Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
All newborns must have a hearing screen by the age of 1 month, a diagnostic audiologic test at 3 months if they do not pass their hearing screen, and a referral for early intervention by 6 months if they are diagnosed with hearing loss.
Genetic factors are the most common causes of hearing loss, accounting for approximately 65% of congenital sensorineural hearing loss (SNHL).
The most common environmental cause of hearing loss is congenital cytomegalovirus (cCMV).
The optimal time to perform cCMV testing is prior to 3 weeks of age.
Ototoxicity is an important cause of hearing loss, especially in children exposed to aminoglycosides.
Clinicians should follow-up after a failed newborn hearing screen in children with middle ear fluid, because approximately 10% may also have SNHL.
Auditory neuropathy will be missed in children who have a hearing screened using otoacoustic emissions only. Because this condition is prevalent in neonatal intensive care infants, hearing screening in these infants should include automated auditory brainstem response testing.
Because progressive SNHL may develop in children despite a normal newborn hearing screen, clinicians should refer children with SNHL risk factors for additional audiologic testing at 24 to 36 months of age.
The most common congenital condition is hearing loss, with a reported prevalence of 3 out of 1000 infants. Prior to the year 2000, only children who were considered high risk underwent hearing screening prior to discharge from the newborn nursery. However, with this “high-risk” screening process, approximately 50% of children with hearing loss were not identified. In 1994 the Joint Committee on Infant Hearing recommended universal newborn hearing screening (UNHS) prior to hospital discharge for all infants. This did not take effect until after the year 1999 in most states, until sufficient evidence was found and it was approved by the US Preventive Services Task Force. Since that time, several states followed suit, and now all states have UNHS programs.
UNHS follows the 1 to 3-6 rule. The infant must undergo hearing screening prior to 1 month of age. Children who do not pass the hearing screen must get diagnostic testing to confirm if they do or do not have hearing loss by the age of 3 months. If the child is diagnosed with hearing loss, they should be referred for early intervention (EI) by 6 months of age. Using the 1 to 3-6 rule, children with congenital hearing loss have been identified and treated earlier.
Since the initiation and acceptance of UNHS, there has been dramatic improvement of early identification of children with hearing loss, because approximately 50% of newborns with hearing loss have no readily identifiable risk factors. Prior to UNHS, the average age of suspicion of hearing loss was 18.8 months, with confirmation at 26 months and hearing aids at 30 months. With the early implementation of UNHS the age of intervention has decreased to 6 months, and in a study performed in Colorado, the average age for fitting hearing aids was 5 weeks. , ,
In addition to UNHS, other advances in diagnosis and management of pediatric sensorineural hearing loss (SNHL) should be noted. The advent of genetic testing panels allows evaluation of more than 100 genes to increase the likelihood of identifying a causative genetic disorder. High-resolution imaging of the temporal bones with computed tomography (CT) and magnetic resonance imaging (MRI) has allowed detailed analysis of inner ear anatomy for children with hearing loss. Furthermore, more birthing hospitals and states are adopting congenital cytomegalovirus (cCMV) testing prior to 3 weeks of age, increasing the possibility of identifying children with cCMV.
Early recognition of hearing loss in young children has led to EI and management with preferential school seating, hearing aids, bone-anchored hearing aids, and cochlear implants. Such EIs recognize the critical period for optimal language skills to develop, and with EI, patients have better outcomes. The literature suggests that diagnosis and intervention prior to 6 months can improve speech and language outcomes. , , In this chapter we will discuss etiology, diagnosis, and management of children who fail their newborn hearing screen.
There are four types of hearing loss: conductive, mixed, sensorineural, and auditory neuropathy. Conductive hearing loss is caused by middle ear fluid or an abnormality of the ossicles and/or the external auditory canal. SNHL is due to malformations or malfunction of the cochlea or cochlear nerve. Mixed hearing loss has components of both conductive hearing loss and SNHL. Auditory neuropathy is due to lack of proper synchronized auditory input from the cochlear nerve to the brain, with or without a normal cochlea. In this chapter we will focus mainly on SNHL.
