Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The congenital and infantile neuropathies are an uncommon and complex group of conditions with broad phenotypic and genetic diversity. The overwhelming majority have a genetic basis, and will be the main focus of this chapter. Rarely, acquired inflammatory causes may occur. Although significant progress has been made in the genetic diagnosis of infantile neuropathies in the past two decades, more than half of children presenting in the first year of life remain without a specific diagnosis. With advances in molecular genetic techniques, the already rapidly expanding number of genes attributed to infantile neuropathies is set to further increase.
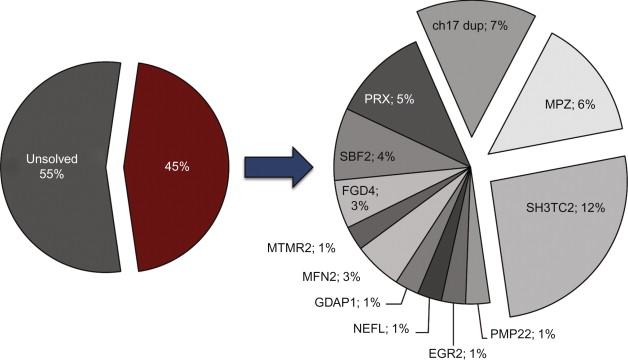
In a large cohort of 260 children with biopsy-confirmed peripheral neuropathy over a 33-year period, 50 (19.2%) were symptomatic in infancy, of whom 48% had a demyelinating and 42% an axonal disorder. While many of these cases presented prior to the era of genetic diagnosis, 20 children fell under the rubric of Charcot-Marie-Tooth disease (CMT)/ hereditary motor and sensory neuropathy (HMSN), 19 of whom had a demyelinating form of CMT/HMSN. Five children had spinal muscular atrophy (with axonal findings on nerve biopsy), three had severe infantile axonal neuropathy with respiratory failure, and four had hereditary sensory autonomic neuropathy. Other etiologies were either single cases (e.g. giant axonal neuropathy) or nonspecific diagnoses, such as peroxisomal or “neurodegenerative” disorders, and many of these infants had co-existent central nervous system involvement. More recently, in a series of 77 children with infantile onset (nonsyndromic) neuropathies (58% demyelinating, 20% axonal, and 22% undefined), 35 children (45%) received a molecular diagnosis involving mutations in one of eleven genes, most of which were associated with demyelinating forms of CMT ( Figure 16.1 ). This emphasizes the genetic heterogeneity of the infantile neuropathies, and that demyelinating phenotypes are more likely to receive a definitive genetic diagnosis, but that over half of children remain without one.
Infants with early onset neuropathies tend to display one of two main phenotypes.
The first group has clear prenatal/neonatal onset and presents at birth with hypotonia, arthrogryposis, and respiratory insufficiency. A history of decreased fetal movements, intrauterine growth retardation, and polyhydramnios may be obtained. Delivery of a hypotonic infant may result in obstetric complications and birth asphyxia, masking the underlying peripheral neuropathy. Ventilatory support is often required and early mortality common. De novo mutations in MPZ , PMP22 , EGR2 , and NEFL are typically found in this cohort ( Figure 16.2 ). Spinal muscular atrophy (SMA) and the non-5q early-onset SMA syndromes are important differential diagnoses for this type of presentation, and, as discussed below, are not always easily differentiated from a classic neuropathy.
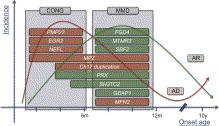
The second (and more common) group presents later in infancy with delayed motor development and/or foot deformities, after an uneventful neonatal period. In contrast to adults, in whom distal weakness and sensory loss are the predominant features of a neuropathy, delayed motor milestones (an indicator of infantile onset) and areflexia are more relevant clinical clues at this age. Proximal weakness, foot deformity, scoliosis and congenital hip dysplasia may be apparent, and the clinical features may mimic myopathy. Facial weakness and tongue fasciculations may be present, reflecting a severe neuropathic process. Recessive mutations in FGD4 , PRX , MTMR2 , SBF2, GDAP1 , and SH3TC2 , and dominant mutations in MFN2 , are strongly represented in this subgroup ( Figure 16.2 ).
It is important to determine: (i) whether the neuropathy is primarily demyelinating/hypomyelinating or axonal; (ii) whether the infant has an isolated peripheral neuropathy or neuropathy in association with a central nervous system (CNS) disorder; (iii) the inheritance pattern; and (iv) if there are any distinguishing features on clinical, neurophysiologic, or neuropathologic examination.
