Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
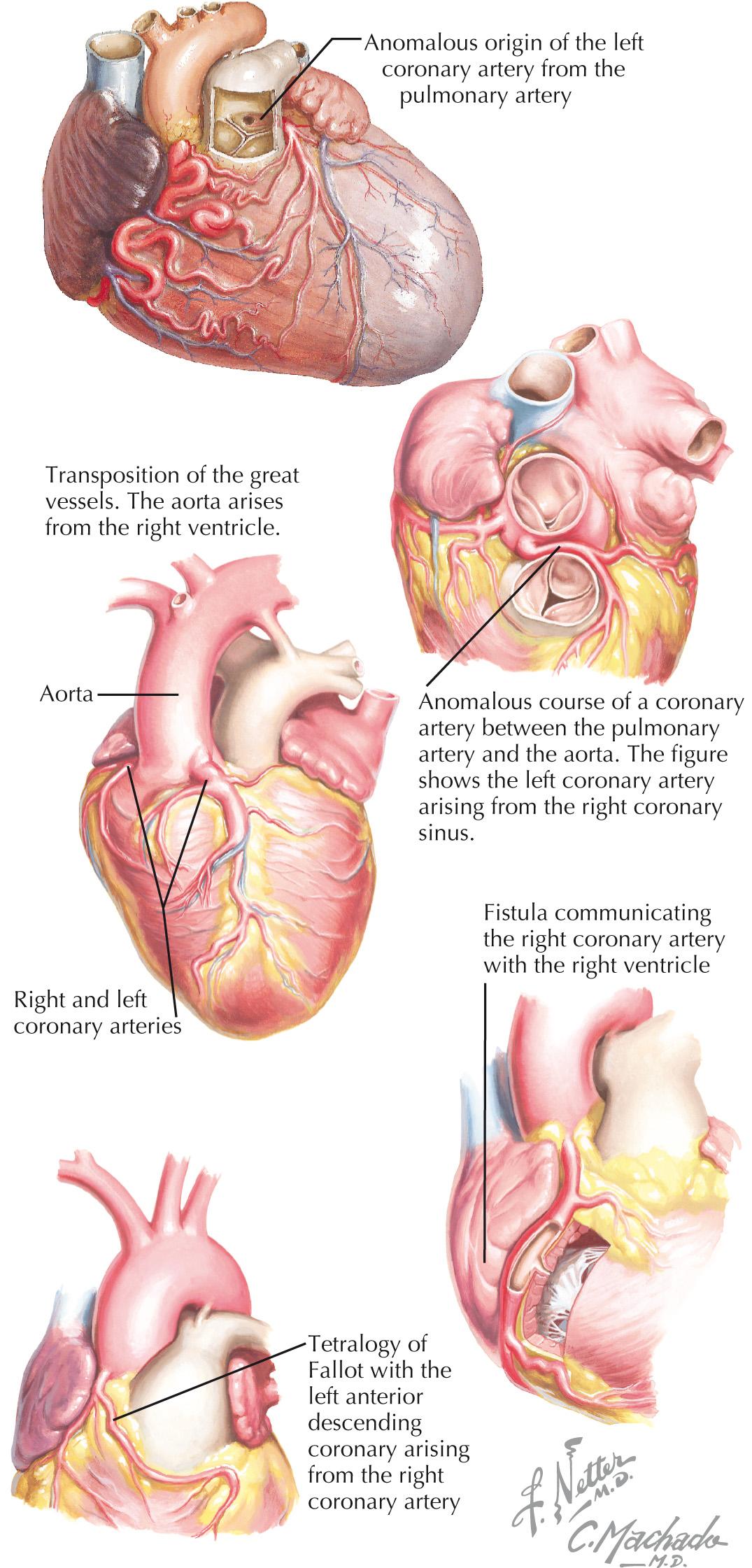
Unfortunately, presentation with cardiac arrest or sudden cardiac death is a common manifestation of congenital coronary anomalies. This clinical relevance underpins the necessity of understanding the anatomy and presentation of congenital coronary anomalies and their treatment options. The two primary congenital coronary anomalies, anomalous connection of the left coronary artery from the pulmonary artery (ALCAPA) and anomalous connection of a main coronary artery to the aorta, as well as two entities associated with coronary artery anomalies—coronary artery fistulas and anomalous coronary circulation—are the focus of this chapter ( Fig. 25.1 ).