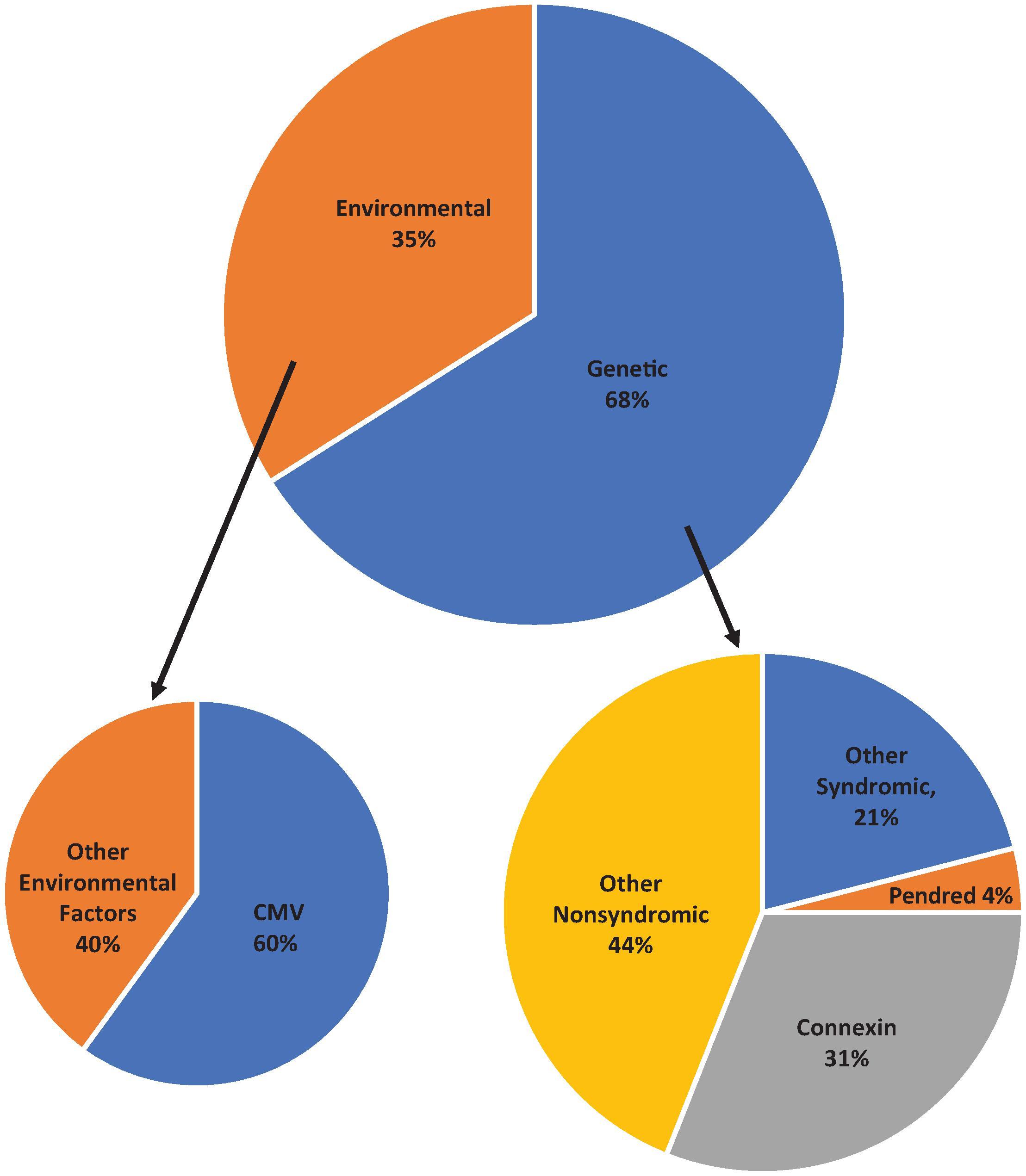
There are several causes of congenital hearing loss ( Fig. 69.1 ). Genetic mutations account for approximately 50% to 68% of congenital hearing loss, and of these, nonsyndromic causes represent approximately 70%. , The most common genetic cause of SNHL is a mutation in the gene coding for connexin 26, a protein responsible for maintaining endolymphatic potential through potassium ion transport. The gene locus coding for this protein is the gap junction beta-2 (GJB2) gene. There are approximately 400 syndromes that are associated with SNHL; some of the common syndromic causes are listed in Table 69.1 .
