Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Complement and immunoglobulins represent the two major arms of humoral immunity and are discussed in detail in respective major subsections of this chapter. The complement section begins with a comprehensive overview of pathway activation, regulation, and effector mechanisms, followed by discussion of the pathological consequences of various complement deficiencies and polymorphisms. Next discussed is complement’s integral role in B-cell and T-cell immunity. Additional topics include the surprisingly detrimental role of complement in cancer control by the immune system, and links between the complement and coagulation systems. The immunoglobulin section similarly begins with an overview of fundamentals and proceeds to discuss how human- and animal-derived immunoglobulins are employed as pharmacologic agents. This is followed by discussion of monoclonal antibody technologies, which have led to a vast array of new drug approvals. To bring this chapter “full circle,” we discuss how monoclonal antibodies that target complement have revolutionized the treatment of paroxysmal nocturnal hemoglobinuria (PNH) and atypical hemolytic uremic syndrome (aHUS). Finally, a promising newly approved anti-complement peptide is described along with a glimpse at some other exciting new complement-targeted drugs on the horizon.
Complement refers to a group of greater than 40 distinct proteins that play a pivotal role in host defense against infection. In the 1880s, the serum factors involved in host response to pathogens were placed into two categories based on sensitivity to heat. Whereas the heat-stable component, antibody, was recognized as being specific for the invading pathogen and arose after immunization, the heat-labile (>56°C) fraction displayed nonspecific killing activity. The heat-labile fraction acted to complement the antibody-mediated lytic killing of targeted organisms.
In addition to its lytic role in the effector arm of the antibody response, the complement system serves several other functions. First, components of the complement system are involved in clearance of targeted microorganisms by the process of opsonization. Opsonization is the coating of a particle with proteins that facilitate phagocytosis of the particle by tissue macrophages and activated follicular dendritic cells (FDCs) as well as binding by receptors on peripheral blood cells. Second, complement promotes inflammation by releasing small peptide fragments from complement proteins. These peptides cause mast cell degranulation, smooth muscle contraction, and directed migration (chemotaxis) of motile cells to sites of inflammation.
Complement can be activated via three distinct pathways: classical, lectin, and alternative. Although all depend on different molecules for their initiation, eventually they converge to generate the same set of effector molecules. Each of these pathways is described here ( Fig. 23.1 ).
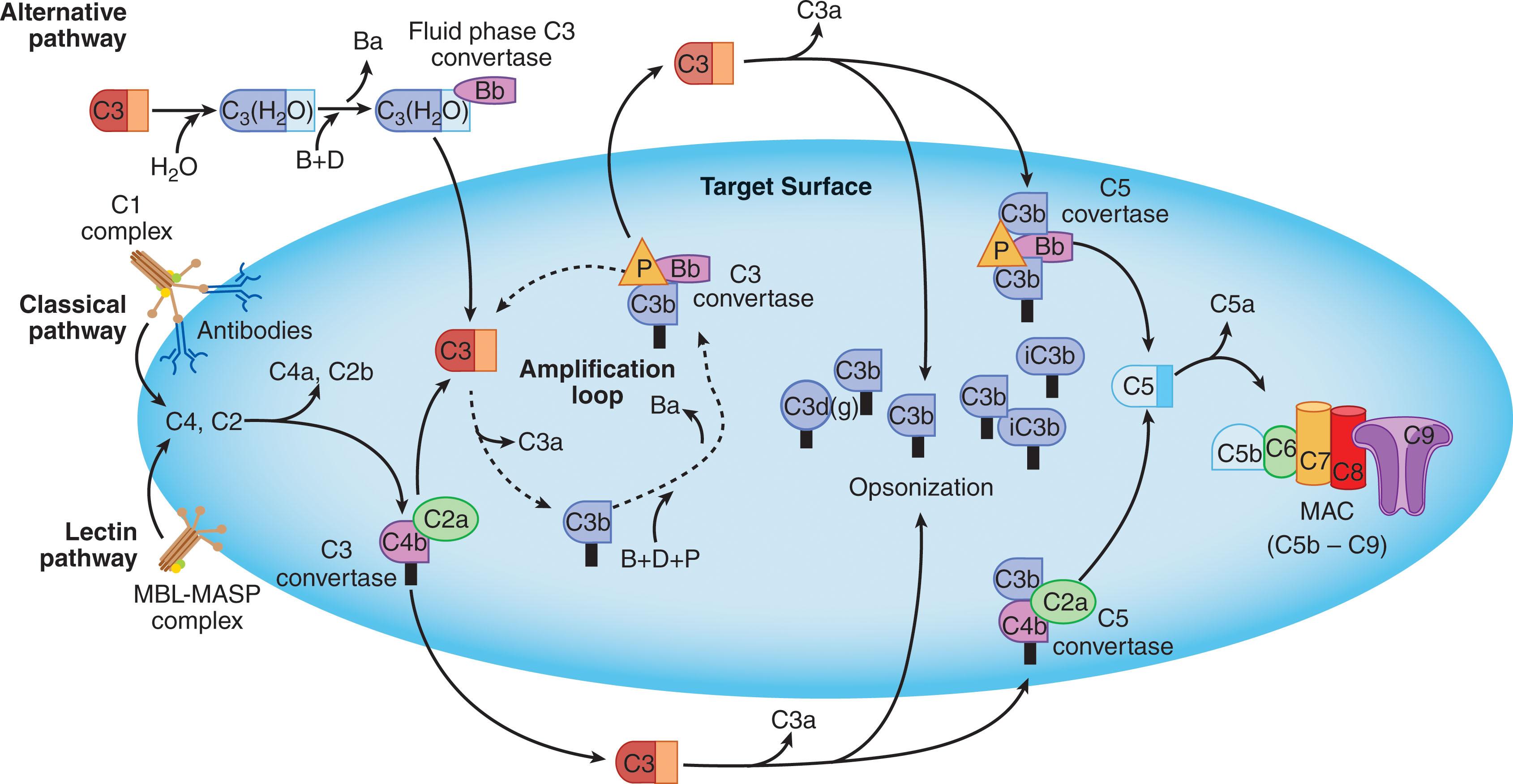
The classical pathway (CP), so called because it was the earliest studied arm of the complement system, directly links the innate and acquired immune systems. There are nine proteins in the CP. As a matter of terminology, each CP component protein is designated with an uppercase C followed by a number. Fragments of these proteins generated by cleavage during the complement cascade are designated with a lower-case letter suffix (e.g., C3a, C3b). In general, the smaller product arising from a proteolytic activation step is given the fragment designation “a,” and the larger product is designated “b.” The sole exception to this rule is for the naming of the C2 proteolytic activation fragments, where, for historical reasons, the larger fragment is C2a and the smaller one is C2b.
C1, the first component of the CP, binds and is activated by the Fc portion of the antibody molecule. C1q is a macromolecule complex composed of three individual protein subunits: C1q, C1r, and C1s. The largest of these subunits, C1q, is an 18-chain molecule with six copies each of three chains: A, B, and C. Structurally, C1q consists of a central core with six radiating arms. Each arm possesses a triple helical structure similar to collagen that is capped at the end with a head region consisting of a quaternary assembly of the globular domains from each of chains A, B, and C. C1q, largely through ionic interactions, links the C1 complex to the antibody molecule. In addition to its capacity for binding to antibody molecules, C1q possesses the capability to bind directly to the surfaces of some microorganisms and apoptotic cells, not unlike mannan-binding lectin (MBL; see Lectin Pathway discussion, later).
Associated with C1q are two molecules each of C1r and C1s. In unactivated C1, C1r and C1s are proenzyme serine esterases. Upon binding of C1q with an array of target-associated immunoglobulin G (IgG) Fc regions, which themselves have a propensity to form hexamers on the target surface, or directly to surface molecules of the pathogen, a conformational distortion of the C1q arms occurs that leads to reciprocal autoactivation of the associated C1r molecules. The active form of C1r then cleaves its associated C1s to generate an active serine protease.