The demyelinating and axonal infantile neuropathies will be presented separately in this chapter. Conditions with isolated peripheral nervous system involvement, versus those with associated CNS involvement, will be distinguished. Most of the isolated peripheral neuropathies in this age group have both motor and sensory involvement, and fall under the umbrella of CMT/HMSN, which is outlined in Chapter 15, Chapter 17 . The hereditary motor neuropathies overlap with both CMT/HMSN and the motor neuronopathies (such as SMA), and will be mentioned only briefly as they are covered in detail in Chapter 8 . The hereditary sensory and autonomic neuropathies (HSAN), with preferential involvement of the sensory and autonomic systems, are discussed in Chapter 18 . In general, the axonal neuropathies are less well characterized and more often associated with CNS or systemic manifestations, which can provide helpful diagnostic clues. Distinguishing clinical features of the congenital and infantile demyelinating and axonal neuropathies are presented in Tables 16.1 and 16.2 .
| Clinical Feature | Clinical Phenotype | Gene |
|---|---|---|
| Pupillary abnormalities | CHN, DSD, CMT1B CMT4C |
MPZ SH3TC2 |
| Multiple cranial neuropathies | CHN, DSD, CMT4E | EGR2 |
| Prominent foot and hand deformities | CMT4A, CMT2H/K CMT4C |
GDAP1 SH3TC2 |
| Severe spinal deformities | CMT4C | SH3TC2 |
| Asymmetric weakness | DSD, CMT4J GBS/CIDP |
FIG4– |
| Post-traumatic rapid progression of weakness | DSD, CMT4J | FIG4 |
| Vocal cord paresis | CMT4A, CMT2H/K CMT4B1 |
GDAP1 MTMR2 |
| Diaphragmatic involvement | CMT4A, CMT2H/K | GDAP1 |
| Glaucoma | CMT4B2 | SBF2 |
| Prominent sensory involvement | DSD, CMT4F | PRX |
| Congenital cataracts | HCC CCFDN |
FAM126A CTDP1 |
| Raised creatine kinase | Merosin-deficient CMD | LAMA2 |
| Clinical Feature | Clinical Phenotype | Gene | Inheritance |
|---|---|---|---|
| Optic atrophy | CMT2A | MFN2 | AD, AR |
| GDAP1 -related neuropathies | GDAP1 | AR | |
| IOSCA | C10orf2 | AR | |
| Infantile neuroaxonal dystrophy | PLA2G6 | AR | |
| Riboflavin transporter deficiency | SLC52A2, SLC52A3 | ||
| Ophthalmoparesis | Mitochondrial disorders | SCO2 | AR |
| C10orf2 | AR | ||
| TK2 | AR | ||
| Skeletal abnormalities | CMT2C, SPSMA, congenital dSMA | TRPV4 | AD |
| Congenital contractures/ arthrogryposis | Congenital dSMA | TRPV4 | AD |
| SMARD1 | IGHMBP2 | AR | |
| X-linked SMA | UBE1 | X-linked | |
| Pontocerebellar hypoplasia type I | EXOSC3 , | AR | |
| VRK1 , | |||
| TSEN54 , | |||
| RARS2 | |||
| SMA with congenital bone fractures | Unknown | Unknown, presumed AR | |
| Congenital fractures | X-linked SMA | UBE1 | X-linked |
| SMA with congenital fractures | Unknown | Unknown, presumed AR | |
| Vocal cord paresis | CMT2A | MFN2 | AD, AR |
| CMT2C , SPSMA, congenital dSMA | TRPV4 | AD | |
| GDAP1 -related neuropathies | GDAP1 | AR | |
| Riboflavin transporter deficiency | SLC52A2, SLC52A3 | AR | |
| Early infantile respiratory failure | SMA1 | SMN1 | AR |
| SMARD1 | IGHMBP2 | AR | |
| X-linked SMA | UBE1 | AR | |
| Pontocerebellar hypoplasia type I | EXOSC3, VRK1, TSEN54, RARS2 | AR | |
| SMA with congenital fractures | Unknown | Unknown, presumed AR | |
| Lethal neonatal AR axonal sensorimotor polyneuropathy | Unknown | AR | |
| Congenital axonal neuropathy with encephalopathy | Unknown | Unknown, presumed AR | |
| Predominant motor involvement | Congenital dSMA, SPSMA | TRPV4 | AD |
| SMA1 | SMN1 | AR | |
| CMT2O / SMA-LED | DYNC1H1 | AD | |
| X-linked SMA | UBE1 | X-linked | |
| Pontocerebellar hypoplasia type I | EXOSC3, VRK1, TSEN54, RARS2 | AR | |
| SMA with congenital fractures | Unknown | Unknown, presumed AR | |
