Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The congenital myopathies are a heterogeneous group of neuromuscular conditions defined by distinctive morphologic abnormalities in skeletal muscle ( Figure 28.1 ). These disorders typically present in infancy or early childhood with hypotonia and muscle weakness, and are usually nonprogressive, or only slowly progressive with age. There is wide variation in clinical severity within each group and marked clinical overlap with other neuromuscular disorders, including the muscular dystrophies, congenital myasthenic syndromes, metabolic myopathies, and spinal muscular atrophy. Therefore, accurate diagnosis requires the combined evaluation of the clinical data, the electromyogram, and histopathologic findings. The congenital myopathies usually have a genetic basis, although for some morphologic entities only sporadic cases have been reported (see later discussion).
Clinically, the congenital myopathies share a number of common features: generalized weakness, hypotonia and hyporeflexia, poor muscle bulk, and dysmorphic features secondary to the myopathy (e.g. pectus carinatum, scoliosis, foot deformities, a high arched palate, and elongated facies) ( Figure 28.2 ). The pathologic process appears to exclusively affect the striated muscle, and thus congenital myopathies may be distinguished clinically from neuromuscular conditions with multisystem involvement, such as facioscapulohumeral muscular dystrophy, which is associated with hearing defects or retinal vascular pathology, myotonic dystrophy with central nervous system involvement, cataracts and cardiac conduction defects, and a subset of patients with congenital muscular dystrophies (CMD) associated with brain pathology (e.g. Walker Warburg syndrome, muscle-eye-brain disease, Fukuyama CMD). Some of the important clinical features that distinguish congenital myopathies from other similar diagnoses and that delineate specific congenital myopathy subtypes are presented in Table 28.1 (for clinical signs in infancy) and Table 28.2 (for clinical features in older children).
| Clinical Features | Congenital Myopathy | Differential Diagnosis |
|---|---|---|
| Facial weakness | NM, CNM ( MTM1 , RYR1 , DNM2 ) | DM1, CMS (rapsyn) |
| Ophthalmoplegia | CNM ( MTM1 , RYR1 , DNM2 ), MmD ( RYR1 ) | CMS, mitochondrial |
| Ptosis | CNM ( MTM1 , RYR1 , DNM2 ), MmD, CCD | CMS, DM1 |
| Facial dysmorphism (long face, dolichocephaly, high arched palate) | NM, CNM ( MTM1 , severe DNM2 ), severe RYR1 | DM1 |
| Bulbar weakness (sucking/swallowing) | NM, CNM ( MTM1 ), severe RYR1 | CMS, DM1, PWS, SMA |
| Severe respiratory involvement at birth | NM, CNM ( MTM1 ), severe RYR1 | DM1, SMA 0, CMS, Pompe |
| Predominant axial hypotonia | RYR1 , SEPN1 | LMNA |
| Severe congenital hypotonia | NM, MTM1 , RYR1 | DM1, PWS, Down Syndrome |
| Orthopedic deformities | RYR1 , NM | COL6, CMS |
| Hip dislocation | RYR1 | COL6 |
| Fetal akinesia/severe arthrogryposis | NM ( ACTA1 , NEB ), severe RYR1 , KLHL40 | CMS, SMA 0, CHS |
| Club feet | NM, RYR1 | CMS, DM1, CHS |
| Clinical Features | Congenital Myopathies | Differential Diagnoses |
|---|---|---|
| Scoliosis | SEPN1 , RYR1 , NM | COL6, LAMA2 |
| Rigid spine | SEPN1 , RYR1 | |
| Cardiomyopathy | TTN , MYH7 , rarely ACTA1 | Pompe disease |
| Foot drop/pes cavus | NM ( NEB , TPM3 , TPM2 ), DNM2 , MYH7 | Peripheral neuropathy |
| Malignant hyperthermia | CCD, MmD and CNM ( RYR1 only) | |
| Respiratory involvement and axial involvement out of proportion to skeletal muscle weakness | SEPN1 , NM ( NEB , TPM3 , ACTA1 ) | LMNA , CMS, Pompe disease |
The first report of a congenital myopathy was by Shy and Magee in 1956. They described a hereditary, nonprogressive disorder that subsequently became known as central core disease. Prior to this, the spinal muscular atrophies were the only well-defined neuromuscular disorders of infancy and childhood. Most patients with congenital myopathies were confusingly classified under such descriptive terms as “amyotonia congenita” or “benign congenital hypotonia.” In 1969, Dubowitz clarified the classification of these disorders with his delineation of the “new myopathies,” later termed congenital myopathies. The pathologic changes associated with congenital myopathies appear to originate within the myofiber itself. The structural diversity of the striated muscle fiber allows for an enormous spectrum of myopathologic features rarely encountered in pathology of any other mammalian cell. Damaging forces from outside the muscle fiber, such as inflammation or denervation, rarely result in the morphologic changes associated with congenital myopathies. Exceptions include target formation in denervation, nemaline bodies secondary to tenotomy, and tubulofilamentous aggregates in inclusion body myositis.