Activated C1s is responsible for cleaving C4 and C2, the next two proteins in the complement pathway. Cleavage of C4 yields two fragments: C4a and C4b. C4b possesses a highly reactive thioester group within its C4d/TED region (structurally analogous to the C3d/TED region of C3 discussed further below) that allows it to bind covalently to molecules in the immediate vicinity of its active site. Only a small proportion of the C4b produced binds to proteins or carbohydrates on the targeted surface; the rest is inactivated by reaction with water in the surrounding milieu. This helps to prevent inadvertent C4b binding to surrounding host cells.
C2, the next substrate in the CP cascade, is susceptible to cleavage by C1s. Upon association with C4b, C2 is cleaved by activated C1s into two fragments: C2a and C2b. C2a, which is now an active serine protease, remains bound to C4b, thereby confining it to the targeted surface. C4b2a is termed the C3 convertase , an enzymatic complex that is responsible for binding and cleaving C3, the next component in the cascade. The function of the C3 convertase is to cleave large numbers of C3 molecules to produce C3b and C3a. Nascently activated C3b, similar to C4b, also possesses a highly reactive thioester bond, allowing a portion of the nascent C3b to covalently bind to the targeted surface (opsonization of the target) and thereby mark it for phagocytosis. The activated thioester in the bulk of the nascent C3b becomes water hydrolyzed and can no longer bind to a target. By contrast, all of the C3a fragment remains in solution, where it initiates a local inflammatory response.
An Ig-independent mechanism for activation of C1q has been identified. The lectin protein Sign R1, which is expressed on a subset of macrophages within the outer marginal zone sinus of the spleen, is capable of capturing to the surface of the marginal zone macrophage both C-polysaccharide–containing bacteria and C1q. The recruited C1 becomes activated and propagates the CP to deposit C3b on the captured bacterium. Sign R1 was also identified on the surface of resident dendritic cells (DC) in draining lymph nodes (LN) and shown to be important in capture and transport of inactivated influenza virus. This novel pathway provides an alternative innate recognition of pathogens leading to activation of the CP of complement.
Before continuing with the discussion of the complement cascade at the point of C3 cleavage by convertase, we turn our attention to the other two complement-activating pathways: the lectin pathway (LP) and the alternative pathway (AP). What will become evident is that all of these pathways converge at C3.
The LP is a relatively recently described pathway for complement activation. MBL, similar to C1q, is a triple helical structure with collagen-like arms coupled to C-type lectin globular domains, which form carbohydrate recognition domains (CRDs) that bind repeating polysaccharides present on the surfaces of many microorganisms. Most commonly MBL circulates as an oligomer of three or four collagenous stem-associated subunits, where each subunit consists of a homotrimer of MBL chains. The MBL CRDs attach to the termini of polymeric carbohydrate chains in the following order: mannose > GlcNAc > fucose > glucose. The greatest avidity appears to be for repeating mannose-based structural patterns typical of microbial surfaces. On vertebrate cells, these sugars are not as dense as on microbial surfaces, thus decreasing the avidity of the MBL-binding interaction, and furthermore, they often are covered by sialic acid residues, thus limiting recognition by MBL. The MBL-associated serine proteases MASP-1 and MASP-2, whose domain architecture is similar to C1r and C1s, predominantly bind as homodimeric zymogens to separate MBL oligomers. Upon MBL binding to polysaccharides on a pathogen surface, MASP-1 and MASP-2 become activated. The mechanism of activation has recently been clarified and is distinctly different from the conformational distortion-based intracomplex mechanism described above for the C1 activation. Although it had originally been thought that MASP-2 was capable of autoactivating itself when MBL bound to its target, and that MASP-1 played a non-essential and ill-defined augmentary role, under physiological conditions autoactivated MASP-1 has now been firmly established to be the obligatory activator of MASP2. Although the relationship between MASP-1 and MASP-2 parallels that of C1r to C1s in the C1 activation mechanism, what is decidedly different is that MASP-1 autoactivation is intercomplex, as is MASP-2 activation by autoactivated MASP-1. Specifically, it is the juxtaposition through clustering of the MBL–MASP-1 and MBL–MASP-2 complexes brought about by MBL binding to the target surface that leads to the intercomplex autoactivation of MASP-1, and the subsequent cleavage of MASP-2 by autoactivated MASP-1 that is on a neighboring complex. Activated MASP-2 acts similar to C1s, cleaving C4 and C2 and thereby forming a C3 convertase, C4b2a, as found in the CP. Besides its role in cleaving zymogen MASP-2, activated MASP-1 also cleaves C2, but not C4. Nevertheless, given the approximately 24-fold higher serum concentration of MASP-1 relative to MASP-2, this would ensure the efficiency of C2 activation on C4b deposited near an MBL-MASP2 complex.
While MBL was the microbial pattern recognition molecule initially identified in the LP, collectin-10 (also known as collectin-liver 1, CL-L1), collectin-11 (also known as collectin-kidney 1, CL-K1), and the ficolins M, L, and H (also known as ficolins-1, -2, -3) are collagen triple helix-containing paralogues of MBL in serum, which also associate with MASP-1 and MASP-2 and which undergo activation in a similar manner to the MBL-MASP complexes. CL-L1 and CL-K1, like its collectin family member MBL, have C-type lectin CRDs, and have a carbohydrate binding spectrum that largely overlaps that of MBL. However, unlike the homotrimeric subunit composition of MBL, CL-K1 and CL-L1 mostly circulate as heteromeric subunit complexes, referred to as CL-LK, with each arm consisting of CL-K1 and CL-L1 in a 2:1 ratio. In contrast to the collectins, the globular regions of the respective ficolin chains bear a fibrinogen-like domain fold and they recognize acetyl groups, be they on carbohydrate (e.g., N -acetylglucosamine) or non-carbohydrate entities (e.g., N -acetyl-glycine or acetylcholine).
MBL serum concentration can differ by up to 1000-fold among individuals, with those having low circulating MBL apparently more vulnerable to infections. MBL insufficiency appears to be a particular risk factor for infections in infants and individuals undergoing chemotherapy or immunosuppression treatment.
Gene-targeted knock-out mouse models deficient in MBL components have been described. In general, in pathogenic microbe infection models, such as Candida albicans or Staphylococcus aureus , MBL knock-out mice showed increased susceptibility to systemic infection and relatively much higher mortality compared to wild type.
The AP may represent one of the earliest forms of innate immunity. Unlike the CP or LP pathway, the AP can be fully activated in the absence of specific pathogen binding by a “recognition” equivalent to C1q or MBL. In fact, the AP is always “on” at a low level. In addition, the AP forms and uses the distinct C3 convertase C3bBb.