It was initially believed that the two main coronary arteries arise from separate ostia within the sinuses of Valsalva. However, current embryological information suggests the proximal coronary arteries grow from the peritruncal area into the aorta, and a single orifice is individually formed for the left and right coronary arteries. The left coronary artery (LCA) then divides into the left anterior descending artery, which traverses the anterior interventricular groove, and into the left circumflex coronary artery, which courses into the left atrioventricular groove. Normally, the right coronary artery (RCA) originates anteriorly from the right aortic sinus and courses along the right atrioventricular groove, commonly giving rise to the posterior descending artery.
ALCAPA is a rare congenital anomaly, usually an isolated lesion, that occurs from 1 in 30,000 to 1 in 300,000 live births ( Fig. 25.2 ). The clinical spectrum of ALCAPA is also known as Bland-White-Garland syndrome. Infants with myocardial ischemia typically present with failure to thrive, profuse sweating, dyspnea, pallor, and atypical chest pain upon eating or crying, usually at between 4 and 6 weeks of age. By 2 to 3 months of age, heart failure is apparent, and patients have persistent tachypnea and tachycardia. Malignant arrhythmias leading to sudden cardiac death are the most extreme presentation of myocardial ischemia in ALCAPA. During the neonatal period, high pulmonary vascular resistance ensures anterograde flow from the pulmonary artery through the LCA. However, as this resistance diminishes, there is an eventual reversal of flow, with left-to-right shunting through the pulmonary artery. The result is the phenomenon of “coronary steal,” with left ventricular (LV) perfusion becoming dependent on collateral circulation from the RCA.
Because infantile circulation has little or no coronary collateral development, ALCAPA leads to severe myocardial ischemia, with resultant LV dysfunction and dilation. Dilation of the LV is due not only to the effects of ongoing myocardial ischemia but also to mitral valve regurgitation, because papillary muscle ischemia is common in ALCAPA. Without surgical intervention and correction of the anomaly, 90% of patients die by age 1 year. Patients who survive to adulthood, secondary to the presence and formation of collateral circulation, may remain asymptomatic despite subclinical, ongoing ischemia. Arrhythmic sudden death purportedly occurs in 80% to 90% of patients by 35 years of age.
Although ALCAPA is rare, a high index of suspicion should be present for infants presenting with signs of myocardial ischemia or dysfunction, or for adolescents presenting with syncope, chest pain, or cardiac arrest after strenuous exercise. Dilated cardiomyopathy and endocardial fibroelastosis are two conditions with clinical signs that are difficult to distinguish from ALCAPA. All three conditions may present with cardiomegaly, mitral regurgitation that results in an apical pansystolic murmur and apical gallop rhythm, and ECG evidence of myocardial ischemia and LV hypertrophy. If an enlarged RCA with global hypokinesis and LV dilation are revealed with two-dimensional and pulsed Doppler echocardiography, ALCAPA must be considered in the differential diagnosis. The gold standard in diagnosing ALCAPA is cardiac catheterization and cineangiography. Hemodynamic data show a low cardiac output despite elevated filling and pulmonary arterial pressures. Magnetic resonance angiography has emerged as a noninvasive diagnostic tool, with similar sensitivity and specificity in comparison to cardiac catheterization. Pulsed and color-flow Doppler examination may delineate a left-to-right shunt. The degree of mitral regurgitation and LV function can be assessed using a left ventriculogram in addition to illustrating coronary anatomy. Coronary angiography also assists in excluding other anatomic etiologies for ischemia and ventricular dysfunction.
Surgical correction remains the gold standard of therapy and is necessary in all patients with ALCAPA due to risk of further myocardial ischemia and subsequent death. Important changes in technique have resulted in improved outcomes. Surgical repair involves direct reimplantation of the anomalous LCA into the aorta by transferring it with a button of the pulmonary artery ( Fig. 25.3 ). There are several options to customize the surgical approach to overcome anatomic challenges of the length and course of the LCA for reimplantation. They include the Tunnel operation and/or Takeuchi repair, LCA translocation, and subclavian-to-LCA anastomosis. In adults in whom reimplantation is more technically challenging, bypass grafting with the left internal thoracic artery is an equally effective approach. If LV function does not improve following revascularization, cardiac transplantation may be indicated.
