, the Pa o 2 increases to greater than 150 mm Hg after exposure to 100% oxygen for 10 to 15 minutes.
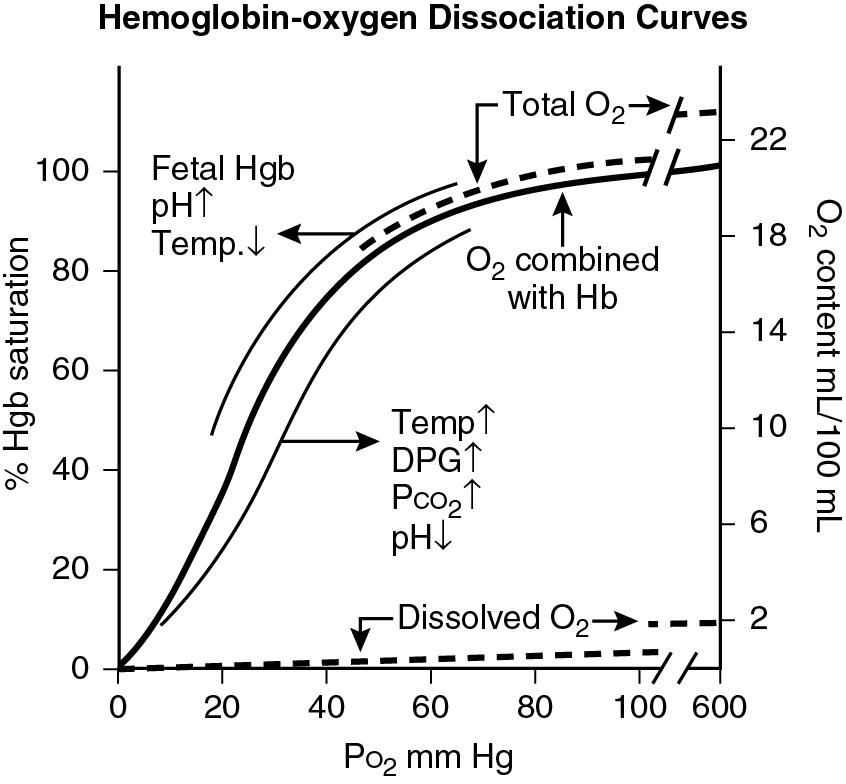
Babies with cyanotic CHD are hypoxemic primarily due to right-to-left shunting of systemic venous blood into the systemic arterial circuit. The systemic arterial circuit therefore has an admixture of pulmonary venous blood (with Pa o 2 of 100 mm Hg and oxygen saturation of 100%) and systemic venous blood (with Pa o 2 of approximately 40 mm Hg and oxygen saturation of approximately 70%). Administering 100% oxygen to patients with cyanotic CHD and no lung disease will increase alveolar and pulmonary venous partial pressure of oxygen (to above 600 mm Hg) but will not increase the oxygen saturation of pulmonary venous blood. As mentioned previously, due to the sigmoidal properties of the hemoglobin-oxygen dissociation curve, increasing alveolar partial pressure of oxygen beyond 100 mm Hg does not cause a significantly greater binding of oxygen to hemoglobin. As noted in Fig. 16.6 , neither oxygen saturation nor the oxygen content at Pa o 2 of 100 mm Hg and at 600 mm Hg is remarkably different. Hence, as administering 100% oxygen does not significantly alter the oxygen saturation and content of the pulmonary venous blood, the net oxygen saturation and Pa o 2 in the arterial circuit in babies with cyanotic CHD is not changed significantly. Typically, the Pa o 2 in babies with cyanotic CHD remains below 100 mm Hg despite exposure to 100% oxygen. The one exception to the rule is persistent fetal circulation where Pa o 2 may remain below 100 mm Hg despite a normal intracardiac anatomy.
Examples of congenital heart lesions likely to present with central cyanosis include those that involve restriction of blood flow to the lungs, e.g., severe pulmonary valve stenosis or atresia, and tetralogy of Fallot with severe valvar and/or subvalvar pulmonary stenosis. In these lesions, a combination of right-to-left shunting of desaturated blood across a patent foramen ovale into the left side of the heart and into the aorta and decreased blood flow into the lungs cause arterial hypoxemia and central cyanosis. Typically, these defects are diagnosed when constriction of the ductus arteriosus causes further decrease in pulmonary blood flow. Defects without restriction to pulmonary blood flow but characterized by the admixture of desaturated blood in the aorta may also present with central cyanosis. These include d-TGA and truncus arteriosus, where there is a single arterial trunk. Table 16.1 lists congenital heart lesions likely to present with central cyanosis.
| A. Right Ventricular Inflow/Outflow Abnormality | Defect |
| Tricuspid valve stenosis/atresia | Stenosis/atresia of tricuspid valve |
| Ebstein’s anomaly | Inferior displacement of tricuspid valve |
| Pulmonary atresia with intact ventricular septum | Atresia of pulmonary valve |
| Pulmonary stenosis | Subvalvar, valvar, or supravalvar obstruction to pulmonary blood flow |
| Tetralogy of Fallot with pulmonary stenosis or atresia | Anterior malalignment of the conal septum leading to variable degree of obstruction to pulmonary blood flow, overriding aorta, ventricular septal defect, and right ventricular hypertrophy |
| B. Without Right Ventricular Inflow/Outflow Abnormality | Defect |
| Transposition of the great arteries | Ventriculoarterial discordance: aorta arises from the right ventricle, pulmonary artery arises from the left ventricle |
| Truncus arteriosus | Single arterial trunk arises from the ventricles with variable origins of the pulmonary arteries from the trunk |
| Totally anomalous pulmonary venous connection with obstruction | Abnormal connection of all the pulmonary veins to the systemic venous system |
Indicate whether the following statement is true (T) or false (F):
Pulse oximetry sensor on the left hand is the ideal location to measure preductal hemoglobin oxygen saturation in infants with a left aortic arch and normal head vessel branching.
False. Preductal and postductal hemoglobin oxygen saturation measurements are critical in the evaluation of a neonate for CHD. A pulse oximetry sensor placed on the right hand in an infant with a presumed left aortic arch and a normal branching pattern of the head vessels measures preductal hemoglobin oxygen saturation; a sensor on either leg measures postductal hemoglobin oxygen saturation. A pulse oximetry sensor on the left hand is not an accurate reflection of preductal oxygen saturation because the origin of the left subclavian artery is close to the region where the ductus arteriosus connects to the aorta.
In which conditions would you expect the preductal hemoglobin oxygen saturation to be higher than the postductal hemoglobin oxygen saturation value, and in which congenital heart lesion would you expect the reverse to be true?
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here