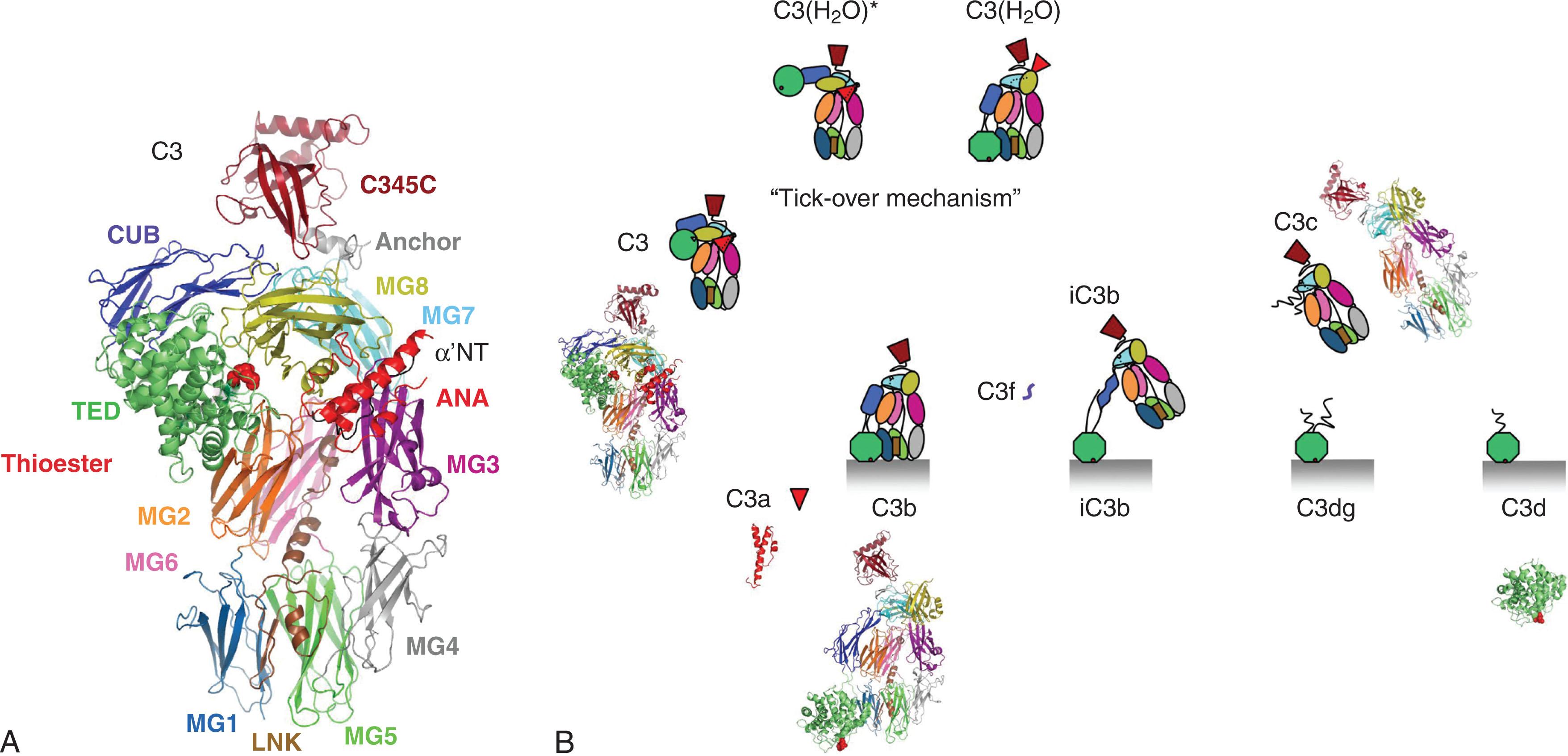
Complement C3 is a disulfide-linked two-chain protein, denoted as α and β, and having a combined apparent molecular weight of approximately 200 kDa. The crystal structure of native C3, shown as a domain-colored ribbon model in Fig. 23.2A , identified 13 distinct domains, including the thioester domain (TED), which contained the covalent binding site. In the native molecule, the intramolecular thioester bond, formed between the side chains of cysteine and glutamine residues within the sequence CGEQ, is buried within a hydrophobic interface formed between the TED and MG8 domains, which is nevertheless close to the protein’s surface. The subsequent determination of the atomic structure of the activated form of C3 (i.e., C3b) demonstrated a dramatic shift in the location of the TED. Proteolytic cleavage releases the C3a anaphylatoxin peptide, and the TED becomes fully exposed to engage potential targets (see structure-based depiction of C3b in Fig. 23.2B ). Thus the dramatic shift in structure also exposes potential binding sites for factor B of the AP and competing sites for regulators of C3b, such as factor H (FH), membrane cofactor protein (MCP/CD46), complement receptor type 1 (CR1/CD35), and decay accelerating factor (DAF/CD55; all described later in this section). At a low so-called “tick-over” level, the thioester bond undergoes spontaneous hydrolysis, forming C3(H 2 O). This conformationally altered C3b-like form of C3 (see Fig. 23.2B ) allows for binding to factor B, a plasma protein. Factor B is a serine protease that is approximately 30% identical to C2. The binding of factor B by C3(H 2 O) allows factor D, another protease, to cleave factor B to form Ba and Bb. Bb remains associated with C3(H 2 O) to form the C3(H 2 O)Bb complex. Factor D appears to function as a serine protease in its native state but can cleave factor B only when bound to C3. Recently, there has been an interesting connection found between factor D and a component of the LP. MASP-1 and MASP-3 are alternative splice products of the same gene with the difference being in the exons encoding the serine protease domain. It was found that a MASP-1/MASP-3 knockout mouse completely lacked AP functionality. Upon further investigation, it was determined that the secreted factor D in this mouse possessed a five-residue propeptide at its amino terminus, suggesting that either MASP-1 or MASP-3 mediated its removal. Subsequently, it was determined that under physiologic conditions, it was solely MASP-3 that was responsible for removing the propeptide from zymogen FD and further, that unlike MASP-1 or MASP-2, MASP-3 circulated predominantly in a proteolytically active form.
C3(H 2 O)Bb is an enzymatic complex capable of cleaving native C3. This complex is a fluid-phase C3 convertase. Although it is formed only in small amounts, it can cleave many molecules of C3. Much of the C3b produced in this process is inactivated by hydrolysis, but some attaches covalently to the surface of host cells or pathogens. C3b bound in this way is able to bind factor B, allowing its cleavage by factor D to yield Ba and Bb. The result is the formation of C3bBb, a C3 convertase akin to C4b2a found in the classical and MBL pathways, with the capability of initiating an amplification cascade (see Fig. 23.1 ).
In light of the nonspecific nature of C3b binding in the AP, it is not surprising that a number of complement regulators exist both in the plasma and on host cell membranes to prevent complement activation on self-tissues. Some of these regulatory components are mentioned now for the sake of clarity; more detailed attention is provided later in this chapter ( Table 23.1 ). CR1 (CD35) and DAF (CD55) compete with factor B for binding to C3b on the cell surface and can displace Bb from a convertase that has already formed. Factor I (FI), a serum protease, in concert with CR1 or MCP (CD46) can prevent convertase formation by converting C3b into its inactive derivative, iC3b. CR1 is unique among the FI cofactors in facilitating an additional proteolytic cleavage of iC3b to yield C3c and C3dg (see Fig. 23.2B ). Trimming of the latter by noncomplement proteases yields the proteolytic limit fragment C3d, which structurally corresponds to the TED domain (see Fig. 23.2B ). Another complement regulatory protein found in the plasma is FH. FH binds C3b and is able to compete with factor B and displace Bb from the convertase. FH also acts as a cofactor for FI to convert C3b to iC3b. In addition to interaction sites for C3b, FH possesses two distinct binding sites for polyanionic molecules, particularly various sulfated glycosaminoglycans (e.g., heparan sulfate) or arrays of sialic acid (e.g., from membrane surface glycoproteins) found on host surfaces in contact with blood plasma. Although these polyanion binding sites are not required for FH to regulate fluid phase AP C3 convertase, they are required for its activity on surface-bound C3bBb. In fact, this is the basis for FH being able to discriminate between AP C3 convertase adventitiously deposited on host tissue versus that deposited on a microbial surface because the latter do not possess either the sulfated glycosaminoglycans or the sialic acid arrays.