The congenital myopathies are rare disorders, although precise epidemiologic data of frequency and geographic distribution are available in only limited circumstances. A recent comprehensive examination of prevalence was performed using a well-delineated pediatric population in a small but ethnically diverse region of the United States (southeastern Michigan). These data estimated the point prevalence of myopathies to be 1:26,000, with core myopathies the most common histopathologic subtype and RYR1 -related myopathies the most prevalent genetic subtype. Additional information comes from several case series. In one series of 250 patients with severe neonatal hypotonia, 34 of the 250 (14%) were diagnosed as having a congenital myopathy. Eight cases (3% of the total) had nemaline myopathy, which is perhaps the most common type presenting in infancy. A more recent series of 66 consecutive patients seen at one center in the United Kingdom identified over half (54%) with core myopathies, 17% with nemaline myopathies, and 13% with centronuclear myopathies, together accounting for the majority of diagnoses.
Over the past three decades, the number of morphologically distinct congenital myopathies has grown rapidly, paralleling the advent and refinement of myopathologic techniques such as electron microscopy, enzyme histochemistry and immunocytochemistry, and more recently, molecular genetic techniques. Recent efforts have focused on defining and classifying the distinct and “real” disease entities. The current clinico-pathological classification is based on the specific structural abnormalities seen on light and electron microscopy ( Box 28.1 ), although improved genetic diagnoses are revealing extensive genetic heterogeneity within morphological subtypes, and considerable overlap between subtypes sharing common genetic etiologies. Certain congenital myopathies are well defined clinically, morphologically, and genetically ( Table 28.3 ). These classical congenital myopathies are nemaline myopathy, core myopathies (encompassing central core disease and multiminicore myopathy), the centronuclear myopathies (including X-linked myotubular myopathy), and congenital fiber type disproportion and aggregation myopathies (including the myofibrillar, or desmin-related, myopathies and the autophagic vacuolar myopathies) and will be reviewed in detail ( Figure 28.1 ). There are several more rare forms of congenital myopathy, many of which have been described in only a very small number of patients. Some of these entities are now recognized as variant forms of the more common subtypes (for example, cap myopathy is likely an allelic variant of nemaline myopathy), while some continue to be unique and poorly understood conditions. A list of the rare myopathies not discussed further in this chapter, along with some of their distinguishing features, is presented in Table 28.4 .
Central core disease
Multiminicore disease
Myofibrillar lysis myopathy/hyaline body myopathy
Actinopathy
Cap disease
Trilaminar fiber myopathy
Broad A-band myopathy
Nemaline/rod myopathy
Desmin-related/myofibrillar myopathies
Cytoplasmic body myopathy
Spheroid body myopathy
Mallory body-like myopathy
Sarcoplasmic body myopathy
Granulofilamentous myopathy
Myotubular myopathy
Centronuclear myopathy
Intranuclear rod myopathy
Fingerprint body myopathy
Zebra body myopathy
Reducing body myopathy
Cylindrical spirals myopathy
Sarcotubular myopathy
Tubular aggregate myopathy
Lysosome-related vacuolar myopathies
Minimal change myopathy
Nonspecific congenital myopathies in syndromes
Rod-core myopathy
Cytoplasmic body–reducing body myopathy
Multiminicore–centronuclear myopathy
Congenital fiber type disproportion
Microfiber myopathy
Type 1 fiber predominance
Type 1 fiber uniformity
Type 1 myofiber hypotrophy
Type 2A fiber uniformity and smallness
Type 2 muscle fiber hypoplasia
| Subtype | Gene | OMIM Designtion | Chromosome Location | Inheritance Pattern |
|---|---|---|---|---|
| Nemaline Myopathy | ACTA1 | NEM3 | 1q42.1 | AD, AR |
| CFL2 | NEM7 | 14q13.1 | AR | |
| KBTBD13 | NEM6 | 15q22.31 | AD | |
| KLHL40 | NEM8 | 3p33.1 | AR | |
| KLHL41 | NEM9 | 2q31.1 | AR | |
| NEB | NEM2 | 2q23.3 | AR | |
| RYR1 | 19q13.2 | AR | ||
| TNNT1 | NEM5 | 19q13.42 | AR | |
| TPM2 | NEM4 | 9p13.3 | AD | |
| TPM3 | NEM1 | 1q21.3 | AD, AR | |
| Cap Disease (NM Variant) | ACTA1 | 1q42.1 | AD | |
| TPM2 | CAPM2 | 9p13.3 | AD | |
| TPM3 | CAPM1 | 1q21.3 | AD | |
| Zebra Body Myopathy | ACTA1 | 1q42.1 | AD | |
| Hyaline Body Myopathy (Myosin Storage Myopathy) | MYH7 | 14q11.2 | AD | |
| Core-Rod Myopathy | KBTBD13 | 15q22.31 | AD | |
| NEB | 2q23.3 | AR | ||
| RYR1 | 19q13.2 | AD, AR | ||
| TPM2 | 9p13.3 | AD | ||
| Central Core Disease | RYR1 | CCD | 19q13.2 | AD, AR |
| Multiminicore Disease | RYR1 | 19q13.2 | AR | |
| SEPN1 | 1p36.11 | AR | ||
| MYH7 | 14q11.2 | AD | ||
| Centronuclear Myopathy | BIN1 | CNM2 | 2q14.3 | AR |
| CCDC78 | CNM4 | 16p13.3 | AD | |
