Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The integrity of the soft tissue (ST) envelope is of paramount importance for any and all foot and ankle reconstructions. Although injury to the ST envelope is most often associated with trauma-related care, elective surgical procedures also are prone to ST complication, as well as certain disease states (e.g., Charcot arthropathy with ulceration, diabetic ulcerations, vascular disease, and infections). This chapter will discuss the reconstructive options for both the ST envelope and critical size bone defects of the foot and ankle. The burgeoning subspecialization of “orthoplastics” has evolved, focusing concentration on the reconstruction of both the ST envelope and bone.
The spectrum of ST deficiency may be broadly divided into complications related to surgery, traumatic wounds, infection, diabetes-related complications, secondary to limb deformity, and prolonged recumbency (decubiti ulcers). Each category has corresponding considerations that impact the ST/osseous reconstruction. Wounds secondary to dermatologic conditions will not be discussed in this chapter.
Prevention is the basic tenet to wound management. Appropriate vascular examination should occur in an escalating fashion: pulses and capillary fill time (CFT), Doppler, arterial-brachial index (ABI), and CT- and MR-angiography or formal angiography. Transcutaneous oxygen pressures (TcPO 2 ) and fluorescein skin imaging play roles in chronic wound management and intraoperative assessments of skin perfusion. A nutritional evaluation is indicated in medically compromised patients, as well as glycemic control in diabetics. Presurgical skin management in elective cases, such as control of edema and dermatoses, must be considered ( Table 42-1 ).
| Lab Value, Normal Range | ||
|---|---|---|
| Nutritional | ||
| Albumin | 3.4–5.0 g/dL | |
| Total protein | 6.4–8.2 g/dL | |
| Hemoglobin | 13.2–18.0 g/dL | |
| Hemoglobin A1C | 4.8–5.6% (HI) | |
| 25-Hydroxy vitamin D | 20–50 mg/mL | |
| Serum calcium | 8.5–10.1 mg/dL | |
| Vitamin A | 20–60 mcg/dL | |
| Physiologic states | ||
| Edema | ||
| Arterial and venous states | ||
| Cardiac output | ||
| Renal function | ||
| Pulmonary (smoking, COPD) |
By far, the most common physical ST complications after a surgical incision are one of three minor healing problems: wound dehiscence, “marginal necrosis,” or slow healing. These lesser, yet very troubling, problems may present simultaneously or with infection. In any setting, infection must be actively treated and addressed, preferably by culture-specific antibiotics. The expectation of wound healing in the face of infection is erroneous; an attempt at a major ST reconstruction in the face of an active uncontrolled infection may end with calamitous results.
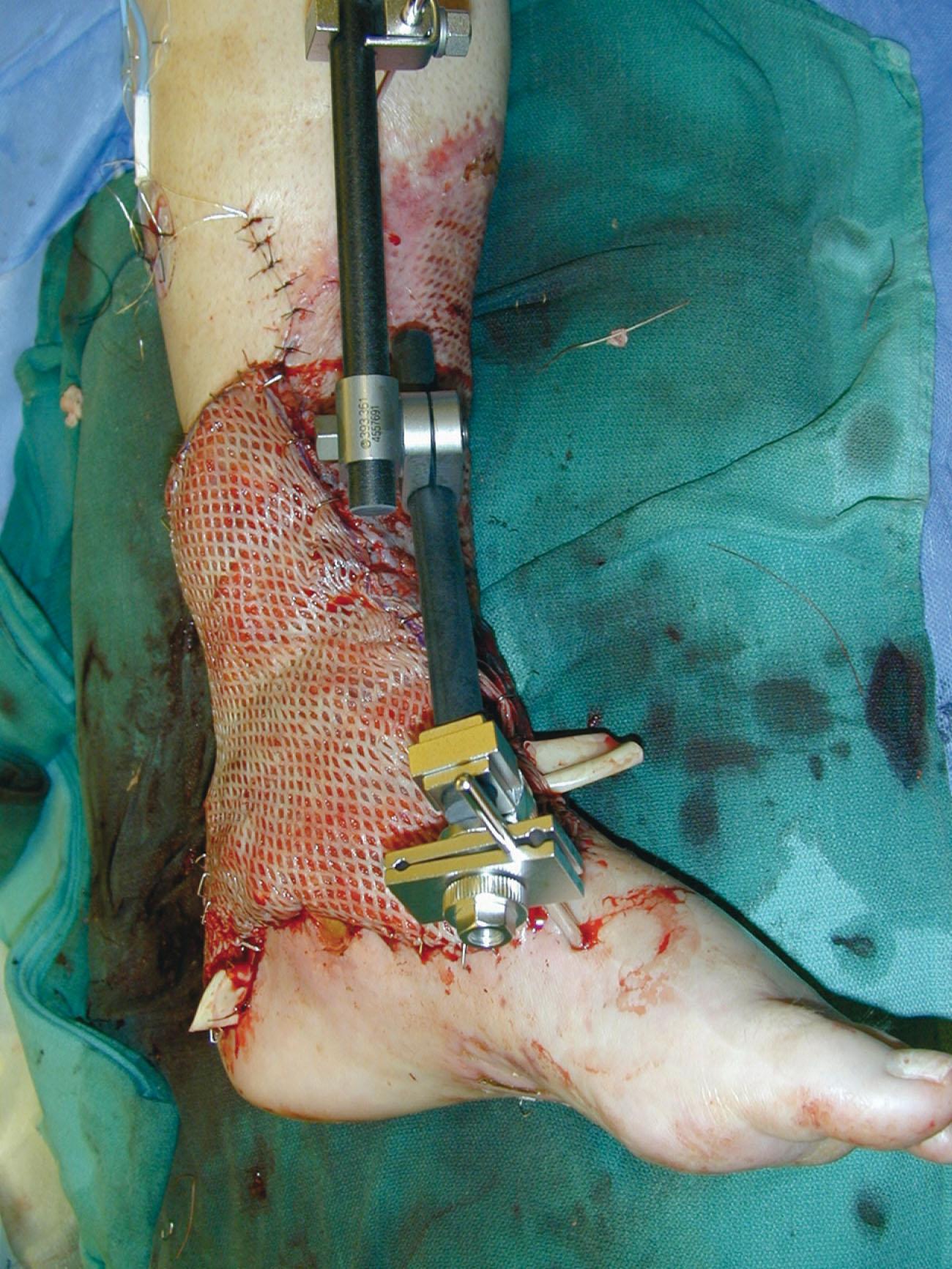
Local wound care should always promote an acute wound environment and prevent bacterial overgrowth. Normal sterile saline dressings remain an excellent first dressing choice. Dressings should be moist to damp (not dry) and performed three to four times per day. Antibiotic ointments, silver-impregnated dressings, or even washing with a bactericidal/bacteriostatic soap are also helpful. When a wound is subject to motion, such as over a joint, a limiting motion and thus “resting the incision” without tensile stresses may also promote angiogenesis. The use of an incisional negative pressure dressing may help “splint” the edges of the incision as well as provide the opportunity for unimpeded angiogenesis across the surgical wound ( Fig. 42-1 ).
In broad terms, an incision for an elective surgery implies a relatively “healthy” or maximized patient with an incisional zone that is amenable to accepting the incision. A myriad of reasons may cause an incision to develop a problem that ranges from minor to catastrophic. Examples include rough handling of ST, unexpected intraoperative or postoperative edema, poor closure, infection, prolonged tourniquet time, improper incision placement, an overlooked item, and simply bad luck. Standard measurements must be obtained in the perioperative period to mitigate the occurrence of preceding scenarios. Any developing problem must be immediately addressed. Blind neglect will always result in a greater problem. This maxim also holds true for urgent and emergent surgical cases.
Pressure wounds may occur in healthy patients, such as from a poorly constructed splint or cast, or in pressure phenomenon in a patient with Charcot arthropathy. Charcot or general neuropathic ulcerations are often hostile, with heavy bacterial burden and the features of a chronic wound. The acral distribution fosters poor local circulation. Pressure wounds of any etiology involving the Achilles region and malleoli, especially the plantar and posterior heel, are particularly difficult to reconstruct and often require a flap. In the compromised host, pressure wounds typically become chronic wounds. Successful management includes various surgical techniques as well as appropriate padding and proper bracing techniques.
Traumatic wounds differ from fresh surgical wounds by the very nature of their creation, the former being birthed via forces that result in a crush of tissue, degloving, and contamination, thus impacting an extended zone of injury greater than what is visible to the naked eye. Very often, tissue trauma exists from the skin to the bone. In the past, aggressive plating with tissue undermining and erroneous placement of incisions for open reduction internal fixation (ORIF) surgery would significantly contribute to additional tissue injury. Fortunately, refinements in intramedullary nailing and external fixation have provided techniques to reduce further ST injury after trauma. Still, the zone of injury must be respected. This region must be debrided and allowed to “declare itself” over time, a process which could range from days to weeks.