| Name | Role in the Regulation of Complement Activation |
|---|---|
| C1 inhibitor (C1INH) | Binds to activated C1r, C1s, removing it from C1q |
| C4-binding protein (C4BP) | Binds C4b, displacing C2a; cofactor for C4b cleavage by factor I |
| Complement receptor 1 (CR1/CD35) | Binds C4b, displacing C2a, or C3b displacing Bb; cofactor for FI |
| Factor H (FH) | Binds C3b, displacing Bb; cofactor for factor I |
| Factor I (FI) | Serine protease that cleaves C3b and C4b: aided by factor H, MCP, C4BP, or CR1 |
| Decay-accelerating factor/CD55 | Membrane protein that displaces Bb from C3b and C2a from C4b |
| Membrane cofactor protein/CD46 | Membrane protein that promotes C3b and C4b inactivation by factor I |
| CD59 | Membrane protein that prevents formation of membrane attack complex on autologous cells |
Pathogen surfaces are normally not afforded the protection offered by these regulators. Persistence of the C3bBb convertase on microbial surfaces may additionally be favored by the positive regulator properdin (P). The positive modulation of the AP by properdin has traditionally been thought to be attributable to its ability to prolong the lifetime of the AP C3 convertase by forming a C3bBbP complex in which properdin contacts segments in both C3b and Bb (see Fig. 23.1 ). This mechanism may indeed be the dominant one exhibited by properdin, however, there is also evidence for properdin displaying pattern recognition functionality for some, but not all, AP targets. For example, native properdin, which circulates predominantly as a trimer, binds to zymosan (yeast cell walls), Chlamydia pneumoniae , and necrotic, or late apoptotic, mammalian cells, but not to Neisseria meningitidis or Neisseria gonorrhoeae . Because of properdin’s trimeric nature, even if it uses two of its subunits to bind to the target surface, one is still left that can recruit C3b, or C3(H 2 O), from the fluid phase to the target surface. The properdin-bound C3b/C3(H 2 O) may then act as a platform for recruiting factors B and D, thereby forming a surface-bound AP C3 convertase. Although this mechanism can be demonstrated to function in vitro, its physiologic relevance has yet to be established.
After forming, the C3bBb convertase rapidly cleaves more C3 to C3b, which can participate in the formation of more molecules of C3bBb convertase. The AP thereby activates an amplification loop that can proceed on the surface of a pathogen but not on a host cell (see Fig. 23.1 ). An additional point regarding amplification by the AP is that C3b deposited on a target as a result of activation of either the CP or the LP can act as a nidus for the formation of an AP C3 convertase. It has been argued that this AP augmentation mechanism is responsible for upwards of 80% of the downstream complement effector mechanisms initiated via the classical or LPs.
Although specific antibody is not required for AP activation, many classes of immunoglobulin can facilitate AP activation. The mechanism by which this occurs remains elusive, although some evidence indicates that C3b covalently bound to IgG displays a reduced rate of inactivation to iC3b by factors H and I. However, in contrast to CP activation, which requires Fc, AP activation can occur with F(ab)′ 2 fragments.
An instructive demonstration for the role of antibody in continuing the AP cascade, with possible ramifications for human disease, comes from a murine model of rheumatoid arthritis. Mice do not spontaneously develop rheumatoid arthritis. However, a murine model has been developed in which expression of antibodies specific for the ubiquitously expressed cytoplasmic protein glucose-6-phosphate can cause joint destruction reminiscent of human rheumatoid arthritis. Interestingly, the disease state, through complement-mediated joint destruction, can occur even if the specific antibodies are of isotypes incapable of fixing complement through the CP. The response may be localized to the joints because of the absence of complement cascade regulators on cartilage.
The formation of the C3 convertase, C4b2a (CP and LP) and C3bBb (AP), is the point at which the three pathways converge (see Fig. 23.1 ). The function of these complexes is to convert C3 to C3a and C3b. C3 is the most abundant complement protein in plasma, occurring at a concentration of 1.2 mg/mL, and up to 1000 molecules of C3b can bind in the vicinity of a single C3 convertase.
The covalent attachment of C3b to either C4b2a or C3bBb converts this enzyme into a trimeric complex (C5 convertase) capable of binding and cleaving C5 into C5a and C5b. Mechanistically, the “adduct” C3b molecule increases the binding affinity of the C5 convertase for its substrate C5 such that its Michaelis constant (KM) is now well below the physiologic concentration of C5 in plasma. In essence, the adduct C3b molecule “primes” C5 for cleavage by the respective C3 convertase enzymatic entities. C5b generation is the initiating event of terminal component assembly into the membrane attack complex (MAC). The MAC is a multiprotein complex whose components are C5b, C6, C7, C8, and multiple C9s.
In addition to the above discussed canonical mechanisms of C5 activation leading to MAC assembly, two non-canonical routes have recently come to light that bear mentioning here as they are relevant to a subsequently discussed clinical observation of breakthrough terminal pathway activity even in cases of ordinarily complete pharmacologic inhibition of either C3 or C5, but under conditions where there is strong complement activation occurring in the individual. Mannes et al. have shown that even in the absence of C3b deposition on a target, if there is robust CP activation leading to a dense array of C4b molecules on the target, these C4b molecules have sufficient affinity for C5 to prime it for cleavage by the CP C3 convertase, C4b2a. These authors also present data suggesting that when C5 cleavage is precluded by a pharmacologic agent, in the presence of a dense array of C3b molecules on a target surface, the binding of C5 to this priming array induces, in at least a portion of the C5 molecules, a conformational change to a C5b-like state (i.e., with C5a still present) which can bind C6 and initiate MAC assembly. As the domain architectures, and indeed respective overall structures of the C3/C4/C5 family of molecules are highly similar in essence the proposed C5b-like molecule is structurally analogous to the thioester-cleaved, but peptide chain-intact, C3(H 2 O) molecule discussed above in the context of AP initiation.
The MAC, when viewed by electron microscopy, resembles a cylinder that possesses a hydrophobic outer face and a hydrophilic central core. If assembled near a lipid bilayer, such as a cell or the bacterial membrane of a gram-negative strain, the MAC can associate with and insert into the lipid bilayer. Such insertion can be thought as “punching holes” into the membrane, allowing for passage of water and small ions into the cell. Osmotic equilibrium is thereby lost, leading to eventual lysis of the targeted cell or bacterium. C5b678 are sufficient to form small pores in the target membrane. The role of C9 appears to be to enlarge the channel through multiple C9 polymerization, thereby causing more rapid loss of membrane function and lysis. Deficiencies in complement components C5 to C9 have only been associated with increased susceptibility to Neisseria species–based infections, such as gonorrhea and bacterial meningitis. Also, the extended cell wall peptidoglycan layer of gram-positive strains of bacteria make them resistant to the lytic arm of complement. It can be concluded from these observations that the requirement for MAC is limited in host protection.
As described in the previous section, complement can act by the direct lysis of targeted cells. Another important function of complement in host protection is facilitating the uptake and destruction of pathogens by phagocytic cells. This occurs by the specific recognition of C3b/C4b–coated (opsonized) particles by complement receptors.
The best characterized complement receptor for the uptake of C4-coated immune complexes is CR1 (CD35). CR1 binds C4b/C3b–bearing immune complexes. CR1, similar to most proteins that bind activation products of C4 and C3 molecules, shares a structural motif known as the short consensus repeat (SCR). Each SCR consists of approximately 60 amino acids. CR1 in humans is composed of 30 linked SCRs and possesses three binding sites for C4b and two for C3b.
CR1 is expressed on a wide variety of cell types in humans, including erythrocytes, macrophages, polymorphonuclear leukocytes, B cells, monocytes, and FDCs. The role of CR1 expression on B cells and FDCs in activating and maintaining the adaptive immune response is detailed subsequently. For now, the focus is on the other cell types that express CR1.
Because CR1 is not directly associated on its cytoplasmic side with any intracellular signaling molecules, binding of C3b by CR1 expressed on phagocytic cells is not in itself capable of inducing endocytosis of the C3b-opsonized target. A secondary signal is required to induce phagocytosis. This second signal can be provided by IgG binding to the phagocyte's Fc receptor, by carbohydrates commonly found on bacterial surfaces, or by exposure of the phagocytic cell to the appropriate cytokines. In addition, some phagocytic cells, such as macrophages, are activated by binding of C5a through C5a receptor (C5aR1, [CD88]) (see Biologic Activity of C3a and C5a, later). What these secondary ligands have in common is that they all bind to receptor domains that are the ligand recognition units of a cell signaling molecule or complex.