| DNM2 | CNM1 | 19p13.2 | AD | |
| MTM1 | CNMX | Xq28 | XL | |
| MYF6 | CNM3 | 12q21.31 | AD | |
| RYR1 | 19q13.2 | AR | ||
| TTN | 2q31.2 | AR | ||
| SPEG | CNM5 | 2q35 | AR | |
| Congenital Fiber Type Disproportion | ACTA1 | 1q42.1 | AD | |
| MYH7 | 14q11.2 | AD | ||
| RYR1 | 19q13.2 | AR | ||
| SEPN1 | 1p36.11 | AR | ||
| TPM2 | 9p13.3 | AD | ||
| TPM3 | 1q21.3 | AD | ||
| Autophagic Vacuolar Myopathies | VMA21 | MEAX | Xq28 | XL |
| LAMP2 | Danon Disease | Xq24 | XL | |
| GAA | Pompe Disease | 17q25.3 | AR | |
| Myofibrillar Myopathies | DES | MFM1 | 2q35 | AD, AR |
| CRYAB | MFM2 | 11q23.1 | AD | |
| MYOT | MFM3 | 5q31.2 | AD | |
| ZASP | MFM4 | 10q23.2 | AD | |
| FLNC | MFM5 | 7q32.1 | AD | |
| BAG3 | MFM6 | 10q26.11 | AD |
| Rare Myopathy | Histopathology | Clinical Features | Genetics |
|---|---|---|---|
| Cylindrical spirals myopathy ( Figure 28.20 ) | Parallel spiraled lamellae as cylindrical bodies | Minimal weakness, myotonia, cramps | Dominant familial cases |
| Tubular aggregate myopathy ( Figure 28.21 ) | Tubular aggregates in hexagonal patterns | Myalgias, mild proximal weakness, cramps | STIM1 ORAI1 |
| Fingerprint body myopathy | Fingerprint bodies, type I fiber hypotrophy | Hypotonia, kyphosis, delayed ambulation | Familial cases |
| Hexagonal crystalloid body myopathy | Hexagonally cross-linked tubular arrays, caveolin 3-positive | Post-exercise myalgias | Familial cases |
While the congenital myopathy subtypes share many common clinical features, some specific signs and symptoms may be present to help distinguish them ( Tables 28.1 and 28.2 ). For example, ophthalmoparesis is common in centronuclear myopathies and in recessive RYR1-related myopathies, but far less common in nemaline myopathy. Foot drop may be observed in nemaline myopathy, DNM2 -related centronuclear myopathy, and MYH7 related myopathies. Specific disease features are provided in greater detail in the specific subsections following.
Most commonly, congenital myopathies are distinguished by their features on muscle biopsy ( Figure 28.1 ). Routine laboratory studies are typically not helpful, though serum creatine phosphokinase (CPK) levels are useful in that very high levels (>1000) are more typically associated with muscular dystrophies (though there are rare exceptions). Adjunct studies, in addition to clinical features, that can aid in the differential diagnosis include especially muscle MRI (see Figure 28.3 and Table 28.5 ). Electromyography may be helpful in distinguishing congenital myopathies from congenital myasthenic syndromes but not in delineating myopathy subtypes. In the majority of cases, a genetic diagnosis can be achieved, and ultimately represents the defining characteristic for a given patient. More than 15 genes have now been discovered. It is estimated that approximately 25–50% of cases remain genetically unsolved, though this number is rapidly dwindling with the application of new genomic technologies.
| Gene | Muscles | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AL | AM | BF | GSL | GSM | GMa | GMi | GRA | PER | PT | PTB | RF | SAT | SMM | ST | SOL | TA | VIM | VL | VM | |
| ACTA1 | S | A | A | S | A | S | A | A | A | |||||||||||
| BIN1 | S | A | A | S | S | A | ||||||||||||||
| DNM2 | A | A | A | A | S | S | S | A | A | A | ||||||||||
| MTM1 | S | A | S | A | S | A | ||||||||||||||
| MYH7 | S | A | A | A | ||||||||||||||||
| NEB | A | S | A | A | ||||||||||||||||
| RYR1 | A | A | S | S | A | A | A | |||||||||||||
| SEPN1 | S | A | A | A | S | A | ||||||||||||||
| TPM2 | A | A | S | S | S | A | ||||||||||||||
Nemaline myopathy is characterized by the presence of electron dense rod-shaped structures in the muscle fibers. The first descriptions of the disorder are generally attributed to Shy and colleagues and Conen and colleagues in 1963. However, it is now recognized that a patient with congenital myopathy and rod bodies was described by Dr. Douglas Reye, a pathologist in Sydney, Australia, in 1958. The term nemaline was first applied by Shy and colleagues because of the threadlike appearance of the rod bodies (the Greek word nema means “thread”). Nemaline myopathy is a rare disorder with an estimated incidence of 0.02 per 1000 live births.
The existing classification of nemaline myopathy is based on age of onset and severity of motor and respiratory involvement with severe congenital, intermediate congenital, typical congenital, childhood-onset, and adult-onset forms, as reviewed by North and associates. However, there is marked overlap between each of these groups. With the recent confirmation of both autosomal dominant and recessive forms of nemaline myopathy, and the discovery of at least nine genetic loci for the disorder (see later discussion), further molecular studies may eventually result in a meaningful classification that permits prediction of prognosis or determination of the mode of inheritance in singleton cases.