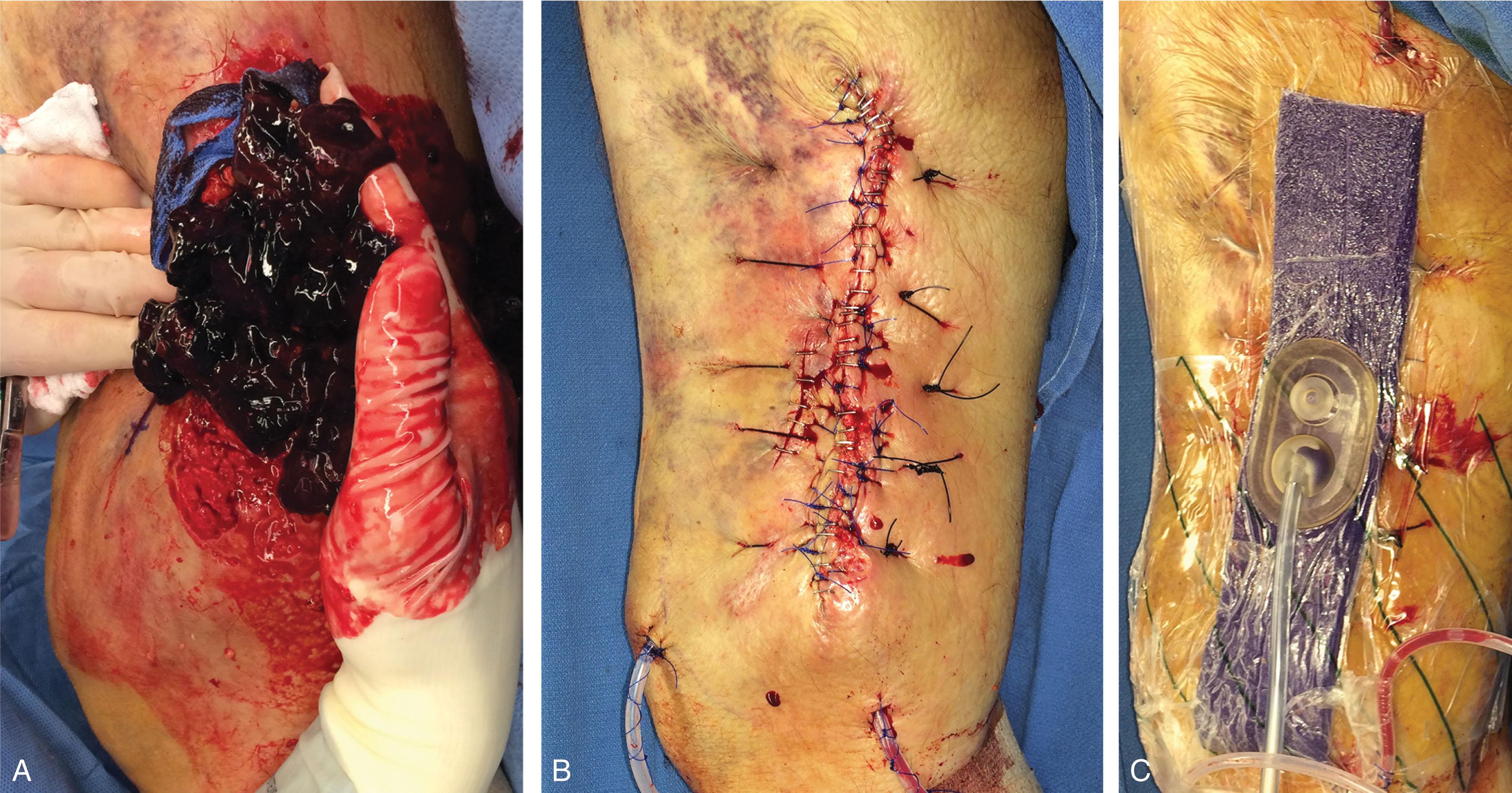
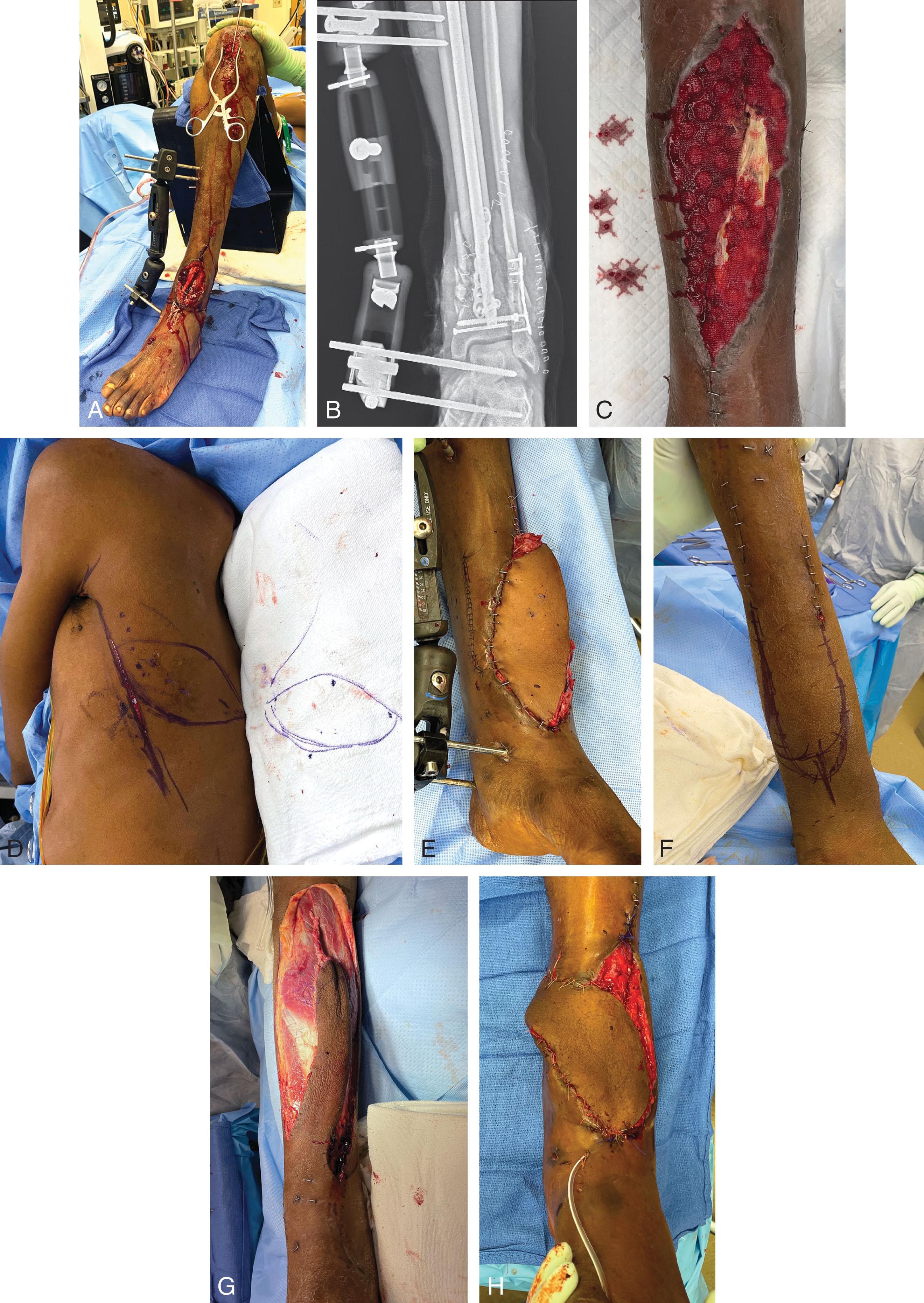
The timing of coverage of an ST defect has been a topic of discussion for decades. For ST injuries that are created by a sharp object (essentially an excision by the trauma), ST coverage may be performed immediately. However, ST defects that result from direct blunt impact, crush, shearing, degloving, or via a ballistic mechanism very often cannot be definitively covered immediately. The zone of viable versus nonviable tissue must declare itself, and repeat ST debridements must be performed. These scenarios may require traditional local dressings, antibiotic “bead pouches,” and negative pressure wound dressings (NPWDs). The use of NPWDs is an excellent temporary technique to bridge to the final ST reconstruction. Caution should be exercised because as the duration of an NPWD increases, so too does the bacterial burden of a wound. Although an NPWD is most often invaluable, the prolonged use of an NPWD may result in an unstable colonized wound bed that is unsuitable for a skin graft ( Fig. 42-2 ), requiring advanced NWPD techniques (e.g., inflow/outflow NWPD [Veraflo; 3M, Saint Paul, MN]) or attempting early wound coverage. The balance between an attempt at early coverage in an inflammatory state versus timing for development of a chronic wound (with a heavy bioburden) requires experience and keen clinical judgment. In some instances, the reconstructive surgeon is forced to prolong ST coverage ( Fig. 42-3 ).
During ST reconstruction, all surrounding ST has been recognized as the vascular envelope responsible for nurturing bone back to health. The importance of its reconstruction early in the posttraumatic course cannot be overemphasized; neither can the importance of surgical anatomy, internervous planes, vascular territories, and atraumatic techniques of dissection. Rather than dissection techniques that result in devascularization of bone and ST, a keener awareness of delicate ST handling and atraumatic technique by the orthopaedic surgeon contributes to the prevention of adverse iatrogenic sequelae after injury. Proper ST handling includes tools such as skin hooks that permit manipulation of skin and tissue flaps without causing further damage to the ST. To reconstruct the ST envelope, the surgeon must identify which layers are deficient as well as the size of the deficiency. Subsequently, the surgeon must outline a treatment plan that treats both bone and ST synergistically. Reconstructive strategies for ST should not burn any bridges—a “Plan B” should be drafted to accompany the optimal “Plan A.”
The ST envelope is composed of several tissue layers, each with a specific function and its own vascular supply. Skin, which consists of epidermis and dermis, is the first ST layer violated in the open injury, and its disruption defines the open fracture. The next layer, subcutaneous tissue, is less vascular than the dermal plexus and carries skin perforators that arise through or between muscle. Additionally, subcutaneous fat contains important veins and cutaneous nerves, provides a cushion around bony prominences, and may be quite specialized, such as on the plantar surface of the foot.
The third layer is the deep fascia. This surrounds the muscle compartments that contain a rich vascular plexus that may function as a support tissue for skin flaps. The flaps can then be transposed or transferred as a microvascular free flap with greater security.
The fourth layer consists of the muscles. These richly vascularized structures power the locomotor system. Muscles are categorized by their five varying blood supplies, as outlined by Mathes and Nahai. Muscles may be manipulated as transposition flaps, island pedicle flaps, and free tissue transplantations (free flaps). The major feature of muscle flaps is their robust blood supply and large metabolic demand. Unlike a skin flap (skin, subcutaneous fat, fascia), a muscle flap will require an intense blood supply to maintain viability. A greater venous return is required for a skin flap when compared with the arterial blood supply that is necessary to meet the metabolic demands of skin. These are important considerations when selecting a flap, as well as during the flap’s surgical execution.
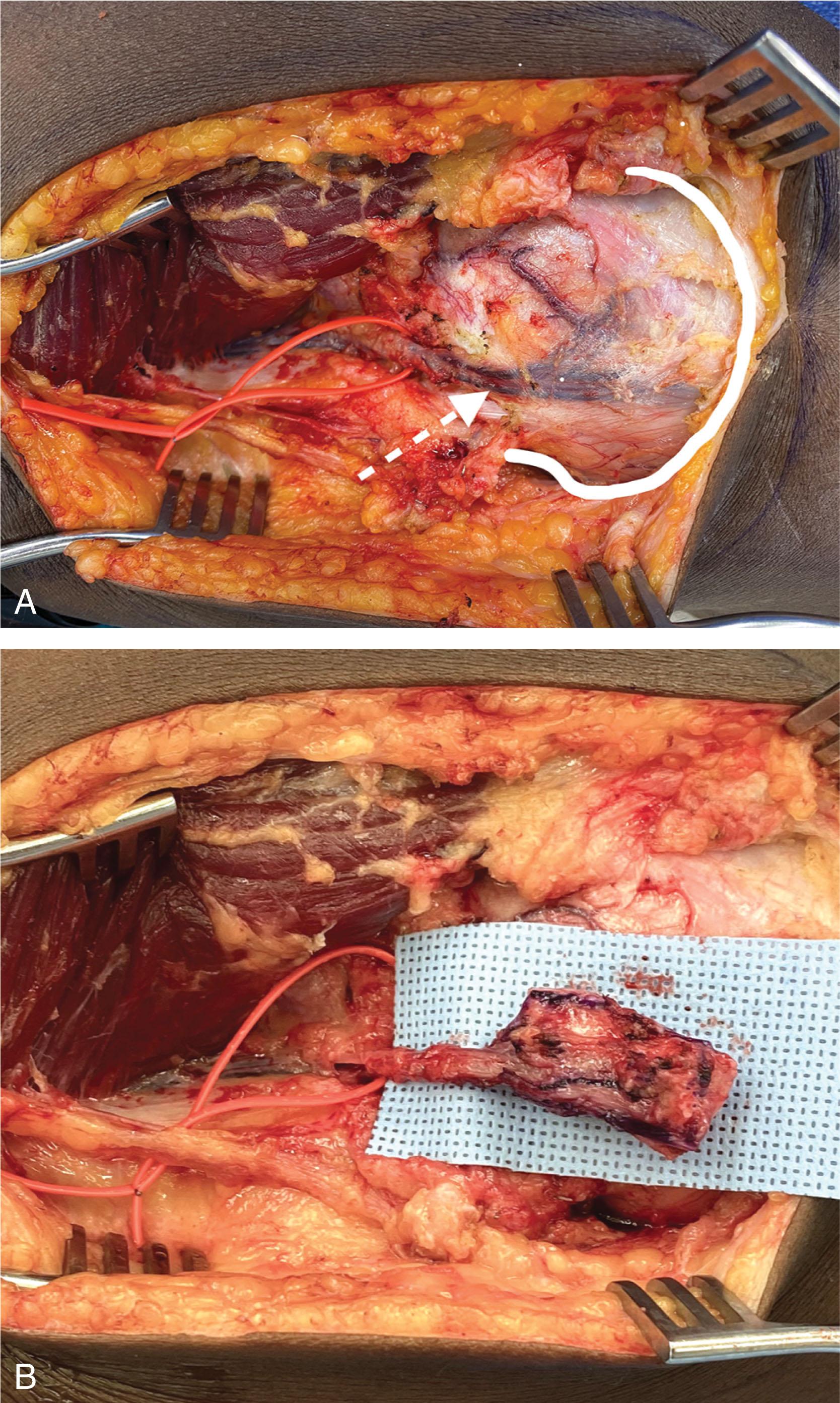
The deepest layer of the ST envelope is the periosteum, a highly vascularized membrane surrounding all bones and is most robust over the cortex of long bones. The periosteum is the blood supply to the cortical bone and is vital to the bone’s injury response and repair. Recent advances in anatomic dissection and free tissue transplantation, based on the medial geniculate artery case, have resulted in use of the periosteum as a free flap to augment conventional methods of bone grafting ( Fig. 42-4 ).