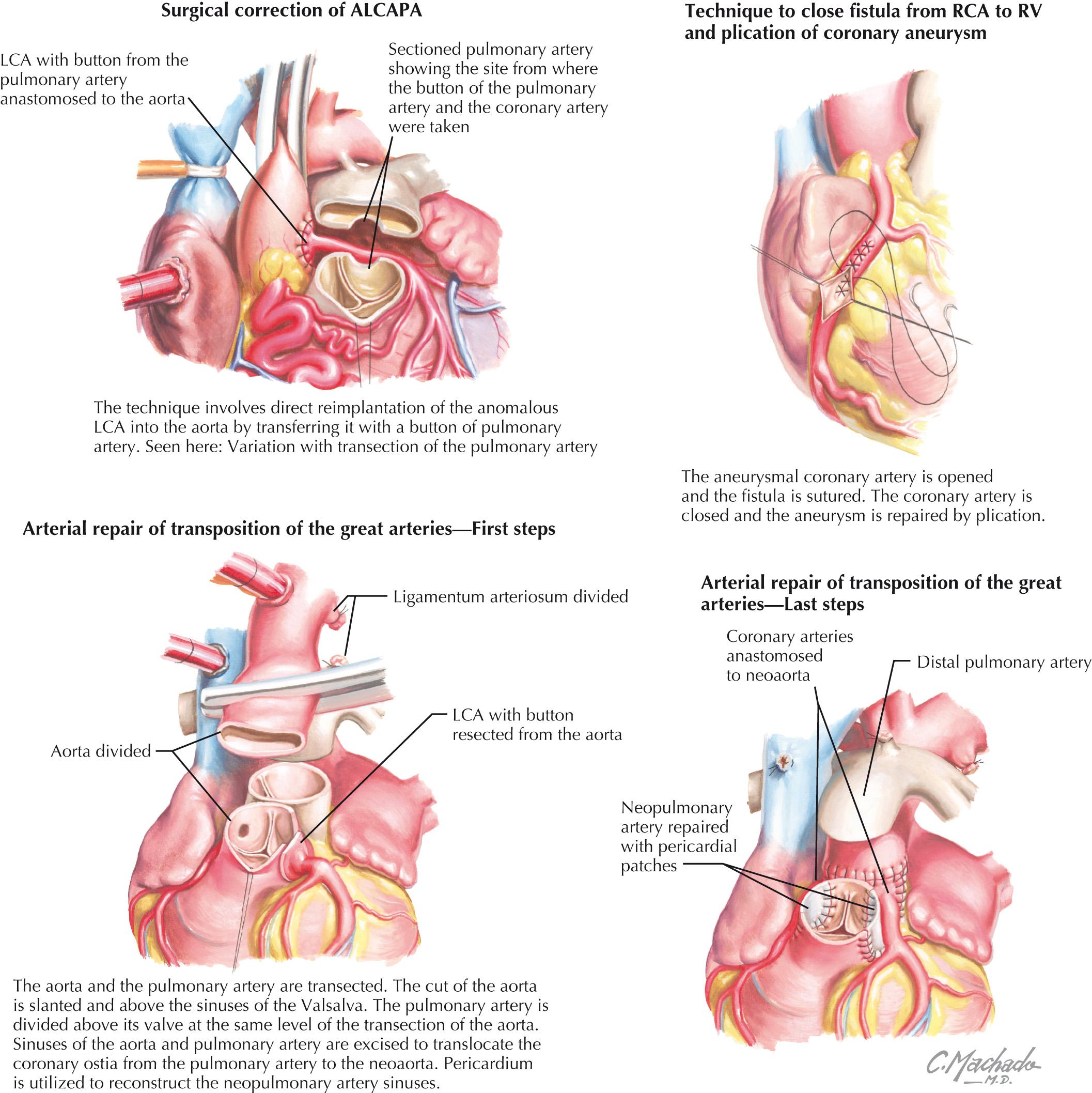
After reestablishment of a two-coronary system, the previously dilated RCA will generally return to normal size, and the intercoronary collateral network that developed before surgery will regress. Operative mortality for all surgical techniques has markedly decreased. Operations resulting in a two-coronary system have been reported to have a mortality rate of 0% to 14%, and represent a vast improvement compared with the mortality rates reported in the early 1980s (75%–80%). One study of long-term survival reported no late deaths in 21 patients with a range of 2 months to 18 years of follow-up and a total of 145 patient-years of follow-up. No differences in LV function or the late mortality rate have been shown comparing the various reimplantation or revascularization techniques used today. Although the previous approach of directly ligating the anomalous coronary was abandoned because of poor outcomes, it is suggested as an option to help stabilize a critically ill patient before revascularization is undertaken.