The severe neonatal form presents at birth with severe hypotonia and muscle weakness, little spontaneous movement, difficulties with sucking and swallowing, gastroesophageal reflux, and respiratory insufficiency. Decreased fetal movements and polyhydramnios may complicate the pregnancy, and death in utero associated with fetal akinesia has been described. Dilated cardiomyopathy occurs infrequently. Arthrogryposis may be a presenting feature in some individuals, and can be quite severe. Death due to respiratory insufficiency or recurrent pneumonia is common during the first weeks or months of life. However, even patients with severe floppiness and lack of spontaneous respiration at birth have been known to survive, some of them with little residual disability.
The mild congenital or classical form of nemaline myopathy usually presents at birth or during the first year of life with hypotonia, weakness, and feeding difficulties; however, the severity of muscle involvement is often less marked than in the severe neonatal form. Some cases present later with delayed attainment of motor milestones, waddling gait, or speech abnormalities. There is commonly distal involvement in addition to the proximal muscle weakness, and some patients have initially been thought to have peroneal paresis because of their foot drop. The respiratory muscles are usually involved, although hypoventilation may not be clinically obvious; cardiac involvement is rare. The course of the disease is often static or only very slowly progressive and most patients will be able to lead an active life. Others may experience deterioration during the prepubertal period of rapid growth and some will start using a wheelchair at this time. See Case Example 28.1 .
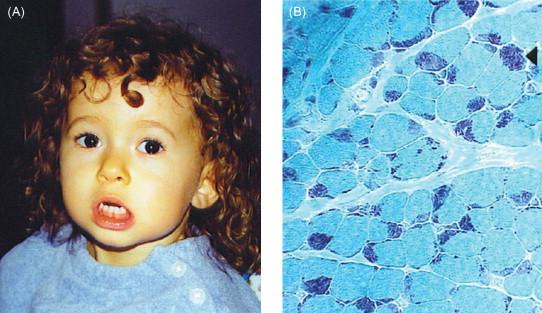
MLL is the first child of a nonconsanguineous Caucasian couple with no family history of neuromuscular disease. She presented at birth with generalized weakness, and particularly bulbar weakness resulting in poor suck and swallow. She had characteristic lower facial weakness ( Figure 28.4 ). During infancy, frequent aspiration led to recurrent pneumonias. Because of her bulbar weakness, MLL required gastrostomy tube feeding until the age of 2.5 years. Through intensive therapy, her swallowing improved, eventually allowing for independent feeding. However, she has had persistent and profuse drooling, which has required frequent suctioning throughout her life.
At 6 months of age, MLL began physical, occupational, and speech therapies. She was never able to lift her head while supine and could never crawl. Her first steps were taken at 13.5 months and independent walking was achieved at 15 months. She has subsequently maintained the ability to walk (though with reduced exercise tolerance), but has never been able to run.
At 4 years of age, MLL was fitted with a prosthetic lift to treat her immobile soft palate and hypernasal speech. At 13 years of age, a pharyngeal flap procedure was performed to improve velopharyngeal incompetency. At 13.5 years of age, she developed scoliosis that was treated with bracing. To date, she has not required scoliosis surgery. Also, breathing assistance has never been required. Of note, MLL has advanced intellectual ability confirmed through standardized neuropsychological assessment.
At her most recent examination (age 16 years), she had marked lower facial weakness with preserved eye movements. She was able to walk independently, but used a mobility scooter for long distances. She was able to jump with assistance but not run. She had a positive Gowers’ maneuver.
Muscle biopsy at 5 years of age demonstrated nemaline bodies on light and electron microscopy. Sequencing of the nebulin ( NEB) gene at 14 years of age revealed a novel heterozygous variant (c.3252_3255+3delTGACGTA). The variant is predicted to abolish the splice donor site in intron 32. A NEB array was also performed and identified a 2.7 kb deletion encompassing exon 77. The approximate genomic locations are 152,469,299 in intron 77 and 152,471,995 in intron 76. Parental studies showed that the two mutations were inherited from different parents, and thus present in trans . Together, the clinical findings, muscle biopsy, and genetic testing results are consistent with a diagnosis of nemaline myopathy due to compound heterozygous mutation in NEB . As both parents are carriers and the condition is autosomal recessive, the risk of recurrence for future pregnancies is 25%.
The childhood-onset form of nemaline myopathy was first described in a large autosomal dominant Australian kindred by Laing and colleagues. Early motor development was normal, with the development of symmetrical weakness of ankle dorsiflexion in the late first or early second decade. Weakness was slowly progressive with eventual involvement of all ankle movements, and more proximal limb musculature. Two older family members were wheelchair-bound by age 40 years. Cardiac muscle was spared.
The late-onset or adult-onset form of nemaline myopathy is heterogeneous in terms of clinical presentation and disease progression. Patients with a true isolated adult-onset form of nemaline myopathy have no family history and no preceding symptoms, and onset of proximal and distal weakness occurs in the third to sixth decades. Respiratory and cardiac involvement occurs in the minority of cases, but in these patients the disease often follows a clearly progressive course.