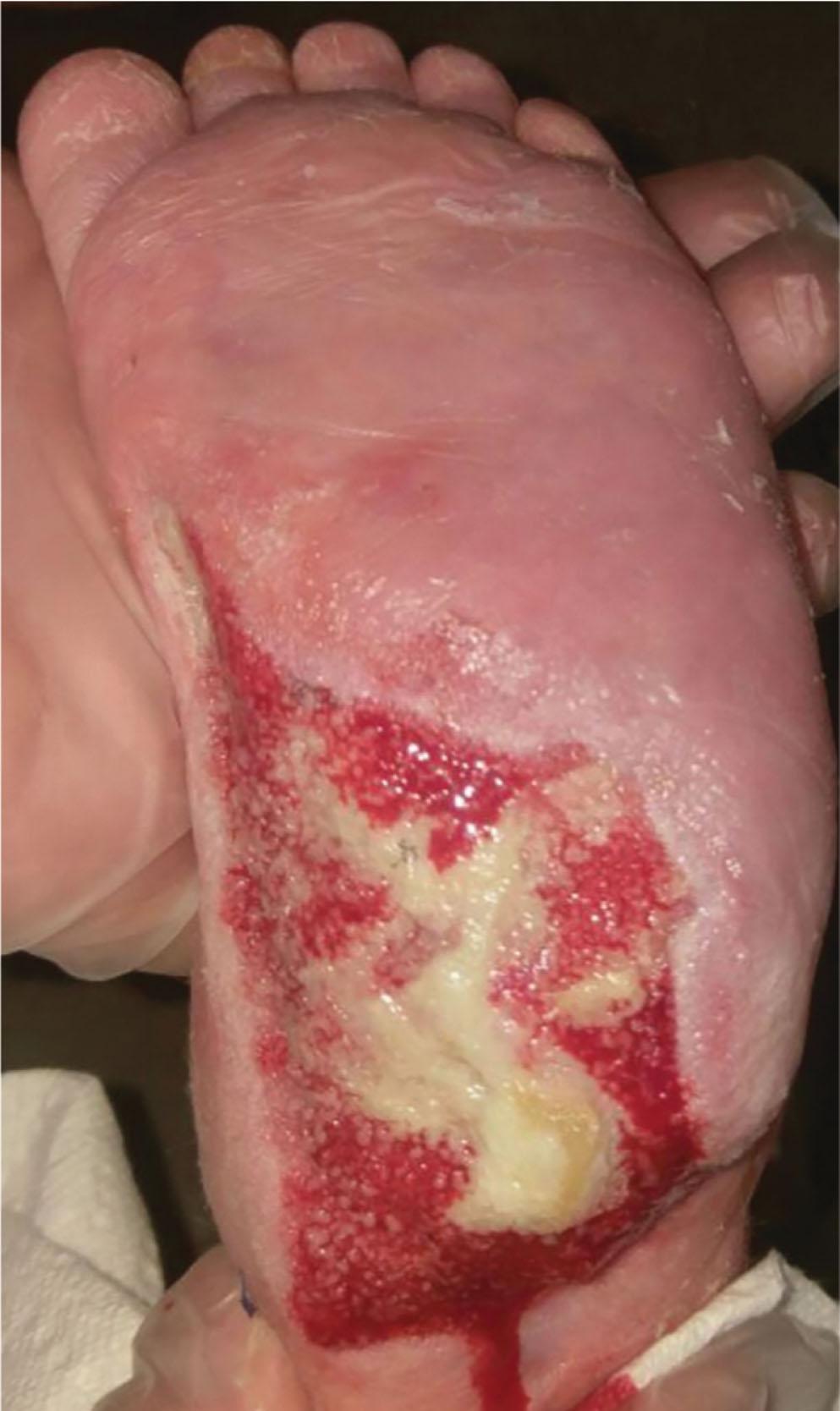
All five layers of ST will accept a split-thickness skin graft ( Fig. 42-5 ). Despite contour irregularity, a skin graft can be applied to seal the open wound if these layers are healthy and well-vascularized. Reconstituting the epithelial surface of the extremity is the first goal in wound management.
A logical method of ST reconstruction must be developed that allows bone to heal and limbs to function normally. The algorithm should be capable of being used by the foot surgeon in the setting of acute or chronic ST injury with or without fractures. In addition, it should be applicable to chronic conditions, such as osteomyelitis, nonunion, or tumor.
Reconstruction is a coordinated effort among orthopaedic surgeons, vascular surgeons, traumatologists, and plastic surgeons. It is not uncommon in practice for an orthopaedic traumatologist to stabilize a fracture, a vascular surgeon to perform an arterial interposition graft, and a microsurgeon to do a free tissue transfer. Communication and careful preoperative planning are important to ensure successful reconstruction. The ability of the reconstructive microsurgeon to deliver the appropriate tissue at the correct time enhances limb salvage.
In instances of bone fracture, the algorithm can be followed with primary hardware placement or external fixator placement, such as an Ilizarov or an Arbeitsgemeinschaft für Osteosynthesefragen (AO) external fixator. If a bone graft is needed because of missing or devitalized bone, tobramycin beads or silicone spacers can be placed in the bone defect, with subsequent ST coverage. This is then followed by secondary bone grafting with an iliac crest graft, allograft, vascularized graft, intercalary vascularized transplant such as the free fibular graft, or periosteal vascularized flaps.
The reconstructive ladder represents increasingly complex solutions to correspondingly complex problems with the same goal: reconstitution of the ST envelope and bone. The lowest rungs of the ladder are often as important as the highest rungs. Understanding the relationship between the needs of the wound and the various techniques offered by the reconstructive ladder is important. In essence, the foot and ankle surgeon must acquire an understanding of the approach used by the plastic or reconstructive microsurgeon to reconstruct deficiencies in ST. This approach, coupled with knowledge of treatment for bone deficiencies or deformities, serves as the foundation of the orthoplastic philosophy of musculoskeletal reconstruction.
Wound management follows a sequence of principles and techniques beginning with the simplest (e.g., local wound care) to the most sophisticated (e.g., free flaps). Often, clinical judgment and the reconstructive surgeon’s clinical experience may indicate the immediate implementation of more complex techniques (“reconstructive elevator”). Regardless, the ST reconstruction must consider the full exoskeleton, tendon and ligaments, bone and neurovascular tissues. The principles of meticulous tissue handling, using “like-tissue to reconstruct like-tissues,” and the utilization of “spare tissue” still apply. The ST (and bone) reconstructive effort is limited only by the creativity of the surgeon.
Evaluation of the patient with an ST injury includes assessing the overall medical state (see above), determining the time of injury, the reason for ST defect, energy absorption, fracture configuration, systemic injuries, damage to the ST envelope, vascularity of the extremity, sensibility, ultimate ability to salvage the foot (both functional and sensate), and the underlying medical conditions of the patient. These principles apply whether in the outpatient clinic, emergency department, or trauma unit.
Evaluating the traumatized limb’s perfusion is of paramount importance, and if vascular (arterial) injury is suspected, a vascular surgery or microsurgery consultation should be obtained, especially in patients who are diabetic and/or elderly. Compartment syndrome should be considered and ruled out in any injured extremity, particularly after crush injuries. A general motor examination, including both active and passive range of motion, and a detailed sensory examination should be performed. A nerve deficit may be secondary to spinal cord injury, nerve laceration, compartment syndrome, traction injury, or entrapment between bone fragments. The radiologic evaluation starts with a standard (“plain”) radiographic examination. The presence of vital implants (e.g., ankle replacement, plates/screws) must be noted. Computed tomography (CT) is indicated in instances of complex injuries, suspected bone loss, or vascular compromise (CT angiogram) and provides valuable information regarding ST damage as well.
The wound should be inspected, with the wound pattern, infection, or foreign body contamination noted. The next inspection of the wound should then be in the operating room under sterile conditions. Repetitive examination of open wounds in the emergency department has led to higher rates of wound infection and osteomyelitis and should therefore be avoided. In cases of open fractures in patients with polytrauma, workup of other injuries can take several hours, not to mention the need for emergent lifesaving visceral surgery that can precede definitive care for open fractures. Prophylactic antibiotics are administered and given on a regular basis until definitive wound debridement and fracture stabilization can be performed.
A universal ST injury classification system does not currently exist. As such, a defect’s description must be thorough and include location, depth, size, mechanism for the ST defect, vascular status, and contamination. Wounds should be categorized as occurring due to trauma, after an elective procedure, resulting from pressure wounds (such as in Charcot deformities), and occurring in medical conditions (e.g., ichthyosis and Niemann Syndrome, ulcerative melanomas). Special circumstances are given to radiation, chemical (e.g., chemotherapy), and thermal wounds.
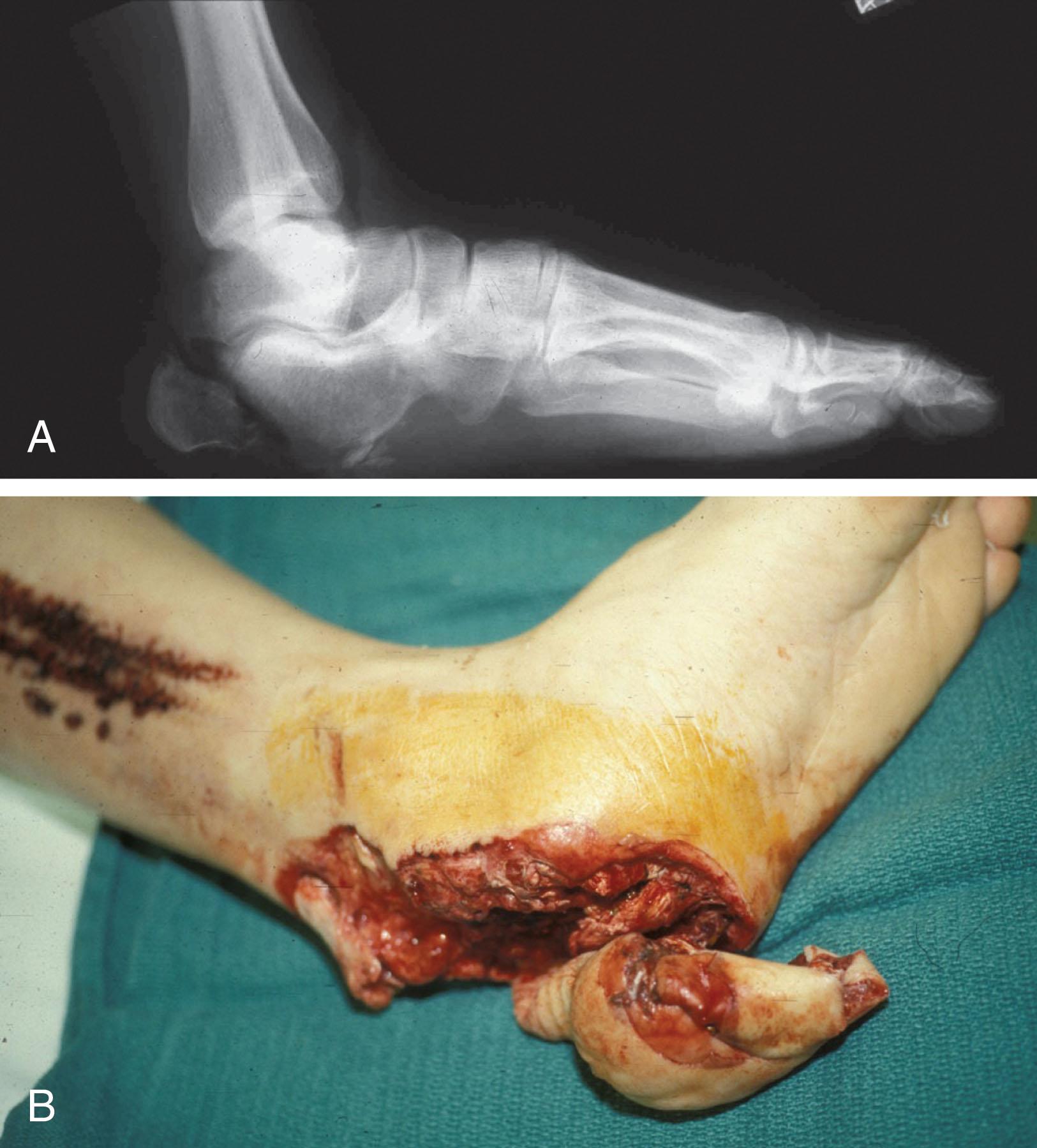
In orthopaedic trauma, assessment of the ST injury is necessary for both open and closed fractures. The Gustilo and Anderson classification system of open fractures is a three-grade classification. Type 1 fractures have a clean wound smaller than 1 cm, type 2 fractures have a laceration greater than 1 cm and without extensive ST damage, and type 3 fractures are severe ST lacerations with segmental or severely comminuted fractures in high-energy trauma. Because of variations and frequency of type 3 injuries, this group was subsequently divided into three subgroups. Type 3A injury has a large ST laceration or flaps but allows for adequate ST coverage of bone. Also included in type 3A are fractures with severe comminution or segmental fractures, regardless of the extent of ST damage. Type 3B fractures are more severe and have extensive periosteal stripping and soft tissue loss with significant bone exposure and massive contamination ( Fig. 42-6 ). Type 3C fractures have an arterial injury requiring repair.