The largest pool of CR1-expressing cells is erythrocytes. Erythrocytes bearing opsonized material are removed from the circulation presumably to prevent deposition in tissue sites such as the renal glomerulus. Erythrocytes bearing opsonized material traverse the sinusoids of the liver and spleen, where they come into close contact with fixed phagocytic cells. These phagocytic cells affect the transfer of opsonized material from the erythrocyte onto their own membranes. The transfer of complexes is enhanced by cleavage of C3b to iC3b by FI, as iC3b is a poor ligand for CR1, but is a good ligand for CRIg, a complement receptor of the Ig superfamily present on tissue-resident phagocytic cells (see later for further discussion of CRIg).
Given its central position in the complement cascade, the presence of C3b is tightly regulated. This regulation is brought about by cleaving C3b into inactive derivatives that cannot participate in forming an active convertase. One of the conformationally altered inactive derivatives of C3b, iC3b (see Fig. 23.2B ), can act as an opsonin in its own right for complement receptors CR2 (CD21), CR3 (CD11b/CD18), and CR4 (CD11c/CD18). CR3 binds iC3b and plays a major role in inducing phagocytosis but probably not activation in the absence of a second signal (e.g., Fc receptor or pattern recognition receptor). CR4 also binds iC3b-opsonized particles, resulting in direct endocytosis. Although its role as a phagocytic receptor is not well characterized, CD11c is the major marker for DCs. It is important to understand the functional importance of this complement receptor on DC and how it participates in uptake of antigen for presentation to T cells.
CR2 expressed on B cells augments cognate antibody receptor signaling (see later section). This receptor recognizes targets that are coated with iC3b, as well as the subsequent degradation products C3dg and C3d, all of which remain covalently bound to the target (see Fig. 23.2B ). CR2 is the only well-defined complement receptor that recognizes C3d/TED on its own as its functionally-relevant ligand. However, the CR2 binding site on TED only becomes accessible after degradation of C3 to at least the iC3b stage. Activation of complement plays a contributing role in producing a strong antibody response. An interesting aside is that CR2 is the cell surface receptor on human B cells that is recognized by the Epstein-Barr virus.
CRIg is a more recently described complement receptor that plays an important role in the clearance of C3b opsonized complexes by phagocytic cells of the liver. It is also expressed on subsets of macrophages, but less is known about this role.
The role of the complement fragments C3a and C5a in the immune response is to produce localized inflammation. C3a and C5a are anaphylatoxins and are structurally similar to chemokines and they bind to their respective receptors, C3aR and C5aR1 (CD88), which in turn are canonical G protein–coupled receptor (GPCR) signaling molecules. When produced in large amounts or injected systemically, C3a and C5a induce a generalized circulatory collapse and shock-like syndrome similar to that seen in a systemic allergic reaction involving IgE antibodies.
Of the two fragments, C5a is the most stable and possesses the best characterized and possibly highest specific biologic activity. Both C3a and C5a induce smooth muscle contraction and increased vascular permeability. C5a and C3a also act on endothelial cells lining blood vessels to induce adhesion molecule expression. In addition, C3a and C5a can activate the mast cells that populate submucosal tissues and line vessels throughout the body to release histamine, tumor necrosis factor α (TNF-α), and protease. The changes induced by C3a and C5a recruit antibody, complement, and phagocytic cells to the site of infection, thereby hastening immune clearance. C5a also induces the upregulation of CR1 and CR3 on the surfaces of these cells. C5a is the only complement chemotactic agent for neutrophils, macrophages, and basophils. By contrast, both C3a and C5a possess chemotactic activity for mast cells. Although a similar fragment, C4a, is produced in the course of C4 activation, its physiologic relevance as a “classical” anaphylatoxin has long been questioned. Specifically, human C4a binds to neither C3aR nor C5aR1 and a specific C4a-binding entity has not been identified.
There is a second high-affinity receptor for C5a, namely C5aR2 (previously known as C5L2), whose function has not been fully delineated. Although highly related in protein sequence to C5aR1, C5aR2 nevertheless contains several critical sequence differences that preclude it from binding G proteins, and thus it does not act as a canonical GPCR signaling entity. It seems that at least one of its functions is to be a downregulator of C5aR1-mediated inflammatory function, both by sequestering excessive C5a as a “decoy” receptor, and through heterodimerization with C5aR1, which facilitates internalization from the cell surface of both cargo-loaded receptors.
Activation of the complement system must be tightly regulated to prevent autologous tissue damage (see Table 23.1 ). Some of the proteins involved in regulating complement action have been described (see Alternative Pathway, earlier). In addition to these regulators, a number of other checkpoints limit the scope and target of complement activation.
As a result of binding to antibody or pathogen, conformational changes to C1q induce the enzymatic activity of C1r and C1s. Both of these enzymes are regulated by the C1 inhibitor (C1-INH). C1-INH is a member of a family of ser ine p rotease in hibitors termed serpins . Serpins provide a bait sequence that mimics the active site of the substrate. When C1r or C1s proteolytically attacks this sequence, the net result is that their respective active site serine hydroxyls become permanently covalently bound to the C1-INH bait site, thereby destroying their proteolytic activity. C1-INH works in a similar fashion in regulating the activated MASP proteases of the LP. Finally, C1-INH is also responsible for preventing spontaneous fluid-phase activation of C1 in plasma, but this activity can be overridden by immune complexes.
Although C1 is capable of cleaving multiple C4 molecules, only approximately 10% of the produced C4b clusters about the targeted antigen, the rest being released into the fluid phase. C4b in the fluid phase is rapidly bound by C4 binding protein (C4bp), which is a cofactor for FI. Factor I cleaves C4b into two fragments, C4c and C4d, which are quickly cleared from the circulation.
In addition to their FI cofactor activities, the soluble regulators C4bp and FH, respectively, promote the dissociation of the CP (C4b2a) and AP (C3bBb) C3 convertases into their constituent components. This decay-dissociation is unidirectional because neither C2a nor Bb can reassociate on its own with their respective C3 convertase subunits. The membrane-bound regulators CR1 and DAF similarly possess decay-accelerating functionality toward both the CP and AP C3 convertases. The importance of CR1 or CR1-like molecules in curbing the complement response can be witnessed in a rather unexpected condition. Complement receptor 1–related gene ( Crry ) is a murine homologue of the human CR1 gene, although its near-ubiquitous tissue distribution more closely resembles that of MCP (a somewhat more distant homologue). Mice lacking Crry are unable to properly regulate C3. Crry-deficient mice spontaneously abort because of C3-dependent injury to the fetus. This presumably is the result of uncontrolled C3 deposition on the placenta. This observation in mice sheds light on the possibility that defective MCP (or perhaps CR1) plays a role in recurrent fetal loss manifest in patients with antiphospholipid syndrome.
The important role of the complement system in preventing disease is witnessed in cases in which components of the system are absent either because of random mutation in the human population or by design in gene-targeted “knock-out” mice. Some complement cascade deficiencies have been described. This section focuses on deficiencies in complement cascade activation that have profound biologic consequences followed by a discussion on deficiencies in complement regulatory proteins.
Homozygous deficiencies in C1q, the most common form of C1 deficiency in humans, is a powerful susceptibility factor for the development of systemic lupus erythematosus (SLE). Patients lacking C1q nearly always present with SLE. They have increased susceptibility to viral and bacterial infections, but it is not nearly as pronounced as in C3 deficiency (see later discussion). C1q knock-out mice show increased mortality, with up to 25% of mice having histologic evidence of glomerulonephritis.