The cardinal features of nemaline myopathy are generalized weakness and hypotonia. Muscle weakness is usually most severe in the face, the neck flexors, and the proximal limb muscles. In some patients there is an additional distal involvement. The extraocular muscles are most often spared. In congenital forms of nemaline myopathy, the face is often elongated and expressionless, the upper lip tented, and the palate high-arched ( Figures 28.2A and 28.4 ). The gait is usually waddling. The build is slender but muscle bulk is not necessarily reduced, especially in young children. The spine is hyperlordotic and sometimes rigid. In some patients, chest deformity is evident at birth. Tendon reflexes are depressed or absent. Gross motor development is slow, whereas fine motor activity is normal. Many patients have hypermobile joints, and joint contractures commonly develop with time. Severe arthrogryposis is not a characteristic feature of the classical or later onset forms of nemaline myopathy, but is more common (and may be quite severe) among patients with the severe neonatal form of the disease.
Respiratory problems are a common feature of congenital nemaline myopathy, not only in the neonatal period but throughout life. The degree of skeletal muscle weakness does not necessarily reflect the degree of respiratory muscle involvement. Even if asymptomatic, most patients will show restricted respiratory capacity on testing. Sleep studies are an important part of the management plan, as patients are at risk of insidious nocturnal hypoxia even in the absence of morning symptoms, and several patients have experienced sudden respiratory failure. Infants with the congenital onset form of nemaline myopathy commonly have feeding difficulties, and older patients may present with isolated swallowing difficulties. Cardiac contractility is usually normal in congenital nemaline myopathy, but cardiac involvement, particularly dilated cardiomyopathy, may rarely occur. The central nervous system is not usually affected in nemaline myopathy and intelligence is usually normal.
Laboratory examinations are of little help in making the diagnosis of nemaline myopathy. The serum CK concentration is usually normal or only slightly elevated (up to five times higher than normal). EMG may be normal in young patients and mild cases but usually shows polyphasic motor unit potentials with small amplitude, and a full interference pattern during weak effort (early recruitment). In addition to these “myopathic” features, electromyographic signs often interpreted as neurogenic (large motor unit potentials with discrete pattern on full effort, and abnormal jitter) may develop with time, especially in distal muscles. However, most patients will have normal findings on examination of peripheral nerves, including normal conduction velocities. Ultrasonography often shows abnormally high echogenicity in affected muscles, computed tomography (CT) will show low density of muscles with preservation of volume, and magnetic resonance imaging (MRI) will commonly reveal fatty infiltration of the muscle tissue. Muscle MRI (particularly T1 imaging) is increasingly used as an adjunct diagnostic modality, and certain nemaline myopathy subtypes (particularly due to NEB mutations) have relatively specific patterns of selective muscle involvement ( Figure 28.3 and Table 28.5 ).
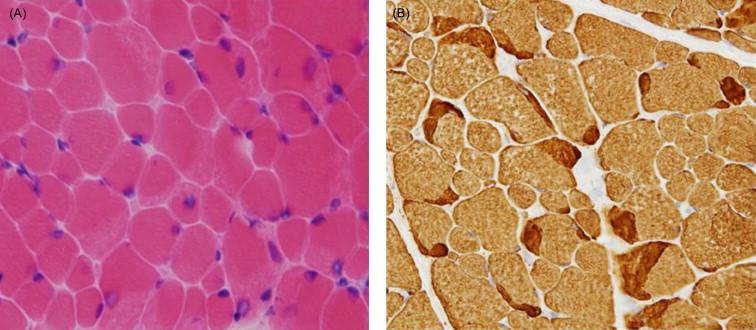
The pathologic changes of all genetic forms of nemaline myopathy are much the same irrespective of the severity of the clinical manifestations or the age of onset. The diagnostic hallmark of these disorders is the presence of distinct rod-like inclusions, so-called nemaline bodies, in the skeletal muscle fibers of affected patients ( Figure 28.1A , Figure 28.4 , and Figure 28.5 ). The rods are not visible on hematoxylin and eosin (H&E) staining, but appear as red or purple structures against the blue-green myofibrillar background with the modified Gomori trichrome stain. The distribution of rods within myofibers may be random, but they show a tendency to cluster under the sarcolemma and around nuclei. The proportion of fibers containing rods varies from case to case and from muscle to muscle, and the number of rods in a muscle specimen does not appear to correlate with disease severity. Replacement of muscle fibers by fat and fibrous tissue may be seen in advanced cases, but necrotic and regenerating fibers are uncommon. Inflammation is not a feature. There may be internal nuclei and occasional fiber splitting.