A more comprehensive soft tissue scale, albeit more difficult to use, is the AO soft tissue scoring system. It incorporates five grades of severity and three categories of tissue (skin, muscle and tendon, and neurovascular structures). Closed fractures involving only skin are graded into four subgroups. For open fractures, four grades are given. A new feature of this system is the evaluation for muscle and tendon injuries. Because of the prognostic value, knowledge of the extent of muscle damage and tendon involvement is essential. A common approach in all classification schemes is a determination of the length of laceration of the skin. As treatment methods have become more comprehensive, and more systemic factors are taken into account when treating open fractures, the presence or absence of muscle injury, nerve injury, and vascular injury have become more prognostically important. Acute systemic factors have similarly been recognized as important prognostic indicators, such as shock, associated injuries, and old age. These influence the acute treatment of fractures and the treatment of complications. For example, debilitating factors such as smoking and malnutrition affect the feasibility of reconstruction in osteomyelitis.
Ruedi et al have developed a classification system that characterizes ST injury by addressing several layers of the ST envelope. This scale determines whether the integument is open or closed. Injuries to muscle, tendon, nerve, and vessels are graded in order of severity. Although this may be more complex than the Gustilo and Anderson classification of open fractures, it is an attempt similar to Tscherne’s classification to define in greater depth the deficiency and defects of the ST. Factors such as contusion or ecchymosis to skin and muscle must be identified to prevent further damage to these tissues in surgical dissection. Such damage of muscle or fascia territories can make these sites unreliable as replacement tissue.
Acute wounds must be thoroughly debrided to fresh tissue. Likewise, chronic wounds must be “transformed” into an acute wound. Chronic wounds are best described by the wound milieu, rather than a time scale. A mixed bacterial bioburden takes over early in the microvascular impaired wound site, producing proteinases in 24 to 48 hours that elevate the pH above the physiologic normal, rapidly converting the “normal” functional type 1 macrophage into the “unproductive” type 2 macrophage. The migration of fibroblasts and production of platelet-derived growth factor (PDGF) is also inhibited. The wound exists in this unfavorable environment until local vascular issues and the bioburden are resolved via excisional debridement. Following debridement, type 1 macrophages become predominant, PDGF becomes abundant, and endothelial progenitor cells begin to populate. Angiogenesis then ensues, and an appropriate healing cascade commences.
In the limb with compromised integument and a break in the epithelial surface, underlying subcutaneous tissue, muscle, fascia, bone, and periosteum are exposed, predisposing them to desiccation with inevitable cell death and risk of infection. To help prevent infection, a sterile moist bandage should be applied to the wound as soon as possible, bathing the damaged tissues in a physiologic medium, such as saline or Ringer’s lactate solution. A biologic dressing is the first step up the reconstructive soft tissue ladder. Preventing desiccation of tissue may reduce the extent of debridement and preserves simpler options for closure, such as skin grafts.
After local wound care and debridement, the general hierarchy of wound management is followed by revision closure, delayed primary closure, healing by secondary intent (with expectant re-epithelialization), skin grafts (with or without regenerative dermal matrices or negative pressure wound dressings), local flaps, regional flaps, free flaps, and amputation. The selection of a particular reconstructive technique is dependent upon the nature of a wound, the original goals of the surgery, and both the immediate and the long-term ST reconstructive goals.
When simple local wound care fails to produce acceptable results, debridement and expectant management with the goal of producing a good granulation bed followed by re-epithelialization can be expected for wounds slightly greater than 2 cm 2 . Wounds larger in size will require skin grafting.
Skin grafting may either be a split-thickness skin graft (STSG), a full-thickness skin graft (FTSG), or a dermal graft. Skin grafts can vary in size from a small pinch graft to a vast area of skin graft ( Fig. 42-7 ). Skin graft donor site morbidity (pain, healing, cosmesis) is to be considered when selecting a skin graft technique and especially holds true for more extensive ST reassignments.
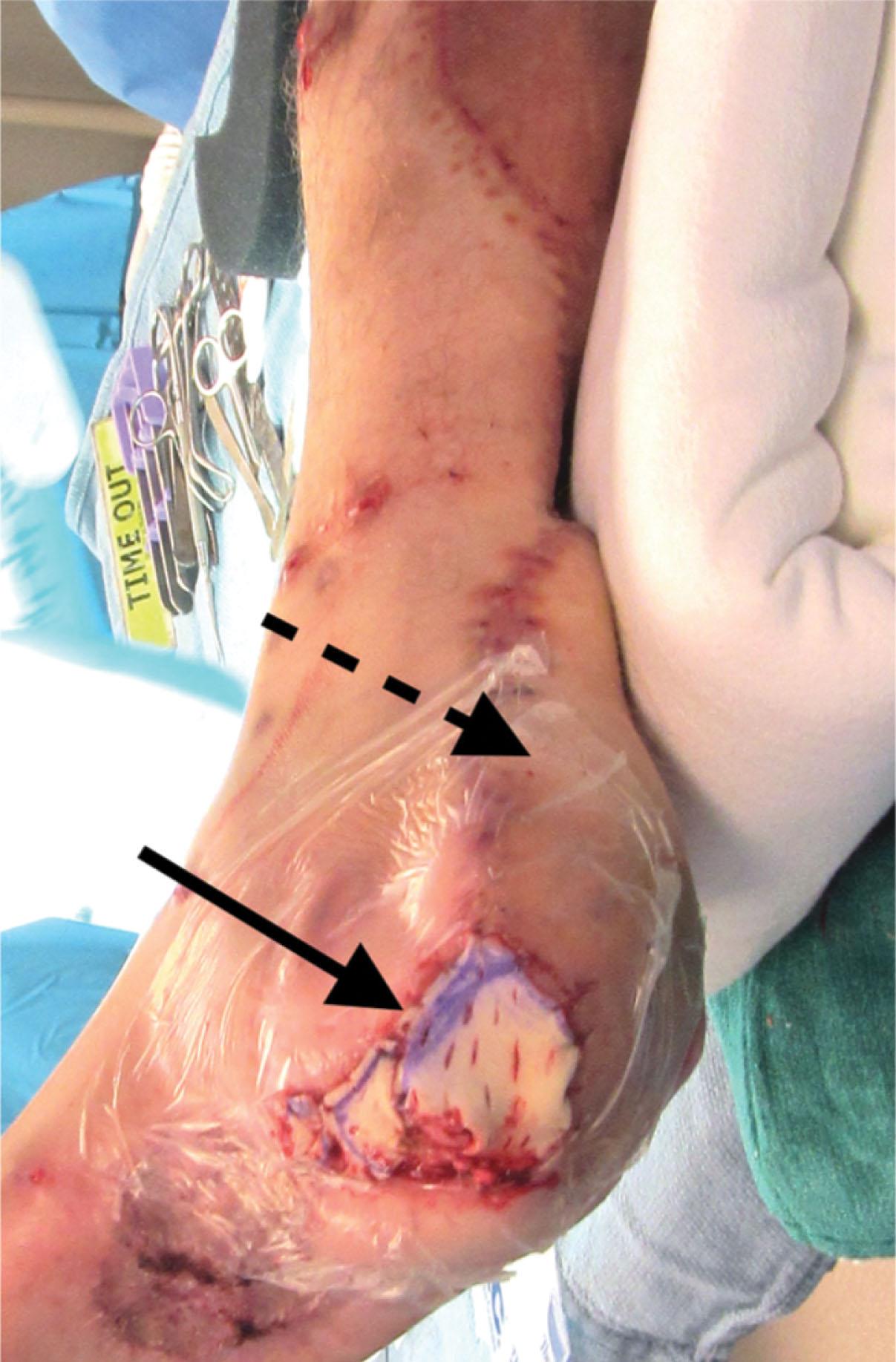
An FTSG is the best choice for areas of high shear and pressure ( Fig. 42-8 ), as well as in exposed areas, and with younger patients where skin growth is required (late contracture is minimal). The downsides of an FTSG are the limited availability of full-thickness skin (namely for the sake of donor site management) and the slightly lower rate of FTSG healing. However, in the patient with a pendulous abdomen, a relatively large FTSG may be harvested that allows for easy closure of the donor site. The thigh may also provide a sizeable FTSG, assessed by the “pinch test.” The leg, and then the foot, in that order, provide lesser sources for an FTSG. The FTSG requires meticulous de-fatting, performed with a scalpel and scissors or a hydrojet scalpel (Versajet; Smith & Nephew, Memphis, TN).
There is a greater selection of sites from which to harvest an STSG; namely the foot, thigh, buttock, abdomen, and back. They may be expanded to increase surface area (meshing), and the donor site could be used again after 3 to 6 months. The STSG is generally considered to be cosmetically unappealing as well as less durable than the FTSG. Split-thickness skin graft donor site pain and poor healing in the compromised patient may arise but can be successfully managed with in-growth substrates, such as DermaPure (Tissue Regenix, Houston, TX). Late reconstruction of a problematic STSG site may be managed with the use of tissue expanders, excision of the graft, and primary closure. This technique is very useful in patients after skin grafting of compartment syndrome defects (discussed later in detail).
Local tissues may be used to close wounds that are otherwise unable to be closed by similar techniques. Local tissue reattachments consist of undermining and elevating wide areas of skin. Combined with relaxing incisions, the ST envelope may be closed utilizing skin perforator-sparing fascial meshing with scoring of the peri-incision skin ( Fig. 42-9 ). This technique borders upon the definition of a flap, but there is not a portion of tissue that is elevated on a vascular pedicle or an island of issue that is reassigned to a remote location.