| Name | Inheritance Pattern | Gene | Phenotype | Expected Hearing Loss |
|---|---|---|---|---|
| Pendred | AR | SLC26A4 | Bilateral enlarged vestibular aqueduct, enlarged thyroid | Severe to profound Mild to moderate progressive |
| Alport | X-linked or AR | COL4A3 , COL4A4 , COL4A5 | Kidney disease and eye abnormalities | Late-onset high frequency |
| Usher type 1 | AR | USH1A , MYO7A , USH1C , CDH23 , USH1E , PCDH15 , USH1G | Vestibular symptoms, retinitis pigmantosa | Profound |
| Usher type 2 | AR | USH2A , USH2B , USH2C , ADGRV1 , WHRN | Retinitis pigmentosa | Mild to severe |
| Usher type 3 | AR | USH3 , CLRN1 | Variable vestibular symptoms, late-onset retinitis pigmentosa | Progressive |
| Waardenburg type 1 | AD | PAX3 | White forelock, heterochromic irises, dystopia canthorum | Normal or moderate to profound |
| Waardenburg type 2 | AD or AR | MITF , SNAI2 | White forelock, heterochromic irises | Normal or moderate to profound |
| Waardenburg type 3 | AD | PAX3 | Same as type 1 with limb defects | Normal or moderate to profound |
| Waardenburg Type 4 | AR | SOX10 , EDN3 , EDNRB | Increased risk of Hirschsprung disease in carriers and homozygotes | Normal or moderate to profound |
| Jervell and Lange-Nielsen syndrome | AR | KCNE1 , KCNQ1 | Prolonged QT | Profound |
| Branchio-otorenal | AD | EYA1 | Renal abnormalities, preauricular pits or other external/middle ear abnormalities, branchial fistula/cyst | SNHL/CHL/MHL, mild to profound |
Environmental etiologic factors cause 35% to 50% of SNHL, of which cCMV infection is the most common. We will discuss cCMV in further detail later in this chapter. Other prenatal infections such as toxoplasmosis, rubella, herpes, and syphilis can cause SNHL. Furthermore, prematurity, hypoxia, hyperbilirubinemia, meningitis, and ototoxic medications have been identified as environmental factors associated with congenital SNHL.
Ototoxicity is a special consideration in newborns, given that antibiotic regimens used to treat neonatal sepsis often contain aminoglycosides. The proposed mechanism of ototoxicity is from free-radical formation that damages the hair cells in the cochlea. Initially, high-frequency hearing is impacted, and then it may progress to include lower frequencies. Factors associated with aminoglycoside ototoxicity are coadministration of vancomycin, loop diuretics, or neuromuscular blockers; underlying disease; prior aminoglycoside exposure; and drug peak and trough concentration. Furthermore, individuals who have mitochondrial mutations, especially mtDNA A155G, are highly susceptible to ototoxicity from these medications even without high drug concentration levels in the blood. , This mutation has been observed in 17% of Caucasians and 10% to 30% of Asians with aminoglycoside ototoxicity.
There is currently no standard for monitoring infants for ototoxicity. Although the American Speech and Language Association recommends obtaining a baseline hearing test, the frequency and urgency of empiric aminoglycoside treatment may make such baseline testing impractical prior to starting treatment. Furthermore, the UNHS protocols do not test higher than 4K Hz, which is an early characteristic of ototoxic hearing loss. However, distortion product otoacoustic emissions can be measured at high frequencies and may have better potential in ototoxic monitoring in this population. Another challenge is the difficulty in interpreting results in very premature neonates due to an immature auditory system, stenotic ear canals with debris, and noise in the neonatal intensive care unit that can interfere with results. Further research is needed to identify the best protocol for evaluating these children and identifying how to decrease potential ototoxicity in this population.