C4 in humans is encoded by two separate loci giving rise to two distinct, albeit highly similar (>99% sequence identity), protein products, C4A and C4B. Complete C4 deficiency correlates with a 75% prevalence of SLE in humans. However, at least in certain human populations, the absence, or even haploinsufficiency, of C4A, but not C4B, is associated with elevated risk for development of autoimmune diseases such as SLE and other lupus-like autoimmune disease. The protective role of C4A with respect to SLE has recently been confirmed through genetic analyses of very large cohorts of European ancestry (6748 cases, 11,516 controls) and African American ancestry (1458 cases, 5908 controls) individuals and it was also confirmed to be independent of any linkage disequilibrium to neighboring HLA alleles. This study found that the protective effect of the expression of a C4A allele copy was equivalent to the expression of 2.3 C4B allele copies. The relative protective effect of C4A gene expression over C4B expression was found to be identical (i.e., 2.3-fold) in a European ancestry cohort of Sjögren syndrome individuals (673 cases, 1153 controls), this being a systemic autoimmune disease targeting mainly exocrine glands. The reason for the protective effect of C4A is not settled, but it is worth noting that the one indisputable functional difference between C4A and C4B is in the nature of the covalent bond formed upon target deposition. Whereas C4A transacylates onto amino group nucleophiles, forming amide bonds, C4B shows a strong preference for forming ester linkages to hydroxyl group nucleophiles. The nature of the covalent bond is in turn determined by the C-terminal-most residue of the so-called isotypic region sequence, a sequence located ~110 residues C-terminal to the thioester-forming residues of C4’s TED domain and is respectively 1101 PC PV LD 1106 in C4A and 1101 LS PV IH 1106 in C4B. The presence of the His1106 imidazole side chain in C4B isotype allows for a catalytic transacylation mechanism to hydroxyl group nucleophiles involving an initial formation of an intramolecular acylimidazole intermediate with the thioester carbonyl of nascently-activated C4b fragment. By contrast, the corresponding Asp1106 carboxylate side chain of C4A isotype cannot form such a covalent intermediate and thus only amino groups are nucleophilic enough to directly attack the exposed thioester carbonyl of nascently-activated C4A isotype. It therefore follows that the approximately threefold greater propensity of C4A, relative to C4B, to bind to amino group–rich C1-bearing IgG aggregates, as would be present in immune complexes in need of complement-dependent clearance, is one possible reason for the association of C4A null states with SLE.
As with C1q, mice deficient in C4 are also predisposed to SLE-like disease. Mice only express one complement pathway-functional isoform of C4, with the corresponding isotypic region sequence being a hybrid of the human isotypic sequences, namely PC PV IH . Nevertheless, the presence of a C-terminal His residue dictates that mouse C4 displays a human C4B-like covalent binding preference. Novel strains of mC4A and mC4B mice were constructed using CRISPR-cas 9 to replace the mouse isotypic region with the corresponding human C4A or C4B nucleotides, generating mC4A and mC4B “like” strains, respectively. Breeding the mice with a lupus mouse (564 Igi) showed that autoreactive B cells reactive to nuclear antigens in the mC4A mice were more efficiently eliminated than in the mC4B strain. Thus, the C4A isotypic form of mouse C4 was more protective in lupus mice. These results suggest one explanation for the finding that C4A is more protective than C4B in lupus patients may be due to its more efficient elimination of autoreactive B cells specific for nuclear antigens (see also “Autoimmunity and Complement Deficiencies” section below regarding potential mechanisms).
C2 deficiency appears to be relatively benign. Humans lacking C2 appear to have a normally functioning immune system, although autoimmune disorders and, less commonly, infections are observed with increased frequency.
In light of the central role of C3 in the complement cascade, it is not surprising that C3 deficiency has dire consequences for the host organism. Of all known cases of C3 deficiency among humans, no patients have been reported as disease free. Infectious complications, predominantly pyogenic in nature, occur frequently and recurrently. Streptococcus pneumoniae and N. meningitidis are the major pathogens reported. In addition, SLE, vasculitic syndromes, and glomerulonephritis have been documented in up to 21% of C3-deficient patients. Mice deficient in C3 show, similar to humans, greatly increased susceptibility to streptococcal infection and death. The 50% lethal dose (LD 50 ) is 50-fold less for C3-deficient mice than for C3-sufficient control subjects. This may be attributable in large part to the inability of mice deficient in C3 to effectively opsonize the bacteria. Moreover, the deficient mice have an impaired humoral response (see later section).
Deficiencies in C1-INH have been observed in the human population. C1-INH deficiency can be inherited as an autosomal dominant trait or can result from autoantibodies that recognize C1-INH, blocking its function. The inherited form of this deficiency is the cause of hereditary angioedema (HAE). Patients with HAE experience chronic spontaneous complement activation leading to the production of excess cleaved fragments of C4 and C2. Although the increased vascular permeability and edema symptoms that are the hallmarks of HAE were initially thought to be mediated by a C2a secondary degradation peptide referred to as C2 kinin, it is now recognized that the primary mediator of these effects is in fact not complement-derived, but rather is bradykinin, a product of the kallikrein-kinin contact system. Bradykinin is produced in an uncontrolled fashion in this disease as a result of the lack of inhibition of another plasma protease, kallikrein, which is activated by tissue damage and is also regulated by C1-INH. Although C1 is unregulated in patients with HAE, large-scale cleavage of C3 is prevented by complement regulatory mechanisms that proteolytically degrade C4b and that dissociate any CP C3 convertase that has formed in solution or on host cells. An increased risk of infection is therefore not associated with C1-INH deficiency. HAE flare-ups can be fully corrected by infusion of purified human serum-derived, or recombinant, C1-INH. A bradykinin-B2-receptor antagonist (icatibant), and inhibitors of kallikrein (ecallantide, lanadelumab, and berotralstat) represent additional treatment options. Plasmin is important in the generation of bradykinin, and this may explain the effect of tranexamic acid in preventing attacks. Attacks can be precipitated by female hormones and especially pregnancy, which can be managed by plasma derived C1-INH. Conversely, danazol is effective but limited to specific groups due to side effects, and its use may be obviated by newer approaches.
Acquired C1-INH deficiency may be associated with lymphoproliferative disorders and in most cases represents development of an autoantibody that binds to and neutralizes C1-INH. In two examined cases, autoantibodies abrogate C1-INH activity by preventing formation of the C1s–C1-INH complex. However, after the complex formed, the autoreactive antibodies had no effect on C1-INH function. Icatibant is an effective therapy for these patients, as is C1-INH concentrate, and treatment of the underlying lymphoma.
The role of FI in complement cascade regulation can be witnessed in patients with FI deficiency. In the presence of a cofactor protein, FI cleaves C3b, producing iC3b, the inactive form of C3b. iC3b is incapable of reacting with factor B to form the AP C3 convertase, thereby preventing uncontrolled AP activation. In the absence of FI, unrestrained C3 consumption occurs secondary to accelerated spontaneous AP turnover. Patients with FI deficiency have recurrent infections caused by pyogenic organisms, including meningococcal meningitis.
Likewise, mice deficient in the central protein FH exhibit unrestrained C3 activation via the AP, leading to near total depletion of serum C3. An important outcome of the failure to regulate C3 activation is glomerulonephritis. Strikingly, mice deficient in FH develop a disease resembling the human disorder membrane glomerulonephritis. The phenotype of the mice confirms the general notion that the AP is always “on” and that failure to regulate activated C3 results in consumption of circulating C3 and tissue injury.