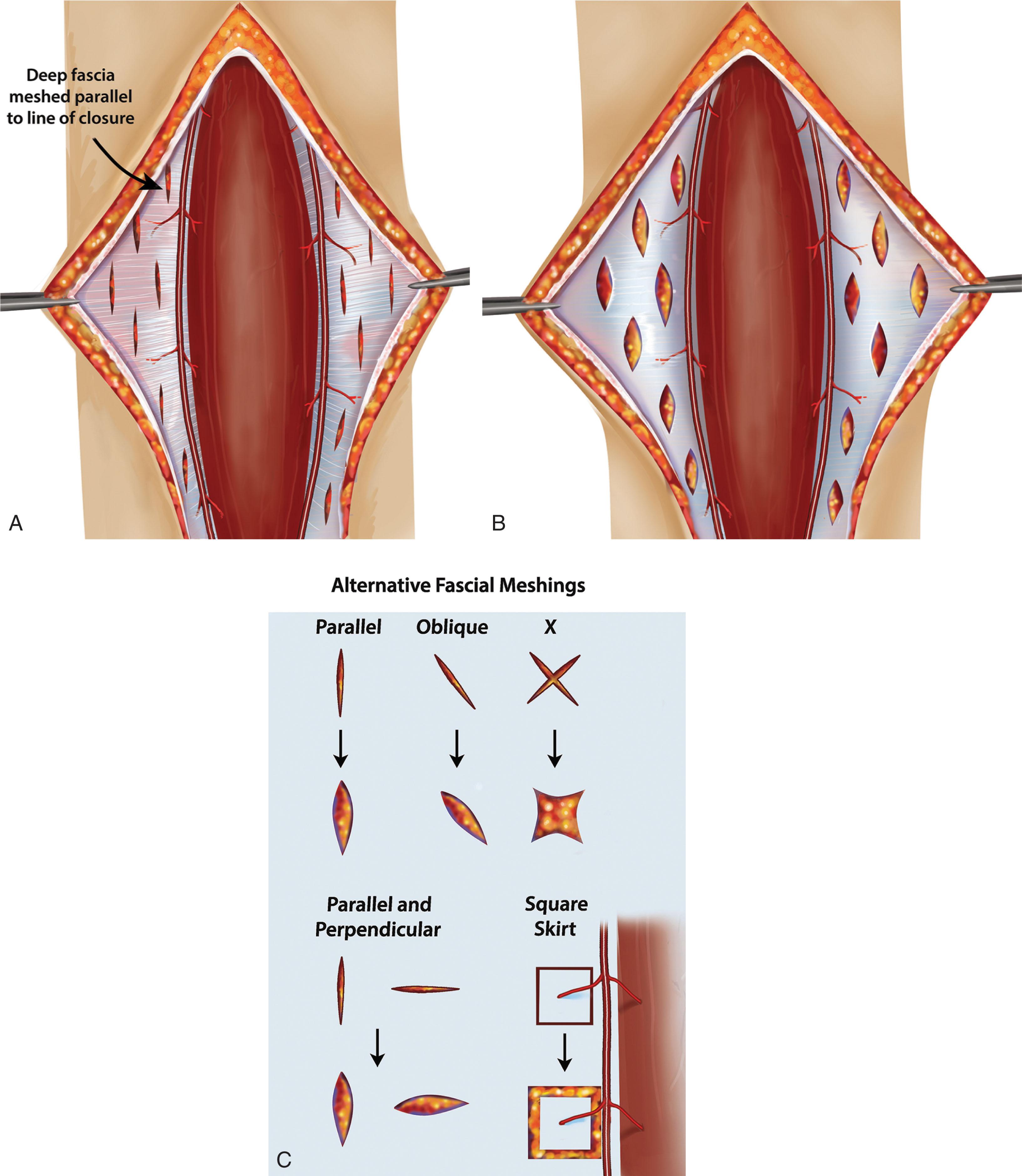
The need for flap reconstruction is indicated when the local tissue reattachment does not solve the defect, there is a need for several tissue composites (e.g., combined skin and muscle, or skin and bone), or a deep defect needs to be filled. In a broad sense, indications for a flap may be thought of when a defect exists that requires fill and resurfacing not unlike a street pothole, or when loss of function needs to be addressed (e.g., functional muscle free flap). Specific areas of the foot and ankle pose special challenges, such as wounds associated with calcaneal fractures.
Flaps may be described by the composite of tissues, the elevation and type of rotation, the anatomic region, vascular pedicle, and technical features (e.g., microsurgical anastomosis free flap). In practice, all these descriptors are used to define a flap. It is typically easiest for the nonspecialist to categorize flaps as nonfree flaps and free flaps, with what tissue comes along with that flap. These two broad classifications will then have several subtypes, based on tissue type, how the flap is elevated, and anatomic/vascular anatomy.
Because foot and ankle surgeons utilize surgical devices that may reach to the proximal tibia (e.g., circular external fixation), this region will be also discussed. A table of foot and ankle flaps is listed in Table 42-2 .
| Local Foot Flaps | Tissue Composite(s) | Coverage Areas |
|---|---|---|
| Plantar medial artery | Fasciocutaneous; sensate | Plantar heel * ; medial ankle; dorsal medial foot |
| Abductor hallucis | Muscle | Medial foot; fill bone defects |
| Abductor digiti minimi | Muscle | Lateral foot |
| Intrinsic muscle | Muscle | Fill calcaneal defects |
| Extensor digitorum brevis | Muscle | Anterior/anterolateral ankle |
| Dorsalis pedis | Skin | Mid/distal dorsal foot |
| Regional Leg Flaps | ||
| Reverse sural | Fasciocutaneous; fascia only | Posterior ankle * ; ankle; anterior leg |
| Reverse hemisoleus | Muscle | Medial ankle * ; medial hindfoot |
| Reverse peroneus brevis | Muscle; myocutaneous | Lateral ankle * ; lateral calcaneus; anterior ankle; posterior achilles |
| Propeller flaps (lateral) | Fasciocutaneous | Anterior ankle; anterolateral foot |
| Peroneus tertius | Muscle | Midline/para-midline ankle (limited) |
| Soleus | Muscle | Distal medial ankle (limited) |
| Free Flaps | ||
| Anterolateral thigh | Fasciocutaneous; sensate | Entire foot and ankle and leg |
| Latissimus dorsi | Muscle; myocutaneous | Entire foot and ankle and leg |
| Gracilis | Muscle; myocutaneous; functional | Small defects entire foot and ankle |
| Radial forearm | Fasciocutaneous (thin) | Small defects dorsal foot; achilles |
| Rectus abdominus | Muscle; myocutaneous | Entire foot and ankle and leg |
| Lateral arm | Fasciocutaneous (not recommended) | Ankle (High donor site morbidity) |
| Scapular and parascapular | Fasciocutaneous | Foot; ankle |
| Iliac crest | Bone; osteocutaneous | Midfoot and ankle fusions |
| Fibula | Bone; osteocutaneous | Entire foot and ankle and leg |
| Medial femoral condyle | Bone; osteocutaneous | Medium defects foot and ankle |
Adequate debridement of damaged tissue is paramount in management of any wound at any stage and of any etiology. The most universal and best debridement technique for most wounds is with a scalpel. In most instances, debridement results in additional loss of tissue, depending on the degree of contamination, which is an “evil necessity.” Debridement must be repeated until all necrotic tissue is removed.
New tools have evolved for debriding wounds. Ultrasound debridement, for example, has been used for chronic wounds. The ultrasonic methods have proved to be less painful than the conventional methods of sharp debridement when used in the outpatient clinic setting. The main advantage of the technique is that debridement can be done more precisely in areas where there are patchy areas of granulation tissue that the surgeon wants to preserve; this is used primarily in wound clinics.
The Versajet Hydrosurgery System ( Fig. 42-10 ) uses a high-velocity stream of sterile saline that jets across the hand piece into a vacuation collector. The Versajet requires less irrigant than traditional techniques, and it confines the irrigant to the wound area. This system has several power settings that can be used depending on the degree of debridement needed. The major advantage of the Versajet is that thin layers of tissue can be removed in a controlled fashion. Compared with conventional pulsate irrigation, the Versajet leaves significantly less bacteria in the wound.
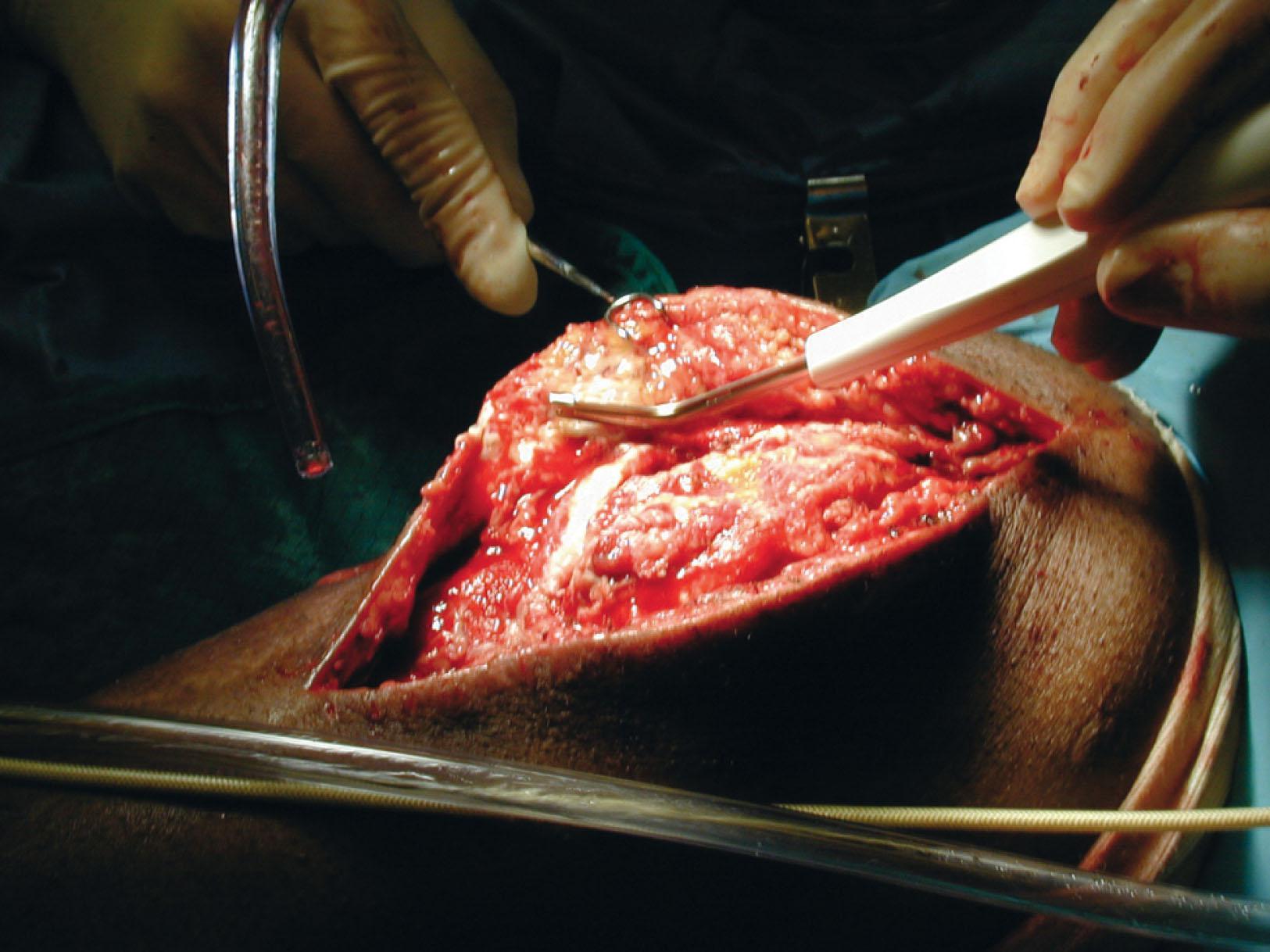
Debridement of the large fresh foot and ankle wound (such as an open type 3B or type 3C pilon fracture) should be performed in an exsanguinated extremity. This permits the surgeon to carefully observe the appearance of the tissue and detect pockets in the wound, assist with clear visualization of foreign material, and decrease blood loss. Healthy tissue in the exsanguinated limb is bright and homogeneous in color. Subcutaneous tissue is yellow, muscles are bright red, and the tendons and fascia are white and shiny. Damaged tissues are recognized by the presence of foreign bodies, irregular tissue consistency, and irregular distribution of dark red stains, which are hematomas.
After debridement is completed and the tourniquet is released, tissues are assessed for vascularity, followed by hemostasis. The edge of the debridement should be in healthy tissue. Intraoperative fluorescein imaging may be helpful at the final stages of debridement to assess microvascular blood flow to the skin and subcutaneous fat. Avulsed skin and muscles should be removed from the base of the avulsed flap. These tissues are contused; the anatomic pattern of the skin’s vascularity is not “axial” because of avulsion of perforators, and the vascularity is therefore insufficient to maintain viability of such flaps. Denuded tendon, if not frayed, should be cleaned. Disrupted tendons can be sutured or can be fixed with a suture to surrounding tissue for later reconstruction.