There are two types of hearing screen tests for young infants: otoacoustic emissions (OAEs) and automated auditory brainstem response (AABR). All newborns must undergo this evaluation prior to 1 month of age, and it is usually performed in the birth hospital prior to discharge. One type of screening is the evoked OAE (EOAE), which tests the outer hair cells and is a measure of only cochlear function. EOAE testing does not evaluate the entire auditory pathway. There are two main types of EOAEs performed: transient (TEOAE) and distortion product (DPOAE). Small microphones are placed into the external auditory canal, and a series of clicks at a sound pressure level of approximately 80 dB are used to elicit a response when performing TEOAEs. If emissions are present, this indicates hearing of at least 20 to 40 dB hearing loss in the 500 to 4K Hz range, essentially excluding hearing losses greater than mild. When two pure-tone stimuli, one low frequency and the other high frequency, are presented at moderate levels (55–65 dB sound pressure level) to the ear, the cochlea generates DPOAE, which occurs somewhere between the low- and high-frequency stimuli. EOAEs are only accurate when both the external ear and the middle ear are clear of debris and/or fluid.
Another type of screening test is the AABR. This screening test evaluates the entire auditory pathway, from the cochlea to the brainstem. When performing the AABR, transient acoustic stimuli are generated and detected with probes that are placed in the ear canal and surface electrodes that are placed in the head and neck region (e.g., vertex, mastoid region). A rapidly repeated click stimulus is presented at 30 to 45 dB hearing loss to evaluate the auditory pathway. The automated test will give a pass or fail result. Because the AABR is actually a measurement of a summated evoked potential over time, background noise and movement of the infant can adversely affect testing. ,
Currently there is no consensus on which screening test should initially be performed in the newborn nursery. The type of test used varies across states, cities, and even at the hospital level. The Joint Committee on Infant Hearing recommendations note that the type of screening program will depend on what is practical and suitable for the program, based on both cost and availability of resources. The only instance where AABR must be performed in all children is in the neonatal intensive care unit (NICU), because auditory neuropathy occurs more frequently in this population. The first hearing screen should be performed at least 12 hours after birth, preferably as close to discharge as possible in order to decrease the referral rate from amniotic fluid or debris in the ear canal. For infants who “refer” after the first screen, a repeat screen is recommended prior to diagnostic testing. Several studies have shown that testing infants at least 24 to 48 hours after birth will lead to decreased false-positive results.
In general, EOAEs are easier and faster to perform; however they only evaluate the cochlea and are adversely impacted by middle and outer ear pathology such as vernix, cerumen, and middle ear fluid. AABR is more time consuming, but it is generally reported to have a lower false-positive rate. In a study by Lin and colleagues, the referral rate was 5.8% for TEOAE, 1.6% for two-step TEOAE and AABR, and 0.8% for AABR only. Clarke et al. compared the results of AABR and TEOAE in 81 newborns undergoing NHS. They found the probability of a newborn passing the AABR was much higher than for TEOAE on both the first and second screens. Despite the lower referral rates with AABR only, the increased technical difficulty and cost are barriers to implementation in many settings.
A two-stage protocol using EOAEs and subsequent AABR in cases where the newborn refers the EOAEs is also used at many birthing centers. A number of investigators have reported that this approach reduces the false-positive rate and will minimize unnecessary referrals, although other studies have shown that infants who have undergone the two-stage NHS protocol may be at increased risk of a missed hearing-loss diagnosis. Optimizing the screens and decreasing false positives will decrease the need for subsequent follow-ups, superfluous testing, cost, and the potential of parental anxiety.