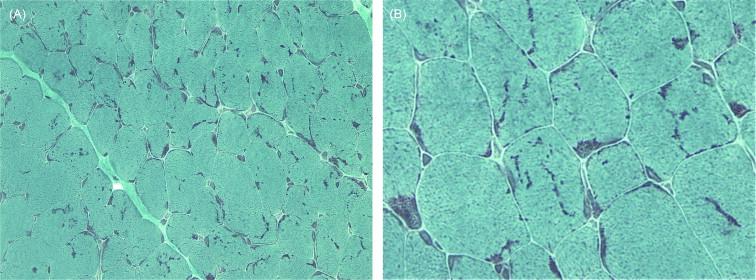
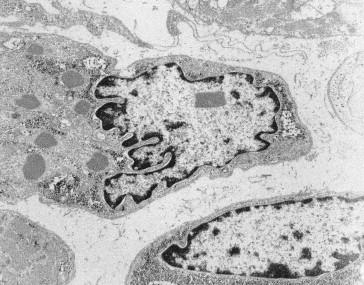
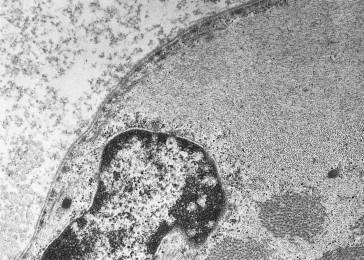
The rods are considered to be derived from the lateral expansion of the Z-line, based on their structural continuity with Z-lines and electron density and criss-cross pattern on electron microscopy (EM), and positive staining with antibodies to α-actinin-2 and α-actinin-3 which are major components of the Z-line in skeletal muscle ( Figure 28.6 ). Focal disruption of the myofibrillar pattern is often seen on EM, and there may be accumulations of thin filaments in areas devoid of sarcomeric structure. Predominance of type 1 fibers is a common feature of nemaline myopathy, and some patients show exclusively type 1 fibers or poor fiber type differentiation. In many biopsies, the mean diameter of the type 1 fibers is smaller than that of the type 2 fibers. Fiber type 1 predominance and atrophy, i.e. fiber type disproportion, tend to become more prominent with age, and there is often a complete deficiency of type 2B (fast glycolytic) fibers. Rods are usually present in both type 1 and 2 fibers, though are restricted to type 1 fibers in patients with TPM3 mutations. Biopsies from patients with severe forms of the disease and extensive myofibrillar disruption often have numerous small rods, and sometimes these rods are visible only on electron microscopy.

Whereas nemaline bodies typically occur in the sarcoplasm of the muscle fiber, occasional instances of intranuclear nemaline bodies ( Figures 28.7 and 28.8 ) have been observed in muscle biopsies from patients with severe neonatal myopathy, often caused by ACTA1 mutations and in some adult-onset cases (so-called SLONM or sporadic late-onset nemaline myopathy), presumably reflecting an autoimmune condition with a progressive course. Intranuclear rods are generally considered to be associated with more severe muscle involvement and a worse prognosis. Rarely, a combination of rods and cores (described below) are noted, and are described as core-rod myopathy. Genes associated with this finding include ACTA1 , TPM2 , NEB , RYR1 , and KBTBD13 .
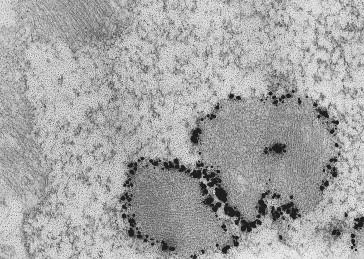
Two rare variants of nemaline myopathy are cap myopathy and zebra body myopathy. Cap myopathy is defined by well-delineated areas on muscle biopsy ( Figure 28.9 ), which are eosinophilic on hematoxylin and eosin stain (giving the appearance of caps on the myofiber). Cap myopathy has been observed most commonly in patients with TPM2 and TPM3 mutations and reported once in a patient with an ACTA1 mutation. Zebra bodies are extremely rarely encountered, and have been observed in association with nemaline bodies, suggesting that they may represent a variant form of the condition. The identification of ACTA1 mutations in some individuals with zebra body myopathy strengthens the hypothesis that zebra body myopathy is likely a variant of NM.
Involvement of cardiac muscle is unusual in nemaline myopathy, although occasional cases have been reported of dilated cardiomyopathy, most often in the setting of ACTA1 mutations. There is a single report of cardiac dysfunction with TPM2 mutation as well. However, in most autopsy studies, nemaline bodies are not present in heart muscle, supporting the assumption that most individuals with NM are not at risk of cardiac disease. Atypical cases have been documented with rods present in both the diaphragm and cardiac muscle, but these are exceptional.
Nemaline bodies may occur as a secondary phenomenon in a number of conditions, including tenotomized rat muscle, mitochondrial myopathy, and human immunodeficiency virus (HIV) infection. Combinations of histologic abnormalities characteristic for different congenital myopathies, particularly cores, have been encountered within families, sometimes even in the same patient. It is not justified, therefore, to make the diagnosis of hereditary nemaline myopathy solely on the basis of pathology in the absence of a typical clinical picture.
Nemaline myopathy is genetically heterogeneous. Mutations in nine different genes have been identified in a subset of nemaline myopathy patients: α-tropomyosin slow ( TPM3 ), nebulin ( NEB ), skeletal muscle α-actin ( ACTA1 ), β-tropomyosin ( TPM2 ), KBTBD13 (exclusively in core-rod myopathy, discussed later), CFL2 , KLHL40 , KLHL41 , and troponin T ( TNNT1 ) (see Table 28.3 ). Five of these genes encode protein components of the thin filaments of muscle fibers, while the others likely participate as regulators of the degradation/turnover of the thin filament apparatus. Because of this, nemaline myopathy is broadly considered a disorder of the thin filament. There are additional cases of nemaline myopathy that do not link to any of the identified loci, suggesting further genetic heterogeneity. The mode of inheritance of nemaline myopathy is variable, with autosomal recessive and autosomal dominant families, as well as sporadic cases. All mutations identified to date in the NEB gene are inherited in an autosomal recessive fashion. Both autosomal dominant and autosomal recessive families have been identified with mutations in TPM3 and ACTA1 . Many of the mutations identified in the skeletal muscle α-actin gene represent new dominant mutations appearing as sporadic cases. The proportion of new mutations and the incidence of germline mosaicism in patients with nemaline myopathy are unknown.