| Mitochondrial disorders | SCO2, TK2 | AR | |
| Kinky hair | Giant axonal neuropathy | GAN | AR |
| Hepatopathy | Mitochondrial disorders | DGUOK | AR |
| C10orf2 | AR | ||
| MTP/LCHAD deficiency | HADHA/HADHB | AR | |
| Cardiomyopathy | Mitochondrial disorders | SCO2 | AR |
| TK2 | AR | ||
| DGUOK | AR | ||
| MTP/LCHAD deficiency | HADHA/ HADHB | AR | |
| CNS involvement | Pontocerebellar hypoplasia type I | EXOSC3, VRK1, TSEN54, RARS2 | AR |
| Giant axonal neuropathy | GAN | AR | |
| Infantile neuroaxonal dystrophy | PLA2G6 | AR | |
| HMSN/ACC | KCC3 | AR | |
| IOSCA | C10orf2 | AR | |
| CMTX1 | GJB1 | X-linked | |
| CMT2O / SMA-LED | DYNC1H1 | AD | |
| With encephalopathy | |||
| Congenital axonal neuropathy | Unknown | Unknown, presumed | |
| AR | |||
| Mitochondrial disorders | SCO2 | AR | |
| DGUOK | AR | ||
| TK2 | AR | ||
| RMND1 | AR | ||
| With cerebellar signs/involvement | |||
| Pontocerebellar hypoplasia type I | EXOSC3, VRK1, TSEN54, RARS2 | AR | |
| Giant axonal neuropathy | GAN | AR | |
| Infantile neuroaxonal dystrophy | PLA2G6 | AR | |
| IOSCA | C10orf2 | AR |
Nerve conduction studies are central to determining whether a neuropathy is primarily demyelinating or axonal. As discussed in Chapter 3 , nerve conduction velocities increase during the first 2 to 3 years of life, so normative values should be referenced. As nerve conduction studies, particularly sensory studies, can be technically challenging in infants and neonates, absent sensory responses should be interpreted with caution. Infants with severe neuropathies may have unobtainable motor and sensory responses, making it difficult to discriminate between a primarily demyelinating or axonal process. Testing of more proximal nerves (such as the phrenic nerve) can be helpful in this instance, while needle electromyography (EMG) helps to distinguish myopathies from neuropathies. Conduction slowing may be so severe in some demyelinating neuropathies that the time base for nerve conduction testing needs to be adjusted to identify very delayed responses.
It is important to appreciate that the distinction between axonal neuropathies and neuronopathies is not always clear. Involvement of both peripheral motor and sensory nerves is described in neuronopathies such as classic SMA and spinal muscular atrophy with respiratory distress type 1, while mutations in genes such as TRPV4 and DYNC1H1 can cause both an SMA phenotype and axonal CMT. Motor responses may be unobtainable, and reliance on absent sensory responses to localize pathology to the nerve rather than anterior horn cell (as may be done in adults or older children) is not always appropriate. Combined with the inability of electromyography to differentiate between axonal motor neuropathies and neuronopathies, this means that it can be difficult to distinguish axonal sensorimotor neuropathies from motor neuronopathies in early childhood. Motor neuronopathies should therefore form part of the differential diagnosis of infants presenting with hypotonia, weakness, areflexia, and neurogenic electromyography findings (and vice versa).
Nerve biopsy can be very informative in determining the basis of prenatal and infantile neuropathies, particularly if neurophysiologic findings are unhelpful. Specific neuropathologic abnormalities may point to particular genetic syndromes. Distinguishing nerve biopsy features of the inherited demyelinating and axonal neuropathies are presented in Table 16.3 . Acquired and potentially treatable causes of neuropathies such as infantile chronic inflammatory demyelinating polyneuropathy (CIDP), although rare, may mimic or occur superimposed on genetic neuropathies, and can be diagnosed on nerve biopsy.