Anomalous aortic origin of coronaries (AAOC) presents much more variably than ALCAPA and is characterized by coronary arteries with anomalies of aortic connection involving courses, stenoses, and compression. Some individuals have myocardial ischemia and can present with sudden death, but in others, this can be an entirely asymptomatic incidental finding at the time of cardiac catheterization or coronary artery imaging. The reasons for this variable presentation involve subtle differences in the anatomy and course of the anomalous coronary artery (see Fig. 25.2 ).The major anomalies of connection that are potentially serious include attachment of the RCA to the left sinus of Valsalva, attachment of the left main coronary artery to the right sinus of Valsalva, attachment of one or both major coronary arteries to the posterior noncoronary or nonfacing sinus of Valsalva, and lastly, a single coronary artery with only one aortic orifice and main stem that divides to supply branches to the entire heart.
Surgical correction is indicated in individuals who present with significant symptoms. In asymptomatic individuals, if the LCA arises from the right coronary sinus and courses between the aorta and the pulmonary artery, surgical intervention is indicated because the risk of sudden cardiac death is high in this group. However, if the RCA arises from the left aortic sinus and is nondominant, such an entity may be benign.
Prevalence of an anomalous course of a coronary artery between the pulmonary artery and aorta (ACCBPAA) is unknown, due to a lack of consistent pathognomonic and clinical features. Current estimates range from 0.1% to 0.3% of the general population. One review of 126,595 cardiac catheterizations revealed an incidence of 1.15%, constituting 87% of all coronary artery anomalies within this series. The most important review of this abnormality described sudden death in 59% of the 242 patients. The diagnosis should be considered in young patients with exercise-induced myocardial ischemia, aborted sudden death, or sudden death. It has recently been suggested that screening of first-degree relatives with echocardiography be performed because of potential familial link. Transthoracic echocardiography with color Doppler will provide valuable information, such as evaluation of heart function, identification of both coronary artery connections, and demonstration of the direction of blood flow within the aortic wall. Despite coronary angiography remaining the gold standard to accurately delineate the anatomy and exclude other associated coronary disease, it is not preferred in children. In those cases, noninvasive imaging with CT and MRI may be used to identify the coronary artery connections and/or confirm diagnosis.
Surgical intervention is indicated in patients with an interarterial and intramural course of the anomalous coronary artery, with signs and/or symptoms of myocardial ischemia or ventricular arrhythmias. Asymptomatic patients with anomalous connection and course of the left main coronary artery should also undergo surgical intervention due to a high risk of sudden death. Choice of surgical technique will depend on specific morphological details, as determined by preoperative imaging. The preferred procedure for patients with an interarterial and intramural course of the anomalous coronary artery is the unroofing procedure. A transverse aortotomy is essential to assess the coronary ostia. If the anomalous coronary artery arises from the opposite sinus, it is necessary to detach the aortic valve commissure. The slitlike ostium, which is characteristic of AAOC and partially responsible for ischemic symptoms, is opened along its longitudinal axis, and a portion of the common wall between the aorta and the coronary artery is excised, with reapproximation of the intimal surfaces. The valve commissure is subsequently resuspended with a pledgeted suture. This unroofing procedure creates a neo-ostium and obliterates the intramural course of the coronary artery.
Other surgical options include revascularization with an internal mammary artery or a saphenous vein bypass graft, direct reimplantation, or reconstruction of the orifice. However, an important issue to consider is that revascularization may lead to competitive flow between the bypass graft and the native circulation, thus increasing the likelihood of bypass graft failure. The advantage of reimplantation is that competitive flow is not an issue, because a single conduit vessel provides flow to the myocardium in that distribution. Even so, reimplantation may be more technically difficult because kinking may occur, or there may be insufficient tissue to create a button around the orifice.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here