Another example of the importance of FH regulation is the reports of genetic association between variant alleles of FH and the human diseases age-related macular degeneration (AMD) and aHUS. Whereas AMD is a fairly common condition—indeed, it is the leading cause of blindness in the Western world—it has been the elucidation of the etiology of the much rarer aHUS condition (two cases per million) that has led to a fuller appreciation of the diverse ways through which dysregulation of the AP of complement can give rise to severe pathology. Classically, HUS is a clinical triad of microangiopathic hemolytic anemia, thrombocytopenia, and acute renal failure. The disease is characterized by a precipitating injury of endothelial cells. In contrast to the fairly common classical form of HUS, which is diarrhea-associated and is usually caused by a Shiga toxin–secreting pathogen, the atypical form of HUS is nondiarrheal and, although it can be idiopathic, or result from autoantibodies to FH (see Chapter 132 ), it is more frequently caused by genetic predisposition. Even haploinsufficiency of variants of FH, MCP, and FI resulting from either loss of expression—or more commonly, loss of regulatory function—results in disease pathology. In addition, gain-of-function variants of factor B have been described that either form the AP C3 convertase more efficiently than wild-type factor B or are more resistant to decay-dissociation by FH or DAF. Finally, several C3 variants have been described in aHUS patients that are gain of function in the sense that as C3b there is decreased binding affinity for MCP and FH and thus AP C3 convertases formed with this C3b as subunit would have a prolonged lifetime relative to wild-type C3b.
Because FH mutations account for at least 30% of reported aHUS cases and approximately 70% of these are caused by missense mutations in SCR domains 19 and 20, the molecular basis for this disease association has been intensively investigated, and the findings of these studies are best understood in the context of a structure-based domain model of FH bound to C3b on a nonactivator (i.e., host) surface ( Fig. 23.3 ). FH consists of 20 SCR domains, where some domains in the middle of the molecule appear to play mainly a structural role, likely allowing the molecule to bend back on itself, but domain clusters near the ends mediate specific functions. SCRs 1 to 4 bind to C3b and mediate both decay-accelerating and FI-cofactor functionalities. Indeed, FH (SCR1 to 4) on its own is able to regulate a fluid-phase AP C3 convertase, but it cannot do so for surface-bound AP C3 convertases. For regulation of the latter, there are three additional binding interactions that become relevant. Two of these are located within SCRs 19 to 20, specifically, a site localized mainly to SCR19 binds to the C3d/TED domain of the surface-bound C3b molecule, and a site within SCR20 binds to surface-associated polyanions such as sulfated glycosaminoglycans or sialic acid arrays. The aHUS-associated missense mutations found within SCRs 19 to 20 affect one or other of these two binding functions and lead to dysregulation of the AP C3 convertase at the surface of host tissue. In particular, complement-mediated damage to the kidney basement membrane is often a hallmark of aHUS. As a tissue devoid of the membrane-associated complement regulators MCP, DAF, or CR1, but rich in sulfated glycosaminoglycans, the functionality of the soluble AP regulator FH becomes even more crucial for host protection and likely explains the high incidence of missense mutations within SCRs 19 to 20 in aHUS patients. Interestingly, missense mutations in FH SCRs 19 to 20 do not result in systemic C3 consumption, as would be the case for complete deficiencies of FH. This is because SCRs 1 to 4 of the mutant molecule are still capable of regulating spontaneously formed AP C3 convertases in the fluid phase.
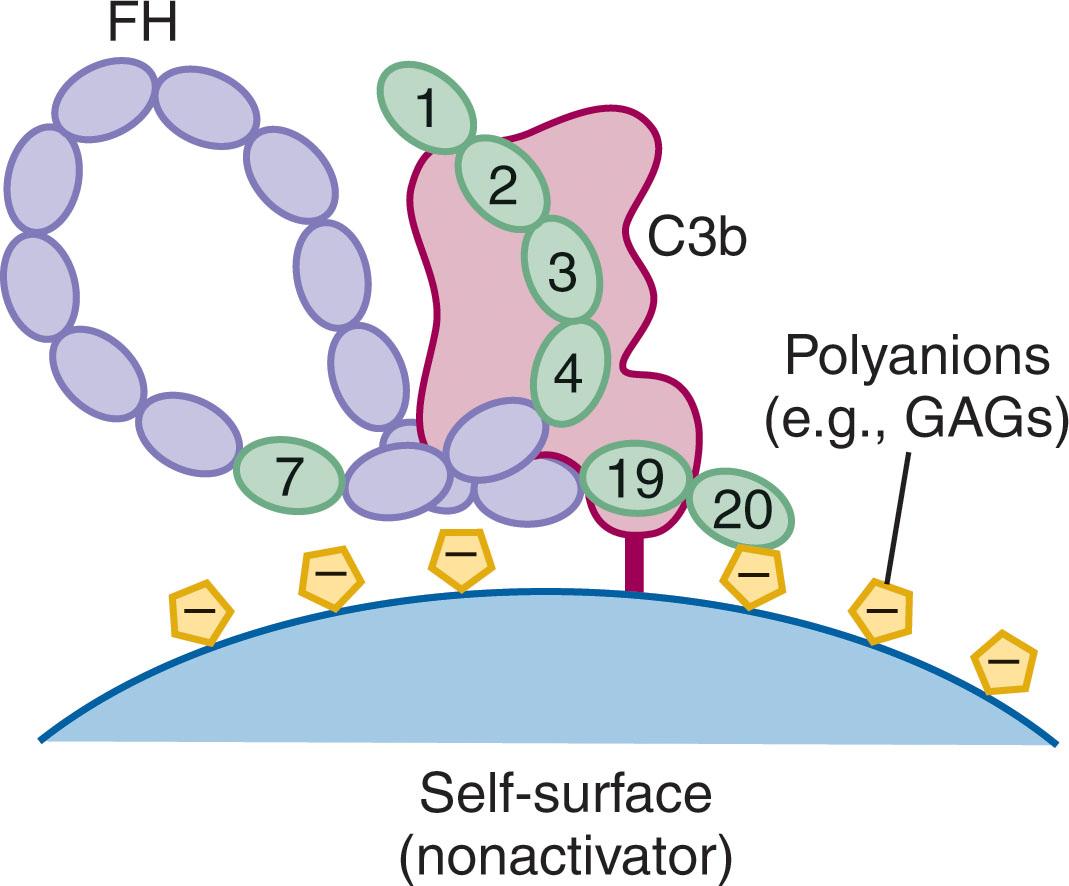
In addition to the polyanion binding site in FH SCR 20, there is also one in SCR 7. This SCR is the site of an amino acid polymorphism in FH (tyrosine to histidine at residue 402, Y402H) that is a significant risk factor for AMD, but interestingly does not correlate with disease susceptibility for aHUS. Heterozygotes and homozygotes for H402 are respectively 2.7-fold and 7.4-fold more at risk for AMD than homozygous Y402 individuals, and this single polymorphism can account for up to 50% of the risk of AMD. Two significant functional differences have been observed for the Y402 and H402 variants of FH. First, the affinity and specificity for a spectrum of sulfated glycosaminoglycans is different for the two variants of FH. Secondly, the affinity of the H402 variant of FH for C-reactive protein (CRP), an acute-phase protein that binds to damaged tissue, is substantially lower than that of the Y402 variant. It is notable that Bruch’s membrane of the macula, similar to the kidney basement membrane, is devoid of membrane-associated complement regulators and so is expected to be highly dependent on FH for local AP regulation. Indeed, the spectrum of sulfated glycosaminoglycans found on the Bruch membrane appear to be more dependent on the polyanionic binding site in SCR 7 for the interaction than that in SCRs 19 to 20 because even with non-AMD eye tissue, there is preferential binding of the Y402 variant to the Bruch membrane.