| Neuropathologic Feature | Clinical Phenotype | Gene |
|---|---|---|
| Demyelinating infantile neuropathies | ||
| Focal myelin folding | Frequently present MPZ -related CHN/DSD CMT4B1 CMT4B2 CMT4B3 CMT4F CMT4H Occasionally present EGR2 -related neuropathies GDAP1 -related neuropathies CMT4C |
Frequently present MPZ MTMR2 SBF2 SBF1 PRX FGD4 Occasionally present EGR2 GDAP1 SH3TC2 |
| Uncompacted myelin | MPZ -related CHN/DSD | MPZ |
| Long Schwann cell cytoplasmic processes | CMT4C | SH3TC2 |
| Node of Ranvier disorganization | CMT4C | SH3TC2 |
| Paranodal abnormalities | CMT4F CMTX1 |
PRX GJB1 |
| Axonal infantile neuropathies | ||
| Giant axons | Giant axonal neuropathy CMT4C CMT2E / CMT1F Myofibrillar myopathy |
GAN SH3TC2 NEFL BAG3 |
| Axonal spheroids | Infantile neuroaxonal dystrophy | PLA2G6 |
| Mitochondrial abnormalities | CMT2A | MFN2 |
Cerebral neuroimaging is not always required in the work-up of infants presenting with a purely peripheral process. However, magnetic resonance imaging (MRI) can sometimes provide important diagnostic clues, and is especially helpful in the investigation of syndromic disorders affecting the central nervous system.
Despite the genetic heterogeneity of infantile neuropathies, they tend to share typical management issues. Respiratory insufficiency may be related to diaphragmatic weakness (caused by involvement of the phrenic nerve), intercostal muscle weakness, or spinal deformity. Ventilatory support may be required from birth or later in the disease course, and assessment for respiratory insufficiency is warranted in most cases. Bulbar weakness may cause poor feeding and recurrent aspiration. Gastroesophageal reflux is probably more common than is recognized. Skeletal deformities including foot deformity, developmental hip dysplasia, and scoliosis are common and should be appropriately managed. Visual disturbance due to optic atrophy or cataracts occurs in some conditions, as does sensorineural hearing loss.
The long-term prognosis of individuals with early-onset demyelinating neuropathies is variable and not well documented. Outcomes are dependent not only on the underlying genetic mutation, but likely also environmental and epigenetic factors, given the inter- and intrafamilial variability often present. Individuals with significant respiratory involvement tend to have a poorer prognosis than those without respiratory compromise. Appropriate genetic counselling should always be provided.
Congenital hypomyelinating neuropathy (CHN), Dejerine-Sottas disease (DSD, also referred to as CMT3 or HMSN III) and CMT4 (also referred to as autosomal recessive CMT1, ARCMT1) are all forms of demyelinating CMT with early onset. The nomenclature is very confusing, as the original descriptions and classification were made prior to the availability of genetic testing, and use of the terms (in particular, DSD and CHN) is inconsistent. A single gene can cause multiple phenotypes of variable severity, and a single phenotype can be caused by mutations in more than one gene.
The features of DSD were defined by Dyck and Lambert and then by Ouvrier et al . as: recessive or sporadic inheritance, onset in infancy or the first two years of life, delayed motor development, extremely slow nerve conduction (motor nerve conduction velocity, MNCV<12 m/s), elevated cerebrospinal fluid (CSF) protein, and nerve biopsy features of a marked reduction in myelinated fiber density, thin myelin sheaths, and onion bulb formations. Most children are areflexic. Foot deformity is common.
Many children with DSD have de novo dominant mutations in MPZ , PMP22 , or EGR2. In addition, individuals with CMT4 (autosomal recessive CMT) sometimes have a DSD phenotype. Thus the term DSD is probably best used to describe the clinical syndrome of a severe demyelinating genetic neuropathy manifesting before two years of age with hypotonia or delayed motor development, with a median nerve MNCV of 12 m/s or less.
CHN is rare and less well characterized than DSD. It is thought to reflect a defect in myelin synthesis rather than degeneration and/or demyelination of preexisting myelin. In its classic form, CHN is characterized pathologically by complete absence of peripheral nerve myelin without onion bulb formations, and the term congenital amyelination has also been used in these cases. Some individuals with CHN have severe hypomyelination and/or unusual “basal lamina onion bulbs” consisting of concentric whorls of double-layered Schwann cell basement membrane. In these “atypical” onion bulbs, Schwann cell processes are thought to have degenerated, leaving behind their basal laminae. Evidence of myelin degeneration is absent. In comparison, nerve biopsies in DSD show evidence of both hypomyelination and demyelination-remyelination, classic onion bulb formation, and myelin breakdown products.