As yet, no definitive clinical or pathologic markers for the various genetic forms of nemaline myopathy have been identified, although a clue to the presence of TPM3 mutations may be the selective involvement of type 1 fibers in these patients. Congenital and childhood-onset forms of nemaline myopathy have been described in association with mutations in the TPM3 and ACTA1 genes, while only severe cases have been uncovered with mutations in KLHL40 . Patients with mutations in the NEB gene were initially thought to largely fall into the typical congenital category, but recent studies have identified cases with a variety of clinical presentations including the neonatal, severe form of the disease. Detailed pathologic studies may provide morphologic clues to guide mutation analysis. For example, α-tropomyosinSLOW ( TPM3) is expressed only in type 1 (slow) fibers, and fiber atrophy and nemaline bodies in patients with the TPM3 mutation occur preferentially in type 1 fibers. Similarly, abnormal accumulation of actin filaments, and abnormal distribution of actin staining have been observed in patients with ACTA1 mutations whose clinical presentations can range from the most severe, with neonatal lethal presentations associated with fetal akinesia, to mild congenital forms compatible with full lifespans and reproductive fitness ( Figure 28.10 ). The number of patients that have been studied in detail is still too small to conclude whether these findings are specific. Of note, ACTA1 mutations are most commonly encountered in dominantly inherited disease and NEB mutations are the most common recessive cause. Of the remaining genes, KLHL40 , TPM3 , and TPM2 are the next most frequently encountered, while CFL2, TNNT1 , and KLHL41 mutations appear extremely rare. KLHL40 mutations are particularly common in severe autosomal recessive NM. The del7lys mutation in TPM2 is a highly recurrent dominant mutation described in more than 50 individuals.
Core myopathies are defined by the presence, in myofibers, of regions devoid of oxidative staining, which may appear as large longitudinally oriented central cores in central core disease (CCD) ( Figure 28.1, B and E ), or multiple smaller “minicores” in multiminicore myopathy. Cores seen in association with other characteristic findings, such as nemaline rods, can lead to hybrid diagnoses, often termed core-rod myopathies, but genetic analysis may allow delineation based on the underlying etiology; for example, there are nemaline myopathies with cores associated with mutations of ACTA1 , NEB , TPM2 , or KBTBD13 and CCD or minicores with nemaline bodies caused by mutations of the skeletal muscle ryanodine receptor, RYR1 . The “pure core myopathies,” CCD and multiminicore myopathy, are most often associated with mutations in RYR1 (see Case Example 28.2 ), though it is not the only cause. SEPN1 mutations are the next most frequently encountered, and are associated with minicore myopathy without ophthalmoplegia. This subsection will discuss both types of core myopathy and associated conditions.
KL is the first child of a nonconsanguineous Caucasian couple with no family history of neuromuscular disease. During the pregnancy, decreased fetal movements were noted. Fetal hydrops was identified on ultrasound but later resolved. KL was born at 38 weeks by Caesarean section for a breech position. Birth weight was 3.055 kilograms. At birth he had mild respiratory distress, hypotonia, severe weakness, and multiple joint contractures (bilateral clubfeet and bilateral knee and wrist contractures), as well as bilateral hip dislocations.
Soon after early infancy, he developed kyphoscoliosis, which first required bracing and eventually (by age 5) surgical correction. He never achieved the ability to sit, crawl, or walk. He did not, however, have significant eye muscle or bulbar weakness. In addition, he could feed adequately despite having significantly reduced body mass.
At 9 years of age, KL had mild lower facial weakness, proximal greater than distal weakness, decreased muscle bulk, hypotonia, hip and knee contractures, and absent deep tendon reflexes. He was nonambulatory and had severe restrictive lung disease, but did not require continuous respiratory support.
KL had a comprehensive diagnostic workup in the first years of life. His karyotype was normal. He had two muscle biopsies. The first, done in infancy, showed only atrophy. The second biopsy, done after 1 year of age, showed central cores ( Figure 28.11A and B ), increased adipose tissue, atrophy, and endomysial fibrosis. Based on the second biopsy, sequencing of the entire coding sequence of the ryanodine (receptor gene RYR1 ) was performed. A novel variant was identified, c.14759C>T (p.Thr4920Ile), in a conserved amino acid located in close proximity to several well-established central core disease-associated mutations. Together, the clinical findings and diagnostic testing results are consistent with a diagnosis of central core disease due to RYR1 mutation. Parental RYR1 testing was not performed. However, this variant is likely de novo , with a probable risk of recurrence of <1%.
CCD was the first congenital myopathy defined on the basis of specific morphologic changes in skeletal muscle. As noted earlier, the disorder was first described by Shy and Magee in 1956, and the term “central core disease” coined by Greenfield, Cornman, and Shy in 1958. The characteristic ultrastructural abnormality of CCD is the presence, in the majority of muscle fibers, of centrally placed cores. A core is a circumscribed lesion of disorganized myofibrils with absent mitochondria and absent staining for oxidative enzymes, phosphorylase, and glycogen. The important and potentially fatal association of CCD with malignant hyperthermia was first noted by Denborough and colleagues in 1973. CCD is a rare condition, but its true incidence is not known.