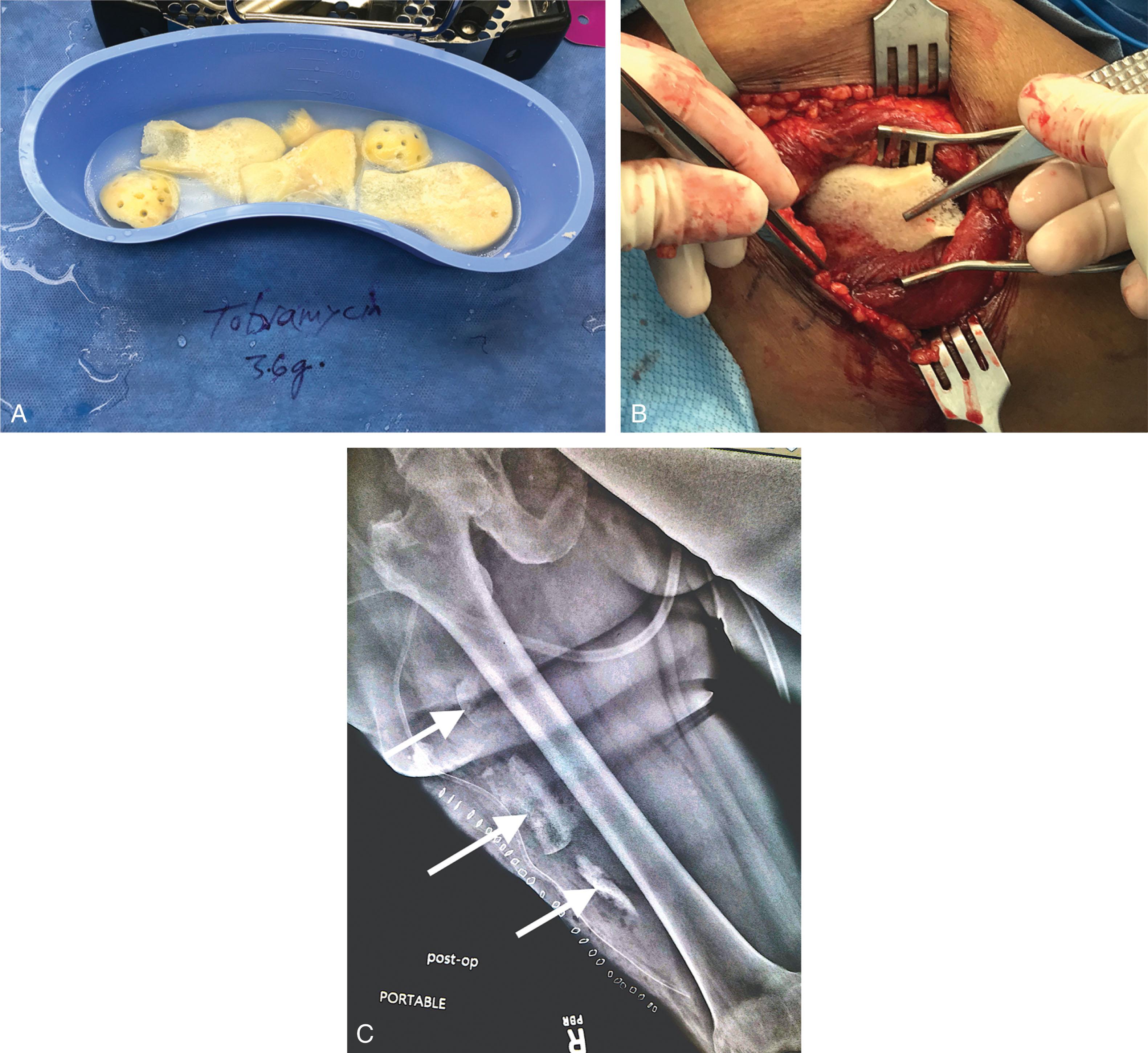
Exposed bones are washed with antibiotic solution and mechanically cleaned with bone rongeurs. Free bone fragments are usually removed, and if sections are quite large, they can be stored in the bone bank as bone autografts for later use when soft tissue coverage has been obtained. Orthoptic bone banking has also been described, where “clean” bone that is stropped from the blood supply is banked in the patient’s thigh or leg ( Fig. 42-11 ) and is later retrieved for use as an autologous bone graft.
Nerves are the only structures where debridement is not radical. Nerve repair should be performed without tension, and nerve grafting should be done in close temporal relationship to the final reconstruction. Those parts that are destroyed without any doubt are removed, and major nerve stumps are anchored in the wound to prevent retraction and allow later reconstruction with nerve grafts. It is recommended that a large cystoclip or hemoclip be applied to the nerve stump, so it may be viewed via radiograph, and appropriate secondary planning can be done in regard to surgical approach and location of the nerve stump. Subacute and chronic nerve repairs include allograft repair, autograft repair, and nerve free flaps. Late peripheral nerve-related pathology also includes nerve transfers and functional free-tissue transfers.
Acute, subacute, and late nerve repairs include allograft repair, autograft repair, nerve transfers, or combinations thereof ( Fig. 42-12 ). Simultaneous repair of large intercalary segments of nerve may be performed with a reverse flow-through nerve free flap, with or without the need for limb bypass revascularization ( Fig. 42-13 ).
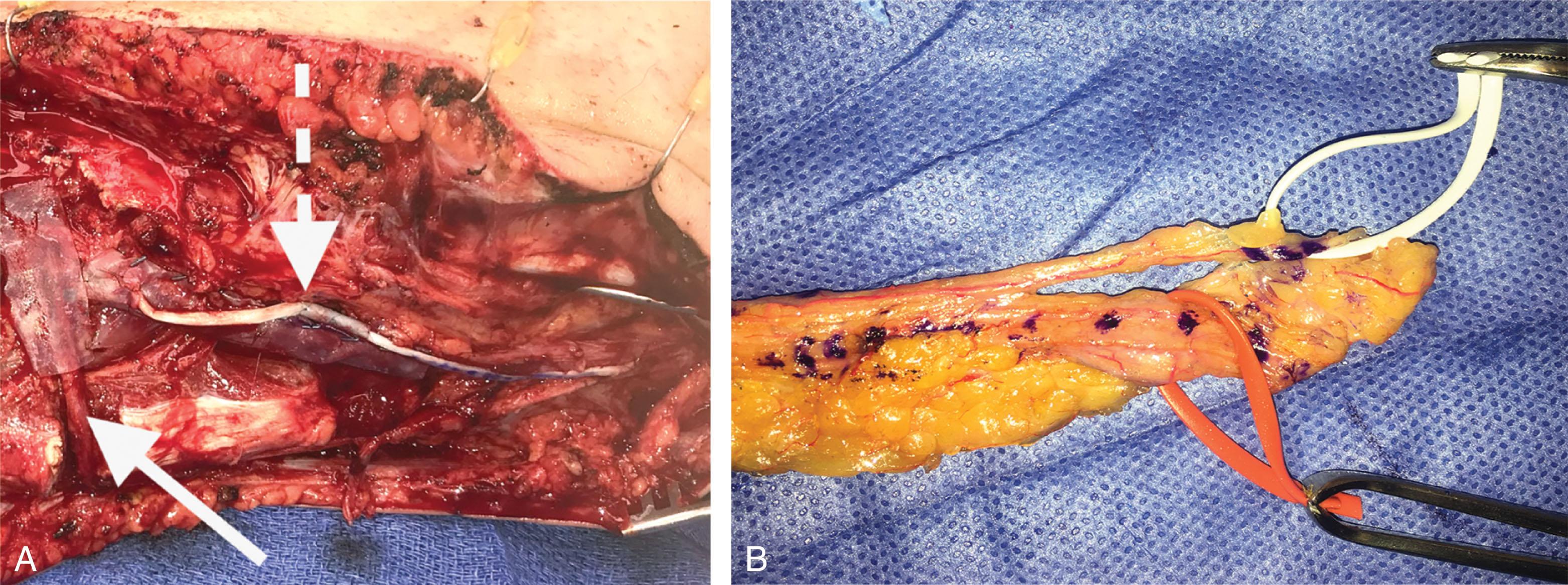
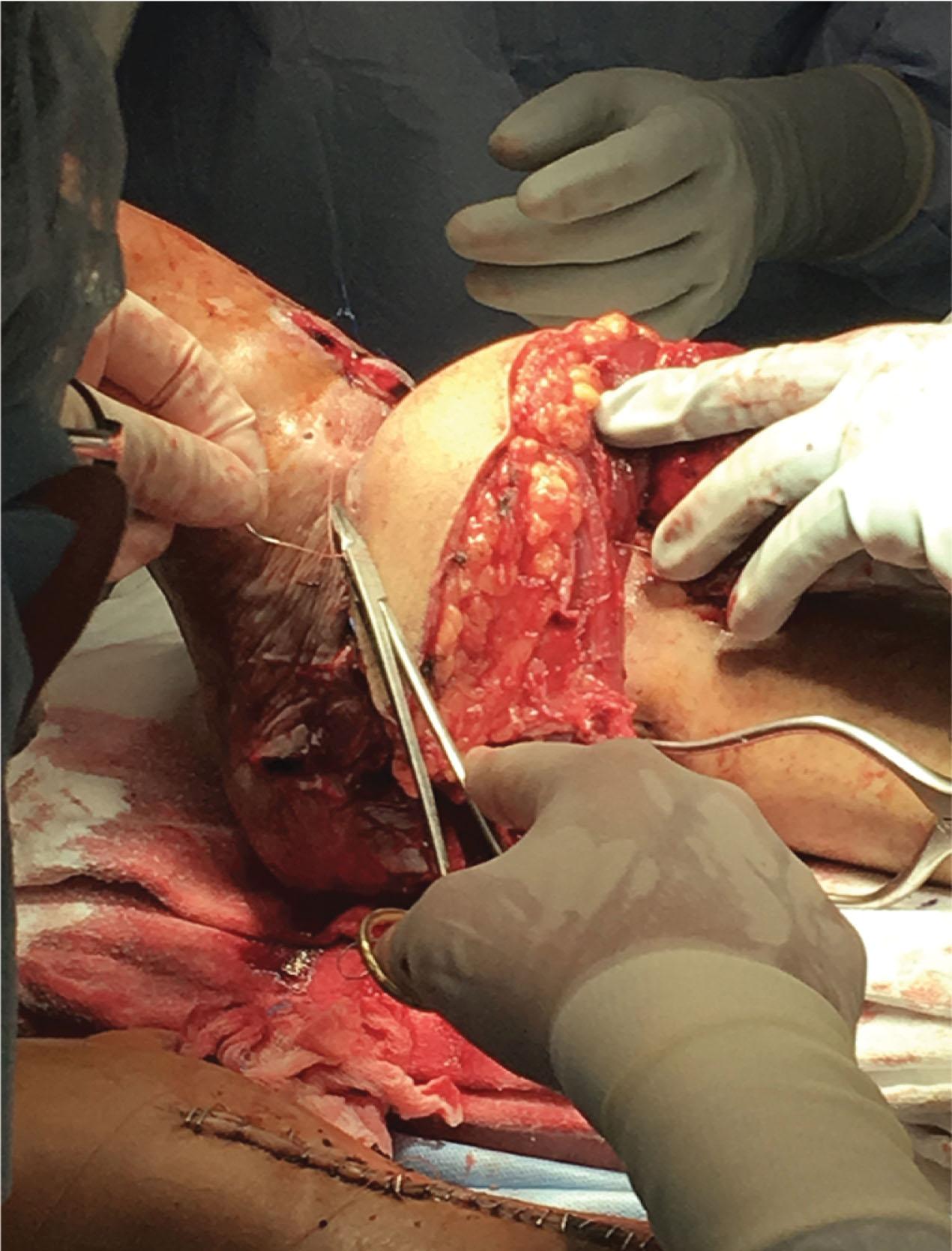
Lower extremity vascularization for severe traumatic foot and ankle wounds (e.g., 3C pilon fracture) and wounds associated with foot and ankle replantation must be addressed expediently in order to avoid desiccation of repaired vessels, nerve, and tendons. These may require emergency local flaps or even free flaps to protect the vascular anastomosis (see Fig. 42-12 ). Occasionally, the bed of the wound remains irregular after debridement. With additional incisions, a more natural shape is achieved, which serves to eliminate dead space, permits better flap placement, and help reduce formation of tenacious scars.
Debridement of a chronic wound is also done with the limb exsanguinated. Superficial scar is excised completely so that the wound margins are in healthy skin and subcutaneous tissue appears normal. The goal is to treat the chronic wound like a tumor and excise it in its entirety down to normal tissue planes. All scars in the wound should be removed in the same manner as cancer surgeons operate; that is, the knife should always cut through healthy tissues. If functionally important structures are entrapped in the scar, the dissection should commence in the healthy surrounding tissue and pass toward the scar entrapment, where the structures such as nerves or tendons are carefully dissected and preserved.
This portion of the procedure is extremely difficult, and it is necessary to anticipate the changes in the anatomy caused by the injury, previous operations, and the traction exerted by scar tissue. Bone debridement is also very difficult. Although clearly necrotic parts of bone can be recognized, more experience is required to identify the viable parts of the bone callus and necrotic and inflamed areas in bone. Studies such as CT scans, bone scans, and magnetic resonance imaging (MRI) may be helpful in preoperative planning for bone and soft tissue debridement.