Severe cases of CHN present at birth with profound weakness, hypotonia, arthrogryposis, and respiratory insufficiency. Nerve conduction is very slow or responses are absent. Tongue fasciculations may be present. Less severe cases present in infancy with hypotonia and motor delay. Complete absence of peripheral nerve myelination does not always predict prenatal or neonatal onset, or early demise, and survival into adulthood is documented. Hence the discrepancy between biopsy findings and clinical features, as well as overlap in genetic causation, makes the distinction between CHN and DSD of limited utility in the diagnosis and management of these patients, particularly in the era of genetic testing in which nerve biopsy may not be required. CHN and DSD are best thought of as a spectrum of early onset “myelinopathies.” Variable mutations (sometimes even identical mutations) in MPZ , PMP22 , and EGR2 can cause both phenotypes. It may be more appropriate to refer to these early onset disorders as infantile- or prenatal-onset neuropathies associated with a particular genetic cause if known. See Case Example 16.1 .
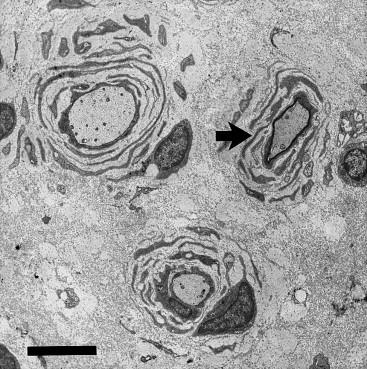
This male infant was born at term via normal delivery after a pregnancy complicated by decreased fetal movements. He had early respiratory distress requiring ventilatory support. He was severely hypotonic, had a marked reduction in spontaneous movement, and was areflexic. Nerve conduction studies were consistent with a severe demyelinating neuropathy with a median nerve MNCV of 10 m/s. Sensory responses were unobtainable. Nerve biopsy showed a moderate reduction in myelinated fibers, and thinned/incomplete myelination of existing myelinated fibers. Electron microscopy showed generalized uniform thinning of the myelin sheaths in intermediate and large fibers ( Figure 16.4 ). The patient died from respiratory failure at 6 weeks of age. Genetic testing for mutations in MPZ , PMP22 , and EGR2 was negative. While this infant had a severe congenital phenotype, myelin was still present, illustrating the discrepancy sometimes seen between clinical severity and degree of hypomyelination.
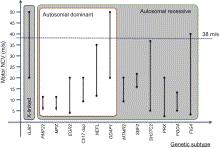
A summary of the clinical and neurophysiologic phenotypes associated with the demyelinating CMT genes is presented in Table 16.4 . Figure 16.3 illustrates the nerve conduction velocities and inheritance patterns associated with these genes.
| Gene | Clinical Phenotypes in Infancy | Inheritance | MNCV (m/s) | Other Features | Other Clinical Phenotypes |
|---|---|---|---|---|---|
| PMP22 (point mutation) | CHN, DSD | AD, AR | <12 | SNHL, facial weakness, nystagmus, vestibular dysfunction | CMT1A, HNPP |
| PMP22 (duplication) | DSD | AD AR |
9–20 10–17 |
CMT1A | |
| PMP22 (deletion) | DSD | AR a | <10 | SNHL | HNPP |
| MPZ | CHN, DSD | AD, AR | <12 | Pupillary abnormalities, SNHL, facial weakness | CMT1B, Intermediate CMT, CMT2 |
| EGR2 | CHN, DSD, CMT4E | AD, AR | 3–20 | Cranial neuropathies frequent | CMT1D |
| NEFL | DSD, CMT1F, CMT2E | AD, AR | 12–35 | Intellectual disability, pyramidal signs, SNHL | CMT1F, CMT2E |
| GJB1 | CMTX1 | X-linked | 20–50 | SNHL, tremor, transient CNS disturbances | CMTX1 |
| GDAP1 | CMT4A, CMT2H, CMT2K (AR) | AR | 20–60 | Vocal cord paresis and diaphragmatic involvement. Severe distal weakness, prominent foot deformity and claw hands | CMT2K (AD) |
| MTMR2 | CMT4B1 | AR | 9–20 | Facial weakness | |
| SBF2/MTMR13 | CMT4B2 | AR | 16–21 | Congenital or juvenile-onset glaucoma | |
| SH3TC2 / KIAA1985 | CMT4C | AR | 4–37 | Early-onset spinal deformities. Foot deformities, SNHL, abnormal pupillary responses | |
| PRX | DSD, CMT4F | AR | 2–20 | Prominent sensory involvement | |
| FGD4 | DSD, CMT4H | AR | 5–13 | Slow progression | |
| FIG4 | DSD, CMT4J | AR | 3–41 | Asymmetric weakness, rapid progression following trauma |
a Only compound heterozygous deletions associated with infantile neuropathies.