The original case report of Shy and Magee exemplifies the typical clinical manifestations of CCD. The disorder usually presents in infancy with weakness and hypotonia. Motor milestones are delayed; many patients do not walk until 3 to 4 years of age. Muscle weakness is symmetrical and mild; active movement of all muscle groups against gravity and resistance is usually present, and most patients achieve ambulation. Weakness is more severe in the lower limbs and preferentially affects proximal musculature. Usually patients are only mildly disabled, and weakness is nonprogressive or only slowly progressive. However, a subset of individuals may present with severe neonatal weakness, arthrogryposis, and respiratory failure. These individuals usually do not walk independently, and often continue to require respiratory support.
Patients with CCD often have poor muscle bulk, but muscle atrophy is not conspicuous. Fasciculations are absent, and deep tendon reflexes are reduced or absent. Mild weakness of facial and neck muscles may occur, but the extraocular muscles are usually spared. Muscle cramps were a predominant feature in one family, and many patients complain of muscle pain or stiffness on moderate exertion. Musculoskeletal deformities such as kyphoscoliosis, congenital dislocation of the hip, pes cavus, pes planus, and thoracic deformities occur frequently. The severity of the deformity often does not correlate with the degree of muscle weakness; in some individuals, skeletal deformities are the sole manifestation of disease. Significant respiratory insufficiency is unusual, cardiac abnormalities occur extremely rarely, and intellectual performance is not impaired.
Although the majority of patients reported display the “typical” clinical phenotype, central cores may be found in individuals who are asymptomatic, in patients with mild weakness or skeletal deformity, and in patients with a raised CK as their only manifestation of the disorder. As mentioned above, a small subset of patients with CCD can have more severe muscle weakness and motor impairment. Amburgey et al. describe two cases in a larger series that typify this severe presentation. Both children had weakness, hypotonia, and arthrogryposis noted at birth. Neither achieved independent ambulation, and both had respiratory difficulties and severe musculoskeletal complications (including severe scoliosis requiring corrective surgery in the first decade of life). Muscle biopsy in both cases demonstrated typical central cores. Mutation analysis revealed de novo heterozygous sequence changes in the CCD hotspot area of RYR1 .
CCD is associated with an increased risk of malignant hyperthermia (MH). MH is an autosomal dominant disorder characterized by an increase in skeletal muscle metabolism in response to certain inhalational anesthetics (particularly halothane) and depolarizing muscle relaxants (particularly succinylcholine). It can also be seen in response to extreme heat or exercise in susceptible individuals. The association between CCD and MH is complex. Most patients with MH have histologically normal muscle, and less than 30% of patients with CCD have MH susceptibilty. Both CCD and MH are most commonly caused by mutations in the RYR1 gene. As such, MH is considered a disorder of calcium metabolism; this is further confirmed by the fact that the other known genetic cause of MH is mutations in the L-type calcium channel.
In anesthesia-related MH, the triggering agent increases the sarcoplasmic calcium concentration, resulting in an increased production of heat. The major symptom is generalized hyperthermia, which may be fatal if untreated. Despite general recognition among anesthesiologists, MH is still considered to be one of the most frequent causes of death during anesthesia. At present, susceptibility to MH can be diagnosed only by means of the pharmacologic in vitro contracture test and/or by demonstration of one of the proven MH mutations in RYR1 . Triggering anesthetic agents should be avoided in susceptible individuals. As the first exposure to the trigger substances elicits an event in only 50% of MH-susceptible patients, a previous history of tolerance of halothane or succinylcholine does not ensure that these anesthetic agents can be used safely in the future.
Two unique MH-related syndromes are well described and worth noting. The first is King-Denborough syndrome, which is characterized by the triad of myopathy (including mild proximal weakness and a distinctive gait), dysmorphic features (including hypertelorism and plagiocephaly), and susceptibility to MH. Muscle biopsy may be normal or may demonstrate nonspecific myopathic changes. All genetically solved cases to date are due to RYR1 mutations. However, some individuals with the King-Denborough phenotype are RYR1 mutation negative, indicating genetic variability. Also, the possibility that in some instances King-Denborough syndrome is due to multiple simultaneous gene mutations (i.e. double trouble) has not been excluded. The other syndrome is Native American myopathy (NAM), which has to date only been identified in Lumbee Native Americans in North Carolina (USA). Like King-Denborough syndrome, this condition is characterized by mild facial dysmorphism, skeletal abnormalities, and mild extremity weakness. All individuals with NAM identified to date harbor recessive mutations in STAC3 . STAC3 is a protein associated with the triad that serves as a regulator of excitation-contraction coupling.
In patients with CCD the serum CK is normal or slightly raised, and the EMG is usually normal or myopathic with short-duration, small-amplitude polyphasic motor unit potentials. The average number of fibers innervated by a single anterior horn cell is increased, resulting in abnormal single-fiber EMG studies. Muscle ultrasound may also be used to demonstrate increased echogenicity associated with the primary myopathic process. Muscle MRI is useful for diagnostic assessment, as individuals with CCD due to RYR1 mutations typically have selective sparing of rectus femoris ( Figure 28.3 and Table 28.5 ).
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here