SCR 7 is also present in a splice variant of the FH gene, FHL-1 (factor H-like 1), which encodes a truncated protein consisting of SCRs 1 to 7, followed by a unique tetrapeptide C-terminus. The question was then asked whether it is FHL-1, or the parent FH, which was responsible for the association of the Y402H polymorphism with AMD. It was found that not only is FHL-1 more highly expressed by retinal pigment epithelium cells than is FH, but owing to its smaller size, only FHL-1, and not intact FH, can diffuse into and across the Bruch membrane of the human macula. Also, the 402H variant of FHL-1 was confirmed to show lower binding to two different tissue sources of heparan sulfate than did the 402Y variant. Finally, the binding of FHL-1 to Bruch’s membrane was shown to be heparan sulfate-dependent. It was therefore suggested that the Y402H polymorphism association with AMD is predominantly driven by the locally-produced FHL-1 splice variant, and not by full-length FH. The lower binding affinity of the H402 FHL-1 variant, coupled with age-related changes in the biosynthesized spectrum of sulfated glycosaminoglycans on Bruch’s membrane, could account for the dysregulation of the AP in the macula with the ensuing inflammation of the macula seen in AMD patients. There may also be a contribution from the differential binding of the FH/FHL-1 Y402H variants to CRP present on the particulate debris (drusen) residing in between the retinal pigment epithelium and the Bruch membrane.
Before leaving the topic of AP dysregulation in the context of complement-mediated pathologies, it is necessary to discuss the emerging role of the factor H-related proteins, FHR-1, -2, -3, -4A, -4B, and -5. These proteins, which generally consist of 4 to 5 SCR domains each (FHR-4B is the exception having 9 SCRs), are encoded by the CFHR1-5 genes, which themselves originated from the CFH parent gene by tandem gene duplication events and are arrayed just downstream of the CFH gene. The sequence similarities of the SCR domains within the FHRs are highest to the C-terminal domains 19 to 20 of FH and to domains 6/7, these being precisely the SCR domains of FH which possess its surface recognition sites. Indeed reflecting these sequence similarities, the FHRs all bind to C3b/iC3b/C3d and most bind to the model sulfated glycosaminoglycan heparin. Importantly, none of the FHRs possess domains corresponding to FH SCRs 1 to 4, which mediate the decay acceleration and FI cofactor activities of FH. Thus the emerging picture (as reviewed in Jozsi et al., 2015) is that the FHRs do not act as supplementary regulatory molecules of the AP, but rather they can act as modulators of FH-mediated regulation by competing with it for its binding sites on cell surface structures and for the C3d binding site located in FH SCRs 19 to 20. When C3b is adventitiously deposited on normal host tissue, the competition of the FHRs with FH is thought to be quite limited for a number of reasons, including their much lower circulating concentrations relative to FH, the total absence of the largest contact site for C3b, i.e., that mediated by SCRs 1 to 4, and a much lower affinity than FH for normal host surface structures, such as sialic acid arrays. However, the nature of the tandem array of the CFHR genes in proximity to the CFH gene sometimes results in the formation of hybrid molecules through homologous recombination events resulting in either loss of function in FH or gain of function in the CFRs, both of which become risk factors for complement-mediated pathologies. For example, a hybrid molecule in which SCR 20 of FH is replaced by its homologue in FHR-1 (namely SCR5) results in the loss of complement regulation on human host cell surfaces and is a risk factor for aHUS. Conversely, the substitution of FHR-1 SCR 5 by FH SCR 20 confers upon this hybrid the host surface binding properties of FH, allowing it to compete with FH and thus being a risk factor for aHUS. Another type of gene rearrangement involves the complete deletion of the adjacent genes encoding FHR-1 1nd FHR-3. This is actually a fairly common polymorphism and has been found to be protective with respect to the development of AMD, presumably because these FHRs will not compete with FH and FHL-1 to regulate the AP at the Bruch membrane. In contrast to the AMD situation, the deletion of the CFHR1 and CFHR3 genes is a risk factor for SLE, possibly indicating that overregulation of complement leads to a decrease in the opsonophagocytic removal of autoantigens present in the blebs of apoptotic cells. A final example of a disease-associated gain of function mutation in an FHR protein involves the formation of higher order oligomers of FHR-1, FHR-2, and FHR-5, which would increase their avidity for ligand and thus be able to compete with FH. These three FHR proteins share near identical SCR 1 to 2 domains and these normally act as head-to-tail dimerization domains, forming both homo- and heterodimers. The gain of function mutation involves the duplication of the exons encoding SCRs 1 to 2 in FHR-1, -2, or -3, which in turn will mediate the formation of higher homo- and hetero-oligomers. These higher oligomers are in turn risk factors for C3 glomerulonephritis (C3G), which involves massive C3 deposition on the glomerular basement membrane. The MAC is one mechanism used by the host to rid itself of certain microorganisms. Host cells are protected from MAC-mediated lysis by CD59 (protectin), a membrane-bound protein. CD59 performs its function by inhibiting the binding of C9 to the C5b–C6–C7–C8–C9 complex. CD59 and DAF (CD55) are linked to the cell surface by a glycosylphosphatidylinositol glycolipid (GPI) anchor. PIG-A, the enzyme responsible for the first step in the synthesis of GPI, is encoded on the X chromosome. An acquired somatic mutation of this gene in hematopoietic stem cells leads to a failure to synthesize the GPI anchor and with it an inability to express CD59 or CD55 on the derived blood cell surfaces. This results in the complement mediated hemolysis and thrombosis seen in PNH, as discussed below and in Chapter 32 . Rare individuals with biallelic mutations of CD59 can display a similar phenotype, but can also develop neuropathy. In contrast, biallelic inactivation of CD55 results in the CHAPLE syndrome (hyperactivation of complement, angiopathic thrombosis, and protein-losing enteropathy) rather than hemolysis, as well as loss of the Cromer blood group antigens. In contrast to PNH, where the disorder is limited to blood cells, germline mutations of PIG-A, or other genes involved in the biosynthesis of GPI, results in a severe neurologic phenotype.
There exists a strong correlative relationship between the lack of certain components of the complement system (i.e., C1 and C4) and autoimmune disease, particularly SLE. Two general non-mutually exclusive hypotheses have been put forward to explain the increased incidence of SLE among complement deficient individuals: the clearance hypothesis and the tolerance hypothesis. The clearance hypothesis is based on the known role of the CP of complement in binding to foreign antigens and transporting them to the liver and spleen for degradation and removal from the circulation. Thus defects in clearance of apoptotic cells or debris would lead to inappropriate accumulation of self-antigen and over-stimulation of self-reactive lymphocytes.
The tolerance model proposes that innate immunity protects against SLE by delivering lupus autoantigens to sites where immature B lymphocytes are tolerized, thereby facilitating their negative selection. SLE is characterized by high-affinity antibodies specific for autoantigens such as double-stranded DNA (dsDNA), ribonuclear proteins, and histones. Validation of the model comes in part from studies with human B cells demonstrating that self-reactive B cells are eliminated or anergized at two major checkpoints, bone marrow (BM) and spleen. Thus counterselection of potentially pathogenic B cells is an active process and most likely involves components of innate immunity.
Recent studies in a lupus mouse model (strain 564 Igi) in which the B cells express an Ig receptor specific for the lupus antigens such as Ro-60 and SSB/LA suggests a third possible explanation for why C4 is critical for protection against SLE. Accordingly, this hypothesis suggests that C4-dependent defects in clearance of immune complexes leads to a loss of tolerance of certain autoreactive B cells. Thus failure to clear immune complexes composed of lupus antigens or apoptotic cells that bear DNA or ribonucleoprotein (RNP) ligands that trigger Toll-like receptors (TLRs) TLR 7 and TLR 9 may induce myeloid cells to release excess type I interferon (IFN-α). In a feed-forward loop, IFN-α release induces increased sensitivity of TLR 7 and 9 receptors, in particular on B cells, such that the combined effects of engagement of DNA or RNP self-antigen by the B-cell receptor (BCR) and increased TLR 7 and 9 signaling leads to escape of B-cell tolerance.
The preceding parts of the complement section familiarized the reader with the general aspects of the complement system. Much of the remainder of this section focuses on the role of the complement system in the initiation and propagation of the adaptive immune response, beginning with a description of natural antibody. Two final complement biology sections of this chapter deal with this system’s surprisingly detrimental role in immune-mediated control of cancer and with the interplay between the complement and coagulation systems of blood.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here