Heterozygous duplications involving PMP22 cause CMT1A, the most frequent type of CMT, while the reciprocal deletion causes hereditary neuropathy with pressure palsies (HNPP). In the context of infantile neuropathies, heterozygous (often de novo ) point mutations in PMP22 are associated with DSD and, less commonly, CHN. PMP22 duplications may also manifest in infancy.
Almost all children with DSD/CHN due to PMP22 point mutations have delayed development; many do not walk until 3–4 years of age, and some only with assistance. A small number never achieve independent ambulation. Increasing weakness may result in wheelchair dependency in later life. Scoliosis and restrictive lung disease may occur ; rarely respiratory involvement leads to early death. Other features reported with PMP22 mutations include sensorineural hearing loss, facial weakness, and rarely nystagmus, vestibular dysfunction, ptosis, limited extraocular movements, and bladder symptoms.
On nerve conduction studies MNCV is almost always <10–12 m/s. Sensory responses are often absent and compound muscle action potentials (CMAPs) are usually low amplitude, reflecting secondary axonal loss. Findings on nerve biopsy are usually typical of DSD with thin myelin sheaths, myelinated fiber loss, increased total transverse fascicular area (due to increased collagen fibers), and prominent onion bulb formations, composed of thin Schwann cell lamellae or basal membranes or both ( Figure 16.5 ). In those cases designated as CHN, hypomyelination is more marked.
Less commonly recessive PMP22 mutations (homozygous point mutations or compound heterozygous deletions ) cause classic DSD. Hence in children with a severe phenotype carrying a PMP22 deletion, analysis for mutations in the other PMP22 allele should be performed.
Heterozygous PMP22 duplications (usually associated with a classic CMT1 phenotype) can also cause an early-onset phenotype, accounting for 7% of all infantile neuropathies in the series by Baets et al . , compared to 1% for PMP22 point mutations ( Figure 16.1 ), although the MNCV may lie in the CMT1A rather than DSD range. Congenital foot deformities in the presence of otherwise normal early development are common in these infants. Nerve biopsies have a lower g-ratio (thicker myelin sheaths) compared to PMP22 point mutations. Homozygous duplications of PMP22 , albeit rare, can also cause DSD, although curiously, the homozygous state is not always associated with greater clinical severity or additional slowing of motor nerve conduction. See Case Example 16.2 .
A 2-year-old girl was born at term with bilateral foot deformities and mild generalized hypotonia. She had delayed motor development and walked at 19 months of age. At 2 years of age she had bilateral pes cavus, normal muscle bulk, hypotonia of the lower limbs, a steppage gait, and absent ankle reflexes. On nerve conduction studies her median nerve MNCV was 17 m/s. Chromosomal microarray testing revealed the typical 17p11.2 duplication seen in CMT1A. Testing of both parents indicated her father was also affected. He did not complain of any neurologic symptoms but on examination had bilateral pes cavus and absent ankle reflexes.
While most individuals with a PMP22 duplication present during childhood or adolescence, some present during infancy with congenital/early onset foot deformities and/or motor delay. A tendency to toe-walk may be a feature. On nerve conduction studies the MNCV tends to lie in the CMT1A rather than DSD range. In infants, lower limb wasting may not be evident, or it may be obscured by subcutaneous fat. The CMT1A duplication should be considered in any child with an inherited demyelinating neuropathy, even in those presenting during infancy.
PMP22, a 22-kDa glycoprotein, accounts for 2–5% of peripheral nerve compact myelin, and may play a role in proliferation and differentiation of Schwann cells. Missense mutations within the transmembrane domains (where the majority of point mutations lie) are thought to result in toxic gain-of-function effects including impaired intracellular protein trafficking, accumulation of mutant PMP22 in the endoplasmic reticulum (ER), inhibition of post-endoplasmic reticulum protein degradation, and cell apoptosis.