Swiontkowski has proposed the use of the laser Doppler to determine bone blood flow in planning for debridement of infected bone. However, this tool has not gained widespread use, and the clinical intraoperative evaluation of bone viability (bleeding, large areas of soft tissue attachment) remains the best tool to estimate bone viability. Historically, bone not covered with periosteum were removed. However, revascularization and biologically active membrane may occur in short segments of bone or bone defects and over longer segments if a robust muscle bed surrounds the bone; this is the basis of the Masquelet technique. Exposed bone may be evaluated with an iced saline bur or drill. If punctate bleeding is encountered from the cortical bone, the bone should remain in situ. If a sequestrum is in the medullary canal, the anterior part of the bone cortex (such as the metatarsal) should be removed to provide a window for access to the medullary canal for placement of muscle flaps, which eliminates dead space and helps control infection.
The technique is as follows:
The extremity is pre-scrubbed to remove grime and surface dirt, and then it is prepared and draped. Nails should be cut and nail plates cleaned.
The leg is elevated for 5 minutes to exsanguinate it (rather than wrapping the extremity with an Esmarch bandage), and the tourniquet is inflated.
The wound is washed with either betadine solution or preferably chlorhexidine-saline solution. Loupes are recommended for debridement. Half-strength peroxide can be used as a first rinse solution to lyse the clot and gain access to the true depths of the wound. Half-strength peroxide will bubble and should be washed away with normal saline solution.
A no. 10 or 15 scalpel blade or very sharp scissors is used to excise the skin and dermis, particularly around the edge of the wound, back to normal tissue.
The subcutaneous layer is inspected and debrided sharply with a no. 15 scalpel blade to the level of fascia. All fascia that is stripped, avulsed, or contaminated should be removed.
The next layer encountered is muscle. Muscle should be resected down to healthy tissue, regardless of the amount of muscle removed. Leaving unhealthy necrotic muscle is one of the surest ways to initiate an infection.
Periosteum that is elevated from bone should be excised to the level from which it is elevated. Small bone fragments devoid of periosteum or free-floating large segments, even structural ones, should be removed for fear of colonization, contamination, and infection.
At the conclusion of debridement, the wound is again irrigated. The tourniquet is released and all tissue planes, particularly the muscle, are observed for bleeding as the arterial pressure increases within the limb.
Areas that remain nonviable, particularly the dermis, skin, and muscle, are re-excised. Excision then can be done sequentially, watching for punctate bleeding from the dermis or the muscle. When large flaps have been avulsed, excision is carried out through the skin to the level of bright red blood coming from dermis on incision.
No attempt should be made to close the wound defect under any tension, for fear of further ischemic damage to already compromised tissue.
The optimal time for soft tissue reconstruction in severe open fractures remains controversial. Godina and others changed the concept of primary repair and reconstruction of damaged tissue by advancing the phase of reconstruction from a delayed elective procedure to the day of injury. The argument favoring a staged method is the need for a second-look debridement. If there is uncertainty about traumatized and devascularized tissue, a second look is performed. The main argument for early reconstruction is to reduce the nosocomial contamination and secondary necrosis of exposed tissues. Late soft tissue reconstruction is associated with a significantly higher infection rate and flap complication rate when compared with early (within 72 hours) soft tissue coverage but still remains a decision that needs to be individualized.
Assuming that an adequate primary debridement is feasible, the outcome should be improved by immediate soft tissue closure to avoid bone infection. Immediate reconstruction improves the time to definitive fracture union, decreases the number of operations that are performed, and reduces infection rates.
Patients with Gustilo and Anderson 3B and 3C open fractures of the foot and ankle whose general condition was suitable for debridement, followed by stable internal fixation and immediate soft tissue reconstruction, demonstrated a better outcome and a shorter period of convalescence. However, the higher incidence of infection in the delayed group may also be due to greater injury and higher contamination. In these instances, early ST reconstruction may be at a higher failure rate if the inflammatory milieu is high and debridements have been inadequate.
Fracture wounds with avulsion of the dermal surface but without damage to the underlying muscle may be treated successfully with several techniques. First is emollient coverage. Emollient-type soft tissue coverage may also be indicated to temporize a wound before soft tissue coverage. This may take the form of silver sulfadiazine, a hydrogel such as Vigilon (Bard Medical, Covington, GA), an antibiotic-impregnated occlusive dressing such as Scarlet Red (Kendall Healthcare, Mansfield, MA), or a simple semipermeable film such as OpSite (Smith & Nephew). A copious layer of antibiotic ointment covered by a Vaseline dressing and gauze can also temporize wounds before soft tissue coverage.
There are four general types of newer wound dressings: semipermeable films (e.g., OpSite, Tegaderm [ 3M] and bio-occlusive), hydrogels (e.g., Vigilon), occlusive hydrocolloids (e.g., Duoderm; ConvaTec, Oklahoma City, OK), and synthetic skin substitutes (e.g., Epigard [Synthes, West Chester, PA] or Integra [Integra LifeSciences, Plainsboro, NJ]). These newer dressings are best applied when the wound site is surrounded by a border of healthy tissue.
Semipermeable films and semiocclusive hydrogels are impermeable to water and bacteria but permeable to oxygen and water vapor. Occlusive hydrocolloids are impermeable to even water vapor and oxygen. For example, DuoDerm has an inner adherent surface with an outer impermeable polyurethane foam. Epigard, one of the synthetic skin substitutes, is a nontextile open matrix polyurethane composed of two layers and backed by a microporous polytetrafluoroethylene (Teflon) film. This matrix allows new microcirculation to develop in its interstices. Newer dressings are not without drawbacks, however, particularly the accumulation of exudate, hematoma, and seroma beneath them.
In addition to these wound dressings, there is an isolated report on the efficacy of honey as a broad-spectrum antimicrobial found to be effective in controlling Staphylococcus aureus, Escherichia coli, Pseudomonas aeruginosa , and Klebsiella pneumoniae . Although this finding needs further clinical verification, honey shows promise for use as a first-line wound dressing agent.
The goal of wound dressings is to assist with obtaining a “clean” bed prior to a final reconstruction. In types 1 to 3 open fracture wounds, the standard dressing technique was to pack the wound with normal sterile saline (NSS)-soaked gauze dressings, which helps eliminate dead space and prevents soft tissue desiccation. If performed correctly, NSS damp to moist dressings can be an effective dressing technique. The disadvantages of saline-soaked gauze dressings include inadvertent soft tissue desiccation, nosocomial bacterial contamination, poor dead space management, and often, significant patient discomfort. Similar antibacterial benefits are obtained with dressings of gauze soaked in Dakin solution (sodium hypochlorite) and half-strength povidone-iodine (Betadine; Avrio Health, Stamford, CT). Although tissue is toxic, Dakin solution and povidone-iodine can significantly reduce the bacterial load of a wound. In general, the authors prefer to utilize this dressing for an average 24 to 48 hours. Negative pressure wound dressings may also be instilled with Dakin solution or normal saline. It is noteworthy to mention that Vashe Wound Solution (dilute hypochlorous acid) (Urgo Medical North America, Fort Worth, TX) has proven to the lead author, via in vitro culture and sensitivity testing, to be inferior to Dakin solution (unpublished data).
Antibiotic beads have been used effectively to prevent or to control infection and can be used after the first debridement until the patient returns to the operating room for a second-look debridement ( Fig. 42-14 ). Advantages of antibiotic beads are that they can deliver antibiotics at high concentration to a compromised wound without systemic effects. The bead pouch, popularized by Henry et al, seals the wound so that transudate from the wound surface is captured, bathing exposed tissues in physiologic fluids that also contain bactericidal levels of antibiotics. Wounds do not desiccate, cell death is avoided, and infection risk is reduced.
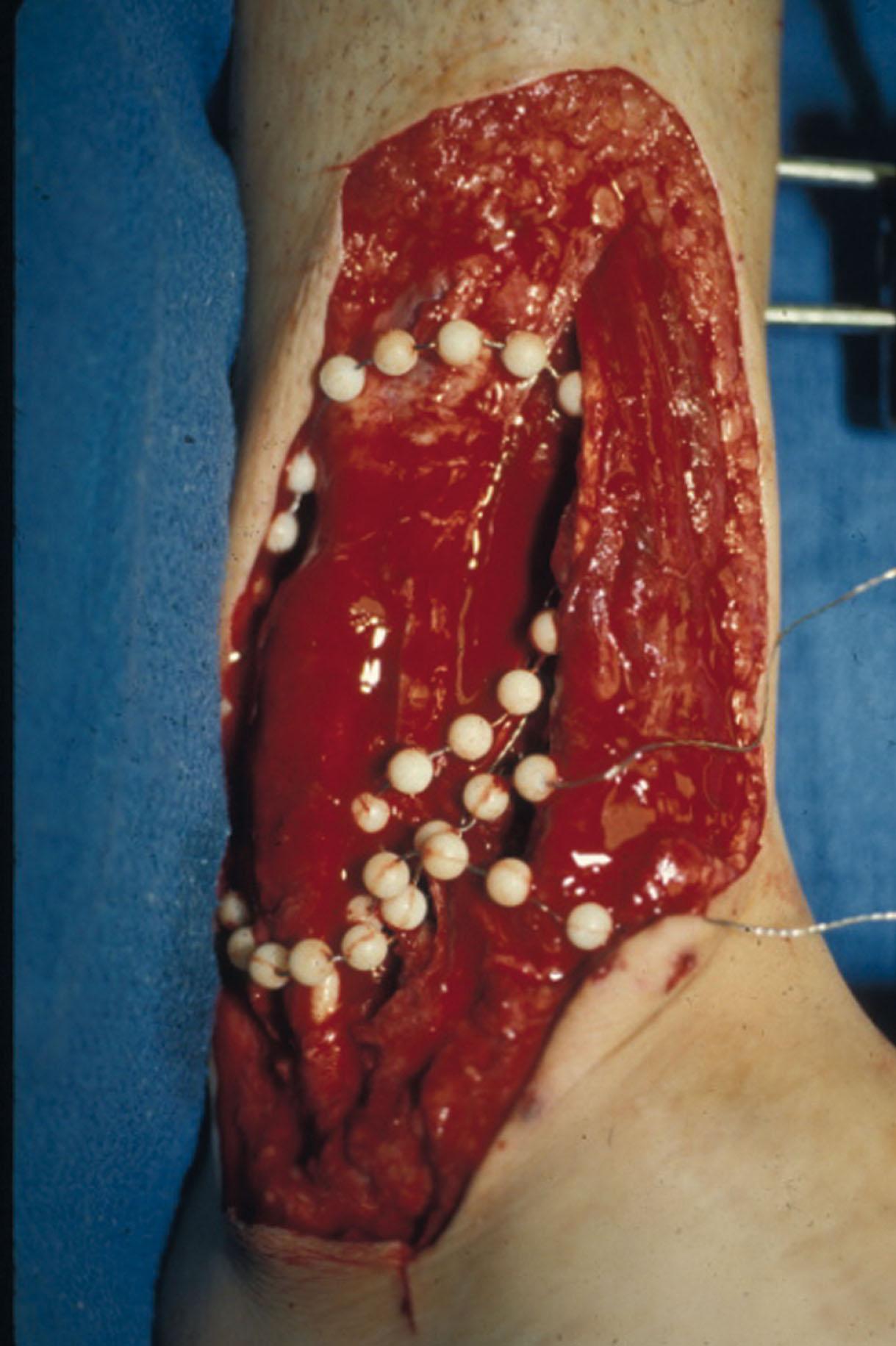
The technique of local antibiotic therapy achieved via antibiotic-impregnated polymethyl methacrylate (ABX-PMMA) originated with Büchholz in Germany. It was an application that used antibiotic-impregnated bone cement to treat infected arthroplasties.
The amount of antibiotic per pack of PMMA should be supracidal: e.g., 4 g of vancomycin plus 3.6 mg of tobramycin. The custom-made versus commercial fabrication processes differ, as the custom-made beads are polymerized in a mold whereas the commercial variety is formed in a press with a corresponding increase in temperature and pressure. Forming ABX-PMMA beads by hand is easy and the size of the beads can be customized. The beads are strung with a nonabsorbable monofilament suture. The ABX-PMMA beads are strung on strong suture or made via a bead mold, using as many as possible to fill soft tissue and bone voids. The ABX-PMMA bead pouch technique is useful for very large wounds (e.g., type 3 open fractures) that present with a high level of contamination and a negative pressure therapy (NPT) dressing is contraindicated (exposed nerve, vessels).