A pass on the NHS does not mean that the child will continue to have normal hearing. Currently, if children pass their NHS, they will not undergo a repeat hearing screen until the age of 4. The literature reports that up to 22% to 30% of children diagnosed with hearing loss had previously passed their NHS. For this reason, it is important to reevaluate children at risk for future SNHL by the age of 24 to 30 months ( Table 69.2 ). With this second screen, children would be identified prior to preschool, and intervention could be started earlier to improve speech and language outcomes. Additionally, per the American Academy of Pediatrics, all children should be monitored during their well-check visits at 9, 18, 24, and 30 months for middle-ear disease, auditory and speech skills, and developmental milestones, using the validated global screening tool. If there is parent or physician concern for hearing, speech, or language delay, the assessment should be performed earlier.
| Caregiver concern regarding speech, language, or developmental delay |
| Family history of permanent childhood hearing loss |
| Neonatal intensive care (NICU) of >5 days |
| NICU for any period of time with the following: Extracorporeal membrane oxygenation (ECMO), assisted ventilation, exposure to ototoxic medications or loop diuretics, and hyperbilirubinemia requiring exchange transfusion |
| In utero infections: Toxoplasmosis, rubella, cytomegalovirus, herpes, syphilis |
| Craniofacial anomalies including those that involve the pinna or ear canal, ear tags, ear pits, and temporal bone anomalies |
| Physical findings associated with syndromes known to include sensorineural or permanent conductive hearing loss |
| Syndrome associated with hearing loss |
| Neurodegenerative disorders |
| Culture-positive postnatal infections associated with sensorineural hearing loss (meningitis) |
| Head trauma, especially basal skull/temporal bone fracture requiring hospitalization |
| Chemotherapy |
Each state has a local Early Hearing Detection and Intervention chapter responsible for ensuring that the NHS guidelines are followed. NHS data are reported to the national chapter, which is part of the Centers for Disease Control and Prevention (CDC), and these data are aggregated and reported, most recently in 2017. The average percentage of newborns who were screened by the recommended 1 month was 97.1% (ranging from 94.3% to 100%), which is reassuring. The most common reasons for no documented hearing screen were infant death, home birth, or the family declining testing. After the screening, an average of 24.6% of infants nationally (range, 0%–86.7%) did not undergo diagnostic testing due to loss to follow-up/documentation. The average rate of infants with diagnosed hearing loss being enrolled into EI by 6 months was reported to be quite low at 43.4% (range, 8.1%–83.3%). Reasons identified by Early Hearing Detection and Intervention were mainly due to families declining EI, families being contacted for follow-up but not responding, inability to contact families, families being nonresidents or moving out of state, or families being ineligible for Part C Medicaid. Overall, even though screening is most likely performed in almost all newborns in the United States, improvements still need to be made in achieving diagnostic testing by 3 months and starting EI for hearing loss by 6 months of age.
Due to the improvement in modern technology, we are fortunate to sustain life in extremely premature infants. However, there are no guidelines detailing when to evaluate hearing and interpretation of diagnostic tests in this group. Myelination of the cochlear nerve and auditory pathway does not begin until the third trimester of pregnancy (27 weeks). At this time, the fetus may start moving to sounds, although this response may be more consistent at 28 to 29 weeks, when evoked responses may be present. Full cochlear maturity is achieved a few weeks prior to term birth. Given the time course for development of the auditory system, it is important to consider gestational age when interpreting the auditory brainstem response (ABR) results. Delayed maturation of the auditory pathway in premature infants may lead to abnormal ABR results, which may improve with time in this population. It is important to consider obtaining repeat evaluations in this population. Hof and colleagues described a series of nine infants with improved hearing prior to 80 weeks’ gestation. Another case series described 23 infants diagnosed with severe to profound hearing loss at 3 months who significantly improved within the first year of life. Although there are no strong data at this time, both studies identified that severely preterm infants should have frequent hearing evaluations until at least 80 weeks of gestational age, especially when evaluating cochlear implantation candidacy. ,
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here