Clinical phenotypes associated with MPZ mutations include CHN/DSD, CMT1B, intermediate CMT, and a CMT2-like phenotype. Most subjects fall into one of two phenotypes—an early onset demyelinating neuropathy, or a later onset neuropathy with axonal features. Mutations are generally phenotype-specific. MPZ mutations accounted for 6% of all infantile neuropathies in a large series ( Figure 16.1 ).
Children with early-onset demyelinating MPZ neuropathies usually have a DSD phenotype, presenting with hypotonia or delayed motor development. Most do not walk until three to four years of age, and some as late as ten years. Increasing weakness and scoliosis may develop, and some become wheelchair-dependent. A few cases designated as CHN have been reported , including two presenting at birth with hypotonia, arthrogryposis, respiratory failure, and almost no myelin on nerve biopsy. Pupillary abnormalities may be seen with both early- and late-onset MPZ mutations, providing an important diagnostic clue. Facial weakness, sensorineural hearing loss, bulbar problems, and, rarely, ptosis with limited extraocular movements are reported. While data on long-term outcomes are limited, most subjects survive into adulthood, albeit requiring mobility assistance.
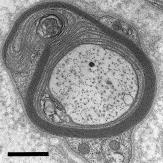
Motor conduction velocities are usually less than 12 m/s. CMAP and SNAP (sensory nerve action potential) amplitudes are reduced (and sometimes unobtainable) in all patients. Apart from the expected neuropathologic findings of myelinated fiber loss, myelin thinning (or absent myelin in some cases of CHN), and onion bulb formations, two distinct and mutually exclusive ultrastructural abnormalities have been described in some cases: focally folded myelin sheaths, or areas of uncompacted myelin, reflecting the role of MPZ in myelin adhesion and compaction ( Figures 16.6 and 16.7 ).

While the overwhelming majority of early onset neuropathies associated with MPZ are due to dominant heterozygous (often de novo ) mutations, recessive homozygous or compound heterozygous mutations are occasionally identified. In these cases, heterozygous parents have a milder neuropathy, with MCNVs ranging from 29 to 54 m/s.
MPZ is a member of the immunoglobulin gene superfamily. It functions as a homophilic adhesion molecule, playing an important role in myelin adhesion and compaction. Most mutations causing early-onset demyelinating neuropathies are in the extracellular domain and probably alter the MPZ tertiary structure and thus myelination. A dominant-negative effect of mutant MPZ at the plasma membrane has been demonstrated, as has impaired mutant protein trafficking with ER accumulation, which can induce apoptosis and protein degradation. See Case Example 16.3 .
An 11-month-old infant was referred with a 5-month history of generalized hypotonia and decreased movement of the left upper limb. Over the following 6 months he developed proximally predominant weakness of the other extremities. Language and social development were normal. He had generalized hypotonia but was able to sit without support when placed. He had no movement around the left shoulder girdle, reduced power in the lower limbs, and was areflexic. Nerve conduction studies indicated a sensorimotor demyelinating neuropathy, with MNCVs of 5–12 m/s, marked increase in distal motor latencies, and absent sensory responses. The CMAPs were of very low amplitude. The CSF protein was mildly elevated (0.48 g/L).
Sural nerve biopsy showed severe depletion of large myelinated fibers, with focal thickening of myelin sheaths. No onion bulbs or inflammatory cell infiltrates were seen. Numerous tomacula-like structures were identified on the teased fiber preparation. Focally folded myelin sheaths were noted on electron microscopy. Genetic testing revealed a de novo heterozygous Lys130Arg mutation in the MPZ gene. At 6 years of age he can ambulate short distances with a walker but otherwise requires a wheelchair for mobility. He has scoliosis and restrictive lung disease and has had a number of respiratory infections.
The initial presentation with an upper limb monoplegia in this case is unusual for an inherited neuropathy, and a diagnosis of infantile CIDP was considered in the first instance. A mildly elevated CSF protein is not specific for inflammatory neuropathies, as this finding may also be present in inherited neuropathies. The severe generalized slowing of nerve conduction and absence of inflammatory infiltrates on the nerve biopsy were more consistent with an inherited neuropathy, and the presence of focally folded myelin sheaths on electron microscopy provided an important diagnostic clue and directed sequencing of the MPZ gene. Excessive myelin outfoldings are seen in demyelinating neuropathies due to mutations in the MPZ , SBF1 , SBF2 , MTMR2 , PRX , and FGD4 genes ( Table 16.3 ). As the most common causes of infantile-onset demyelinating neuropathies are mutations in the MPZ , PMP22 , and SH3TC2 genes, the MPZ gene was sequenced in the first instance.