Using a block of ABX-PMMA for the infected retrograde tibiotalocalcaneal fusion rod and an antibiotic rod technique has been well described. Absorbable antibiotic “pellet-beads” composed of calcium phosphates are recommended only for a final reconstructive setting within a contained bone defect.
The technique is as follows:
All necrotic, avascular, and contaminated tissue is removed from the fracture site during the initial irrigation and debridement. Wound margins are extended to appropriate widths.
A thorough lavage consisting of bacitracin and normal saline is performed.
Depending on the severity of the fracture, reduction and stabilization are accomplished with either external or internal fixation.
One standard packet of PMMA is mixed by hand or under vacuum with 4 to 6 g vancomycin and 3.6 g tobramycin powder. The antibiotic-impregnated PMMA is made into beads (via a mold or by hand) and strung on a heavy gauge nonabsorbable suture. One or more chains of antibiotic beads are inserted into the wound surrounding the fracture site. Placement should be such as to fill the soft tissue cavity but leave adequate room to attempt wound closure with interrupted sutures.
A suction drain (0.32-cm diameter) is placed in the wound. The drain should be positioned so that it exits the hematoma site through normal tissue.
If an ABX-PMMA bead pouch is used, drains are clamped for 48 to 72 hours to allow antibiotic elution, creating an “antibiotic soup.”
Wound coverage is achieved with an adhesive polyethylene wound film, such as OpSite or Ioban, to prevent leakage of wound fluid, with a second layer wrapped around the entire wound.
Hyperbaric oxygen (HBO) can be used to promote formation of granulation tissue and stimulate angiogenesis in wounds that are compromised, usually by impaired arterial inflow or venous outflow. In addition to exposure to hyperbaric oxygen, wound dressings may be changed under sterile conditions by chamber personnel under sedation, avoiding discomfort to the patient. Patients who have gas gangrene associated with fractures require emergent debridement, hyperbaric oxygen, antibiotics, and ultimately fracture and soft tissue management. Hyperbaric oxygen therapy is a very useful adjunct modality for wound care but is never a stand-alone.
Normal tissue oxygen levels are approximately 40 mm Hg. When tissue levels fall below 30 mm Hg, normal metabolic activity is significantly impaired. In infected wounds and traumatized tissue, oxygen levels often fall to less than 30 mm Hg. Hyperbaric oxygen enhances oxygen delivery to ischemic and hypoxic wounds, and even when it causes local vasoconstriction, the overall increase in blood oxygen content results in a net gain so that the net oxygen concentration at the wound increases. Hyperbaric oxygen improves neutrophil function, facilitates fibroblast cell division, increases collagen formation, and encourages new capillary budding. The promotion of angiogenesis by HBO is thought to be one of the major factors in promoting the healing of chronic hypoperfused wounds.
Negative pressure therapy exposes a wound to subatmospheric pressure. It has proved to be extremely effective in treating a wide spectrum of wounds, including traumatic wounds as well as dehisced incisions with or without exposed hardware.
The wound cavity is dressed with a cell foam dressing that is connected to an adjustable vacuum source with a negative pressure of up to 125 mm Hg. The level of negative pressure is determined by the wound bed condition and fragility of the tissues. The use of Adaptic (3M) between the bed and foam can improve results and decrease pain at dressing changes. The foam dressing and wound site are sealed with a thin adhesive film, converting the open wound to a controlled closed wound. The periwound skin should be protected with a covering. Pressure is applied continuously or cyclically to the wound ( Fig. 42-15 ). The removal of excess interstitial fluid from the wound periphery results in a decrease in the local interstitial pressure, thus restoring blood flow to compressed or collapsed vessels. Along with removal of the chronic fluid, factors that inhibit healing are also removed. An additional mechanism of action of NPT is the mechanical stimulation of cells by tensile forces placed on the tissue because of the collapse of the foam dressing by negative pressure.
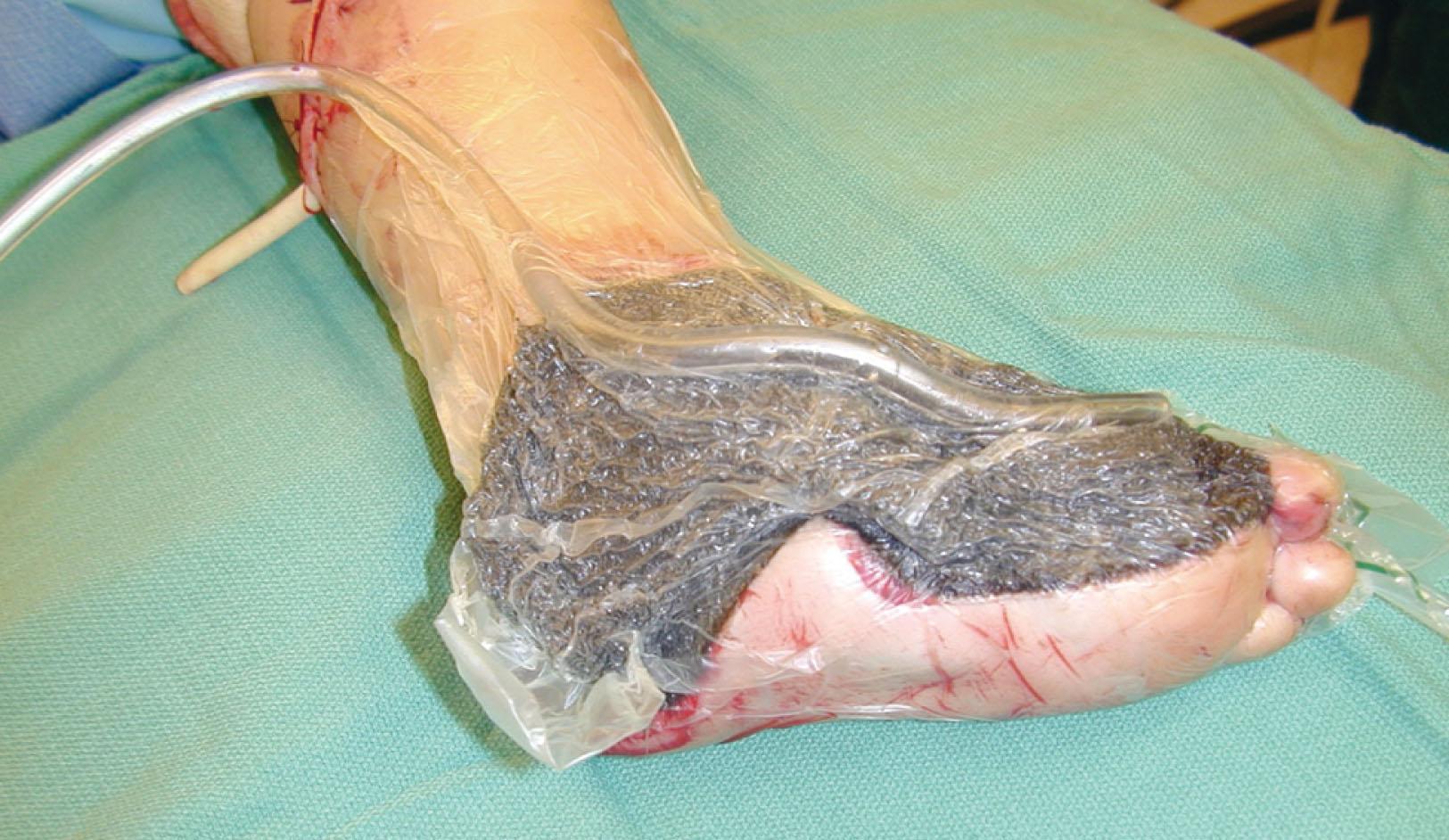
Negative pressure therapy closure has become an important adjunct to foot and ankle care. This device decreases edema, increases wound vascularization, fights infection, and promotes granulation tissue through proliferation of capillaries and fibroblasts.
Negative pressure therapy has several applications in foot and ankle surgery. It can be used for soft tissue injuries after initial debridement. If a relatively clean wound can be established, a wound vacuum-assisted closure can be applied. This device serves as a barrier dressing, and it isolates the wound from the hospital environment. Simultaneously, it encompasses all of the physiologic benefits that have been mentioned. It establishes an environment for granulation to occur. The wound NPT relies more on physiologic response of the host tissue, but therapeutic solutions (e.g., Dakin solution, dilute acetic acid) can be instilled manually or via the V.A.C Veraflo system (3M).
Another application of wound NPT has been in the treatment of fasciotomy sites. Traditionally, fasciotomy sites are covered with materials such as biosynthetic skin substitute (Biobrane; Smith & Nephew) or saline dressings that require frequent dressing changes to prevent wound desiccation. In an era when hospitals are trying to diminish global costs, particularly in the trauma population, the wound NPT can remain on a wound for 2 or 3 days, and sometimes longer, depending on the clinical circumstances. For example, the wound NPT has been a very effective stabilizer of skin grafts that are used for definitive coverage of open wounds or be skin grafted to fasciotomy sites. In those cases, the wound NPT stays on for approximately 5 days with a pressure of 75 to 125 mm Hg.
After a second-look procedure, NPT may be used as definitive wound care treatment, usually for large cavitary wounds or wounds with irregular surface topography; a wound NPT can also stabilize the wound until definitive soft tissue treatment can occur. For example, an open calcaneus fracture that has a large cavitary soft tissue component can benefit from a wound NPT to serve as a barrier dressing from the hospital environment. When the patient is taken back to the operating room, NPT can be removed and appropriate soft tissue reconstruction can be performed, typically with a flap.
Although it is possible to definitively treat certain wounds to completion with a wound NPT, meaning complete epithelialization, a word of caution should be added regarding the use of wound NPTs with exposed hardware, such as fixation plates and screws. If the NPT is applied long enough, it is possible that a wound will granulate such that the granulation grows over exposed plates and screws. The granulation tissue contains bacteria that can colonize the plates and screws, resulting in chronic infection. In addition, the ultimate healing of soft tissue using NPT creates a healed wound, and the wound has a large degree of fibrosis associated with it. If one needed to reoperate on a foot that was treated with NPT and a skin graft, compared with supple soft tissue such as a muscle flap or regional transpositional flap, working through the scarred bed would be difficult, resulting in complications after secondary procedures, such as tendon transfers, bone grafting, or hardware exchange or removal.
In complex extremity injuries, the treating physician must first determine whether limb salvage is feasible prior to embarking on complex and prolonged reconstructions. Every patient deserves consideration for the ability of bipedal locomotion. Ideally, a salvaged limb should function as well as a prosthesis. A functional limb via a well-fitted prosthesis should be viewed as an excellent therapeutic option, and early amputation should not be considered a failure. Lange and Hansen have delineated a sound algorithm for these difficult cases and stress that the benefits of limb salvage should outweigh the risks. Thus, enthusiasm for limb-salvage techniques, especially of the traumatized foot and ankle, must be tempered by a realistic assessment of the results, not just for the injured part but for the patient as a whole. Patient evaluations need to be individualized.
Indicators of a poor prognosis for limb salvage, in order of significance, are massive crush injuries, other high-energy soft tissue injuries, a warm ischemia time longer than 6 hours, severely comminuted or segmental fracture patterns, infrapopliteal arterial injury, prolonged severe hypovolemic shock, and age older than 50 years.
Occupational demands and the presence or absence of underlying diseases, such as diabetes, are also important considerations. The same anatomic injury can require a different treatment decision in a 20-year-old laborer than in a 60-year-old diabetic patient.
Similarly, a given tibial injury might need to be treated differently if there is unreconstructible ipsilateral foot trauma that precludes reasonable limb function even if the leg is salvageable. Although several authors stated complete disruption of the posterior tibial nerve is a functional liability significant enough to warrant amputation, decreased sensation on presentation, in reality, is not an indication for amputation.
Of note, expedient amputation of a massively traumatized limb, even if it appears salvageable, may also be necessary in the multiply injured patient who cannot tolerate the reconstructive time or metabolic demands of the reconstruction. This is an extremely difficult judgment that is highly individualized and impossible to quantitate.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here