Mutations in EGR2 account for less than 1% of CMT cases overall and 1% of infantile neuropathies. Approximately 11 cases of CHN/DSD have been reported, the majority due to de novo heterozygous missense mutations. Recessive inheritance was identified in one family with three affected siblings (designated CMT4E), and a clinical phenotype similar to the heterozygous cases. CMT1D is also associated with EGR2 mutations.
Most cases present in the first few months of life with hypotonia and delayed motor development. Peculiar to EGR2 -related neuropathies is a high frequency of cranial nerve involvement. Ptosis, eye movement abnormalities, tongue fasciculations, and facial weakness are described. The course is slowly progressive in most cases. Motor conduction velocities are usually <12 m/s but may be as high as 20 m/s. Nerve biopsies show a range of hypomyelination (from almost complete myelin absence to thinly myelinated axons), as well as myelinated fiber loss, demyelination/remyelination, and onion bulb formations. Focally folded myelin sheaths were described in one patient.
EGR2 is a zinc finger transcription factor which binds regulatory domains of target genes, including those critical for myelin formation and maintenance such as MPZ , PMP22 , GJB1 , and PRX . Most mutations lie in the zinc finger domains, likely affecting DNA binding and transcription of these genes.
Dominant mutations in neurofilament light chain polypeptide ( NEFL ) cause CMT1F and CMT2E. While most individuals with NEFL mutations become symptomatic in childhood or adolescence, a number of severe early-onset cases have been caused by dominant, or occasionally recessive, mutations. NEFL mutations accounted for 1% of infantile neuropathies in one series. Affected children present before the age of two years with hypotonia, delayed motor development, or foot deformity. Distal weakness and wasting can be severe, and proximal weakness may be present. Additional features in early-onset patients include intellectual disability, pyramidal signs, ataxia, tremor, and hearing loss.
Neurophysiologic findings are variable in NEFL -related neuropathies. Mixed axonal and demyelinating features may be present. In those with the early-onset phenotype, MNCVs are between 12 and 35 m/s. CMAPs are usually moderately to severely reduced.
NEFL mutations probably do not cause a true “demyelinating” neuropathy, but rather an axonal neuropathy with secondary myelin loss. NEFL assembles into heteropolymers with heavy and medium neurofilaments, forming the major intermediate filament in axons. Neurofilaments determine axonal diameter and therefore conduction velocity. NEFL mutations disrupt neurofilament assembly and axonal transport, resulting in decreased axonal diameter, contributing to slowed conduction. On nerve biopsy the predominant finding is loss of large myelinated fibers with regenerating clusters. Some individuals have giant axons containing accumulations of irregularly oriented neurofilaments.
In infancy, recessive GDAP1 mutations are associated with an early onset neuropathy with demyelinating and/or axonal features which has been designated CMT4A, CMT2H/K, and recessive “intermediate” CMT. In reality these conditions probably lie on a pathologic spectrum. GDAP1 mutations accounted for 1% of infantile neuropathy cases in Baets et al.
The clinical phenotype associated with recessive GDAP1 mutations is fairly homogeneous. Children present in the first year of life with prominent foot deformities, gait difficulties, and distal weakness. Some are hypotonic at birth but many have normal early motor development and walk by 2 years of age, although are often limited by foot deformity. Progression is relatively rapid, leading to severe distal weakness and wasting of the feet and hands. Many patients develop “claw hands” within the first or second decade, and foot deformities are often severe. Progressive proximal weakness renders many subjects wheelchair-dependent by the end of the second decade. A more slowly progressive course is less common. Vocal cord paresis, diaphragmatic dysfunction, and restrictive lung disease are common. Facial weakness is described and one patient had optic atrophy.
Both axonal and demyelinating features are apparent. MNCVs range from 20 to 60 m/s, with MNCVs in the demyelinating, axonal (most commonly), and intermediate range in individual patients. CMAPs are low amplitude or unrecordable. On nerve biopsy some patients have a predominantly demyelinating process, with a decrease in the number of myelinated fibers, segmental demyelination, and frequent onion bulb formations. Focal myelin folding is occasionally noted. More commonly, a primarily axonal process is present, with axonal degeneration, regeneration, and pseudo-onion bulbs. In some, the picture is mixed.
The GDAP1 protein is expressed in axons, localized to the outer mitochondrial membrane, and involved in mitochondrial fission and regulation of the mitochondrial network.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here